2. 齐全农牧集团股份有限公司,遂宁 629000
2. Complete Agriculture and Animal Husbandry Group Co. Ltd., Suining 629000, China
我国是世界第一养猪大国,生猪产业是否健康发展关乎民生。后备母猪的繁殖生产性能直接影响整个猪群的生产性能和经济效益[1-2]。当前,国内能繁母猪因为发情等繁殖障碍导致淘汰率居高不下,来自一个规模猪场的生产数据表明:统计2018年全年度淘汰的后备/断奶母猪21 497头,在后备状态由于发情障碍导致的淘汰占比25.66%,第一胎猪由于发情障碍导致的淘汰占18.96%,这两个阶段由于发情障碍导致的淘汰最高。母猪发情与卵巢密切相关,卵泡经由原始卵泡、初级卵泡、次级卵泡、窦状卵泡、优势卵泡的发育过程,最终排卵。伴随着卵泡的发育,雌激素分泌量上升,促进母猪出现发情行为。淫羊藿是我国一种传统中草药,被中医药列入“催情和催乳药物”类别。淫羊藿是多年生草本药物,含有多种黄酮类物质,全草入药,全国大部分地区有分布,具有补肾壮阳,祛风止痛,催情等功效。相关的研究表明,淫羊藿能治疗女性卵巢早衰[3]。淫羊藿的主要成分淫羊藿苷能够在猪卵泡的体外培养中抑制颗粒细胞的凋亡从而抑制卵泡的闭锁[4]。淫羊藿已经广泛在生产中作为母猪促发情药物使用[5-6],而淫羊藿对母猪发情的具体作用及作用机制尚不明晰。本试验旨在通过活体试验结合转录组学、代谢组学及网络药理学,揭示淫羊藿对后备母猪发情和卵巢的作用和潜在机制,为淫羊藿提高后备母猪发情利用率提供理论依据。
1 材料与方法 1.1 试验动物试验选择发育成熟且符合配种条件的后备待配二元母猪32头(对照组16头、试验组16组),随机分成两组,体重(99.547±1.987)kg,210~220日龄。基础日粮参考NRC2012哺乳期营养进行配制(每日定量3.0 kg),对照组饲喂基础日粮,试验组在饲喂基础日粮的基础上补充淫羊藿提取物(四川恒瑞通达生物)50 mg·d-1。试验饲喂28 d,期间统计发情情况。饲喂结束每组屠宰4头,收集血液、卵巢用于后续检测。其余猪配种,跟踪母猪繁殖性能。
1.2 生殖激素检测颈静脉采血,通过ELISA试剂盒检测血清中FSH(H101-1-2,南京建成)、E2(H102-1-2,南京建成)和LH(H206-1-2,南京建成)的含量。
1.3 卵巢转录组测序RNA提取:使用TRlzol试剂(Life technologies,California,USA)提取卵巢总RNA。使用NanoDrop 2000(Thermo Fisher Scientific,Wilmington,DE)测量RNA浓度和纯度。使用安捷伦生物分析仪2100系统的RNA Nano 6000检测试剂盒(安捷伦技术公司,CA,USA)评估RNA完整性。使用NEBNext UltraTM RNA生成测序文库。用AMPure XP系统纯化PCR产物,并在Agilent Bioanalyzer 2100系统上评估文库质量。使用DESeq2进行差异表达分析,使用Benjamini和Hochberg的方法来调整得到的P值。通过DESeq2发现的调整后P<0.05的基因为差异表达基因。依据差异基因表达量,利用R语言的Pheatmap软件包对差异基因进行聚类分析。利用R语言的ggplot2包进行绘图。
1.4 卵巢代谢组测序代谢组学分析的LC/MS系统由Waters Acquity I-Class PLUS超高性能液体串联Waters Xevo G2-XS QTof高分辨率质谱仪组成。通过MassLynx V4.2采集一次和二次质谱数据。原始数据由Proggenesis QI软件处理,进行峰提取、峰比对等数据处理操作,基于Proggenesis QI软件在线METLIN数据库和Biomark自建库进行鉴定。使用总峰面积对原始峰面积信息进行归一化,使用MetaboAnalystR包进行差异分析。利用R语言的ggplot2包进行绘图。
1.5 淫羊藿潜在有效成分、潜在靶点筛选与网络构建利用TCMSP数据库(https://old.tcmsp-e.com/)以淫羊藿为关键词进行检索,以口服利用度(OB)与类药性(DL)为指标进行筛选(OB>30%,DL≥0.18),筛选得到淫羊藿潜在有效成分。以筛选得到的淫羊藿潜在有效成分为对象,利用swiss数据库(http://swisstargetprediction.ch/)预测成分的潜在作用靶点(Probability>0.1)。以卵巢早衰(POI)和月经不调(MI)为检索词,利用OMIM数据库(https://www.omim.org/)与GeneCards数据库(https://www.genecards.org/)分别进行检索,得到POI和MI相关靶点。将得到的淫羊藿潜在靶点与卵巢早衰相关靶点分别在UniProt数据库(https://www.uniprot.org/)规范化后,利用venny2.1(https://bioinfogp.cnb.csic.es/tools/venny/)进行交集分析,即得淫羊藿作用卵巢的潜在靶点。将筛选得到的淫羊藿潜在靶点录入STRING数据库(https://cn.string-db.org/)绘制PPI(protein protein interaction)网络。将其数据导入Cytoscape 3.7.1软件中进行拓扑分析,根据等级值(degree) 排名,得到这个网络中的枢纽蛋白,即为淫羊藿作用卵巢的核心靶点。
1.6 分子对接在RCDB PDB数据库(https://www.rcsb.org/)下载候选靶点蛋白质三维结构,在TCMSP数据库(https://old.tcmsp-e.com/)下载候选化合物三维结构。通过CB-DOCK2在线软件(https://cadd.labshare.cn/cb-dock2/php/index.php)进行分子对接,得到分子对接图与Vina Score。Vina Score得分越低,表明分子结合能力更强。
1.7 KEGG与GO富集分析用在线软件DAVID(https://david.ncifcrf.gov/)对筛选的差异基因进行GO和KEGG分析,根据校正P值进行筛选。
1.8 数据分析试验数据使用GraphPad Prime 8进行分析。结果用“平均值±SEM”表示,并包括至少3个独立的生物重复(n=3)。双尾非配对Student t检验用于比较两个试验组。Dunnett检验和Sidak检验分别用于单因素方差分析的事后检验。P<0.05和P<0.01的统计学显著值分别用(*)和(**)表示。
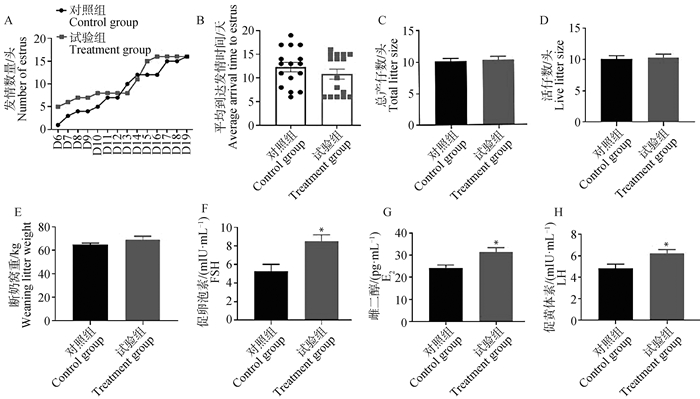
2 结果 2.1 淫羊藿对后备母猪繁殖性能及血清激素的作用饲喂淫羊藿28 d后,统计后备母猪的发情率。收集血液进行激素测定。结果发现,试验组后备母猪整体发情时间提前,在D6-D10的发情数量高于对照组(图 1A)。对照组平均到达发情天数为(12.313±1.003)d,试验组平均到达发情天数为(10.813±1.054)d(图 1B)。对于配种母猪进行生产数据追踪,结果发现,淫羊藿饲喂后母猪的产仔数、活仔数与仔猪断奶窝重均无显著变化,但存在上升趋势(图 1C,D和E)。血液中的FSH、E2、LH均显著高于对照组(P<0.05)(图 1F,G和H)。以上结果表明淫羊藿饲喂能够促进母猪发情,并可能存在改善母猪繁殖性能的潜在作用。
|
A. 饲喂淫羊藿后出现发情时间统计;B. 对照组与试验组平均到达发情时间;C、D、E. 饲喂淫羊藿25 d后,母猪该胎次产仔数(C)、活仔数(D)、仔猪断奶窝重(E);F、G、H. 饲喂25 d后,母猪血清FSH(F)、E2(G)、LH(H)水平。*. P<0.05 A. Statistics of estrus occurrence time after feeding epimedium; B. The average arrival time to estrus in the control group and the treamtment group; C, D, E. After feeding epimedium for 25 days, the litter size (C), live litter size (D), and weaning litter weight (E) of the gilts at that parity were determined; F, G, H. After 25 days of feeding, the serum hormone levels of FSH (F), E2 (G), LH (H) were measured in gilts.*. P < 0.05 图 1 淫羊藿饲喂对后备母猪繁殖性能与血清激素的影响 Fig. 1 Effect of epimedium feeding on reproductive performance and serum hormones of gilts |
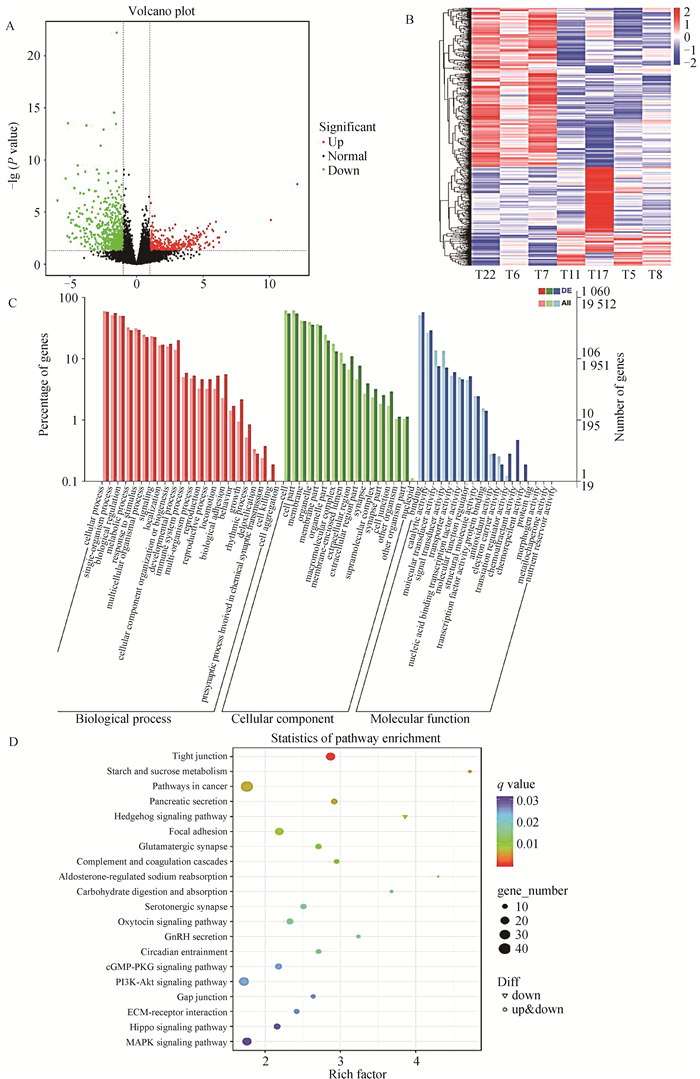
为了揭示淫羊藿对母猪发情促进作用的潜在机制,收集母猪卵巢进行转录组测序。通过edgeR包进行差异表达分析,将P<0.05,并且|Fold Change|>1的基因认为是显著差异基因。根据差异基因的表达量绘制mRNA火山图(图 2A)和差异mRNA热图(图 2B)。其中检测出差异mRNA共1 231个,其中477个上调,754个下调。为了进一步明确淫羊藿对卵巢的功能,本研究对差异mRNA进行了功能富集分析(图 2C)和信号通路富集分析(图 2D)。结果,GO富集分析发现,差异的mRNA主要富集在运输囊泡、脂肪细胞分化、节律行为、发情周期等过程;KEGG富集分析发现,差异的mRNA主要富集在紧密连接、碳水化合物代谢、刺猬信号通路、GnRH分泌和PI3K-AKT信号通路等信号通路。
|
A. 差异基因火山图;B.差异基因热图;C.差异基因GO分析;D.差异基因KEGG分析 A.Differential genes volcano map; B. Differential genes heatmap; C. Differential genes GO analysis; D. Differential genes KEGG analysis 图 2 淫羊藿饲喂对后备母猪卵巢转录组的影响 Fig. 2 Effect of epimedium feeding on the ovarian transcriptome of gilts |
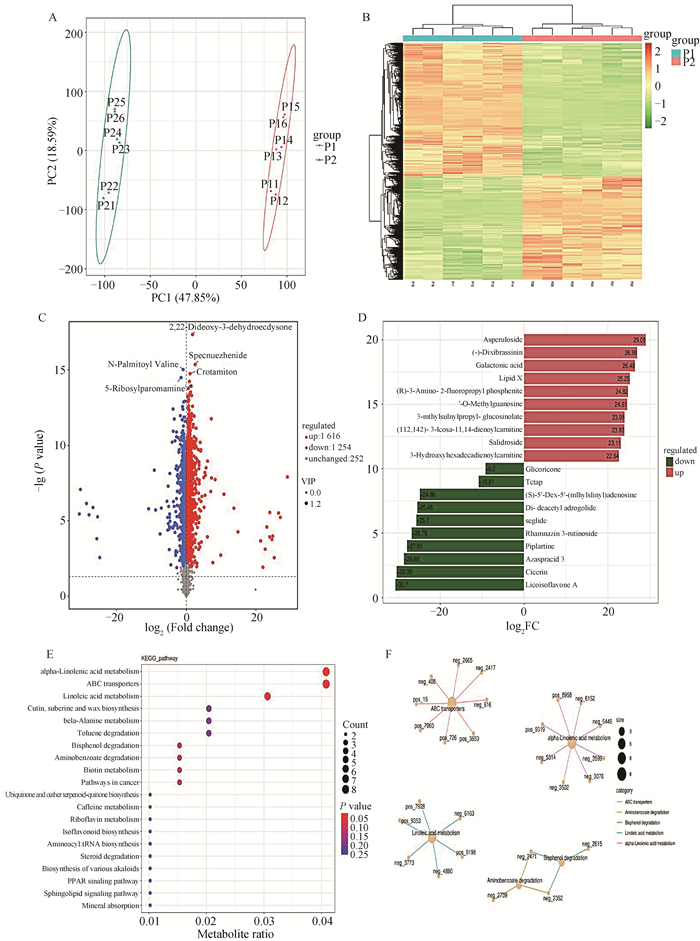
通过卵巢代谢组测序分析淫羊藿对卵巢代谢物的影响。PCoA主成分分析与差异代谢物热图表明,对照组和淫羊藿饲喂组卵巢代谢物组成有明显差异(图 3A和B)。进一步对差异代谢物进行分析,结果表明共有1 616个代谢物上调,1 254个代谢物下调(图 3C)。根据代谢物Fold Change筛选出差异前20名代谢物。其中上调前10代谢物为甘草异黄酮A、西色林、阿扎螺酸3、匹普拉汀、鼠李糖苷3-芸香糖苷、司格列肽、二-脱乙酰基腺内酯、甲硫腺苷亚砜、TCTAP、甘草瑞酮。下调前10代谢物为车叶草苷、二氧苔藓素、半乳糖酸、脂质X、(R)-3-氨基-2-氟丙基磷酸酯、3-O-甲基鸟苷、3-甲硫基异丙基硫代葡萄糖苷、(11Z,14Z)-3-二十碳-11,14-二烯酰肉碱、红景天苷和3-羟基十六碳二烯酰肉碱(图 3D)。KEGG富集分析表明,差异代谢物主要富集在α-亚麻酸代谢、ATP转运蛋白、亚油酸代谢、β-丙氨酸代谢、氨基苯甲酸盐降解、生物素代谢、泛醌和其他萜醌的生物合成、咖啡因代谢、核黄素代谢、异黄酮生物合成、氨基-tRNA生物合成,甾体降解、多种生物碱的生物合成、PPAR信号通路、鞘脂信号通路等通路(图 3E和F)。
|
A. 卵巢代谢组PCoA分析;B. 差异代谢物热图;C. 差异代谢物火山图;D. TOP20差异代谢;E. 差异代谢物KEGG分析;F. 富集KEGG网络图 A. Ovarian metabolomic PCoA analysis; B. Differential metabolites heat map; C. Differential metabolites volcano map; D. TOP20 differential metabolism; E. Differential metabolites KEGG analysis; F. Enrich the KEGG network diagram 图 3 淫羊藿饲喂对后备母猪卵巢代谢组的影响 Fig. 3 Effect of epimedium feeding on ovarian metabolome of gilts |
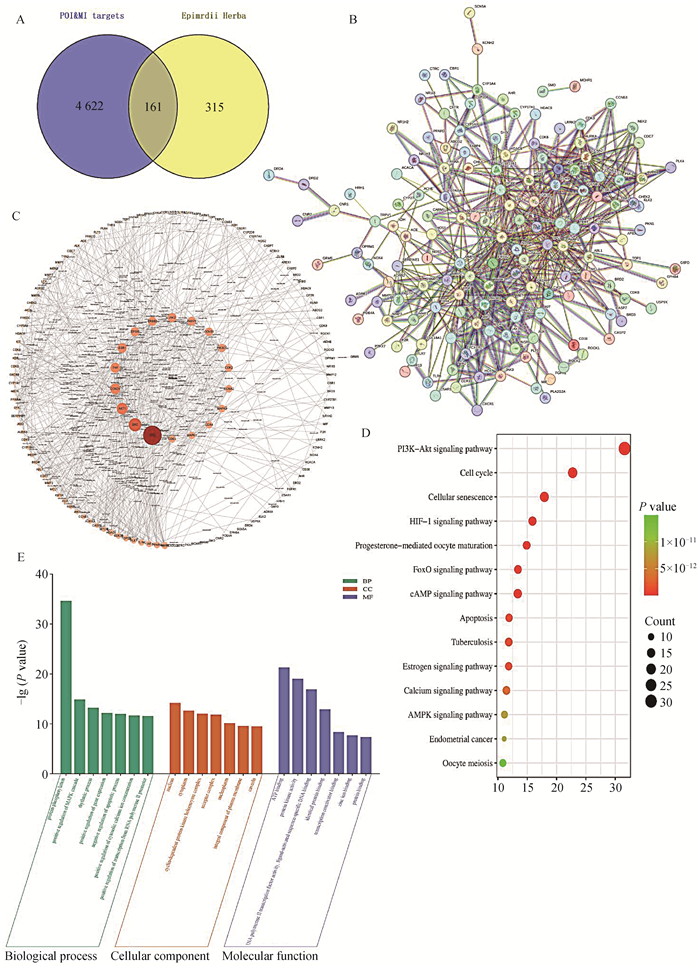
使用TCMSP数据库以淫羊藿为关键词进行检索,筛选后得到22个有效成分(表 1)。使用swiss数据库预测以上22个成分的潜在靶点(Probability>0.1)共478个。与检索所得4 353个卵巢早衰和月经不调相关靶点取交集,即得淫羊藿治疗卵巢早衰的潜在作用靶点161个(图 4A)。通过String软件进行蛋白互作分析得到的网络图中共有161个节点,636条连线,平均节点度7.9,平均局部聚类系数0.526,PPI富集P值<1.0×10-16(图 4B)。通过Cytoscape软件对PPI互作网络进行分析,得到degree大于等于20的蛋白共18个作为核心靶点,根据得分依次为TP53、SRC、AKT1、CCND1、TNF、ESR1、EP300、ERBB2、JAK2、PARP1、CCNB1、PIK3CD、CDK2、CCNA2、MAPK3、CDK4、MAPK1和CDK1(图 4C)对135个作用靶点进行GO分析与KEGG分析,结果表明,淫羊藿参与雌性疾病的靶点主要参与了蛋白磷酸化、MAPK级联反应的正调控、生物节律、基因表达的正调控、细胞凋亡过程的负调控、细胞内钙离子浓度的正调控、RNA聚合酶II启动子转录的正调控等生物学过程;参与核、细胞质、细胞周期依赖性蛋白激酶全酶复合物、受体复合物、核原形质核浆和质膜整体成分等细胞组分;参与ATP结合、蛋白激酶活性、RNA聚合酶II转录因子活性、转录共激活因子结合、锌离子结合和蛋白质结合等分子功能(图 4D)。KEGG分析结果显示,靶点蛋白信号通路富集在PI3K-AKT信号通路、细胞周期、细胞衰老、HIF-1信号通路、孕激素介导的卵母细胞成熟、Fox0信号通路、cAMP信号通路、细胞凋亡、结核病、雌激素信号通路、钙信号通路、AMPK信号通路、子宫内膜癌和卵母细胞减数分裂等途径(图 4E)。进一步对核心靶点进行分子对接预测,根据Vina Score选择每个靶点蛋白最优候选化合物进行分子对接(图 5A-J)。
|
|
表 1 淫羊藿潜在有效成分 Table 1 Potential active ingredients of epimedium |
|
A. 卵巢早衰与月经不调疾病靶点与淫羊藿有效物质靶点韦恩图;B. 共有靶点蛋白的蛋白互作网络图; C. 内圈蛋白质为degree≥20的核心蛋白;D. 共有蛋白的KEGG信号通路富集分析气泡图;E. 共有蛋白的GO分析直方图 A. Venn map of targets for ovarian premature failure and menstrual disorders, as well as effective substances in epimedium; B. Protein interaction network diagram of common target proteins; C. The inner circle protein is a core protein with a degree ≥ 20; D. Bubble plot analysis of KEGG signaling pathway enrichment of shared proteins; E. GO analysis histogram of shared proteins 图 4 网络药理学分析淫羊藿作用母猪卵巢的潜在靶点与机制 Fig. 4 Network pharmacological analysis of potential targets and mechanisms of epimedium on sow ovaries |
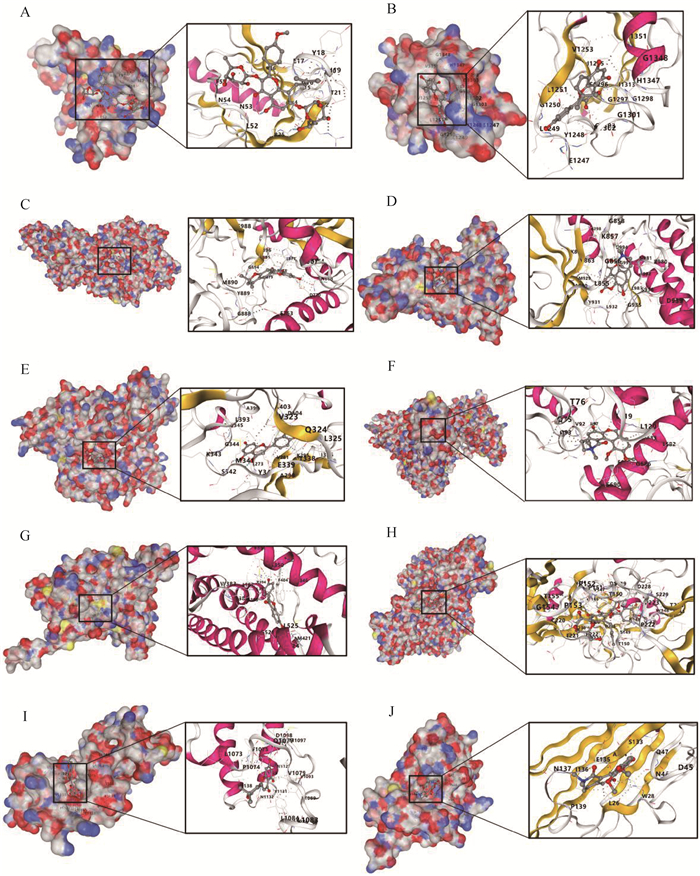
|
A. AKT1与Icariin分子对接示意图;B. ERBB2与8-Isopentenyl-kaempferol分子对接示意图;C. PARP1与YinyanghuoC分子对接示意图;D. JAK2与6-hydroxy-11, 12-dimethoxy-2, 2-dimethyl-1, 8-dioxo-2, 3, 4, 8-tetrahydro-1H-isochromeno[3, 4-h]isoquinolin-2-ium分子对接示意图;E. SRC与luteolin分子对接示意图;F. PIK3CD与6-hydroxy-11, 12-dimethoxy-2, 2-dimethyl-1, 8-dioxo-2, 3, 4, 8-tetrahydro-1H-isochromeno[3, 4-h]isoquinolin-2-ium分子对接示意图;G. ESR1与DFV分子对接示意图;H.TP53与Icariin分子对接示意图;I.EP300与YinyanghuoC分子对接示意图;J. TNF与6-hydroxy-11, 12-dimethoxy-2, 2-dimethyl-1, 8-dioxo-2, 3, 4, 8-tetrahydro-1H-isochromeno[3, 4-h]isoquinolin-2-ium分子对接示意图 A. Schematic diagram of the docking between AKT1 and Icariin molecules; B. Schematic diagram of molecular docking between ERBB2 and 8-Isopentenyl-kaempferol; C. Schematic diagram of PARP1 docking with YinyanghuoC molecules; D. Schematic diagram of molecular docking between JAK2 and 6-hydroxy-11, 12-dimethoxy-2, 2-dimethyl-1, 8-dioxo-2, 3, 4, 8-tetrahydro-1H-isochromeno [3, 4-h] isoquinolin-2-ium; E. Schematic diagram of the docking between SRC and luteolin molecules; F. Schematic diagram of PIK3CD docking with 6-hydroxy-11, 12-dimethoxy-2, 2-dimethyl-1, 8-dioxo-2, 3, 4, 8-tetrahydro-1H-isochromeno [3, 4-h] isoquinolin-2-ium molecules; G. Schematic diagram of the docking between ESR1 and DFV molecules; H. Schematic diagram of TP53 docking with Icariin molecules; I. Schematic diagram of the docking between EP300 and YinyanghuoC molecules; J. Schematic diagram of the docking of TNF with 6-hydroxy-11, 12-dimethoxy-2, 2-dimethyl-1, 8-dioxo-2, 3, 4, 8-tetrahydro-1H-isochromeno [3, 4-h] isoquinolin-2-ium molecules 图 5 靶点蛋白与最优候选化合物分子对接图 Fig. 5 Docking diagram of target proteins and optimal candidate compound molecules |
近年来,后备母猪发情利用率低一直是限制我国生猪产业的关键问题。后备母猪卵巢的发育状态以及性激素分泌水平决定了母猪发情的时间[7]。为了提高后备母猪发情利用率,淫羊藿目前已被广泛应用于母猪发情异常的治疗中,然而淫羊藿对后备母猪发情的具体功能及作用尚不明晰。因此,本研究通过淫羊藿饲喂后备母猪,利用转录组学、代谢组与网络药理学分析淫羊藿对母猪卵巢的作用及其机制,以期为淫羊藿在母猪生产中的应用提供理论依据。前人大量研究发现,淫羊藿苷对雌性生殖功能具有重要影响,本研究发现,饲喂淫羊藿能使后备母猪发情提前,血清FSH、LH和E2含量增高。相关研究报道了淫羊藿饲喂小鼠能提高血清E2和P4含量[8]。淫羊藿苷饲喂大鼠能促进卵泡发育,提高有腔卵泡数量[9]。以上研究与本研究结果一致,即淫羊藿能够提高性激素水平,进而促进动物发情。为了探究淫羊藿是否对其他繁殖性能有影响,本研究进一步统计了饲喂淫羊藿母猪的生产性能,包括总仔数、活仔数和断奶窝重,表明淫羊藿饲喂没有显著作用,但存在一定上升趋势。这是一个有趣的发现,下一步将通过扩大群体规模,持续关注淫羊藿是否对母猪生产性能具有积极作用。
通过对转录组差异蛋白和潜在靶点蛋白的KEGG分析,发现PI3K-AKT信号通路均显著富集。PI3K-AKT信号通路调节细胞生长和增殖,卵巢颗粒细胞中的氧化应激常导致PI3K-AKT信号通路下调,抑制颗粒细胞增殖,从而影响卵巢功能障碍并引起各种疾病[10]。淫羊藿苷能够通过PI3K-AKT-MTOR途径提高POI大鼠血清E2水平和有腔卵泡数量,降低卵泡闭锁水平[11]。卵巢的氧化应激会引起卵泡的异常闭锁,降低卵母细胞质量[12]。线粒体是雌二醇合成的主要场所,维持线粒体稳态对于雌激素合成至关重要,Hu等[13]发现,激活PI3K-AKT信号通路能够促进猪卵巢颗粒细胞的雌二醇合成。淫羊藿具有抗氧化功能,此前研究了淫羊藿苷能提高软骨细胞的SOD,缓解H2O2带来的氧化应激损伤[14]。张越等[4]发现,淫羊藿苷能够激活PI3K-AKT信号通路抑制卵泡体外培养的凋亡水平,减少ROS产生,提高CAT和SOD活性。以上结果表明,淫羊藿对后备母猪发情的促进作用可能通过PI3K-AKT信号通路介导的抗氧化途径来实现的,淫羊藿的有效成分对颗粒细胞雌二醇合成的作用还需进一步验证。
代谢组的差异代谢物TOP20中存在多种天然类黄酮类物质,如甘草异黄酮A、西色林、鼠李糖苷3-芸香糖苷等。这表明,饲喂淫羊藿能够使其在猪体内发生代谢,并进入卵巢发挥功能。KEGG富集分析结果显示, 差异代谢物富集在α-亚麻酸代谢、ABC转运蛋白、亚油酸代谢、β-丙氨酸代谢等信号通路。多不饱和脂肪酸通常分为Ω-3多不饱和脂肪酸(Ω-3PUFA)和Ω-6多不饱和脂肪酸(Ω-6PUFA)。α-亚麻酸属于Ω-3PUFA,小鼠缺失PUFA合成酶,卵泡中颗粒细胞数量显著下降,日粮补充Ω-3PUFA可显著增加颗粒细胞数量,促进卵泡生长[15]。Ω-3PUFA可以使后备母猪的初情期提前[16-17],缩短经产母猪的断奶发情间隔[18-19]。此外,日粮中补充Ω-3PUFA也能够显著提高母牛卵巢的有腔卵泡数和排卵数[20],增加奶牛排卵前卵泡大小,增加血液中及卵泡液中雌激素的含量[21-22]。苏行等[23]发现,雌二醇可以通过影响ABC转运蛋白相关基因的表达水平促进鹅颗粒细胞增殖和脂质沉积。亚麻油属于Ω-6PUFA,前人研究表明,母羊饲喂富含Ω-6PUFA的饲料能够促进颗粒细胞增殖,促进母羊发情[24]。范子玲等[25]发现, β-丙氨酸在乏情奶牛中显著降低,这表明β-丙氨酸可能参与了奶牛的卵泡发育。以上过程提示, 淫羊藿饲喂后备母猪可能通过黄酮类代谢物到达卵巢,影响卵巢脂质代谢和能量代谢,进而影响卵泡发育过程。
通过TCMSP数据库发现了22种淫羊藿的潜在有效成分,2种有效成分数据库中没有对应的靶点信息, 其中许多物质已报道与卵巢发育和功能密切相关。槲皮素能够降低卵巢氧化应激,缓解大鼠卵巢早衰,提高雌二醇的合成[26-27]。山柰酚能够促进蛋鸡卵巢发育[28],另有研究发现山柰酚能调节下丘脑-垂体-卵巢轴缓解PCOS的症状[29],谷甾醇通过PI3K-AKT信号通路促进人KGN细胞增殖,抑制凋亡[30],促进小鼠卵巢的雌二醇合成[31]。唐晓静[32]发现,木犀草素能够通过cAMP信号通路促进颗粒细胞雌二醇合成;张曦倩等[33]发现,木犀草素能够通过MAPK-ERK信号通路降低小鼠卵巢MDA水平,增加SOD和CAT活性,抑制颗粒细胞氧化应激诱导的凋亡。王彦予[34]研究表明,鼠李糖苷能够抑制卵巢癌。淫羊藿苷作为天然的非甾体类化合物,在许多植物和草本植物中广泛存在[35],尤以催情类中草药含量较高。它可与雌激素受体(ER)结合,发挥雌激素样作用,副作用小[36]。以上的研究表明,淫羊藿的有效成分均对卵巢发育或维持卵巢功能具有积极作用。其余有效成分还未见相关报道,可进一步研究其对卵巢细胞的作用。
通过网络药理学分析发现了淫羊藿作用卵巢的18个核心靶点。ESR1、ESR2属于雌激素受体,对于调节卵巢功能具有重要作用。CCNB1、CCNA2、CDK1、CDK2和CDK4属于细胞周期蛋白,与细胞活力和增殖密切相关。TP53是一种转录因子,可以磷酸化MAPK调控细胞功能, 也可作为反式激活剂参与细胞周期[37]。SRC是一种非受体酪氨酸激酶,参与控制多种生物活性的信号通路,包括基因转录、细胞黏附、细胞增殖、细胞凋亡等过程[38]。ERBB2(Erb-B2受体酪氨酸激酶2)在细胞核中参与转录调节,与PTGS5/COX-3启动子中的2′-TCAAATTC-2′序列结合并激活其转录,与CDKN1A的转录激活有关[39]。调节外周微管(MTs)的生长和稳定[40]。肿瘤坏死因子(TNF)可以与其受体TNFRSF1A/TNFR1和TNFRSF1B/TNFBR结合,从而发挥作用。TNF参与调节广泛的生物过程,包括细胞增殖、分化、细胞凋亡、脂质代谢和凝血[41]。EP300(E1A结合蛋白P300)作为组蛋白乙酰转移酶起作用,通过染色质重塑调节转录,在细胞增殖和分化过程中很重要。它通过特异性结合磷酸化的CREB蛋白来介导cAMP基因调控[42]。JAK2通过STATs磷酸化启动JAK-STAT信号通路,参与细胞生长、发育、分化或组蛋白修饰等过程[43]。PARP1(聚(ADP-核糖)聚合酶1)参与调节细胞分化、增殖和肿瘤转化,DNA损伤修复等过程[44]。PIK3CD(磷酸肌醇3激酶,PI3-Ks)是一类脂质激酶,能够磷酸化磷酸肌醇环的3′OH,激活PI3K-AKT信号通路,参与细胞增殖和存活。以上结果表明,本研究预测得到的靶点蛋白对于细胞的正常生理状态具有重要作用。以上蛋白可以作为淫羊藿影响后备母猪卵巢功能的潜在结合靶标,进一步进行机制验证。
综上所述,本研究结合活体试验、多组学与网络药理学,从活体、代谢、转录和分子层面揭示了淫羊藿对后备母猪发情的影响和作用途径,为淫羊藿治疗后备母猪发情异常,提高后备母猪发情利用率提供理论依据。
4 结论本研究从活体、代谢、转录和分子层面揭示了淫羊藿对后备母猪发情的影响和作用途径,发现饲喂淫羊藿能够改变后备母猪卵巢转录与代谢模式,显著提高血清FSH、LH和E2水平,进而促进母猪发情,淫羊藿的主要成分能够与TP53、SRC、AKT1、CCND1、TNF、ESR1、EP300、ERBB2、JAK2、PARP1结合发挥调控作用。本研究为淫羊藿应用提高后备母猪发情利用率提供理论依据。
| [1] |
贾振兴. 后备母猪的饲养管理技术要点[J]. 现代畜牧科技, 2020(9): 47-48. JIA Z X. Key points of breeding and management techniques for reserve sows[J]. Modern Animal Husbandry Science & Technology, 2020(9): 47-48. (in Chinese) |
| [2] |
吴亚凤. 母猪异常淘汰的原因与预防[J]. 现代畜牧科技, 2018(6): 32. WU Y F. Reasons and prevention of abnormal elimination of sows[J]. Modern Animal Husbandry Science & Technology, 2018(6): 32. (in Chinese) |
| [3] |
董若曦, 朱小丹, 张越, 等. 淫羊藿及其主要成分在卵巢早衰治疗中的应用进展[J]. 陕西中医, 2019, 40(5): 678-680. DONG R X, ZHU X D, ZHANG Y, et al. Progress in the application of Epimedium and its main components in the treatment of premature ovarian failure[J]. Shaanxi Journal of Traditional Chinese Medicine, 2019, 40(5): 678-680. (in Chinese) |
| [4] |
张越, 徐亚萍, 徐伟超, 等. 淫羊藿苷在猪卵泡体外培养过程中对颗粒细胞凋亡的抑制作用[J]. 畜牧与兽医, 2017, 49(12): 60-65. ZHANG Y, XU Y P, XU W C, et al. Anti-apoptosis property of icariin (ICA) to granulosa cells in porcine ovarian follicles cultured in vitro[J]. Animal Husbandry & Veterinary Medicine, 2017, 49(12): 60-65. (in Chinese) |
| [5] |
郭沈涛. 影响母猪生产性能的中草药添加剂研究进展[J]. 广东饲料, 2017, 26(5): 25-28. GUO S T. Research progress on Chinese herbal additives that affect sow production performance[J]. Guangdong Feed, 2017, 26(5): 25-28. (in Chinese) |
| [6] |
宋银朵. 后备母猪的饲养管理[J]. 养殖技术顾问, 2013(5): 28. SONG Y D. Breeding management of reserve gilts[J]. Breeding Technology Consultant, 2013(5): 28. (in Chinese) |
| [7] |
BARB C R, HAUSMAN G J, LENTS C A. Energy metabolism and leptin: effects on neuroendocrine regulation of reproduction in the gilt and sow[J]. Reprod Domest Anim, 2008, 43(Suppl 2): 324-330. |
| [8] |
吴炯树, 张毅, 文娱, 等. 淫羊藿苷对去卵巢小鼠血清雌二醇水平的影响[J]. 贵州医药, 2010, 34(1): 79-80. WU J S, ZHANG Y, WEN Y, et al. The effect of icariin on serum estradiol levels in ovariectomized mice[J]. Guizhou Medical Journal, 2010, 34(1): 79-80. (in Chinese) |
| [9] |
张森, 王新, 伊鹏霏, 等. 淫羊藿苷对性成熟雌性大鼠卵巢、子宫发育的影响[J]. 中兽医医药杂志, 2007, 26(2): 15-18. ZHANG S, WANG X, YI P F, et al. Effects of icariin on the development of ovary and uterus in sexually matured female rats[J]. Journal of Traditional Chinese Veterinary Medicine, 2007, 26(2): 15-18. (in Chinese) |
| [10] |
LIU S, JIA Y B, MENG S R, et al. Mechanisms of and potential medications for oxidative stress in ovarian granulosa cells: a review[J]. Int J Mol Sci, 2023, 24(11): 9205. DOI:10.3390/ijms24119205 |
| [11] |
朱波吕, 曹俊岩, 张丽. 淫羊藿苷调控PI3K/Akt/mTOR通路对早发性卵巢功能不全大鼠卵巢结构及功能的保护作用及机制[J]. 西部医学, 2023, 35(5): 632-638. ZHU B L, CAO J Y, ZHANG L. Protective effect and mechanism of icariin regulating PI3K/Akt/mTOR pathway on ovarian structure and function in rats with early-onset ovarian insufficiency[J]. Medical Journal of West China, 2023, 35(5): 632-638. (in Chinese) |
| [12] |
WANG L, TANG J H, WANG L, et al. Oxidative stress in oocyte aging and female reproduction[J]. J Cell Physiol, 2021, 236(12): 7966-7983. DOI:10.1002/jcp.30468 |
| [13] |
HU Y M, XU J J, SHI S J, et al. Fibroblast growth factor 21 (FGF21) promotes porcine granulosa cell estradiol production and proliferation via PI3K/AKT/mTOR signaling[J]. Theriogenology, 2022, 194: 1-12. DOI:10.1016/j.theriogenology.2022.09.020 |
| [14] |
李莉. 代谢组学联合转录组学研究淫羊藿素治疗骨关节炎的作用机制[D]. 长春: 吉林大学, 2023. LI L. Study on the mechanism of Icaritin against osteoarthritis based on metabolomics combined with transcriptomics[D]. Changchun: Jilin University, 2023. (in Chinese) |
| [15] |
STOFFEL W, SCHMIDT-SOLTAU I, BINCZEK E, et al. Dietary ω3-and ω6-Polyunsaturated fatty acids reconstitute fertility of Juvenile and adult Fads2-Deficient mice[J]. Mol Metab, 2020, 36: 100974. DOI:10.1016/j.molmet.2020.100974 |
| [16] |
MOREIRA F, CHEUICHE Z M G, RIZZOTO G, et al. Metabolic and reproductive parameters in prepubertal gilts after omega-3 supplementation in the diet[J]. Anim Reprod Sci, 2016, 170: 178-183. DOI:10.1016/j.anireprosci.2016.05.008 |
| [17] |
PETRONE R C, WILLIAMS K A, ESTIENNE M J. Effects of dietary menhaden oil on growth and reproduction in gilts farrowed by sows that consumed diets containing menhaden oil during gestation and lactation[J]. Animal, 2019, 13(9): 1944-1951. DOI:10.1017/S1751731119000193 |
| [18] |
CHEN J C, XU Q Q, LI Y X, et al. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets[J]. J Anim Sci, 2019, 97(10): 4256-4267. DOI:10.1093/jas/skz284 |
| [19] |
YADAV D, SINGH A K, KUMAR B, et al. Effect of n-3 PUFA-rich fish oil supplementation during late gestation on kidding, uterine involution and resumption of follicular activity in goat[J]. Reprod Domest Anim, 2019, 54(12): 1651-1659. DOI:10.1111/rda.13575 |
| [20] |
MOALLEM U, SHAFRAN A, ZACHUT M, et al. Dietary α-linolenic acid from flaxseed oil improved folliculogenesis and IVF performance in dairy cows, similar to eicosapentaenoic and docosahexaenoic acids from fish oil[J]. Reproduction, 2013, 146(6): 603-614. DOI:10.1530/REP-13-0244 |
| [21] |
AMBROSE D J, KASTELIC J P, CORBETT R, et al. Lower pregnancy losses in lactating dairy cows fed a diet enriched in α-linolenic acid[J]. J Dairy Sci, 2006, 89(8): 3066-3074. DOI:10.3168/jds.S0022-0302(06)72581-4 |
| [22] |
ROBINSON R S, PUSHPAKUMARA P G, CHENG Z, et al. Effects of dietary polyunsaturated fatty acids on ovarian and uterine function in lactating dairy cows[J]. Reproduction, 2002, 124(1): 119-131. DOI:10.1530/rep.0.1240119 |
| [23] |
苏行, 兰刚, 胡深强, 等. 雌二醇对鹅等级卵泡颗粒细胞的效应分析[J]. 农业生物技术学报, 2021, 29(11): 2166-2176. SU H, LAN G, HU S Q, et al. Effect of estradiol on granulosa cells of goose (Anser cygnoides domestica) grade follicles[J]. Journal of Agricultural Biotechnology, 2021, 29(11): 2166-2176. (in Chinese) |
| [24] |
WONNACOTT K E, KWONG W Y, HUGHES J, et al. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos[J]. Reproduction, 2010, 139(1): 57-69. DOI:10.1530/REP-09-0219 |
| [25] |
范子玲, 宋玉锡, 张江, 等. 基于1H-NMR技术的产后卵巢静止奶牛乳清和血清代谢谱分析[J]. 畜牧兽医学报, 2018, 49(12): 2602-2611. FAN Z L, SONG Y X, ZHANG J, et al. Analysis of metabolic profiles of whey and serum in postpartum dairy cows with ovary quiescence based on1H-NMR[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(12): 2602-2611. (in Chinese) |
| [26] |
黄长盛, 贺守第, 管雁丞, 等. 菟丝子黄酮和槲皮素对雷公藤多苷致卵巢早衰大鼠卵巢功能的影响[J]. 中国临床药理学杂志, 2020, 36(6): 667-670. HUANG C S, HE S D, GUAN Y C, et al. Effects of dodder flavonoids and quercetin on the ovarian function in rat model of premature ovarian failure induced by tripterygium glycosides[J]. The Chinese Journal of Clinical Pharmacology, 2020, 36(6): 667-670. (in Chinese) |
| [27] |
严如根, 王雨琦, 何静, 等. 中药单体改善卵巢早衰的作用机制研究进展[J]. 中国药房, 2022, 33(21): 2685-2688. YAN R G, WANG Y Q, HE J, et al. Advances of the mechanism study on Chinese herb monomers in improving premature ovarian failure[J]. China Pharmacy, 2022, 33(21): 2685-2688. (in Chinese) |
| [28] |
王明迪. 淫羊藿总黄酮对蛋鸡卵泡发育的影响[D]. 保定: 河北农业大学, 2022. WANG M D. Effect of total flavonoids of herba epimedii on follicular development in layers[D]. Baoding: Hebei Agricultural University, 2022. (in Chinese) |
| [29] |
赵心瑜, 秦璐璐, 刘文琼. 基于脂代谢异常探讨从痰论治多囊卵巢综合征作用机制[J]. 陕西中医, 2022, 43(7): 921-924. ZHAO X Y, QIN L L, LIU W Q. To explore the mechanism of treating polycystic ovary syndrome from phlegm based on abnormal lipid metabolism[J]. Shaanxi Journal of Traditional Chinese Medicine, 2022, 43(7): 921-924. (in Chinese) |
| [30] |
赵帅, 陈冬梅, 虎娜, 等. β-谷甾醇通过Pl3K/AKT通路影响颗粒细胞增殖及凋亡[J]. 宁夏医科大学学报, 2021, 43(4): 339-344. ZHAO S, CHEN D M, HU N, et al. The effect of β-sitosterol on KGN cell proliferation and apoptosis through PI3K/AKT pathway[J]. Journal of Ningxia Medical University, 2021, 43(4): 339-344. (in Chinese) |
| [31] |
郝海鑫, 谢心美, 何剑斌. 植物甾醇对去卵巢KM小鼠血清生殖激素及乳腺组织孕激素受体的影响[J]. 现代畜牧兽医, 2014(5): 4-8. HAO H X, XIE X M, HE J B. Effect of phytosterol on the serum reproductive hormone and progesterone receptor in mammary tissue of ovariectomized KM mice[J]. Modern Journal of Animal Husbandry and Veterinary Medicine, 2014(5): 4-8. (in Chinese) |
| [32] |
唐晓静. 党参炔苷和木犀草素对卵巢颗粒细胞激素分泌的影响及其作用机制[D]. 北京: 中央民族大学, 2015. TANG X J. The effects and mechanisms of Codonopsis pilosula and Luteolin on hormone secretion in ovarian granulosa cells[D]. Beijing: Minzu University of China, 2015. (in Chinese) |
| [33] |
张曦倩, 姚俐, 罗燕群, 等. 木犀草素缓解双酚A诱导的小鼠卵巢毒性作用研究[J]. 新医学, 2020, 51(7): 539-543. ZHANG X Q, YAO L, LUO Y Q, et al. Effect of luteolin on bisphenol A-induced ovarian toxicity in mouse models[J]. New Medicine, 2020, 51(7): 539-543. (in Chinese) |
| [34] |
王彦予. 北桑寄生化学成分研究[D]. 济南: 山东中医药大学, 2022. WANG Y Y. Study on the chemical constituents of Loranthus tanakae[D]. Ji'nan: Shandong University of Traditional Chinese Medicine, 2022. (in Chinese) |
| [35] |
RIETJENS I M C M, LOUISSE J, BEEKMANN K. The potential health effects of dietary phytoestrogens[J]. Br J Pharmacol, 2017, 174(11): 1263-1280. DOI:10.1111/bph.13622 |
| [36] |
SIROTKIN A V, HARRATH A H. Phytoestrogens and their effects[J]. Eur J Pharmacol, 2014, 741: 230-236. DOI:10.1016/j.ejphar.2014.07.057 |
| [37] |
MOGI A, KUWANO H. TP53 mutations in nonsmall cell lung cancer[J]. J Biomed Biotechnol, 2011, 2011: 583929. |
| [38] |
MA Y C, HUANG X Y. Novel regulation and function of Src tyrosine kinase[J]. Cell Mol Life Sci, 2002, 59(3): 456-462. DOI:10.1007/s00018-002-8438-2 |
| [39] |
SADEK B T, HOMAYOUNFAR G, ABI RAAD R F, et al. Is a higher boost dose of radiation necessary after breast-conserving therapy for patients with breast cancer with final close or positive margins?[J]. Breast Cancer Res Treat, 2015, 154(1): 71-79. DOI:10.1007/s10549-015-3579-9 |
| [40] |
ZAOUI K, BENSEDDIK K, DAOU P, et al. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells[J]. Proc Natl Acad Sci U S A, 2010, 107(43): 18517-18522. DOI:10.1073/pnas.1000975107 |
| [41] |
PROBERT L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects[J]. Neuroscience, 2015, 302: 2-22. DOI:10.1016/j.neuroscience.2015.06.038 |
| [42] |
CHANG K L, XUE R Q, ZHAO M L, et al. EP300/CBP is crucial for cAMP-PKA pathway to alleviate podocyte dedifferentiation via targeting Notch3 signaling[J]. Exp Cell Res, 2021, 407(2): 112825. DOI:10.1016/j.yexcr.2021.112825 |
| [43] |
PERNER F, PERNER C, ERNST T, et al. Roles of JAK2 in aging, inflammation, hematopoiesis and malignant transformation[J]. Cells, 2019, 8(8): 854. DOI:10.3390/cells8080854 |
| [44] |
ALEMASOVA E E, LAVRIK O I. Poly(ADP-ribosyl)ation by PARP1:reaction mechanism and regulatory proteins[J]. Nucleic Acids Res, 2019, 47(8): 3811-3827. DOI:10.1093/nar/gkz120 |
(编辑 郭云雁)



