卵母细胞冷冻技术在家畜繁殖和种质资源保存中具有重要的应用价值。首先,冷冻有效延长了优良母畜卵子的保存时间且提高了卵母细胞运输和利用的便利性[1-4],降低了活体运输带来的疾病传播风险[5],提高了优良种质的利用效率,降低了育种成本[6]。其次,将卵母细胞冷冻技术用于构建卵子资源库,可以永久保存濒危物种的遗传资源,为濒危动物复原提供了可能性[7]。此外,冷冻卵母细胞还可为女性提供自由选择生育时期的机会,保护患癌女性的生育能力[8]。因此,自上世纪70年代末卵母细胞冷冻获得成功以来,该技术得到了大力发展,已被成功用于猪[5]、牛[9]、羊[10]、兔[11]、鼠[12]等哺乳动物。
然而,大量研究表明,由于冷冻会引起质膜破裂[13-17]、活性氧升高[18]、凋亡增加[19]、纺锤体[20]、内质网和线粒体等细胞器功能异常[21-22]等一系列细胞损伤,冷冻卵母细胞的发育能力仍低于新鲜卵母细胞[13-15]。众多研究指出,脂质含量过高是引起家畜卵母细胞冷冻效果下降的重要原因[23-25]。牛卵母细胞中脂质含量非常丰富,是小鼠的2.8倍[26]。卵母细胞中丰富的脂质主要以三酰甘油的形式储存于脂滴中[27]。在冷冻降温过程中,脂质易发生脂质相变[28-30]和脂质过氧化[31],导致内质网、线粒体等细胞器损伤[32],降低卵母细胞发育能力[28, 33-34]。另外,多项研究表明,通过离心和显微操作方法或添加化学物质降低卵母细胞脂质含量后,其冷冻后发育能力有所提高[35-37]。由此可见,冷冻前降低脂质含量是提高家畜卵母细胞冷冻效率的有效途径。
β-烟酰胺单核苷酸(nicotinamide mononucleo tide,NMN)是一种具有生物活性的核苷酸[38]。在哺乳动物体内,NMN是合成烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD)的主要前体物质之一[38],可通过补充NAD+水平参与能量代谢[39]、应激反应[40]、DNA损伤修复[41]和基因表达[42]等重要生理过程。据报道,NMN对糖尿病[43]、肥胖[44]、非酒精性脂肪肝[45]、脑损伤[46]、衰老[39, 47]等疾病均有显著的改善作用。近年来,有研究指出,NMN可以促进细胞脂质代谢,对细胞脂质沉积也具有显著的抑制效果[48],但其对牛卵母细胞是否具有同样的作用仍有待验证。
因此,本试验通过在体外成熟液中添加NMN,对牛卵母细胞进行体外成熟(in vitro maturation,IVM)培养,并对牛卵母细胞脂滴含量、活性氧(reactive oxygen species,ROS)水平、细胞凋亡水平、冷冻-解冻后存活率以及囊胚发育率进行检测,以探究NMN对牛卵母细胞脂滴含量及冷冻效果的影响,以期为提高牛卵母细胞的冷冻效率奠定一定理论基础。
1 材料与方法 1.1 试验分组本试验主要分组如下:体外成熟液未添加NMN为新鲜对照组(fresh control)、体外成熟液添加NMN为NMN组(NMN)、体外成熟液未添加NMN但进行玻璃化冷冻作为玻璃化对照组(vitrification control)、体外成熟液添加NMN且进行玻璃化作为NMN玻璃化组(NMN + vitrification)。
1.2 主要试剂除特别说明外,本试验所用试剂均采购自Sigma公司。TCM-199和胎牛血清(foetal bovine serum,FBS)购自Gibco公司。NMN和尼罗红染料购自北京索莱宝科技有限公司。ROS检测试剂盒和Annexin V-FITC细胞凋亡检测试剂盒购自上海碧云天生物技术有限公司。
1.3 试验方法1.3.1 卵母细胞采集与IVM 本试验所用卵母细胞均来自屠宰场所屠宰动物的卵巢。将屠宰场卵巢置于含青链霉素的37 ℃生理盐水中,2 h内运送到实验室。从直径为2~8 mm的卵泡中抽取卵丘-卵母细胞复合体(cumulus-oocyte complexes,COCs),置于体式显微镜下挑选至少含有3层卵丘细胞的COCs用于后续试验。将符合标准的COCs放入含体外成熟液的4孔板中(50个·孔-1),置于38.5 ℃,5% CO2培养箱中成熟22~24 h。本试验中,NMN浓度为1 μmol ·L-1,对照组不添加NMN。
1.3.2 玻璃化冷冻与解冻 根据Zhao等[49]的方法,采用OPS管进行玻璃化冷冻。将卵母细胞置于预处理液中孵育30 s,转入冷冻液中,并迅速吸入OPS管中,25 s内投入液氮中。玻璃化冷冻溶液根据Hou等[50]的方法配制并稍作改动。预处理液配制:DMSO ∶EG ∶FBS ∶DPBS按1 ∶1 ∶1 ∶7(v/v)配制。玻璃化冷冻液配制:DMSO ∶EG ∶FSF按照2 ∶2 ∶6(v/v)配制,其中FSF由DPBS、0.5 mol ·L-1蔗糖、300 g ·L-1聚蔗糖、20% FBS组成。
解冻处理:从液氮中取出OPS管,快速将卵母细胞吹入0.5 mol ·L-1蔗糖溶液中,孵育5 min,再转移至0.25 mol ·L-1蔗糖溶液中,孵育5 min。将解冻后的卵母细胞置于10% FBS-M199中洗涤3次,放入38.5 ℃,5% CO2培养箱中静置1.5~2 h,恢复细胞形态,挑选细胞形态完整、正常的卵母细胞用于后续试验。
1.3.3 体外受精 根据Brackett和Oliphant[51]的方法稍作改动,进行卵母细胞体外受精操作。首先,将成熟后的卵母细胞置于1 mg ·mL-1的透明质酸酶溶液中,反复吹打,脱去多余的颗粒细胞,仅保留1~2层。随后,将冻精置于38 ℃水浴解冻,7 mL洗精液洗涤两次,离心条件为1 800 r ·min-1,5 min。精子沉淀用受精液重悬,调整精子密度为5×106个·mL-1。取20 μL重悬的精液与80 μL受精液混匀,制备成受精滴,放入恒温培养箱平衡1.5 h。将卵母细胞转移至受精滴中,放入38.5 ℃、5% CO2培养箱中,培养16~18 h。受精结束后的受精卵转移至胚胎前期培养液中继续培养,48 h后统计卵裂率,并转入后期培养液中继续培养,每隔48 h半量换液,直到受精后第7天(受精当天为第0天),统计囊胚率。
1.3.4 脂滴含量检测 成熟22~24 h后,将COCs置于1 mg ·mL-1的透明质酸酶溶液中,反复吹打,脱去颗粒细胞。卵母细胞置于0.1% PVA-PBS溶液中清洗3次,置于10 μg ·mL-1尼罗红染色液中,37 ℃,避光染色10 min。随后,卵母细胞用0.1% PVA-PBS溶液清洗3次,置于倒置荧光显微镜(尼康,日本)下拍照。采集的荧光图像利用EZ-C1 Free Viewer software (尼康,日本)软件进行荧光值统计。
1.3.5 ROS检测 成熟22~24 h后,将COCs置于1 mg ·mL-1的透明质酸酶溶液中,反复吹打,脱去颗粒细胞。根据制造商说明书,利用活性氧检测试剂盒(S0033 S,碧云天)检测卵母细胞ROS水平。卵母细胞经0.1% PVA-PBS洗涤3次后,置于10 mmol ·L-1DCFH-DA染色液中,37 ℃避光染色20 min。染色完成后,卵母细胞用0.1% PVA-PBS溶液清洗3次,置于倒置荧光显微镜(尼康,日本)下拍照。采集的荧光图像利用EZ-C1 Free Viewer software (尼康,日本)进行荧光值统计。
1.3.6 细胞凋亡检测 成熟22~24 h后,将COCs置于1 mg ·mL-1的透明质酸酶溶液中,反复吹打,脱去颗粒细胞。根据制造商说明书,利用Annexin V-FITC细胞凋亡检测试剂盒(C1062 M,碧云天)检测卵母细胞早期凋亡水平。首先,根据说明书将Annexin V-FITC、碘化丙啶染色液和Annexin V-FITC结合液混匀,制备成100 μL染色滴。随后,将卵母细胞用0.1% PVA-PBS洗涤3次后,转移至染色滴中,室温,避光染色20 min。染色完成后,卵母细胞用0.1% PVA-PBS溶液清洗3次,置于倒置荧光显微镜(尼康,日本)下拍照。
1.4 数据统计与分析本试验均重复3次。采用SAS8.2软件进行单因素方差分析,并使用Duncan's检验法进行显著性分析,结果以“平均数±标准差”表示,P<0.05表示差异显著。
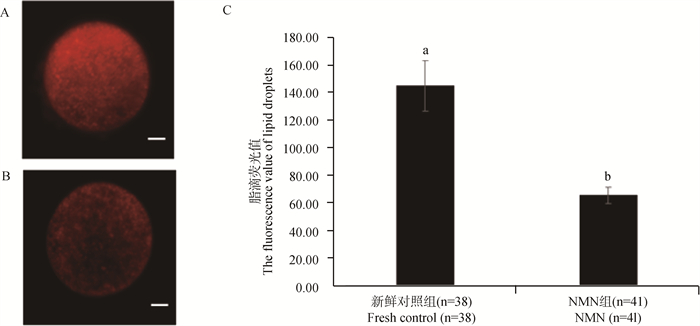
2 结果 2.1 NMN对牛卵母细胞脂滴含量的影响为了研究NMN对牛卵母细胞脂滴含量的影响,本研究采用尼罗红染料检测了牛卵母细胞脂滴含量的变化。图 1A和1B分别为新鲜对照组和NMN组卵母细胞的脂滴染色图。如图 1C所示,与新鲜对照组相比,NMN组的脂滴荧光强度显著降低(P<0.05),表明IVM期间添加NMN可以有效降低牛卵母细胞脂滴含量。
|
A. 新鲜对照组卵母细胞脂滴尼罗红荧光图像,标尺=20 μm;B. NMN处理组卵母细胞脂滴尼罗红荧光图像,标尺=20 μm;C. 卵母细胞脂滴荧光值。不同字母表示差异显著(P<0.05),下同 A. Nile red fluorescence image of lipid droplets in oocytes from the fresh control group, bar=20 μm; B. Nile red fluorescence image of lipid droplets in oocytes from the NMN group, bar=20 μm; C. The fluorescence value of lipid droplets in oocytes. Different letters indicate significant differences(P < 0.05), the same as below 图 1 卵母细胞脂滴检测 Fig. 1 Determination of lipid droplets in oocytes |
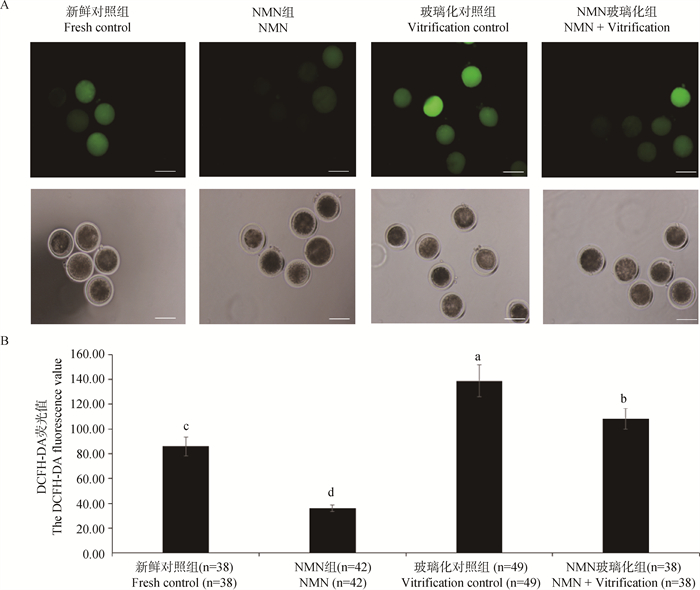
为了研究NMN对牛卵母细胞ROS水平的影响,本研究采用ROS检测试剂盒检测了牛卵母细胞ROS水平。牛卵母细胞DCFH-DA荧光染色图像如图 2A所示。由图 2B可知,NMN组牛卵母细胞的DCFH-DA荧光强度显著低于新鲜对照组(P<0.05),并且NMN玻璃化组卵母细胞DCFH-DA荧光强度也显著低于玻璃化对照组(P<0.05),说明IVM期间添加NMN能有效降低牛卵母细胞ROS水平。
|
A. 卵母细胞DCFH-DA荧光图像,标尺=100 μm;B. 卵母细胞DCFH-DA荧光值 A. The DCFH-DA fluorescence image of oocytes, bar=100 μm; B. The DCFH-DA fluorescence value of oocytes 图 2 卵母细胞ROS水平检测 Fig. 2 Determination of ROS level in oocytes |
为了探究NMN对牛卵母细胞凋亡水平的影响,本研究采用Annexin V-FITC细胞凋亡试剂盒检测了牛卵母细胞早期凋亡水平。卵母细胞Annexin V-FITC荧光图像如图 3A-C所示,其中图 3A为未凋亡卵母细胞,图 3B为早期凋亡细胞,图 3C为坏死细胞。如图 3D所示,NMN组卵母细胞凋亡水平显著低于新鲜对照组(P<0.05),同时,NMN玻璃化组卵母细胞凋亡水平也显著低于玻璃化对照组(P<0.05),说明IVM期间添加NMN可以有效抑制牛卵母细胞凋亡。

|
A. 正常卵母细胞Annexin V-FITC荧光图像,标尺=20 μm;B. 早期凋亡卵母细胞Annexin V-FITC荧光图像, 标尺=20 μm;C. 坏死卵母细胞Annexin V-FITC荧光图像,标尺=20 μm;D. 卵母细胞早期凋亡率 A. The Annexin V-FITC fluorescence image of normal oocytes, bar=20 μm; B. The Annexin V-FITC fluorescence image of early apoptotic oocytes, bar=20 μm; C. The Annexin V-FITC fluorescence image of necrotic oocytes, bar=20 μm; D. Early apoptosis rate of oocytes 图 3 卵母细胞早期凋亡检测 Fig. 3 Detection of early apoptosis in oocytes |
为了探究NMN对牛卵母细胞冷冻效果的影响,本研究统计了玻璃化牛卵母细胞IVF后的卵裂率和囊胚率。如表 1所示,NMN组的卵裂率((89.57±7.58)%)和囊胚率((45.63±3.78)%)均显著高于新鲜对照组((80.77±0.70)%,(36.90±1.20)%,P<0.05),且NMN玻璃化组卵母细胞冷冻后的存活率((97.25±0.11)%)、卵裂率((75.47±1.11)%)和囊胚率((33.75±1.43)%)均显著高于玻璃化对照组((89.29±1.16)%、(52.00±1.26)%、(15.38±1.73)%,P < 0.05),表明NMN显著提高了玻璃化牛卵母细胞的发育能力。
|
|
表 1 添加NMN对牛卵母细胞玻璃化冷冻后发育能力的影响 Table 1 Effect of adding NMN on the developmental ability of bovine oocytes after vitrification |
脂质含量过高一直被认为是家畜卵母细胞低温保存失败的重要原因之一,冷冻前降低脂质含量是提高卵母细胞解冻后发育能力的有效措施之一[25]。卵母细胞中的脂质主要储存在脂滴中,可被尼罗红染料特异性结合[35]。因此,本试验通过尼罗红荧光探针检测了NMN对牛卵母细胞脂滴含量的影响。本研究结果表明,IVM期间添加NMN有效降低了牛卵母细胞的脂滴含量。与本研究结果相似,Wang等[52]也发现NMN处理可有效降低小鼠卵母细胞的脂滴含量,且NMN可通过抑制脂质合成、转运及吸收相关基因的表达,促进脂质氧化相关基因的表达,减少肝脏细胞脂质沉积[48]。以往的研究表明,小檗碱[53]、左旋肉碱[54-55]、共轭亚油酸[56]等物质均可通过降低脂滴含量而改善卵母细胞或胚胎的冷冻效果。本研究表明,NMN可通过减少牛卵母细胞脂滴含量而改善其冷冻效果。
相关研究表明,玻璃化会导致卵母细胞线粒体结构和功能受损,膜电位降低,产生大量的ROS[57]。高浓度的ROS易与脂质结合发生脂质过氧化反应产生过量的脂氢过氧化物,进一步加剧线粒体等细胞器功能损伤,最终导致卵母细胞发育能力下降[58-60]。研究发现,IVM期间添加左旋肉碱不仅降低了猪卵母细胞的脂滴含量,还有效降低了ROS水平[55]。Takahashi等[61]也报道,左旋肉碱可以有效降低牛胚胎脂滴含量且减少2细胞胚胎的ROS含量。Sun等[62]还在大鼠肝脏细胞中发现,小檗碱在减少其脂质沉积的同时抑制了ROS的生成。以上研究表明,降低脂滴含量有助于减少细胞内ROS的产生。因此,本试验检测了IVM期间添加NMN对牛卵母细胞ROS水平的影响。本研究结果表明,与对照组相比,NMN有效降低了牛卵母细胞中的ROS水平。与本研究结果相似,Wang等[52]研究也表明,NMN可以促进抗氧化基因SOD1的表达,有效降低衰老小鼠卵母细胞ROS水平[63]。Song等[64]研究还表明,NMN可通过减少氧化应激从而改善猪卵母细胞的发育能力。本研究表明,NMN能有效降低玻璃化牛卵母细胞ROS水平。
研究表明,脂质过度积累造成的氧化应激和内质网应激最终会引起细胞凋亡[65-68]。同时,有研究报道,去脂物质(小檗碱)可以有效减少高脂引起的细胞凋亡损伤[65]。因此,本试验还检测了NMN对牛卵母细胞凋亡水平的影响。当细胞发生凋亡时,磷酯酰丝氨酸会从细胞内部翻转到细胞外部,这种现象被视为细胞早期凋亡标志之一,广泛应用于卵母细胞早期凋亡水平检测[69-71]。因此,本试验检测了IVM期间添加NMN对玻璃化牛卵母细胞磷酯酰丝氨酸外翻的影响。结果显示,IVM期间添加NMN有效抑制了玻璃化牛卵母细胞的早期凋亡。与本研究结果相似,Meng等[72]报道NMN可以有效降低细胞凋亡水平,Wan等[73]也报道NMN可以显著降低促凋亡基因BAX的表达,促进抗凋亡基因BCL2的表达,从而抑制细胞凋亡。本研究结果表明,NMN能有效减少玻璃化引起的卵母细胞凋亡损伤。
此外,本研究结果表明,IVM期间添加NMN不仅可以促进牛卵母细胞的发育,而且可以提高玻璃化牛卵母细胞的发育能力。与本研究结果相似,小鼠和猪上的研究也表明,NMN可通过降低ROS水平、恢复线粒体功能、减少DNA损伤等途径提高卵母细胞的发育能力[74-75]。
4 结论本研究结果表明,IVM期间添加NMN可有效降低牛卵母细胞脂滴含量、ROS水平和凋亡水平,从而改善牛卵母细胞的冷冻效果。本研究结果可为提高家畜卵母细胞耐冻性和促进卵母细胞冷冻技术的应用与推广奠定一定理论基础。
| [1] |
ARGYLE C E, HARPER J C, DAVIES M C. Oocyte cryopreservation: where are we now?[J]. Hum Reprod Update, 2016, 22(4): 440-449. DOI:10.1093/humupd/dmw007 |
| [2] |
RIENZI L, GRACIA C, MAGGIULLI R, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance[J]. Hum Reprod Update, 2017, 23(2): 139-155. |
| [3] |
THARASANIT T, THUWANUT P. Oocyte cryopreservation in domestic animals and humans: principles, techniques and updated outcomes[J]. Animals (Basel), 2021, 11(10): 2949. |
| [4] |
ANGEL-VELEZ D, DE COSTER T, AZARI-DOLATABAD N, et al. New alternative mixtures of cryoprotectants for equine immature oocyte vitrification[J]. Animals (Basel), 2021, 11(11): 3077. |
| [5] |
SOMFAI T, KIKUCHI K. Vitrification of porcine oocytes and zygotes in microdrops on a solid metal surface or liquid nitrogen[M]//WOLKERS W F, OLDENHOF H. Cryopreservation and Freeze-Drying Protocols. New York: Springer, 2021: 455-468.
|
| [6] |
MORRELL J M, MAYER I. Reproduction biotechnologies in germplasm banking of livestock species: a review[J]. Zygote, 2017, 25(5): 545-557. DOI:10.1017/S0967199417000442 |
| [7] |
MAYER I. The role of reproductive sciences in the preservation and breeding of commercial and threatened teleost fishes[M]//COMIZZOLI P, BROWN J L, HOLT W V. Reproductive Sciences in Animal Conservation. Cham: Springer, 2019: 187-224.
|
| [8] |
WALKER Z, LANES A, GINSBURG E. Oocyte cryopreservation review: outcomes of medical oocyte cryopreservation and planned oocyte cryopreservation[J]. Reprod Biol Endocrinol, 2022, 20(1): 10. DOI:10.1186/s12958-021-00884-0 |
| [9] |
SOMFAI T, HIRAO Y. Vitrification of immature bovine oocytes in protein-free media: the impact of the cryoprotectant treatment protocol, base medium, and ovary storage[J]. Theriogenology, 2021, 172: 47-54. DOI:10.1016/j.theriogenology.2021.05.029 |
| [10] |
MOAWAD A R, CHOI I, ZHU J, et al. Caffeine and oocyte vitrification: sheep as an animal model[J]. Int J Vet Sci Med, 2018, 6(S1): S41-S48. |
| [11] |
JIMéNEZ-TRIGOS E, VICENTE J S, MARCO-JIMÉNEZ F. First pregnancy and live birth from vitrified rabbit oocytes after intraoviductal transfer and in vivo fertilization[J]. Theriogenology, 2014, 82(4): 599-604. DOI:10.1016/j.theriogenology.2014.05.029 |
| [12] |
KAMOSHITA M, FUJIWARA K, ITO J, et al. Highly successful production of viable mice derived from vitrified germinal vesicle oocytes[J]. PLoS One, 2021, 16(3): : e0248050. DOI:10.1371/journal.pone.0248050 |
| [13] |
HAO T, ZHANG P P, HAO H S, et al. The combination treatment of cholesterol-loaded methyl-β-cyclodextrin and methyl-β-cyclodextrin significantly improves the fertilization capacity of vitrified bovine oocytes by protecting fertilization protein JUNO[J]. Reprod Domest Anim, 2021, 56(3): 519-530. DOI:10.1111/rda.13890 |
| [14] |
HOCHI S, IDE M, UENO S, et al. High survival of bovine mature oocytes after nylon mesh vitrification, as assessed by intracytoplasmic sperm injection[J]. J Reprod Dev, 2022, 68(5): 335-339. DOI:10.1262/jrd.2022-053 |
| [15] |
KAGAWA S, HIRAIZUMI S, BAI H, et al. Cattle production by intracytoplasmic sperm injection into oocytes vitrified after ovum pick-up[J]. Theriogenology, 2022, 185: 121-126. DOI:10.1016/j.theriogenology.2022.03.022 |
| [16] |
UM D E, SHIN H, PARK D, et al. Molecular analysis of lipid uptake- and necroptosis-associated factor expression in vitrified-warmed mouse oocytes[J]. Reprod Biol Endocrinol, 2020, 18(1): 37. DOI:10.1186/s12958-020-00588-x |
| [17] |
RUIZ-CONCA M, VENDRELL M, SABÉS-ALSINA M, et al. Coenzyme Q10 supplementation during in vitro maturation of bovine oocytes (Bos taurus) helps to preserve oocyte integrity after vitrification[J]. Reprod Domest Anim, 2017, 52(S4): 52-54. |
| [18] |
GARCÍA-MARTÍNEZ T, VENDRELL-FLOTATS M, MARTÍNEZ-RODERO I, et al. Glutathione ethyl ester protects in vitro-maturing bovine oocytes against oxidative stress induced by subsequent vitrification/warming[J]. Int J Mol Sci, 2020, 21(20): 7547. DOI:10.3390/ijms21207547 |
| [19] |
ANCHAMPARUTHY V M, PEARSON R E, GWAZDAUSKAS F C. Expression pattern of apoptotic genes in vitrified-thawed bovine oocytes[J]. Reprod Domest Anim, 2010, 45(5): e83-e90. |
| [20] |
ARCARONS N, VENDRELL-FLOTATS M, YESTE M, et al. Cryoprotectant role of exopolysaccharide of Pseudomonas sp.ID1 in the vitrification of IVM cow oocytes[J]. Reprod Fertil Dev, 2019, 31(9): 1507-1519. DOI:10.1071/RD18447 |
| [21] |
WANG N, HAO H S, LI C Y, et al. Calcium ion regulation by BAPTA-AM and ruthenium red improved the fertilisation capacity and developmental ability of vitrified bovine oocytes[J]. Sci Rep, 2017, 7(1): 10652. DOI:10.1038/s41598-017-10907-9 |
| [22] |
ZHAO N, LIU X J, LI J T, et al. Endoplasmic reticulum stress inhibition is a valid therapeutic strategy in vitrifying oocytes[J]. Cryobiology, 2015, 70(1): 48-52. DOI:10.1016/j.cryobiol.2014.12.001 |
| [23] |
ZHOU G B, LI N. Bovine oocytes cryoinjury and how to improve their development following cryopreservation[J]. Anim Biotechnol, 2013, 24(2): 94-106. DOI:10.1080/10495398.2012.755466 |
| [24] |
SPRÍCIGO J F, MORATÓ R, ARCARONS N, et al. Assessment of the effect of adding L-carnitine and/or resveratrol to maturation medium before vitrification on in vitro-matured calf oocytes[J]. Theriogenology, 2017, 89: 47-57. DOI:10.1016/j.theriogenology.2016.09.035 |
| [25] |
AMSTISLAVSKY S, MOKROUSOVA V, BRUSENTSEV E, et al. Influence of cellular lipids on cryopreservation of mammalian oocytes and preimplantation embryos: a review[J]. Biopreserv Biobank, 2019, 17(1): 76-83. DOI:10.1089/bio.2018.0039 |
| [26] |
GENICOT G, LEROY J L M R, SOOM A V, et al. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes[J]. Theriogenology, 2005, 63(4): 1181-1194. DOI:10.1016/j.theriogenology.2004.06.006 |
| [27] |
DUNNING K R, RUSSELL D L, ROBKER R L. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation[J]. Reproduction, 2014, 148(1): R15-R27. DOI:10.1530/REP-13-0251 |
| [28] |
ARAV A, ZERON Y, LESLIE S B, et al. Phase transition temperature and chilling sensitivity of bovine oocytes[J]. Cryobiology, 1996, 33(6): 589-599. DOI:10.1006/cryo.1996.0062 |
| [29] |
DE KRUYFF B, VAN DIJCK P W M, GOLDBACH R W, et al. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii[J]. Biochim Biophys Acta, 1973, 330(3): 269-282. DOI:10.1016/0005-2736(73)90232-0 |
| [30] |
YESTE M. Sperm cryopreservation update: cryodamage, markers, and factors affecting the sperm freezability in pigs[J]. Theriogenology, 2016, 85(1): 47-64. DOI:10.1016/j.theriogenology.2015.09.047 |
| [31] |
KIM J G, PARTHASARATHY S. Oxidation and the spermatozoa[J]. Semin Reprod Med, 1998, 16(4): 235-239. DOI:10.1055/s-2007-1016283 |
| [32] |
WU L L Y, DUNNING K R, YANG X, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates[J]. Endocrinology, 2010, 151(11): 5438-5445. DOI:10.1210/en.2010-0551 |
| [33] |
MAREI W F A, VAN DEN BOSCH L, PINTELON I, et al. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model[J]. Hum Reprod, 2019, 34(10): 1984-1998. DOI:10.1093/humrep/dez161 |
| [34] |
AARDEMA H, VOS P L A M, LOLICATO F, et al. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence[J]. Biol Reprod, 2011, 85(1): 62-69. DOI:10.1095/biolreprod.110.088815 |
| [35] |
FU X W, WU G Q, LI J J, et al. Positive effects of forskolin (stimulator of lipolysis) treatment on cryosurvival of in vitro matured porcine oocytes[J]. Theriogenology, 2011, 75(2): 268-275. DOI:10.1016/j.theriogenology.2010.08.013 |
| [36] |
CHANKITISAKUL V, SOMFAI T, INABA Y, et al. Supplementation of maturation medium with L-carnitine improves cryo-tolerance of bovine in vitro matured oocytes[J]. Theriogenology, 2013, 79(4): 590-598. DOI:10.1016/j.theriogenology.2012.11.011 |
| [37] |
REN L, FU B, MA H, et al. Effects of mechanical delipation in porcine oocytes on mitochondrial distribution, ROS activity and viability after vitrification[J]. Cryo Letters, 2015, 36(1): 30-36. |
| [38] |
PODDAR S K, SIFAT A E, HAQUE S, et al. Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule[J]. Biomolecules, 2019, 9(1): 34. DOI:10.3390/biom9010034 |
| [39] |
MILLS K F, YOSHIDA S, STEIN L R, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice[J]. Cell Metab, 2016, 24(6): 795-806. DOI:10.1016/j.cmet.2016.09.013 |
| [40] |
KLIMOVA N, FEARNOW A, LONG A, et al. NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms[J]. Exp Neurol, 2020, 325: 113144. DOI:10.1016/j.expneurol.2019.113144 |
| [41] |
BRAIDY N, BERG J, CLEMENT J, et al. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes[J]. Antioxid Redox Signal, 2019, 30(2): 251-294. DOI:10.1089/ars.2017.7269 |
| [42] |
KISS T, GILES C B, TARANTINI S, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects[J]. GeroScience, 2019, 41(4): 419-439. DOI:10.1007/s11357-019-00095-x |
| [43] |
NAHLE A, JOSEPH Y D, PEREIRA S, et al. Nicotinamide mononucleotide prevents free fatty acid-induced reduction in glucose tolerance by decreasing insulin clearance[J]. Int J Mol Sci, 2021, 22(24): 13224. DOI:10.3390/ijms222413224 |
| [44] |
UDDIN G M, YOUNGSON N A, DOYLE B M, et al. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise[J]. Sci Rep, 2017, 7(1): 15063. DOI:10.1038/s41598-017-14866-z |
| [45] |
DALL M, HASSING A S, TREEBAK J T. NAD+ and NAFLD-caution, causality and careful optimism[J]. J Physiol, 2022, 600(5): 1135-1154. DOI:10.1113/JP280908 |
| [46] |
WANG X N, HU X J, ZHANG L, et al. Nicotinamide mononucleotide administration after sever hypoglycemia improves neuronal survival and cognitive function in rats[J]. Brain Res Bull, 2020, 160: 98-106. DOI:10.1016/j.brainresbull.2020.04.022 |
| [47] |
NADEESHANI H, LI J Y, YING T L, et al. Nicotinamide mononucleotide (NMN) as an anti-aging health product-promises and safety concerns[J]. J Adv Res, 2022, 37: 267-278. DOI:10.1016/j.jare.2021.08.003 |
| [48] |
UDDIN G M, YOUNGSON N A, CHOWDHURY S S, et al. Administration of nicotinamide mononucleotide (NMN) reduces metabolic impairment in male mouse offspring from obese mothers[J]. Cells, 2020, 9(4): 791. DOI:10.3390/cells9040791 |
| [49] |
ZHAO X M, REN J J, DU W H, et al. Effect of vitrification on promoter CpGisland methylation patterns and expression levels of DNA methyltransferase 1o, histone acetyltransferase 1, and deacetylase 1 in metaphase Ⅱ mouse oocytes[J]. Fertil Steril, 2013, 100(1): 256-261. DOI:10.1016/j.fertnstert.2013.03.009 |
| [50] |
HOU Y P, DAI Y P, ZHU S E, et al. Bovine oocytes vitrified by the open pulled straw method and used for somatic cell cloning supported development to term[J]. Theriogenology, 2005, 64(6): 1381-1391. DOI:10.1016/j.theriogenology.2005.03.012 |
| [51] |
BRACKETT B G, OLIPHANT G. Capacitation of rabbit spermatozoa in vitro[J]. Biol Reprod, 1975, 12(2): 260-274. DOI:10.1095/biolreprod12.2.260 |
| [52] |
WANG L Y, CHEN Y R, WEI J R, et al. Administration of nicotinamide mononucleotide improves oocyte quality of obese mice[J]. Cell Prolif, 2022, 55(11): e13303. DOI:10.1111/cpr.13303 |
| [53] |
张紫薇, 于博, 戴佳格, 等. 小檗碱对玻璃化冷冻猪卵母细胞脂滴含量及胚胎发育的影响[J]. 北京农学院学报, 2020, 35(1): 67-74. ZHANG Z W, YU B, DAI J G, et al. Effects of berberine on lipid droplets content and embryo development in vitrified frozen porcine oocytes[J]. Journal of Beijing University of Agriculture, 2020, 35(1): 67-74. (in Chinese) |
| [54] |
LOWE J L, BARTOLAC L K, BATHGATE R, et al. Cryotolerance of porcine blastocysts is improved by treating in vitro matured oocytes with L-carnitine prior to fertilization[J]. J Reprod Dev, 2017, 63(3): 263-270. DOI:10.1262/jrd.2016-141 |
| [55] |
XU H X, JIA C, CHENG W X, et al. The effect of L-carnitine additive during in vitro maturation on the vitrification of pig oocytes[J]. Cell Reprogram, 2020, 22(4): 198-207. DOI:10.1089/cell.2020.0014 |
| [56] |
ABSALÓN-MEDINA V A, BEDFORD-GUAUS S J, GILBERT R O, et al. The effects of conjugated linoleic acid isomers cis-9, trans-11 and trans-10, cis-12 on in vitro bovine embryo production and cryopreservation[J]. J Dairy Sci, 2014, 97(10): 6164-6176. DOI:10.3168/jds.2013-7719 |
| [57] |
AGHAZ F, VAISI-RAYGANI A, KHAZAEI M, et al. Enhanced cryoprotective effect of melatonin and resveratrol by coencapsulation: improved in vitro development of vitrified-warmed mouse germinal vesicle oocytes[J]. Biopreserv Biobank, 2021, 19(3): 184-193. DOI:10.1089/bio.2020.0102 |
| [58] |
MIHALAS B P, DE IULIIS G N, REDGROVE K A, et al. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte[J]. Sci Rep, 2017, 7(1): 6247. DOI:10.1038/s41598-017-06372-z |
| [59] |
ALAM F, SYED H, AMJAD S, et al. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions[J]. Curr Res Physiol, 2021, 4: 119-124. DOI:10.1016/j.crphys.2021.03.002 |
| [60] |
AGARWAL A, MALDONADO ROSAS I, ANAGNOSTOPOULOU C, et al. Oxidative stress and assisted reproduction: a comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment[J]. Antioxidants (Basel), 2022, 11(3): 477. DOI:10.3390/antiox11030477 |
| [61] |
TAKAHASHI T, INABA Y, SOMFAI T, et al. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro[J]. Reprod Fertil Dev, 2013, 25(4): 589-599. DOI:10.1071/RD11262 |
| [62] |
SUN Y X, YUAN X L, ZHANG F F, et al. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells[J]. Sci Rep, 2017, 7(1): 11340. DOI:10.1038/s41598-017-11860-3 |
| [63] |
MIAO Y L, CUI Z K, GAO Q, et al. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes[J]. Cell Rep, 2020, 32(5): 107987. DOI:10.1016/j.celrep.2020.107987 |
| [64] |
SONG M J, LI Y, ZHOU Y H, et al. Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress[J]. J Anim Sci Biotechnol, 2022, 13(1): 68. DOI:10.1186/s40104-022-00716-0 |
| [65] |
XIANG X Y, LIU T, WU Y, et al. Berberine alleviates palmitic acid-induced podocyte apoptosis by reducing reactive oxygen species-mediated endoplasmic reticulum stress[J]. Mol Med Rep, 2021, 23(1): 3. |
| [66] |
RAZA S H A, ABD EL-AZIZ A H, ABDELNOUR S A, et al. The role of forskolin as a lipolytic stimulator during in vitro oocyte maturation and the in vitro embryo production of livestock[J]. Reprod Domest Anim, 2021, 56(12): 1486-1496. DOI:10.1111/rda.14021 |
| [67] |
SU L J, ZHANG J H, GOMEZ H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis[J]. Oxid Med Cell Longev, 2019, 2019: 5080843. |
| [68] |
FERNÁNDEZ A, ORDÓÑEZ R, REITER R J, et al. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis[J]. J Pineal Res, 2015, 59(3): 292-307. DOI:10.1111/jpi.12264 |
| [69] |
GIARETTA E, SPINACI M, BUCCI D, et al. Effects of resveratrol on vitrified porcine oocytes[J]. Oxid Med Cell Longev, 2013, 2013: 920257. |
| [70] |
VALLORANI C, SPINACI M, BUCCI D, et al. Pig oocyte vitrification by cryotop method and the activation of the apoptotic cascade[J]. Anim Reprod Sci, 2012, 135(1-4): 68-74. DOI:10.1016/j.anireprosci.2012.08.020 |
| [71] |
KALO D, ROTH Z. Involvement of the sphingolipid ceramide in heat-shock-induced apoptosis of bovine oocytes[J]. Reprod Fertil Dev, 2011, 23(7): 876-888. DOI:10.1071/RD10330 |
| [72] |
MENG Y F, PU Q, DAI S Y, et al. Nicotinamide mononucleotide alleviates hyperosmolarity-induced IL-17a secretion and macrophage activation in corneal epithelial cells/macrophage co-culture system[J]. J Inflamm Res, 2021, 14: 479-493. DOI:10.2147/JIR.S292764 |
| [73] |
WAN Y X, HE B, ZHU D Y, et al. Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats[J]. Arch Biochem Biophys, 2021, 712: 109050. DOI:10.1016/j.abb.2021.109050 |
| [74] |
BERTOLDO M J, LISTIJONO D R, HO W H J, et al. NAD+ repletion rescues female fertility during reproductive aging[J]. Cell Rep, 2020, 30(6): 1670-1681. DOI:10.1016/j.celrep.2020.01.058 |
| [75] |
MIAO Y L, LI X Y, SHI X Y, et al. Nicotinamide mononucleotide restores the meiotic competency of porcine oocytes exposed to ethylene glycol butyl ether[J]. Front Cell Dev Biol, 2021, 9: 628580. DOI:10.3389/fcell.2021.628580 |
(编辑 郭云雁)



