2. 山西省畜牧技术推广服务中心,太原 030001
2. Shanxi Animal Husbandry Technology Extansion Service Center, Taiyuan 030001, China
猪骨骼肌作为人类动物蛋白的主要来源,对畜牧经济发展有至关重要的作用。骨骼肌的生长发育直接影响猪肉的质量和产量,研究猪骨骼肌生长发育的分子机制对提高猪的生长速度至关重要[1-3]。肌纤维是骨骼肌的基本功能单位,其数量在哺乳动物出生后保持不变,骨骼肌的生长发育是通过已有肌纤维肥大来实现的[4-6]。在肌生成过程中,骨骼肌卫星细胞能在出生后提供大量肌肉来源的干细胞,其经历成肌细胞、多核肌管、肌纤维等阶段后逐渐形成成熟的肌肉组织,在骨骼肌的生长中起着不可或缺的作用。此外,卫星细胞激活后所发生的成肌生成过程可以作为胚胎肌生成的合适模型[7-8]。
MicroRNAs(miRNAs)是一类长度为22 nt左右的单链RNA,不具有编码潜能且广泛存在于自然界中。miRNAs种子序列与mRNA的3′UTR或CDS区碱基配对结合,促进mRNA降解或抑制基因翻译,从而发挥转录后调控作用[9-10]。miRNAs广泛参与各种生物学过程,主要包括动植物发育[11]、癌症发生[12]、细胞分化[13]、凋亡[14]和代谢[15]等。已有研究发现miRNAs作为重要的表观遗传因素,在骨骼肌发育、稳态和修复过程等许多方面发挥调节作用[16]。如肌肉特异性表达的miRNA-133a、miRNA-206和miRNA-1,与肌源性因子MyOD、MRF4、MyOG、MEF2C高度相关[17-22]。其中,miRNA-1和miRNA-206在猪骨骼肌中特异性高表达,通过调控成对框基因7(PAX7)和DNA聚合酶α(DNA pol α)的最大亚基的表达来影响骨骼肌卫星细胞的增殖和分化[23-24]。此外,miRNA-133可通过抑制血清反应因子(SRF)的表达来减弱成肌细胞分化并促进细胞的增殖[25]。以上研究说明,miRNAs在肌生成过程中发挥关键作用,但关于miRNAs参与调控猪骨骼肌卫星细胞增殖和分化的分子机制还有待研究。
本课题组前期对不同阶段晋汾白猪背最长肌进行全转录组测序分析,发现miR-145-5p在晋汾白猪背最长肌中高表达,且在1、90和180 d猪背最长肌中表达量逐渐升高。已有研究表明,miR-145靶向IGF1R基因调控结肠癌、肝细胞癌和胰腺癌等多种癌细胞的增殖[26-28]。此外,在牛骨骼肌卫星细胞中,miR-145靶向IGF1R抑制牛骨骼肌卫星细胞生长,但关于miR-145-5p在猪成肌过程中的研究却未有报道[29]。本研究采用qRT-PCR方法检测miR-145-5p在猪不同组织和不同生长时期背最长肌中的表达情况,阐明其表达规律;采用qRT-PCR、EdU、CCK-8、Western blot和免疫荧光染色法检测过表达和抑制miR-145-5p后对猪骨骼肌卫星细胞增殖和分化的影响;采用双荧光素酶试验、qRT-PCR和Western blot探究miR-145-5p和IGF1R及其下游通路的靶向关系。本研究通过探究miR-145-5p对猪骨骼肌卫星细胞增殖和分化的影响,揭示其对猪骨骼肌生长发育的作用及调控机制,丰富猪骨骼肌形成的分子调控网络,并为猪肌肉性状的分子育种提供依据。
1 材料与方法 1.1 试验样品本研究所用组织样均为实验室前期采集,选用1、90和180 d晋汾白猪背最长肌以及90 d晋汾白猪心、肝、脾、肺、肾和背部皮下脂肪等6种组织,进行miR-145-5p时空表达特性分析。
1.2 主要试剂与仪器TRIzol Ⓡ Reagen、PrimeScript Ⓡ RT Reagent Kit和SYBR Ⓡ PrimeScriptTM RT-PCR Kit均购自日本TaKaRa公司;引物、miRNA第一链合成试剂盒和荧光定量PCR试剂盒购自上海生工有限公司;PBS、TBST、青霉素/链霉素混合液(双抗)、DAPI染液和胰蛋白酶购于北京索莱宝公司;分散酶购自美国Sigma公司;DMEM、胶原酶和FBS购自美国Gibco公司;EdU购自广州锐博公司;Lipofectamine 2000购自美国赛默飞公司;4%多聚甲醛、山羊血清、AKT抗体、磷酸酶抑制剂和CCK-8试剂盒购自北京博奥森公司;蛋白二抗购自美国LI-COR公司;PAX7、MyOD、MyHC抗体购自美国abcam公司;p-AKT购于广州艾菲公司;IGF1R抗体购于上海碧云天公司;β-actin和荧光二抗购于武汉赛维尔公司;RNA寡核苷酸由上海吉玛公司合成;IGF1R-WT和IGF1R-MUT由北京擎科公司合成。
ND-1000核酸蛋白测定仪购自美国Nanodrop公司;7500 Real Time实时荧光定量PCR仪购自美国Thermofisher公司;Synergy H1全功能微孔板检测仪、Leica DMi8 M倒置荧光显微镜购自德国莱卡公司;Odyssey CLX远红外扫描系统购自日本尼康公司等。
1.3 试验方法1.3.1 RNA的提取、反转录与qRT-PCR 使用TRIzol试剂提取组织总RNA,使用PrimeScript RT试剂盒进行RNA的逆转录;使用核酸检测仪测定RNA浓度并将浓度归一为500 ng ·μL-1。使用SYBR Ⓡ PrimeScriptTM RT-PCR Kit进行qRT-PCR反应,总体积为10 μL,包括上、下游引物各0.5 μL,2×SYBR 5 μL,cDNA 1 μL,ddH2O补齐。反应程序为95 ℃ 30 s;95 ℃ 5 s,60 ℃ 30 s,72 ℃ 30 s,40个循环;95 ℃ 15 s,60 ℃ 30 s,95 ℃ 30 s制作熔解曲线。引物序列见表 1,以18S rRNA为内参,评估各基因的相对表达水平。
|
|
表 1 qRT-PCR引物信息 Table 1 Primer information of qRT-PCR |
1.3.2 miRNA的反转录与qRT-PCR 将RNA反转录成cDNA,反应总体积为10 μL(500 ng总RNA,1 μL Enzyme Mix,5 μL Solution Mix,ddH2O补齐),反应程序为37 ℃ 60 min,85 ℃ 5 min。qRT-PCR反应总体积为10 μL(上、下游引物各0.5 μL,cDNA 1 μL,2×miRNA qPCR master mix 5 μL,RNase Free ddH2O补齐),反应程序同上。每个样本技术重复3次,以试剂盒中提供的u6为内参,评估miR-145-5p的表达水平。
1.3.3 猪骨骼肌卫星细胞分离 采集1 d晋汾白猪腿部趾长伸肌组织1 cm3,用含1%双抗的PBS洗涤后剪至肉糜状;用胶原酶(1 mg ·mL-1)和分散酶(1 mg ·mL-1)在37 ℃消化1 h,加入完全培养基终止消化;依次经70和40 μm细胞筛过滤后离心(1 000 r ·min-1,5 min);沉淀相继经红细胞裂解液和PBS重悬后离心(1 000 r ·min-1,5 min);完全培养基重悬沉淀并转移至新培养皿中。待细胞长满后用0.25%胰酶消化,差速贴壁1 h后将悬液转移到新的培养皿或孔板中。
1.3.4 细胞转染 当12孔板中猪骨骼肌卫星细胞密度达到60%左右时进行转染。分别用50 μL的DMEM稀释2 μL 100 nmol ·mL-1RNA寡核苷酸(序列见表 2)和Lipofectamine 2000,混匀后静置5 min;混合两种液体后静置15 min;用DMEM补齐至1 mL后加入到12孔板中,6 h后更换为无抗培养基,每个处理3个重复。
|
|
表 2 miR-145-5p mimics和inhibitor序列 Table 2 The sequences of miR-145-5p mimics and inhibitor |
1.3.5 5-乙基-2′-脱氧尿苷(EdU)染色 将细胞接种于24孔板中,转染48 h后每孔加入100 μL 1 ∶1 000稀释的EdU溶液,孵育2 h后收集孔板。PBS清洗后加入100 μL 4%多聚甲醛固定30 min;加入100 μL甘氨酸(2 mg ·mL-1)孵育10 min;加入100 μL 0.1% Triton X-100,孵育30 min;在每步结束后用PBS洗涤细胞2次,每次8 min;加入100 μL Apollo染色液,避光孵育30 min;加入0.1% Triton X-100脱色摇床清洗2次;加入100 μL DAPI染液避光孵育30 min;PBS清洗2次在荧光倒置显微镜上观察染色细胞,并使用Image J进行数据分析。
1.3.6 CCK-8检测 将细胞接种于96孔板中,在转染完0、24、36和48 h加入10 μL CCK-8溶液,混匀后继续培养3 h,全程避光操作,使用微孔板检测仪测定孔板在450 nm的OD值。
1.3.7 Western blot 转染完后待猪骨骼肌卫星细胞长满,用2%马血清分化培养基诱导分化至有明显的肌管产生,收集孔板加入蛋白裂解液提取蛋白质;蛋白经变性后用5%浓缩胶和12%分离胶电泳(电泳条件:80 V 30 min;120 V 90 min);电泳结束后裁出目的大小胶条转至PVDF膜上;在5 mL 5%奶粉封闭2 h后用抗体(MyOD、IGF1R、AKT、p-AKT均1 ∶1 000稀释)4 ℃孵育过夜;TBST洗去未结合抗体后用蛋白二抗(1:10 000稀释)在室温下避光孵育1 h;用远红外扫描系统观察蛋白条带。
1.3.8 免疫荧光染色 收集转染完并诱导分化6 d的24孔板,用100 μL 4%甲醛固定30 min,加入100 μL 0.1% TritonX-100渗透30 min;加100 μL 2%山羊血清室温封闭30 min;加入1 ∶100稀释的MyHC抗体4 ℃孵育过夜;PBS洗去未结合MyHC抗体后,加入1 ∶200稀释的荧光二抗37 ℃孵育1 h;PBS洗涤后加入DAPI染液染色20 min;使用荧光倒置显微镜拍摄细胞图像。
1.3.9 双荧光素酶报告基因检测 含有IGF1R-WT或IGF1R-MUT的psiCHECK-2双荧光素酶报告载体(500 ng ·孔-1)与0.5 μL miR-145-5p mimics或mimics-NC用Lipofectamine 2000转染到48孔293T细胞中。转染48 h后收集孔板,每孔加入60 μL细胞裂解液,室温裂解10 min后离心:吸取20 μL上清液加入96孔检测板,再加入100 μL Luciferase Reaction Reagent,检测萤火虫荧光素酶活性,再加入100 μL Luciferase Reaction Reagent II,混匀后检测海肾荧光素酶活性;以海肾荧光素酶的荧光值为内参,校正萤火虫荧光素酶的荧光值。
1.3.10 统计分析 试验均有3个重复,使用SPSS 26.0对数据进行统计分析,两组比较采用独立样本t检验,多组间比较采用单因素方差分析,并进行Duncan's多重比较,P < 0.05为差异显著,P < 0.01为差异极显著。
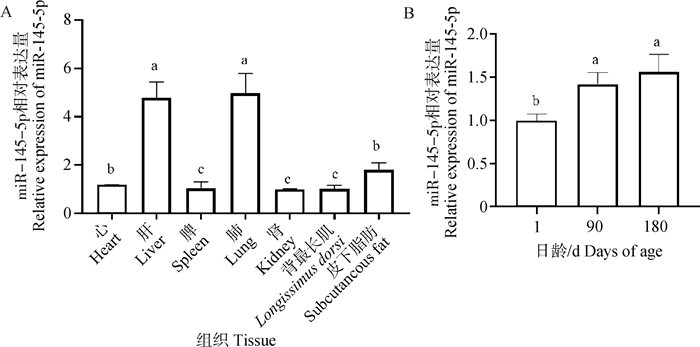
2 结果 2.1 miR-145-5p的表达特性分析miR-145-5p在晋汾白猪各组织中均有表达,在肝和肺中表达量最高,心和背部皮下脂肪中次之(P < 0.05,图 1A);随着晋汾白猪日龄的增加,背最长肌中miR-145-5p的表达量持续升高(P < 0.05,图 1B)。
|
A. 猪不同组织中miR-145-5p的相对表达量;B. 1、90、180 d晋汾白猪背最长肌中miR-145-5p的相对表达量。不同小写字母代表差异显著(P < 0.05),下同 A. The relative expression of miR-145-5p in different tissues of pigs; B. The relative expression of miR-145-5p in the longissimus dorsi muscle of Jinfen White pigs at 1, 90 and 180 d. The different lowercase letters represent significant differences (P < 0.05), the same as below 图 1 miR-145-5p的表达特性 Fig. 1 The expression characteristics of miR-145-5p |
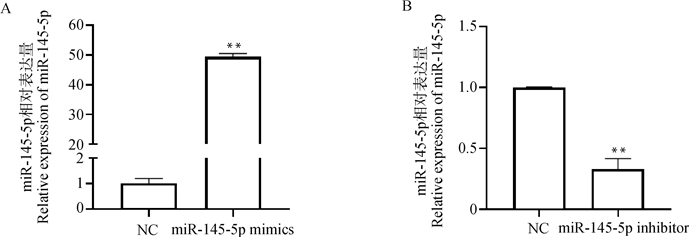
用miR-145-5p mimics和inhibitor分别转染猪骨骼肌卫星细胞,48 h后检测其转染效率。结果显示miR-145-5p mimics和inhibitor可以极显著地升高或降低miR-145-5p的表达量(P < 0.01,图 2A、2B)。
|
A. miR-145-5p mimics的转染效率;B. miR-145-5p inhibitor的转染效率。*. P < 0.05; **. P < 0.01,下同 A. Transfection efficiency of miR-145-5p mimics; B. Transfection efficiency of miR-145-5p inhibitor. *. P < 0.05; **. P < 0.01, the same as below 图 2 miR-145-5p的相对表达量 Fig. 2 Relative expression of miR-145-5p |
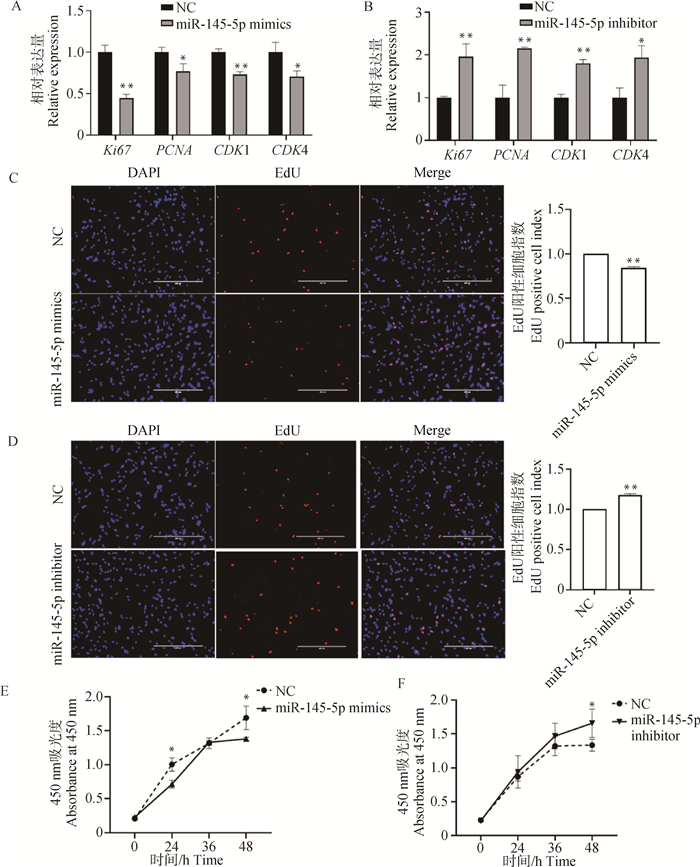
与mimics NC组相比,转染miR-145-5p mimics极显著降低细胞增殖相关基因Ki67和CDK1的mRNA水平(P < 0.01),显著降低PCNA和CDK4的mRNA水平(P < 0.05),阳性细胞指数显著降低(P < 0.01),转染miR-145-5p mimics组在24和48 h的细胞活性也显著降低(P < 0.05,图 3A、3C和3E)。与inhibitor NC组相比,转染miR-145-5p inhibitor极显著提高Ki67、PCNA和CDK1的mRNA水平(P < 0.01),显著提高CDK4的mRNA水平(P < 0.05),阳性细胞指数也极显著上调(P < 0.01),转染miR-145-5p inhibitor组在48 h的细胞活性显著提高(P < 0.05,图 3B、3D和3F)。因此,miR-145-5p抑制猪骨骼肌卫星细胞的增殖。
|
A、B. 转染miR-145-5p mimics和inhibitor后Ki67、PCNA、CDK1和CDK4的相对表达量;C、D. EdU染色结果,比例尺=400 μm;E、F. 转染miR-145-5p mimics和inhibitor后CCK-8结果 A, B. Relative expression of Ki67, PCNA, CDK1 and CDK4 after transfection of miR-145-5p mimics and inhibitor; C, D. EdU staining results, scale bar=400 μm; E, F. CCK-8 results after transfection of miR-145-5p mimics and inhibitor 图 3 miR-145-5p抑制猪骨骼肌卫星细胞增殖 Fig. 3 miR-145-5p inhibits proliferation of porcine skeletal muscle satellite cells |
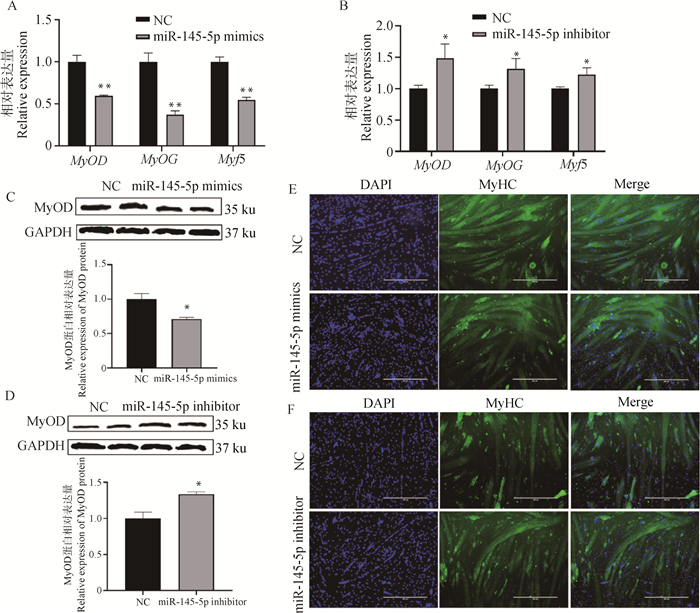
与mimics NC组相比,转染miR-145-5p mimics能极显著降低分化相关基因MyOD、MyOG和Myf5的mRNA表达水平(P < 0.01),MyOD蛋白质水平也显著降低(P < 0.05),过表达组中的MyHC阳性肌管数明显少于对照组(图 4A、4C和4E)。与inhibitor NC组相比,转染miR-145-5p inhibitor能显著提高分化相关基因MyOD、MyOG和Myf5的mRNA表达水平(P < 0.05),MyOD蛋白水平也显著提高(P < 0.05),MyHC阳性肌管数明显多于对照组(图 4B、4D和4F)。以上结果表明,miR-145-5p抑制猪骨骼肌卫星细胞的分化。
|
A、B. 转染miR-145-5p mimics和inhibitor后MyOD、MyOG和Myf5的相对表达量;C、D. 转染miR-145-5p mimics和inhibitor后MyOD的蛋白水平及灰度值分析;E、F. 转染miR-145-5p mimics和inhibitor后肌管MyHC免疫荧光染色,比例尺=400 μm A, B. The relative expression of MyOD, MyOG and Myf5 after transfection of miR-145-5p mimics and inhibitor; C, D. The protein level and gray value analysis of MyOD after transfection of miR-145-5p mimics and inhibitor; E, F. MyHC immunofluorescence staining of myotubes after transfection of miR-145-5p mimics and inhibitor, scale bar=400 μm 图 4 miR-145-5p抑制猪骨骼肌卫星细胞分化 Fig. 4 miR-145-5p inhibits the differentiation of porcine skeletal muscle satellite cells |
使用RNAhybird预测miR-145-5p与IGF1R的3′UTR区结合二级结构,其最小自由能为-35.1 kcal ·mol-1,表明其结构稳定(图 5A)。设计如图 5B所示的IGF1R 3′UTR区序列与miR-145-5p种子序列的突变位点,miR-145-5p mimics和IGF1R 3′UTR WT共转组的相对荧光素酶活性极显著低于其他3组(P < 0.01,图 5C)。已有研究表明,IGF1R能激活AKT通路,因此检测miR-145-5p靶向IGF1R来抑制AKT通路的可能性。转染miR-145-5p mimics后,IGF1R的mRNA水平和蛋白水平显著下降(P < 0.05,图 5D、5E);转染miR-145-5p inhibitor后IGF1R的mRNA水平和蛋白水平表达量极显著上升(P < 0.01,图 5D、5F)。转染miR-145-5p mimics显著降低了AKT的磷酸化水平,而转染miR-145-5p inhibitor促进了p-AKT的表达,且能挽救si-IGF1R对AKT磷酸化水平的抑制作用,p-AKT蛋白水平得到恢复(P < 0.01,图 5G、5H)。综上,miR-145-5p通过负调控IGF1R介导的AKT通路抑制猪骨骼肌卫星细胞的增殖和分化(图 5I)。
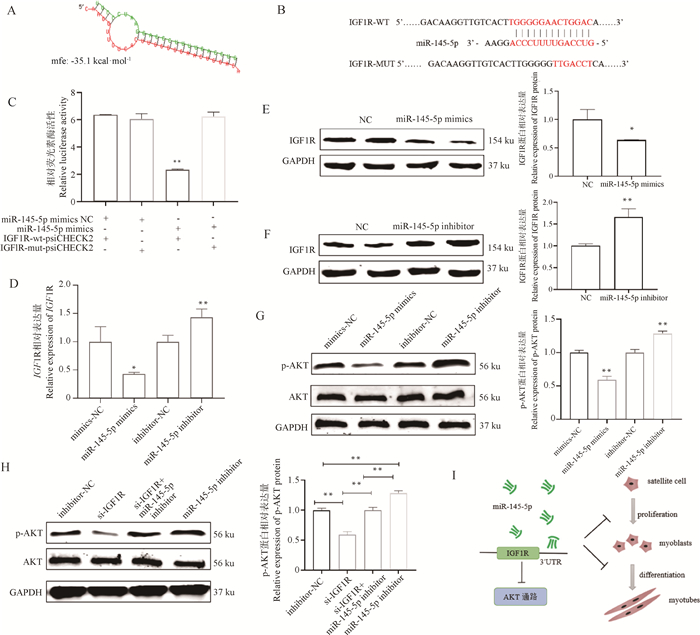
|
A. miR-145-5p与IGF1R的潜在结合位点;B. IGF1R突变位点序列;C. 双荧光素酶报告试验结果;D. 过表达和抑制miR-145-5p后IGF1R的相对表达量;E~G. 转染miR-145-5p mimics和inhibitor后IGF1R、AKT和p-AKT的蛋白水平及灰度值分析;H. si-IGF1R和miR-145-5p inhibitor共转染后AKT和p-AKT的蛋白水平及灰度值分析;I. miR-145-5p抑制猪骨骼肌卫星细胞增殖和分化的示意图 A. The potential binding sites of miR-145-5p and IGF1R; B. Sequence of IGF1R mutation sites; C. Dual-luciferase reporter gene assay results; D. Relative expression of IGF1R after transfection of miR-145-5p mimics and inhibitor; E-G. Analysis of IGF1R, AKT and p-AKT protein levels and gray value after overexpression and inhibition of miR-145-5p; H. Analysis of protein level and gray value of AKT and p-AKT after cotransfection with si-IGF1R and miR-145-5p inhibitor; I. The schematic model of miR-145-5p inhibiting the proliferation and differentiation of porcine skeletal muscle satellite cells 图 5 miR-145-5p靶向IGF1R抑制猪骨骼肌卫星细胞中AKT信号通路 Fig. 5 miR-145-5p targets IGF1R to inhibit the AKT signaling pathway in porcine skeletal muscle satellite cells |
骨骼肌形成是一个复杂的过程,需要对关键肌源性因素进行严格的时空调控。RNA加工对于调节这些因素至关重要,并且多个转录后调节途径相互依赖且独立地工作,以便在整个肌肉发育和修复过程中精确控制转录本[30]。多项研究表明,miRNAs可以调控骨骼肌卫星细胞的增殖和分化,在骨骼肌发育中发挥重要作用。例如miRNA-1和miRNA-206靶向Pax7抑制骨骼肌卫星细胞的增殖和分化[23]。因此探究肌肉发育过程中的新分子调控机制,可为提高畜牧业生产、诊断人类疾病和完善分子治疗方法提供新思路。
miR-145-5p的序列已经在多个物种中被鉴定且保守性高,在免疫、肿瘤和癌症、骨骼肌卫星细胞的增殖分化等许多过程中发挥重要作用。在鼻咽癌中,miRNA-145-5p调节Krüppel-like因子5(KLF5)抑制鼻咽癌细胞增殖、迁移和侵袭[31]。在前列腺癌中,miR-145-5p通过抑制原癌基因(WIP1)的表达来抑制前列腺癌细胞的生长和转移,从而发挥抑癌作用[32]。在C2C12中,miR-145-5p通过灭活PI3K/AKT/mTOR途径促进细胞死亡引起肌肉功能障碍[33]。在山羊骨骼肌卫星细胞中,miR-145-5p通过靶向泛素特异性肽酶13(USP13)编码域序列抑制山羊骨骼肌卫星细胞分化[34]。在牛骨骼肌卫星细胞中,miR-145靶向IGF1R基因抑制PI3K/AKT信号通路的作用,从而抑制细胞生长[29]。
猪miR-145-5p位于2号染色体150580126-150580211区域,前体86 nt,成熟序列24 nt。对比不同物种miR-145-5p的成熟序列,发现miR-145-5p在人、鼠、斑马鱼、牛、犬和猪等物种中高度保守,推测其可能在不同物种中具有类似的功能。本研究中,miR-145-5p在不同发育阶段晋汾白猪背最长肌中均有表达,且在1、90和180 d背最长肌组织中差异显著,推测miR-145-5p可能参与猪骨骼肌生长发育进程。进一步在体外培养的猪骨骼肌卫星细胞,发现miR-145-5p抑制PCNA、Ki67、CDK1和CDK4的表达,减少阳性细胞数,降低细胞活性,从而抑制猪骨骼肌卫星细胞增殖。同时,miR-145-5p抑制MyOD、MyOG和Myf5的表达,降低MyOD蛋白水平,减少MyHC阳性肌管数目,从而抑制猪骨骼肌卫星细胞分化。这些结果与Shen等[29]在牛骨骼肌卫星细胞上的研究结果一致。与牛的miR-145-5p序列相比,猪miR-145-5p的3′端多一个U碱基,其核心种子区序列完全一致。经hybrid预测,猪miR-145-5p的种子序列与IGF1R的3′UTR区完全结合,推测其是通过调控IGF1R的表达参与猪骨骼肌卫星细胞增殖分化过程。
IGF1R是一种跨膜受体,能与IGF1结合激活酪氨酸激酶导致自磷酸化,激活MAPK/ERK和PI3K/AKT信号传导。IGF1R在各种生物过程中发挥重要功能,包括细胞增殖、细胞凋亡和细胞迁移,IGF1R在成肌细胞增殖和正常肌肉质量维持方面也具有重要意义[35-38]。研究表明,IGF1R通过促进细胞周期的G2/M进展调控小鼠精原干细胞的增殖[39]。在猪骨骼肌卫星细胞中,IGF1R促进成肌细胞的增殖,过表达IGF1R上调增殖标志基因CDK4、PCNA和CyclinD1的表达水平[40]。本研究利用RNAhybrid网站预测IGF1R能与miR-145-5p的种子序列完全结合,双荧光素酶试验结果显示miR-145-5p mimics能降低IGF1R 3′UTR WT的相对荧光素酶活性,且过表达miR-145-5p能显著下调IGF1R mRNA和蛋白的相对表达水平,显著降低了AKT的磷酸化水平,而没有改变猪骨骼肌卫星细胞中总AKT的表达;干扰miR-145-5p能极显著上调IGF1R mRNA和蛋白的相对表达水平,挽救了si-IGF1R对AKT的磷酸化水平的抑制作用,p-AKT蛋白表达水平得到恢复。以上结果表明,miR-145-5p靶向IGF1R负调控AKT通路抑制猪骨骼肌卫星细胞的增殖和分化。
4 结论miR-145-5p基因在猪各组织中均有表达,在肝和肺中表达量最高,心和背部皮下脂肪中表达量次之;随着日龄的增加,miR-145-5p在背最长肌中的表达量持续升高。miR-145-5p能靶向IGF1R的3′UTR区,调控IGF1R mRNA和蛋白的相对表达水平,并影响AKT通路,进而抑制猪骨骼肌卫星细胞的增殖和分化。
| [1] |
BERTOL T M, DE CAMPOS R M L, LUDKE J V, et al. Effects of genotype and dietary oil supplementation on performance, carcass traits, pork quality and fatty acid composition of backfat and intramuscular fat[J]. Meat Sci, 2013, 93(3): 507-516. DOI:10.1016/j.meatsci.2012.11.012 |
| [2] |
JUNG J H, SHIM K S, NA C S, et al. Studies on intramuscular fat percentage in live swine using real-time ultrasound to determine pork quality[J]. Asian-Australas J Anim Sci, 2015, 28(3): 318-322. DOI:10.5713/ajas.14.0927 |
| [3] |
POLETI M D, REGITANO L C A, SOUZA G H M F, et al. Longissimus dorsi muscle label-free quantitative proteomic reveals biological mechanisms associated with intramuscular fat deposition[J]. J Proteomics, 2018, 179: 30-41. DOI:10.1016/j.jprot.2018.02.028 |
| [4] |
BAGHDADI M B, TAJBAKHSH S. Regulation and phylogeny of skeletal muscle regeneration[J]. Dev Biol, 2018, 433(2): 200-209. DOI:10.1016/j.ydbio.2017.07.026 |
| [5] |
YANG J J, LIU H, WANG K F, et al. Isolation, culture and biological characteristics of multipotent porcine skeletal muscle satellite cells[J]. Cell Tissue Bank, 2017, 18(4): 513-525. DOI:10.1007/s10561-017-9614-9 |
| [6] |
BENTZINGER C F, WANG Y X, DUMONT N A, et al. Cellular dynamics in the muscle satellite cell niche[J]. EMBO Rep, 2013, 14(12): 1062-1072. DOI:10.1038/embor.2013.182 |
| [7] |
METZGER K, TUCHSCHERER A, PALIN M F, et al. Establishment and validation of cell pools using primary muscle cells derived from satellite cells of pig skeletal muscle[J]. In Vitro Cell Dev Biol Anim, 2020, 56(3): 193-199. DOI:10.1007/s11626-019-00428-2 |
| [8] |
WIGMORE P M, STICKLAND N C. Muscle development in large and small pig fetuses[J]. J Anat, 1983, 137(Pt 2): 235-245. |
| [9] |
BARTEL D P. MicroRNAs: genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-297. DOI:10.1016/S0092-8674(04)00045-5 |
| [10] |
HE L, HANNON G J. MicroRNAs: small RNAs with a big role in gene regulation[J]. Nat Rev Genet, 2004, 5(7): 522-531. DOI:10.1038/nrg1379 |
| [11] |
YU Y, ZHANG Y C, CHEN X M, et al. Plant noncoding RNAs: hidden players in development and stress responses[J]. Annu Rev Cell Dev Biol, 2019, 35: 407-431. DOI:10.1146/annurev-cellbio-100818-125218 |
| [12] |
HILL M, TRAN N. miRNA interplay: mechanisms and consequences in cancer[J]. Dis Model Mech, 2021, 14(4): dmm047662. DOI:10.1242/dmm.047662 |
| [13] |
JIAO P, WANG X P, LUORENG Z M, et al. miR-223:an effective regulator of immune cell differentiation and inflammation[J]. Int J Biol Sci, 2021, 17(9): 2308-2322. DOI:10.7150/ijbs.59876 |
| [14] |
SCHOBER A, BLAY R M, SABOOR MALEKI S, et al. MicroRNA-21 controls circadian regulation of apoptosis in atherosclerotic lesions[J]. Circulation, 2021, 144(13): 1059-1073. DOI:10.1161/CIRCULATIONAHA.120.051614 |
| [15] |
AGBU P, CARTHEW R W. MicroRNA-mediated regulation of glucose and lipid metabolism[J]. Nat Rev Mol Cell Biol, 2021, 22(6): 425-438. DOI:10.1038/s41580-021-00354-w |
| [16] |
HENSLEY A P, MCALINDEN A. The role of microRNAs in bone development[J]. Bone, 2021, 143: 115760. DOI:10.1016/j.bone.2020.115760 |
| [17] |
MCCARTHY J. MicroRNA-206:the skeletal muscle-specific myomiR[J]. Biochim Biophys Acta (BBA) - Gene Regul Mech, 2008, 1779(11): 682-691. DOI:10.1016/j.bbagrm.2008.03.001 |
| [18] |
MCCARTHY J J, ESSER K A. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy[J]. J Appl Physiol, 2007, 102(1): 306-313. DOI:10.1152/japplphysiol.00932.2006 |
| [19] |
MIRETTI S, VOLPE M G, MARTIGNANI E, et al. Temporal correlation between differentiation factor expression and microRNAs in Holstein bovine skeletal muscle[J]. Animal, 2017, 11(2): 227-235. DOI:10.1017/S1751731116001488 |
| [20] |
SWEETMAN D, GOLJANEK K, RATHJEN T, et al. Specific requirements of MRFs for the expression of muscle specific microRNAs, miR-1, miR-206 and miR-133[J]. Dev Biol, 2008, 321(2): 491-499. DOI:10.1016/j.ydbio.2008.06.019 |
| [21] |
DONG W, CHEN X Y, WANG M H, et al. miR-206 partially rescues myogenesis deficiency by inhibiting CUGBP1 accumulation in the cell models of myotonic dystrophy[J]. Neurol Res, 2019, 41(1): 9-18. DOI:10.1080/01616412.2018.1493963 |
| [22] |
GAGAN J, DEY B K, LAYER R, et al. Notch3 and Mef2c proteins are mutually antagonistic via Mkp1 protein and miR-1/206 microRNAs in differentiating myoblasts[J]. J Biol Chem, 2012, 287(48): 40360-40370. DOI:10.1074/jbc.M112.378414 |
| [23] |
CHEN J F, TAO Y Z, LI J, et al. MicroRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7[J]. J Cell Biol, 2010, 190(5): 867-879. DOI:10.1083/jcb.200911036 |
| [24] |
KIM H K, LEE Y S, SIVAPRASAD U, et al. Muscle-specific microRNA miR-206 promotes muscle differentiation[J]. J Cell Biol, 2006, 174(5): 677-687. DOI:10.1083/jcb.200603008 |
| [25] |
WU N Z, GU T T, LU L, et al. Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle[J]. J Cell Physiol, 2019, 234(4): 3490-3499. DOI:10.1002/jcp.26857 |
| [26] |
SU J J, LIANG H W, YAO W Y, et al. MiR-143 and miR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer[J]. PLoS One, 2014, 9(12): e114420. DOI:10.1371/journal.pone.0114420 |
| [27] |
LAW P T Y, CHING A K K, CHAN A W H, et al. MiR-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma[J]. Carcinogenesis, 2012, 33(6): 1134-1141. DOI:10.1093/carcin/bgs130 |
| [28] |
DOBRE M, HERLEA V, VLǍDUŢ C, et al. Dysregulation of miRNAs targeting the IGF-1R pathway in pancreatic ductal adenocarcinoma[J]. Cells, 2021, 10(8): 1856. DOI:10.3390/cells10081856 |
| [29] |
SHEN X M, TANG J, JIANG R, et al. CircRILPL1 promotes muscle proliferation and differentiation via binding miR-145 to activate IGF1R/PI3K/AKT pathway[J]. Cell Death Dis, 2021, 12(2): 142. DOI:10.1038/s41419-021-03419-y |
| [30] |
WESKAMP K, OLWIN B B, PARKER R. Post-transcriptional regulation in skeletal muscle development, repair, and disease[J]. Trends Mol Med, 2021, 27(5): 469-481. DOI:10.1016/j.molmed.2020.12.002 |
| [31] |
YUAN C H, HSU W C, HUANG A M, et al. MicroRNA-145-5p modulates Krüppel-like factor 5 and inhibits cell proliferation, migration, and invasion in nasopharyngeal carcinoma[J]. BMC Mol Cell Biol, 2022, 23(1): 28. DOI:10.1186/s12860-022-00430-9 |
| [32] |
SUN J M, DENG L G, GONG Y. MiR-145-5p inhibits the invasion of prostate cancer and induces apoptosis by inhibiting WIP1[J]. J Oncol, 2021, 2021: 4412705. |
| [33] |
JIN J, LI F Y, FAN C H, et al. Elevated miR-145-5p is associated with skeletal muscle dysfunction and triggers apoptotic cell death in C2C12 myotubes[J]. J Muscle Res Cell Motil, 2022, 43(3): 135-145. DOI:10.1007/s10974-022-09624-2 |
| [34] |
ZHANG Z, DENG K P, KANG Z Q, et al. MicroRNA profiling reveals miR-145-5p inhibits goat myoblast differentiation by targeting the coding domain sequence of USP13[J]. FASEB J, 2022, 36(7): e22370. |
| [35] |
WANG H T, SU X Y, FANG J K, et al. Tanshinone ⅡA attenuates insulin like growth factor 1 -induced cell proliferation in PC12 cells through the PI3K/Akt and MEK/ERK pathways[J]. Int J Mol Sci, 2018, 19(9): 2719. DOI:10.3390/ijms19092719 |
| [36] |
AHMAD S S, AHMAD K, LEE E J, et al. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases[J]. Cells, 2020, 9(8): 1773. DOI:10.3390/cells9081773 |
| [37] |
MANNING B D, CANTLEY L C. AKT/PKB signaling: navigating downstream[J]. Cell, 2007, 129(7): 1261-1274. DOI:10.1016/j.cell.2007.06.009 |
| [38] |
LIN Y B, LIU H Y, WARAKY A, et al. SUMO-modified insulin-like growth factor 1 receptor (IGF-1R) increases cell cycle progression and cell proliferation[J]. J Cell Physiol, 2017, 232(10): 2722-2730. DOI:10.1002/jcp.25818 |
| [39] |
WANG S, WANG X X, WU Y J, et al. IGF-1R signaling is essential for the proliferation of cultured mouse spermatogonial stem cells by promoting the G2/M progression of the cell cycle[J]. Stem Cells Dev, 2015, 24(4): 471-483. DOI:10.1089/scd.2014.0376 |
| [40] |
SONG J, HAO L L, ZENG X F, et al. A novel miRNA Y-56 targeting IGF-1R mediates the proliferation of porcine skeletal muscle satellite cells through AKT and ERK pathways[J]. Front Vet Sci, 2022, 9: 754435. DOI:10.3389/fvets.2022.754435 |
(编辑 孟培)



