肠道健康是动物健康的基础,它决定了动物的免疫系统、生长性能和整体健康,在动物生长发育的各个环节都具有重要作用。益生菌是用于调控动物肠道健康的一种重要途径。但益生菌作为饲料添加剂有其局限性,其益生功能会受环境条件(温度、pH、含氧量、水分等)影响而降低,部分益生菌株存在毒力因子会导致局部炎症反应,益生菌株中的抗生素耐药基因可能增加感染风险[1-2]。相比之下,后生素被认为是一种比益生菌更稳定、更安全的活性物质。后生素指的是对动物健康有益的无生命的微生物细胞和/或其成分的制剂[3]。该后生素的概念由国际益生菌和益生元科学协会(International Scientific Association of Probiotics and Prebiotics,ISAPP)在2021年正式提出。尽管后生素的概念很新,但在动物生产中对后生素的利用远远早于其定义的提出,使用最多的后生素当属乳杆菌源后生素与酵母源后生素。目前后生素在动物应用中以代谢产物为主,对无生命的微生物细胞及菌体成分并没有过多的研究与应用。本文将以无生命的微生物细胞和菌体成分的后生素为主,介绍其主要的来源菌株、作用机制及其在动物中的应用,以期为后生素后续的分类、开发和利用提供参考。
1 后生素概述动物肠道微生物与宿主之间进行着动态且复杂的互作,微生物影响宿主的多种生理功能,宿主通过多种方式调节微生物的组成和功能,微生物与宿主表型和代谢有着密不可分的关系。近年来研究表明,微生物对肠道健康的作用不一定与活菌有关,其菌体成分或代谢产物可能是促进肠道健康的关键[4]。例如,肠道微生物会产生大量生物活性分子,可供宿主与其他微生物利用[5],同时发现一些无生命的菌体及其组成成分(菌毛、磷壁酸等)也对宿主有益处[6-7]。2012年西班牙正式提出后生素“Postbiotics”一词[8],2021年5月ISAPP首次明确后生素的定义,即对宿主健康有益的无生命微生物和/或其成分的制剂[3]。后生素包括无生命的微生物细胞,菌体成分以及代谢产物中的一种或多种。其中菌体成分主要包括细胞壁、细胞膜、脂磷壁酸(lipoteichoic acid,LTA)、脂多糖(lipopolysaccharide,LPS)、细胞外囊泡、菌毛和细胞表层蛋白等;代谢产物主要包括短链脂肪酸(short chain fatty acids,SCFAs)、酶、分泌蛋白、细菌素和胆汁酸等[3](图 1)。有效的后生素须含有无生命的微生物细胞或菌体成分,无论是否含有代谢产物都能够观察到其对宿主的健康益处。后生素的生产方式主要以传统热加工(巴氏杀菌、高温灭菌)为主,但磁场加热、超声波、高压等技术也有可能被应用于生产后生素。
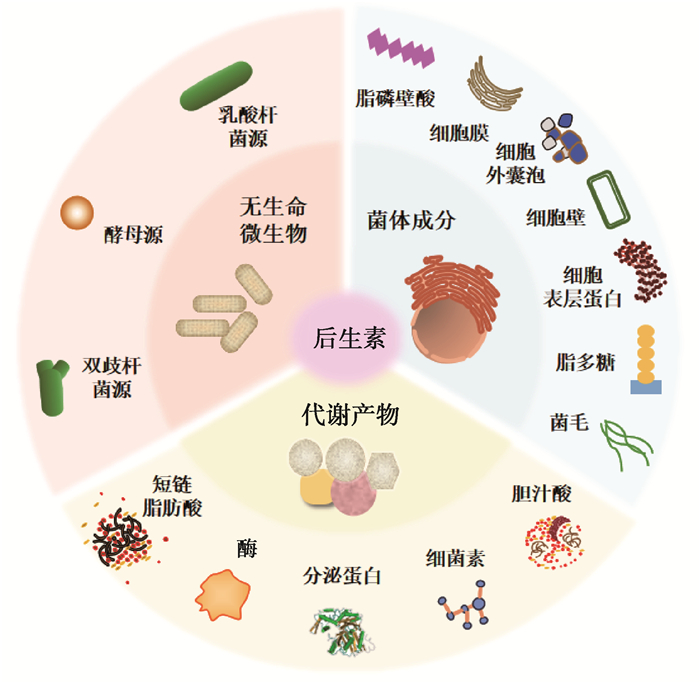
|
图 1 后生素的组成成分 Fig. 1 Components of postbiotics |
与益生菌(probiotics)、益生元(prebiotics)和合生元(synbiotics)不同,后生素不需要定植,能够通过肠道与动物机体直接相互作用。此外,后生素稳定性好,有利于其活性成分被靶向运输至所需肠道部位[9]。目前在人的健康中,尤其是在婴儿和早产新生儿中,后生素产品如热灭活植物乳杆菌、鼠李糖乳杆菌裂解物作为一种新型生物制剂已进入临床使用阶段[10-11]。在动物营养和健康领域,后生素从平衡肠道菌群、提高饲料消化率到增强免疫、促进动物机体生长等方面都具有重要作用和应用潜力。
2 后生素的作用机制后生素能够对肠道微生物产生积极有效的影响,对宿主的健康具有重要的作用。后生素的成分包括许多生物活性分子,其通过许多不同的机制来介导宿主的健康。后生素的作用机制主要分为以下5个方面:1)调节肠道菌群;2)增强肠道屏障功能;3)调节免疫反应;4)调节机体代谢;5)神经传导作用。后生素的作用机制及其利用的效应分子示例见图 2。
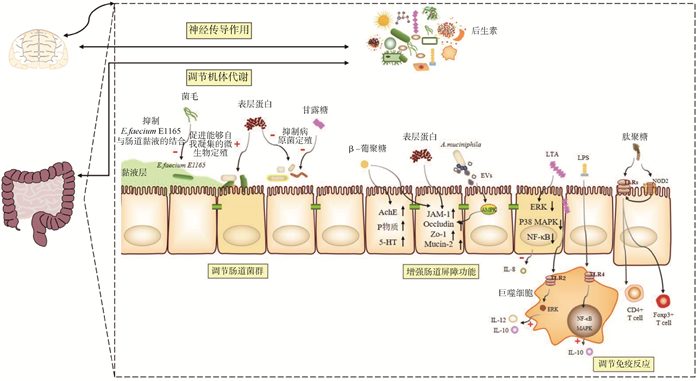
|
E.faecium E1165. 粪肠球菌E1165;A.muciniphila. 嗜黏蛋白阿克曼菌; EVs. 细胞外囊泡; LTA. 脂磷壁酸; LPS. 脂多糖; TLRs. Toll样受体; NOD2. 核苷酸寡聚化结构域2;AchE. 乙酰内胆碱酯酶; 5-HT. 5-羟色胺; JAM-1. 连接黏附分子; Occludin. 闭合蛋白; Zo-1. 紧密连接蛋白1;Mucin-2. 黏蛋白-2;ERK. 细胞外信息调节激酶; P38 MAPK. 丝裂原活化蛋白激酶P38; NF-κB. 核因子κB; TLR2. Toll样受体2;TLR4. Toll样受体4;IL-8.白细胞介素8;IL-10. 白细胞介素10;IL-12. 白细胞介素12;CD4+ T cell. CD4+调节性T细胞; Foxp3+ T cell. Foxp3+调节性T细胞 E.faecium E1165. Enterococcus faecium E11665;A.muciniphila. Akkermansia mucinphila; EVs. Extracellular vesicles; LTA. Lipoteichoic acid; LPS. Lipopolysaccharide; TLRs. Toll-like receptors; NOD2. Nucleotide-binding oligomerization domain 2;AchE. Acetylcholine esterase; 5-HT. 5-hydroxy tryptamine; JAM-1. Junctional adhesion molecule 1; Zo-1. Zonula Occludens Protein 1; ERK. Extracellular signal-regulated kinase; P38 MAPK. Mitogen-activated protein kinases P38; NF-κB. Nuclear factor kappa-B; TLR2. Toll-like receptors 2;TLR4. Toll-like receptors 4;IL-8. Interleukin-8;IL-10. Interleukin-10;IL-12. Interleukin-12;CD4+ T cell. CD4+ Regulatory T cell; Foxp3+ T cell. Foxp3+ Regulatory T cell 图 2 后生素的作用机制及其利用的效应分子示例 Fig. 2 Postulated mechanisms of postbiotics and example effector molecules utilized by them |
后生素能够通过直接作用(通过菌体成分如表层蛋白、菌毛和细胞壁等)或间接作用(通过群体感应效应)来调节肠道菌群。表层蛋白是微生物细胞壁的最外层组成部分,也是分子质量最小的一类蛋白质,仅有25~71 ku[12]。在表层蛋白的氨基酸中疏水性氨基酸和碱性氨基酸占绝大部分,疏水性氨基酸通过促进能够自我凝集的微生物细胞分布在肠上皮细胞,从而抑制致病菌的定殖[13]。另外,表层蛋白作为一种重要的黏附素,其可以通过与致病菌竞争黏附位点或掩盖干扰其识别黏附位点等方式拮抗病原菌对HT-29和Caco-2细胞的黏附[14-15]。菌毛是类似伞状菌的原纤维蛋白质表面附属物,具有良好的黏附能力,能使微生物在宿主肠道中长时间定殖并稳定存在。鼠李糖乳杆菌GG(Lactobacillus rhamnosus GG,LGG)存在SpaCBA-srtC1基因岛,能够编码SpaC、SpaB和SpaA菌毛蛋白(图 3)[16],其中,LGG的SpaC菌毛蛋白(GG-SpaC)是其高黏附表型的主要参与者[17]。GG-SpaC与粪肠球菌E1165(Enterococcus faecium E11665,E.faecium E1165)的PilB菌毛蛋白具有高度的序列同源性[18-19]。GG-SpaC产生的抗体能够与E.faecium E1165的菌毛特异性相互作用,干扰E.faecium E1165与黏液的结合能力[20]。酵母细胞壁中的β-葡聚糖能够破坏微生物的细胞膜和细胞壁,增加微生物细胞膜的通透性,进入细菌内干扰微生物代谢[21]。酵母细胞壁成分甘露糖具有类似于肠道病原菌受体的结构,与病原菌表面的甘露糖特异性凝集素结合抑制病原菌在肠绒毛表面黏附,使病原菌(大肠杆菌、沙门菌)无法在肠壁上生长[22-23]。另外,有研究表明甘露糖提取物能够促进乳酸菌生长[24]。说明酵母细胞壁多糖能够抑制肠道内病原菌增殖,增加有益菌丰度,维护肠道内菌群平衡。
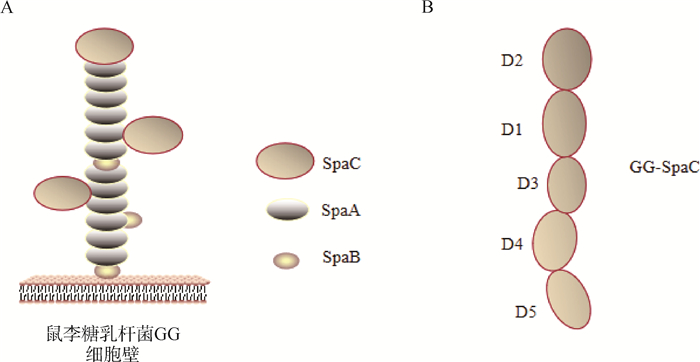
|
A. 鼠李糖乳杆菌GG的SpaCBA菌毛是由3种不同的菌毛蛋白亚基组成异聚物,每种蛋白亚基都有自己的位置和功能:主干SpaA用于长度,基础SpaB用于锚定,尖端SpaC用于黏附。B. GG-SpaC由五个不同的结构域组成,即D1(CnaA)、D2(vWFA)、D3(CnaB)、D4(CnaB)、D5(CnaB) A. SpaCBA pilus of Lactobacillus rhamnosus GG are heteropolymers of three different pilin-protein subunits, each with its own location and function in the pilus: backbone SpaA for length, basal SpaB for anchoring, and tip SpaC for adhesion. B. GG-SpaC consists of five domains with various folds, i.e., D1 (CnaA), D2 (vWFA), D3 (CnaB), D4 (CnaB), and D5 (CnaB) 图 3 鼠李糖乳杆菌GG的SpaCBA菌毛以及GG-SpaC的结构模型图[16] Fig. 3 Structural model for SpaCBA pilus of Lactobacillus rhamnosus GG and GG-SpaC[16] |
肠道屏障功能是维持宿主及微生物之间平衡的关键。后生素中的多种生物活性分子(膜蛋白、多聚糖、LTA、分泌蛋白等)都能够介导高度复杂的肠道屏障系统[25-26]。微整合膜蛋白(micro integral membrane protein,MIMP)是植物乳杆菌表层蛋白中最小的结构域,其能够诱导丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)、蛋白激酶B(protein kinase B,AKT)等信号通路,上调紧密连接蛋白(tight junction protein,TJP),如连接黏附分子(junctional adhesion molecule 1,JAM-1)、闭合蛋白(Occludin)、紧密连接蛋白1(ZO-1)等的表达,降低肠道通透性[27]。酵母细胞壁中的β-葡聚糖通过上调肠道中乙酰内胆碱酯酶(acetylcholine esterase,AchE)、P物质和5-羟色胺(5-HT)等神经递质的表达及TJP如ZO-1和黏蛋白-2(Mucin-2)的表达,维持肠道屏障完整性[20]。罗伊氏黏液乳杆菌(Lactobacillus reuteri)的LTA中的D-丙氨酸(D-Ala)对小鼠胃肠生物膜的形成能力具有调节作用[28]。细胞外囊泡(extracellular vesicles,EVs)是由肠道微生物分泌的一种包裹核酸、蛋白、脂类等分子的磷脂双分子层颗粒。EVs可以直接与肠道上皮细胞相互作用,启动多种信号通路。其中嗜黏蛋白阿克曼菌(Akkermansia mucinphila,A.muciniphila)来源的EVs可以激活腺苷酸活化蛋白激酶(AMPK),诱导AMPK磷酸化,启动细胞-细胞连接处的紧密连接组装,对Occludin进行调节,降低肠道通透性,增加肠道屏障功能[29]。
2.3 调节免疫反应无生命的微生物细胞及菌体成分能够与免疫细胞的特定模式识别受体,如Toll样受体(Toll-like recepter,TLRs)、核苷酸结合寡聚化结构域(nucleotide-binding oligomerization domain,NOD)、C型凝集素等相互作用,从而调节机体的免疫活动[30]。表层蛋白具备调节宿主免疫系统的功能,能够预防血清型鼠伤寒沙门杆菌诱导的Caco-2细胞凋亡[31]。特异性抗原DC-SIGN能被表层蛋白识别并结合,抑制病毒造成的细胞损伤,调节免疫反应[32]。LTA是微生物细胞膜的主要组成部分,由16~40个磷酸甘油残基通过共价键的形式锚定在细胞膜上所形成[33],是发挥其免疫功能的重要表面分子之一。植物乳杆菌(Lactobacillus plantarum,L.plantarum)的LTA的D-Ala支链以及脂质部分能够降低细胞外信息调节激酶(extracellular signal-regulated kinase,ERK)和核因子κB(NF-κB)蛋白的活性,并且抑制丝裂原活化蛋白激酶P38(P38 MAPK)磷酸化,从而抑制猪小肠上皮细胞分泌白细胞介素-8(IL-8)[34]。L.plantarum的LTA也能够激活TLR2依赖的ERK通路,进而激活巨噬细胞以维持白介素10(IL-10)/白介素12(IL-12)的平衡[35]。LPS是革兰阴性菌外壁层中的成分,其自然免疫力对巨噬细胞抗结核自噬和细胞因子的产生有促进作用。A.muciniphila的LPS是激活巨噬细胞TLR4受体的生物活性分子,LPS与TLR4结合激活TLR4使之二聚体化,当信号传入胞内,能够激活NF-κB和MAPK两条通路,促进巨噬细胞分泌IL-10[36-37]。肽聚糖存在于微生物的细胞壁中,由乙酰氨基葡萄糖、乙酰胞壁酸与氨基酸短肽聚合而成,可诱导肠黏膜的免疫应答[38]。如,唾液乳杆菌33(Lactobacillus salivarius 33)的肽聚糖能够激活核苷酸寡聚化结构域2(nucleotide-binding oligomerization domain 2,NOD2)受体后,负向调节TLRs,促进CD4+、Foxp3+调节性T细胞分泌抗炎因子,或直接与TLRs结合促进机体免疫细胞分泌从而诱导免疫反应的发生[39-40]。
2.4 调节机体代谢微生物的代谢产物可直接介导机体代谢反应。在诸多的代谢产物中,SCFAs如丁酸、丙酸、乙酸等对机体代谢功能方面的研究相对成熟[41-42]。丁酸能够提高棕色脂肪细胞中线粒体解偶联蛋白的活性以及上调PGC-1α的表达量来加强脂肪酸的氧化代谢[43]。丙酸可通过以下两种途径对机体代谢进行调控:1)激活外周神经系统游离脂肪酸受体(free fatty acid receptor,FFARs),提高机体胰岛素抗性[44],抑制脂肪代谢;2)调控肠细胞中短肽(peptide YY,PYY)和胰高血糖素样肽(glucagon-like peptide-1,GLP-1)的分泌,参加机体能量代谢。另外,胆盐水解酶(bile salt hydrolase,BSH)作为微生物的一种代谢产物,当其水解结合胆盐后能够产生游离胆汁酸,从而能够对菌群代谢与机体代谢进行调节[45]。研究表明,BSH与游离胆汁酸还与多种宿主受体相互作用,对宿主的一系列代谢过程如脂质、能量、葡萄糖代谢等产生影响[46-47]。例如,游离胆汁酸作为信号传导分子,可通过法尼醇X受体(Farnesoid X receptor,FXR)和G蛋白偶联膜受体5(G-protein-coupled bile acid receptor,TGR5)介导信号,促进肠内分泌细胞生成GLP-1,调节机体的糖脂代谢[48-50]。微生物产生的氨基酸衍生物能够与肠道菌群相互作用,从而调节机体的蛋白质代谢。生物化学上,蛋白质首先被微生物水解为肽和氨基酸[51],然后肠道菌群可进一步利用释放的肽和氨基酸合成细胞成分进而参与机体代谢调节,也可通过不同的途径被分解代谢[52]。
2.5 神经传导作用微生物菌体成分如EVs等在调控血清素水平及相关基因的表达方面发挥重要作用。普氏栖粪杆菌(Faecalibacterium prausnitzii)和A.muciniphila释放的EVs能够上调结肠Tph1基因的表达,增加结肠中血清素的水平,进一步影响胃肠道运动[53]。除菌体成分外,肠道微生物-肠-脑轴迷走神经在中枢神经系统与肠道神经系统及肠道内分泌细胞之间双向传导信息[54],其中肠道微生物的代谢产物在整个系统中起重要作用。研究表明,微生物代谢产物如SCFAs、色胺、胆汁酸等生物活性分子能够通过影响脑肠肽和神经递质(如血清素)合成,进一步调节大脑功能和行为。如SCFAs能够改变胃饥饿素水平,胃饥饿素与生长激素促分泌受体(growth hormone secretagogue receptor,GHS-R)相结合能够刺激生长激素(growth hormone,GH)的产生[55-56]。另外,丁酸、乙酸能够下调血清素转运体Slc6a4表达,使肠染色质细胞产生血清素,当其在黏膜肠嗜铬细胞和肌间神经丛被激活时,能够刺激肠肌丛调节肠道蠕动[57-58]。
3 后生素在动物中的应用与益生菌相比,后生素在配方、安全性和监管方面具有相当大的优势,其在动物养殖中的研究和应用越来越深入。国内外相继开发应用的后生素包括乳杆菌源后生素、酵母源后生素等。这些后生素具有改善肠道内环境,优化新陈代谢及提高动物机体免疫机能、抗氧化等应用功效。
3.1 乳杆菌源后生素在动物中的应用乳杆菌源后生素以无生命乳杆菌(热灭活乳杆菌、高温高压灭活乳杆菌、紫外线灭活乳杆菌等)及其菌体成分(胞外多糖、LTA、肽聚糖等)为主。其具有提高动物机体免疫机能、改善生产性能等应用功效和生物学功能。研究表明,热灭活乳杆菌具有调控动物机体免疫的作用,其作为一种新型天然的生物添加剂在提高免疫力中越来越受关注。如,热灭活植物乳杆菌L-137(Lactobacillus plantarum L-137)可以刺激非特异性免疫产生I型干扰素(IFN),从而增强宿主仔猪对病毒感染的防御能力[59]。Kang等[60]研究发现,在仔猪日粮中添加热灭活的鼠李糖乳酸菌(Lactobacillus rhamnosus)能够降低血清中肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、转化生长因子β(transforming growth factor,TGF-β)和皮质醇(Cortisol,Cor)的浓度,提高仔猪免疫力,降低腹泻率。另外,热灭活乳杆菌能够促进动物机体的生长发育。仔猪口服热灭活的粪肠球菌NHRD IHARA(Enterococcus faecalis NHRD IHARA)能够使小肠绒毛高度升高,从而能够更好地吸收营养物质,改善仔猪生长性[61]。Hyabg等[62]为比较无生命微生物与活性微生物对动物机体的益生作用,通过高压均质的方法来灭活嗜酸乳杆菌(Lactobacillus acidophilus,L.acidophilus),用灭活处理后的菌体与活菌剂分别饲喂肉鸡,结果发现两个添加组在相同浓度下肉鸡增重无差异,且体重随添加量的增加而升高,说明高压均质灭活处理的嗜酸乳杆菌对动物机体的益生功能不亚于嗜酸乳杆菌活菌剂。目前乳杆菌源后生素在我国畜牧行业中的应用仍然较少,尤其是在反刍动物领域,且尚不明确其在日粮中的适宜添加量。
3.2 酵母源后生素在动物中的应用在动物养殖中,酵母细胞壁长期以来一直被用作生物活性化合物的来源,其中酿酒酵母细胞壁已纳入《饲料原料目录》[63]。酵母细胞壁是指将酵母培养增殖后,收集菌种细胞并破碎,再离心分离后提取得到的细胞壁制剂[64]。如今看来,酵母细胞壁完全契合后生素的概念。酵母细胞壁作为一类新型无毒、无污染、无有害残留的绿色生物饲料添加剂,具有抗真菌感染、提供营养物质、促进动物生长、提高机体免疫力等多种作用。酵母细胞壁表面有复杂的结合位点,能够识别并结合真菌毒素(黄曲霉素)[65]。因此,在生产中,常使用酵母细胞壁以减少毒素转移到牛奶中[66]。另外,酵母细胞壁富含β-葡聚糖和甘露糖等多种生物活性物质,可调节和稳定瘤胃内环境,提高生长和生产性能。研究发现,酵母细胞壁能够通过降低瘤胃中LPS浓度[67]以及改善瘤胃菌群结构,提高纤维消化率来改善肉牛的生长性能[68]。Salinas-Chavira等[69]研究表明,添加酵母细胞壁能够提高犊牛的干物质采食量,从而提高平均日增重,改善胴体重量。另外,酵母细胞壁中的β-葡聚糖能激发机体的非特异性和特异性免疫反应,以增强机体抗病力。据报道,在犊牛中添加75 mg·kg-1浓度的β-葡聚糖,能够通过提高免疫球蛋白浓度和刺激碱性磷酸酶来提高机体免疫力[70]。酵母细胞壁在单胃动物中也有所应用,日粮中添加酵母细胞壁能够提高肉鸡饲料转化率。此外,酵母细胞壁能够改善肠道环境,增加肠道水平的糖缀合物分泌,为鸡提供抵抗病原体的能力[71]。在仔猪日粮中添加0.05%的酵母细胞壁可强化仔猪的非特异性免疫,降低TNF-α以及白细胞介素-1β(IL-1β)的水平[72]。
3.3 其他微生物源后生素在动物中的应用除乳杆菌源后生素和酵母源后生素,双歧杆菌、肠球菌、草分枝杆菌以及丁酸梭菌等微生物源后生素在动物中也有所研究与应用。例如,热灭活短双歧杆菌(Bifidobacterium breve)具有调节免疫功能的潜力,能够抑制脾细胞中促炎细胞因子的产生[73]。Sukegawa等[61]研究发现,日粮中添加热灭活粪肠球菌(Enterococcus faecalis)能够通过诱导血清免疫球蛋白A(IgA)的产生、激活肠黏膜以及抑制致病大肠杆菌等病原体在仔猪体内的定殖来促进仔猪的生长健康,并与活菌株具有相同的效果。Zhong等[74]研究证明,热灭活草分枝杆菌(Mycobacterium Phlei)能够通过诱导保护性免疫反应来预防早期断奶仔猪的肠道健康问题。在凡纳滨对虾日粮中添加1×1011 cfu·kg-1热灭活丁酸梭菌CBG01(Clostridium butyricum CBG01)或1×1011 cfu·kg-1超声灭活丁酸梭菌CBG01显著提高血清中过氧化物酶(Perxidase,POD)、超氧化物歧化酶(Superoxide dismutase,SOD)以及溶菌酶的含量,提高机体的非特异性免疫能力,且超声灭活丁酸梭菌的效果优于热灭活丁酸梭菌[75]。
4 小结综上所述,后生素在过去几年取得了快速的发展,后生素介导动物健康效应,主要通过无生命微生物、菌体成分或代谢产物直接或间接地调节肠道菌群、增强肠道屏障功能、调节免疫反应、调节机体代谢和神经传导五种主要作用机制来诱导有益的效果。尽管后生素对动物的益生功能众多,但由于其属于功能性组分的混合物,多种活性成分与动物机体有机协调,进而作用到机体的各个维度,所以目前对其研究仍然处于起步阶段,有关其菌体成分/代谢产物如何与机体细胞(肠道细胞)相互作用以及如何影响下游信号通路,是一个具有前景的研究方向;菌体成分与代谢产物的构效关系,即通过生化策略调整后生素组分结构组成使其功能最优化,对应用于动物也具有重要意义;不同后生素之间存在非常大的差异,不同的菌株以及相同菌株不同发酵工艺参数(pH、温度、发酵底物、发酵时间)、不同剂量都会影响后生素的效果;在菌属选择方面,除了常见的乳杆菌和酵母菌,可增加其他菌属的微生物如拟杆菌、真杆菌以及大肠杆菌等。通过系统性地探索后生素益生功能和效果,优化整合并合理利用各种益生组分,推动后生素在实际生产中的广泛应用。
| [1] |
TERPOU A, PAPADAKI A, LAPPA I K, et al. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value[J]. Nutrients, 2019, 11(7): 1591. DOI:10.3390/nu11071591 |
| [2] |
LERNER A, MATTHIAS T, AMINOV R, et al. Potential effects of Horizontal gene exchange in the human gut[J]. Front Immunol, 2017, 27(8): 1630. |
| [3] |
SALMINEN S, COLLADO M C, ENDO A, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(9): 649-667. DOI:10.1038/s41575-021-00440-6 |
| [4] |
LAVELLE A, SOKOL H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(4): 223-237. DOI:10.1038/s41575-019-0258-z |
| [5] |
FISCHBACH M A, SEGRE J A. Signaling in host-associated microbial communities[J]. Cell, 2016, 164(6): 1288-1300. DOI:10.1016/j.cell.2016.02.037 |
| [6] |
ŻOIKIEWICZ J, MARZEC A, RUSZCZYNSKI M, 等. Postbiotics—A step beyond pre- and probiotics[J]. Nutrients, 2020, 12(8): 2189. |
| [7] |
MUROSAKI S, YAMAMOTO Y, ITO K, et al. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice[J]. J Allergy Clin Immunol, 1998, 102(1): 57-64. DOI:10.1016/S0091-6749(98)70055-7 |
| [8] |
TSILINGIRI K, BARBOSA T, PENNA G, et al. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model[J]. Gut, 2012, 61(7): 1007-1015. DOI:10.1136/gutjnl-2011-300971 |
| [9] |
OUWEHAND A C, TÖLKKÖ S, KULMALA J, et al. Adhesion of inactivated probiotic strains to intestinal mucus[J]. Lett Appl Microbiol, 2000, 31(1): 82-86. DOI:10.1046/j.1472-765x.2000.00773.x |
| [10] |
ATHALYE-JAPE G, RAO S, SIMMER K, et al. Bifidobacterium breve M-16V as a probiotic for preterm infants: a strain-specific systematic review[J]. J Parenter Enteral Nutr, 2018, 42(4): 677-688. DOI:10.1177/0148607117722749 |
| [11] |
KATARIA J, LI N, WYNN J L, et al. Probiotic microbes: do they need to be alive to be beneficial?[J]. Nutr Rev, 2009, 67(9): 546-550. DOI:10.1111/j.1753-4887.2009.00226.x |
| [12] |
ALP D, KULEAŞAN H, KORKUT ALTINTAŞ A. The importance of the S-layer on the adhesion and aggregation ability of Lactic acid bacteria[J]. Mol Biol Rep, 2020, 47(5): 3449-3457. DOI:10.1007/s11033-020-05430-6 |
| [13] |
XUE C H, ZHANG L W, LI H B, et al. Functionality of the S-layer proteins from Lactobacillus in the competitive against enteropathogens infection[J]. Eur Food Res Technol, 2013, 236(2): 249-255. DOI:10.1007/s00217-012-1871-z |
| [14] |
TAVERNITI V, STUKNYTE M, MINUZZO M, et al. S-layer protein mediates the stimulatory effect of Lactobacillus helveticus MIMLh5 on innate immunity[J]. Appl Environ Microbiol, 2013, 79(4): 1221-1231. DOI:10.1128/AEM.03056-12 |
| [15] |
牛钰涵. 表层蛋白对乳杆菌益生性质的影响及其抑菌功能[D]. 无锡: 江南大学, 2019. NIU Y H. Effect of surface layer proteins from lactobacillus on the strains' probiotic properties and their antibacterial function[D]. Wuxi: Jiangnan University, 2019. (in Chinese) |
| [16] |
REUNANEN J, VON OSSOWSKI I, HENDRICKX A P A, et al. Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG[J]. Appl Environ Microbiol, 2012, 78(7): 2337-2344. DOI:10.1128/AEM.07047-11 |
| [17] |
CAPURSO L. Thirty years of Lactobacillus rhamnosus GG[J]. J Clin Gastroenterol, 2019, 53(S1): S1-S41. |
| [18] |
HENDRICKX A P A, BONTEN M J M, VAN LUIT-ASBROEK M, et al. Expression of two distinct types of pili by a hospital-acquired Enterococcus faecium isolate[J]. Microbiology (Reading), 2008, 154(10): 3212-3223. DOI:10.1099/mic.0.2008/020891-0 |
| [19] |
HENDRICKX A P A, VAN WAMEL W J B, POSTHUMA G, et al. Five genes encoding surface-exposed LPXTG proteins are enriched in hospital-adapted Enterococcus faecium clonal complex 17 isolates[J]. J Bacteriol, 2007, 189(22): 8321-8332. DOI:10.1128/JB.00664-07 |
| [20] |
TYTGAT H L P, DOUILLARD F P, REUNANEN J, et al. Lactobacillus rhamnosus GG outcompetes Enterococcus faecium via mucus-binding pili: evidence for a novel and heterospecific probiotic mechanism[J]. Appl Environ Microbiol, 2016, 82(19): 5756-5762. DOI:10.1128/AEM.01243-16 |
| [21] |
CHEN Z Y, LIN S S, JIANG Y, et al. Effects of bread yeast cell wall beta-glucans on mice with loperamide-induced constipation[J]. J Med Food, 2019, 22(10): 1009-1021. DOI:10.1089/jmf.2019.4407 |
| [22] |
ROBERFROID M B. Chicory fructooligosaccharides and the gastrointestinal tract[J]. Nutrition, 2000, 16(7-8): 677-679. DOI:10.1016/S0899-9007(00)00244-6 |
| [23] |
GIBSON G R, ROBERFROID M B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics[J]. J Nutr, 1995, 125(6): 1401-1412. DOI:10.1093/jn/125.6.1401 |
| [24] |
GANAN M, CARRASCOSA A V, DE PASCUAL-TERESA S, et al. Effect of mannoproteins on the growth, gastrointestinal viability, and adherence to caco-2 cells of lactic acid bacteria[J]. J Food Sci, 2012, 77(3): M176-M180. |
| [25] |
YAN F, LIU L P, DEMPSEY P J, et al. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor[J]. J Biol Chem, 2013, 288(42): 30742-30751. DOI:10.1074/jbc.M113.492397 |
| [26] |
SCHIAVI E, GLEINSER M, MOLLOY E, et al. The surface-associated exopolysaccharide of Bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 responses[J]. Appl Environ Microbiol, 2016, 82(24): 7185-7196. DOI:10.1128/AEM.02238-16 |
| [27] |
YIN M M, YAN X B, WENG W H, et al. Micro integral membrane protein (MIMP), a newly discovered anti-inflammatory protein of lactobacillus plantarum, enhances the gut barrier and modulates microbiota and inflammatory cytokines[J]. Cell Physiol Biochem, 2018, 45(2): 474-490. DOI:10.1159/000487027 |
| [28] |
WALTER J, LOACH D M, ALQUMBER M, et al. D-Alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100-23 results in impaired colonization of the mouse gastrointestinal tract[J]. Environ Microbiol, 2007, 9(7): 1750-1760. DOI:10.1111/j.1462-2920.2007.01292.x |
| [29] |
CHELAKKOT C, CHOI Y, KIM D K, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions[J]. Exp Mol Med, 2018, 50(2): e450. DOI:10.1038/emm.2017.282 |
| [30] |
LEBEER S, VANDERLEYDEN J, DE KEERSMAECKER S C J. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens[J]. Nat Rev Microbiol, 2010, 8(3): 171-184. DOI:10.1038/nrmicro2297 |
| [31] |
LI P C, YU Q H, YE X L, et al. Lactobacillus S-layer protein inhibition of Salmonella-induced reorganization of the cytoskeleton and activation of MAPK signalling pathways in Caco-2 cells[J]. Microbiology (Reading), 2011, 157(9): 2639-2646. DOI:10.1099/mic.0.049148-0 |
| [32] |
MARTÍNEZ M G, ACOSTA M P, CANDURRA N A, et al. S-layer proteins of Lactobacillus acidophilus inhibits JUNV infection[J]. Biochem Biophys Res Commun, 2012, 422(4): 590-595. DOI:10.1016/j.bbrc.2012.05.031 |
| [33] |
张铭书, 夏永军, 艾连中, 等. 益生菌磷壁酸引起的免疫反应研究进展[J]. 食品科学, 2022, 43(9): 242-248. ZHANG M S, XIA Y J, AI L Z, et al. A review of studies on immunoregulation induced by probiotic teichoic acid[J]. Food Science, 2022, 43(9): 242-248. (in Chinese) |
| [34] |
KIM K W, KANG S S, WOO S J, et al. Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates poly I: C-induced IL-8 production in porcine intestinal epithelial cells[J]. Front Microbiol, 2017, 8: 1827. DOI:10.3389/fmicb.2017.01827 |
| [35] |
KAJI R, KIYOSHIMA-SHIBATA J, NAGAOKA M, et al. Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages[J]. J Immunol, 2010, 184(7): 3505-3513. DOI:10.4049/jimmunol.0901569 |
| [36] |
OTTMAN N, REUNANEN J, MEIJERINK M, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function[J]. PLoS One, 2017, 12(3): e0173004. DOI:10.1371/journal.pone.0173004 |
| [37] |
LU Y C, YEH W C, OHASHI P S. LPS/TLR4 signal transduction pathway[J]. Cytokine, 2008, 42(2): 145-151. DOI:10.1016/j.cyto.2008.01.006 |
| [38] |
洪亮, 余方流, 黄月娥. 不同乳酸杆菌肽聚糖对小鼠肠道黏膜的免疫调节作用[J]. 皖南医学院学报, 2019, 38(5): 419-420, 424. HONG L, YU F L, HUANG Y E. Immunomodulatory effects of different lactobacillus peptidoglycans on intestinal mucosa in mice[J]. Journal of Wannan Medical College, 2019, 38(5): 419-420, 424. (in Chinese) |
| [39] |
REN C C, ZHAN Q X, DE HAAN B J, et al. Protective effects of lactic acid bacteria on gut epithelial barrier dysfunction are Toll like receptor 2 and protein kinase C dependent[J]. Food Funct, 2020, 11(2): 1230-1234. DOI:10.1039/C9FO02933H |
| [40] |
FERNANDEZ E M, VALENTI V, ROCKEL C, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide[J]. Gut, 2011, 60(8): 1050-1059. DOI:10.1136/gut.2010.232918 |
| [41] |
HAMER H M, JONKERS D M A E, BAST A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans[J]. Clin Nutr, 2009, 28(1): 88-93. DOI:10.1016/j.clnu.2008.11.002 |
| [42] |
MOFFETT J R, PUTHILLATHU N, VENGILOTE R, et al. Acetate revisited: a key biomolecule at the nexus of metabolism, epigenetics and oncogenesis — Part 1:acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases[J]. Front Physiol, 2020, 11: 580167. DOI:10.3389/fphys.2020.580167 |
| [43] |
DONOHOE D R, GARGE N, ZHANG X X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon[J]. Cell Metab, 2011, 13(5): 517-526. DOI:10.1016/j.cmet.2011.02.018 |
| [44] |
KIMURA I, INOUE D, MAEDA T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41)[J]. Proc Natl Acad Sci U S A, 2011, 108(19): 8030-8035. DOI:10.1073/pnas.1016088108 |
| [45] |
FOLEY M H, O'FLAHERTY S, ALLEN G, et al. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization[J]. Proc Natl Acad Sci U S A, 2021, 118(6): e2017709118. DOI:10.1073/pnas.2017709118 |
| [46] |
JIA W, XIE G X, JIA W P. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(2): 111-128. DOI:10.1038/nrgastro.2017.119 |
| [47] |
LONG S L, GAHAN C G M, JOYCE S A. Interactions between gut bacteria and bile in health and disease[J]. Mol Aspects Med, 2017, 56: 54-65. DOI:10.1016/j.mam.2017.06.002 |
| [48] |
HUANG F J, ZHENG X J, MA X H, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism[J]. Nat Commun, 2019, 10(1): 4971. DOI:10.1038/s41467-019-12896-x |
| [49] |
JIANG C T, XIE C, LV Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction[J]. Nat Commun, 2015, 6(1): 10166. DOI:10.1038/ncomms10166 |
| [50] |
LIN S, YANG X M, LONG Y R, et al. Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets[J]. Br J Nutr, 2020, 124(8): 797-808. DOI:10.1017/S0007114520001774 |
| [51] |
JOHN W R. Ruminal microbial metabolism of peptides and amino acids[J]. J Nutr, 1996, 126(Suppl 4): 1326S-1334S. |
| [52] |
DAI Z L, WU G Y, ZHU W Y. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health[J]. Front Biosci (Landmark Ed), 2011, 16(5): 1768-1786. |
| [53] |
YAGHOUBFAR R, BEHROUZI A, ZARE BANADKOKI E, et al. Effect of Akkermansia muciniphila, Faecalibacterium prausnitzii, and their extracellular vesicles on the serotonin system in intestinal epithelial cells[J]. Probiotics Antimicrob Proteins, 2021, 13(6): 1546-1556. DOI:10.1007/s12602-021-09786-4 |
| [54] |
BONAZ B, BAZIN T, PELLISSIER S. The vagus nerve at the interface of the microbiota-gut-brain axis[J]. Front Neurosci, 2018, 12: 49. DOI:10.3389/fnins.2018.00049 |
| [55] |
ABIZAID A, HOUGLAND J L. Ghrelin signaling: GOAT and GHS-R1a take a LEAP in complexity[J]. Trends Endocrinol Metab, 2020, 31(2): 107-117. DOI:10.1016/j.tem.2019.09.006 |
| [56] |
TIAN C L, YE F, XU T J, et al. GHRP-6 induces CREB phosphorylation and growth hormone secretion via a protein kinase Cσ-dependent pathway in GH3 cells[J]. J Huazhong Univ Sci Technol Med Sci, 2010, 30(2): 183-187. DOI:10.1007/s11596-010-0210-5 |
| [57] |
YANO J M, YU K, DONALDSON G P, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis[J]. Cell, 2015, 161(2): 264-276. DOI:10.1016/j.cell.2015.02.047 |
| [58] |
DE VADDER F, GRASSET E, MANNERÅS HOLM L, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks[J]. Proc Natl Acad Sci U S A, 2018, 115(25): 6458-6463. DOI:10.1073/pnas.1720017115 |
| [59] |
ARIMORI Y, NAKAMURA R, HIROSE Y, et al. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs[J]. Immunopharmacol Immunotoxicol, 2012, 34(6): 937-943. DOI:10.3109/08923973.2012.672425 |
| [60] |
KANG J, LEE J J, CHO J H, et al. Effects of dietary inactivated probiotics on growth performance and immune responses of weaned pigs[J]. J Anim Sci Technol, 2021, 63(3): 520-530. DOI:10.5187/jast.2021.e44 |
| [61] |
SUKEGAWA S, IHARA Y, YUGE K, et al. Effects of oral administration of heat-killed Enterococcus faecium strain NHRD IHARA in post-weaning piglets[J]. Anim Sci J, 2014, 85(4): 454-460. DOI:10.1111/asj.12163 |
| [62] |
HYABG M K, CHOI Y J, HOUDE R, et al. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens[J]. Poult Sci, 2004, 83(5): 788-795. DOI:10.1093/ps/83.5.788 |
| [63] |
章亭洲, 朱廷恒, 赵艳, 等. 酵母及其相关产品在饲料行业的应用[J]. 饲料博览, 2021(1): 26-34. ZHANG T Z, ZHU T H, ZHAO Y, et al. Application of yeasts and its relative products in feed industry[J]. Feed Review, 2021(1): 26-34. (in Chinese) |
| [64] |
赵芳芳, 张日俊. 酵母细胞壁生理功能及其应用[J]. 中国饲料, 2003(17): 17-18. ZHAO F F, ZHANG R J. The application and physiological function of the cell wall of yeast[J]. China Feed, 2003(17): 17-18. (in Chinese) |
| [65] |
FIRMIN S, MORGAVI D P, YIANNIKOURIS A, et al. Effectiveness of modified yeast cell wall extracts to reduce aflatoxin B1 absorption in dairy ewes[J]. J Dairy Sci, 2011, 94(11): 5611-5619. DOI:10.3168/jds.2011-4446 |
| [66] |
AAZAMI M H, FATHI NASRI M H, MOJTAHEDI M, et al. Effect of yeast cell wall and (1→3)-β-d-glucan on transfer of aflatoxin from feed to milk in Saanen dairy goats[J]. Anim Feed Sci Technol, 2019, 254: 114191. DOI:10.1016/j.anifeedsci.2019.05.014 |
| [67] |
KHAFIPOUR E, KRAUSE D O, PLAIZIER J C. Alfalfa pellet-induced subacute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation[J]. J Dairy Sci, 2009, 92(4): 1712-1724. DOI:10.3168/jds.2008-1656 |
| [68] |
PENG Q H, CHENG L, KANG K, et al. Effects of yeast and yeast cell wall polysaccharides supplementation on beef cattle growth performance, rumen microbial populations and lipopolysaccharides production[J]. J Integr Agric, 2020, 19(3): 810-819. |
| [69] |
SALINAS-CHAVIRA J, MONTANO M F, TORRENTERA N, et al. Influence of feeding enzymatically hydrolysed yeast cell wall+yeast culture on growth performance of calf-fed Holstein steers[J]. J Appl Anim Res, 2018, 46(1): 327-330. |
| [70] |
MA T, TU Y, ZHANG N F, et al. Effects of dietary yeast β-glucan on nutrient digestibility and serum profiles in pre-ruminant Holstein calves[J]. J Integr Agric, 2015, 14(4): 749-757. |
| [71] |
PASCUAL A, PAULETTO M, GIANTIN M, et al. Effect of dietary supplementation with yeast cell wall extracts on performance and gut response in broiler chickens[J]. J Anim Sci Biotechnol, 2020, 11: 40. |
| [72] |
LEE J J, KYOUNG H, CHO J H, et al. Dietary yeast cell wall improves growth performance and prevents of diarrhea of weaned pigs by enhancing gut health and anti-inflammatory immune responses[J]. Animals (Basel), 2021, 11(8): 2269. |
| [73] |
SUGAHARA H, YAO R, ODAMAKI T, et al. Differences between live and heat-killed bifidobacteria in the regulation of immune function and the intestinal environment[J]. Benef Microbes, 2017, 8(3): 463-472. |
| [74] |
ZHONG J F, WU W G, ZHANG X Q, et al. Effects of dietary addition of heat-killed Mycobacterium phlei on growth performance, immune status and anti-oxidative capacity in early weaned piglets[J]. Arch Anim Nutr, 2016, 70(4): 249-262. |
| [75] |
LUO K, TIAN X L, WANG B, et al. Evaluation of paraprobiotic applicability of Clostridium butyricum CBG01 in improving the growth performance, immune responses and disease resistance in Pacific white shrimp, Penaeus vannamei[J]. Aquaculture, 2021, 544: 737041. |
(编辑 范子娟)



