2. 乌拉特中旗农牧和科技局, 乌拉特中旗 015300
2. Bureau of Agriculture, Animal Husbandry and Science and Technology of Ulat Middle Banner, Ulat Middle Banner 015300, China
苏尼特羊是内蒙古优良的地方绵羊品种之一,具有肉质鲜嫩多汁、无膻味、胴体丰满等优点[1]。随着人们对畜牧业产量需求的不断增长,过度放牧日益严重,草原生态系统不断恶化。为了改善反刍动物的生长性能,缓解过度放牧对草原生态的压力,舍饲饲喂粗饲料和精料的全混合日粮成为传统放牧的替代方式。与传统放牧的肉羊相比,舍饲肉羊的活动面积、摄入牧草种类和营养成分多样性均有所减少,而这可能会影响其胃肠道环境和肉品质[2]。
反刍动物瘤胃是一个稳定且极其复杂的微生态系统,瘤胃液中的微生物对饲料的发酵消化至关重要[3]。瘤胃菌群组成和多样性受饲养方式、品种、寄主年龄、季节和地理区域的影响,这也是反刍动物饲料转化效率不同的主要原因[4]。之前有研究表明,与精料为主的舍饲组相比,采食天然牧草的放牧组牦牛瘤胃细菌多样性更高[5]。李蒋伟[6]研究发现,随着饲粮精粗比的提高,藏羊瘤胃中细菌多样性和丰富度也随之提高,但厚壁菌门的相对丰度显著降低。此外,有研究发现天然牧草可以增加反刍动物肌肉中多不饱和脂肪酸含量,影响肌肉的嫩度和风味,而肌肉中多不饱和脂肪酸的含量主要与瘤胃生物氢化作用有关[7]。因此通过对瘤胃菌群的研究,有助于认识复杂的瘤胃微生态系统,对高效利用饲料、改善动物育肥性能和肉品质具有重大意义。本试验以3月龄苏尼特羊为研究对象,研究放牧和舍饲2种饲养方式对苏尼特羊生长性能、屠宰性能、肉品质和瘤胃菌群的影响,并对舍饲羊肉品质劣化观念进行研判,以期为改善舍饲羊肉品质提供理论依据。
1 材料与方法 1.1 试验设计和饲养管理饲养试验在内蒙古自治区巴彦淖尔市乌拉特中旗哈拉图苏木牧区进行。选取24只体重(20.16±1.56)kg、健康状况良好的苏尼特羊,随机分为2组,每组12只,舍饲组设2个重复,每个重复6只羊,公母各半。放牧组苏尼特羊在天然草场放牧,每天07:00~17:00进行放牧,放牧期间自由采食和饮水。放牧草场属于温性荒漠草原类,年均降水量200~250 mm,主要优势植物为石针茅、芨芨草和矮锦鸡儿等。舍饲组苏尼特羊在圈中集中饲养,每日08:00和16:00饲喂玉米秸秆、葵花饼以及精饲料组成的基础饲粮,自由采食和饮水。预试期为7 d,正试期为90 d。
1.2 饲草的采集和测定采集新鲜天然牧草样品5份,采集方法参照《野生牧草种质资源野外采集技术规范》(DB63/T 882—2010)。每份样品经称重、风干、粉碎、混匀后装入密封袋中,参照国家标准GB 5009和王敏[8]的方法进行牧草营养成分的检测。参考《肉羊饲养标准》(NY/T 816—2004)配制基础饲粮,精粗比为60∶40。天然牧草营养水平、舍饲组精饲料组成及营养水平见表 1。
|
|
表 1 天然牧草营养水平、精饲料组成及营养水平(干物质基础) Table 1 Nutrient levels of natural grass, composition and nutrient levels of basal diets (DM basis) |
1.3.1 生长性能 饲养试验第1天和饲养试验第90天晨饲前分别记录所有羊只体重,用软皮尺测量体高、体长、胸围,根据测量数据计算平均日增重和体重增长率。
1.3.2 屠宰性能 试验期结束禁食24 h、禁水2 h后屠宰。屠宰后根据《鲜、冻胴体羊肉》(GB/T 9961—2008)将胴体分割,测量方法参照赵有璋[9]《羊生产学》。屠宰率、净肉率和肉骨比计算公式如下:
屠宰率(%)=胴体重/宰前活重×100;
净肉率(%)=净肉重/宰前活重×100;
肉骨比(%)=净肉重/骨重×100。
1.3.3 肉品质指标测定 屠宰后,立即采集背长肌进行pH[宰后45 min pH(pH45 min)、4 ℃排酸24 h(pH24 h)]、肉色[亮度(L*)、红度(a*)、黄度值(b*)]、蒸煮损失率和剪切力值的测定和计算,具体参照侯艳茹等[10]的方法。
1.3.4 瘤胃菌群结构 屠宰后,每组选取6只羊取瘤胃内容物用4层纱布过滤出瘤胃液,分装于无菌无酶的5 mL冻存管中,迅速放入液氮罐中速冻,然后转移至-80 ℃冰箱保存。将采集的瘤胃液样品解冻并充分混匀,参考E.Z.N.A.DNA试剂盒说明书对瘤胃样品中总DNA进行提取,用1%琼脂糖凝胶电泳检测DNA的纯度和浓度。利用引物515F(5′-GTGCCAGCMGCCGCGGTAA-3′)和926R(5′-CCGTCAATTCMTTTRAGTTT-3′)扩增16S rRNA基因的V4-V5区。Illumina Miseq测序及生物信息学分析,具体参照王柏辉[11]的方法。
1.4 数据统计分析数据经Excel 2016预处理后,利用SPSS 26.0软件对试验数据采用独立样本t检验进行分析比较,结果用“平均值±标准差”表示,P < 0.05时表示差异显著。
2 结果 2.1 饲养方式对苏尼特羊生长性能的影响由表 2可知,舍饲组苏尼特羊终末体重、平均日增重、体重增长率和胸围显著高于放牧组(P < 0.05),体高和体长较放牧组有所提高,但差异不显著(P>0.05)。
|
|
表 2 饲养方式对苏尼特羊生长性能的影响 Table 2 Effects of feeding regimens on growth performance of Sunit sheep |
由表 3可知,舍饲组苏尼特羊胴体重、净肉重、屠宰率和肉骨比显著高于放牧组(P < 0.05),两组之间其他屠宰性能指标无显著差异(P>0.05)。
|
|
表 3 饲养方式对苏尼特羊屠宰性能的影响 Table 3 Effects of feeding regimens on slaughter performance of Sunit sheep |
由表 4可知,放牧组苏尼特羊背最长肌的L*值、蒸煮损失率和剪切力显著低于舍饲组(P < 0.05)。两组苏尼特羊宰后45 min的pH无显著差异(P>0.05),置于冷库排酸24 h后放牧组pH24 h显著高于舍饲组(P < 0.05)。
|
|
表 4 饲养方式对苏尼特羊肉品质的影响 Table 4 Effects of feeding regimens on meat quality of Sunit sheep |
2.4.1 瘤胃菌群OTUs数量 基于97%物种相似性,将瘤胃液样品中获得的序列进行操作分类单元(operatianal taxonomic units, OTU)聚类,结果如图 1所示。两组共得到1 080个OTUs,放牧组获得870个OTUs,舍饲组获得709个OTUs,组间共有的OTUs有499个,说明两种饲养方式下苏尼特羊瘤胃菌群组成有所差异。
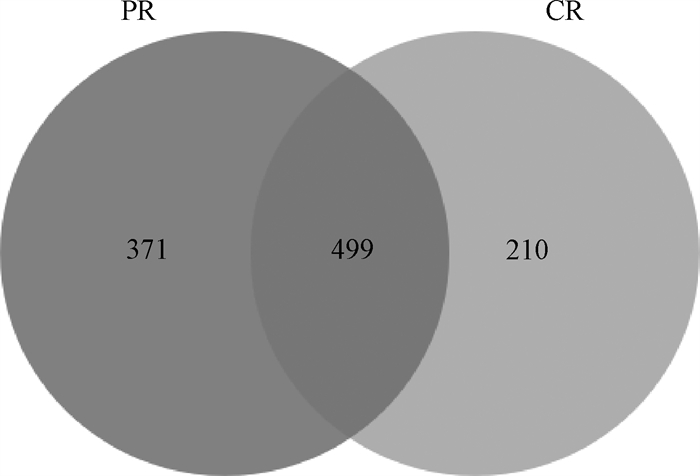
|
“PR”指放牧,“CR”指舍饲 'PR' represents pasture group and 'CR' represents confinement group 图 1 韦恩图 Fig. 1 Venn graph |
2.4.2 Alpha多样性分析 由表 5可知,各组OTUs序列的覆盖率(coverage)均达到0.99,说明测序样品满足后续瘤胃菌群生物信息学分析的要求。其中放牧组的Shannon指数显著高于舍饲组(P < 0.05),Simpson指数、ACE指数和Chao1指数在两组中无显著差异(P>0.05)。
|
|
表 5 饲养方式对苏尼特羊瘤胃菌群Alpha多样性指数影响 Table 5 Effects of feeding regimens on α-diversity index of rumen microbiota of Sunit sheep |
2.4.3 门水平 由表 6瘤胃菌群门水平上相对丰度(排名前5的菌属)可知,放牧组瘤胃厚壁菌门(Firmicutes)的相对丰度显著高于舍饲组(P < 0.05),拟杆菌门(Bacteroidetes)和变形菌门(Proteobacteria)的相对丰度显著低于舍饲组(P < 0.05)。饲养方式对苏尼特羊瘤胃螺旋体门(Spirochaetes)和放线菌门(Actinobacteria)的相对丰度无显著影响(P>0.05)。
|
|
表 6 饲养方式对苏尼特羊瘤胃菌群门水平相对丰度的影响 Table 6 Effects of feeding regimens on relative abundance of rumen microbiota community of Sunit sheep at phylum level |
2.4.4 属水平 由表 7瘤胃菌群属水平上相对丰度(排名前10的菌属)可知,放牧组理研菌科_RC9_肠道群属(Rikenellaceae_ RC9_gut_group)和丁酸弧菌属_2(Butyrivibrio_2)的相对丰度显著高于舍饲组(P < 0.05),而普雷沃氏菌属_1(Prevotella_1)的相对丰度显著低于舍饲组(P < 0.05)。
|
|
表 7 饲养方式对苏尼特羊瘤胃菌群属水平相对丰度的影响 Table 7 Effects of feeding regimens on relative abundance of rumen microbiota community of Sunit sheep at genus level |
影响肉羊生长性能的因素除了遗传因素之外,饲粮营养水平和饲养方式也尤为重要。本试验中,舍饲组苏尼特羊终末体重和平均日增重显著高于放牧组,说明舍饲饲养较放牧饲养可以显著提高苏尼特羊生长性能。吴铁梅[12]以4月龄内蒙古绒山羊为研究对象,发现夏季舍饲育肥的绒山羊终末体重和平均日增重均优于放牧组。Priolo等[13]研究不同饲养方式下法兰西岛羊的生长性能发现,集约化饲养组的生长性能显著优于放牧组。本研究结果与上述研究结果一致,分析原因可能是舍饲羊的饲粮能量较高,活动范围有限导致运动量和能量消耗较少,饲粮分解的营养物质以糖类和脂肪等能量物质形式沉积在体内,使其体重增加[14]。体尺性状和体重之间有极显著的相关性,尤其是胸围与体重的相关性最高[15]。本研究中,舍饲组苏尼特羊胸围显著高于放牧组,并与体重之间的增长趋势相同。
3.2 饲养方式对苏尼特羊屠宰性能的影响屠宰性能可以直观地反映动物的生产性能,也是畜禽经济价值最直接的体现。屠宰率、净肉率和眼肌面积等指标是衡量屠宰性能的常规方法[16]。本研究中,舍饲组苏尼特羊胴体重、屠宰率、净肉重和肉骨比显著高于放牧组,这与荷花[17]对不同饲养方式下绒山羊的屠宰性能研究结果一致。和世春等[18]研究发现,日粮补饲浓缩料或精饲料的舍饲组江城牛屠宰率、净肉率、产肉率等均高于放牧组。王敏[8]研究发现,由于营养水平增加和运动量较少,舍饲后畜禽可能发生应激反应、脂质代谢异常等现象,且舍饲肉牛的胴体重、净肉重、净肉率均显著高于放牧肉牛。
3.3 饲养方式对苏尼特羊肉品质的影响肉的品质可通过肉色、pH、系水力和嫩度等客观性状反映。肉色是最直接的感官品质,肉色的变化来源于肌红蛋白的氧化状态,L*值越大表示肉的颜色越白[10]。本研究中,放牧组背最长肌的L*值显著低于舍饲组,说明放牧饲养能改善肌肉色泽。pH是肉质形成的核心指标,不仅反映肌肉的酸碱度,更是反映动物宰后糖原酵解进程的重要标志[19]。本研究中,放牧组背最长肌pH24 h显著高于舍饲组,说明放牧饲养降低了肌肉中糖原积累和糖酵解速率,宰后成熟的速度更慢[20]。杨昌福等[21]研究表明,与舍饲牦牛相比,全天放牧牦牛的pH24 h显著降低,这与本试验结果相一致。蒸煮损失率反映烹饪过程中肌肉组织维持水分的能力,与肉质的产量和风味有关,蒸煮损失率低可使肌肉保持较好的嫩度和营养[22]。放牧组苏尼特羊肉的蒸煮损失率显著低于舍饲组,说明放牧羊肉具有更好的保水性,其嫩度可能也会因此改善。剪切力值与嫩度呈负相关,可以客观评价肉质嫩度[23]。本研究中,放牧组苏尼特羊肉的剪切力值低于舍饲组,说明放牧组苏尼特羊肉嫩度更好,分析原因可能是舍饲肉羊运动量较少且饲粮精料比例较高,所吸收的营养物质主要用于脂肪沉积和体重增加,导致其肌纤维变粗,嫩度下降[24]。
3.4 饲养方式对苏尼特羊瘤胃菌群的影响Shannon指数和Simpson指数可以反映物种多样性,ACE指数和Chao1指数可以反映物种丰富度和均匀度[25]。本研究中放牧组中观测到Shannon指数显著高于舍饲组,说明放牧组苏尼特羊瘤胃微生物多样性显著高于舍饲组,该结果与张晨光[26]的研究结果一致。出现上述结果可能是由于瘤胃微生物多样性随着饮食多样性的增加而增加,舍饲条件下饮食较为单一,而放牧条件下饮食较多样[27]。
关于反刍动物瘤胃菌群的结构组成,之前的研究表明,厚壁菌门和拟杆菌门在瘤胃样本门水平上占主导地位[28],这与本研究结果一致。拟杆菌门有利于非纤维性碳水化合物的降解,而厚壁菌门则主要由各种纤维和纤维素分解菌组成[29]。本研究中,放牧组苏尼特羊瘤胃内厚壁菌门的相对丰度更高,这与Fu等[30]在放牧滩羊和舍饲滩羊上关于瘤胃中厚壁菌门的丰度研究结果一致。可能的原因是舍饲组苏尼特羊饲粮能量水平较高,而放牧组苏尼特羊采食的天然牧草中粗纤维的含量较高,导致舍饲组中拟杆菌门相对丰度更高,放牧组中厚壁菌门相对丰度更高,而放牧组苏尼特羊获取能量的能力差可能是导致其生长性能差的原因[31]。变形菌门是瘤胃中第三丰富的菌门,参与瘤胃代谢中不溶性糖的消化和生物膜的形成[32]。本研究中,舍饲苏尼特羊瘤胃变形菌门更为丰富,这一发现与之前报道的变形菌门相对丰度与饲粮营养水平呈正相关的说法一致[33]。
在属水平上,苏尼特羊瘤胃中的优势菌属是普雷沃氏菌属、未分类_f__韦荣氏球菌、理研菌科_RC9_肠道群属等。从反刍动物瘤胃中分离培养的普雷沃氏菌,其功能不仅局限于水解淀粉和蛋白质,还参与必需脂肪酸的合成,满足宿主的营养需要[34-35]。本研究中,普雷沃氏菌属_1在舍饲组的相对丰度高于放牧组,分析原因可能是舍饲组苏尼特羊饲粮精料的蛋白质和淀粉含量较高,导致蛋白质和淀粉降解菌群的数量增加[36]。理研菌科RC9_肠道群属可以发酵碳水化合物和蛋白质,并能改善机体脂质代谢[37]。本研究中放牧组理研菌科RC9_肠道群属的相对丰度显著高于舍饲组,与Wang等[38]研究结果一致。此外,放牧组丁酸弧菌属的相对丰度高于舍饲组,由于丁酸弧菌属是参与多不饱和脂肪酸生物氢化最活跃的瘤胃细菌,并且与瘤胃中丁酸的产生及脂质水解和代谢有关,所以丁酸弧菌属相对丰度的增加对于瘤胃中脂肪酸代谢和瘤胃发酵至关重要[39]。综上分析可知,不同饲养模式下瘤胃菌群差异的原因可能是饮食成分不同造成的,除此之外,环境因素、运动方式、季节和温度等也是饲养模式存在的差异[4]。
4 结论与传统放牧相比,舍饲饲养苏尼特羊提高了终末体重、平均日增重、胴体重、屠宰率,改善了育肥增重效果和屠宰性能,提高了苏尼特羊背最长肌的剪切力和蒸煮损失率,但劣化了羊肉的加工品质和食用口感。此外,舍饲降低了苏尼特羊瘤胃微生物的多样性,提高了蛋白质和淀粉降解菌群的相对丰度。因此在进一步的研究中,可以通过调整苏尼特羊的运动方式和饲粮组成这两种差异因素,改善瘤胃菌群组成,进而达到既能提高苏尼特羊生长性能和屠宰性能,又能改善羊肉品质的目的。
| [1] |
旭日干. 中国肉用型羊主导品种及其应用展望[M]. 北京: 中国农业科学技术出版社, 2016: 57-59. BOU S. China's leading breeds of meat-type sheep and their application prospects[M]. Beijing: China Agricultural Science and Technology Press, 2016: 57-59. (in Chinese) |
| [2] |
WANG H R, CHEN Q, CHEN L M, et al. Effects of dietary physically effective neutral detergent fiber content on the feeding behavior, digestibility, and growth of 8-to 10-month-old Holstein replacement heifers[J]. J Dairy Sci, 2017, 100(2): 1161-1169. DOI:10.3168/jds.2016-10924 |
| [3] |
XIAO J X, ALUGONGO G M, CHUNG R, et al. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Ruminal fermentation, gastrointestinal morphology, and microbial community[J]. J Dairy Sci, 2016, 99(7): 5401-5412. DOI:10.3168/jds.2015-10563 |
| [4] |
ZHANG R Y, ZHU W Y, JIANG L S, et al. Comparative metabolome analysis of ruminal changes in Holstein dairy cows fed low-or high-concentrate diets[J]. Metabolomics, 2017, 13(6): 74. DOI:10.1007/s11306-017-1204-0 |
| [5] |
谭子璇, 柏雪, 罗璠, 等. 不同饲养方式对牦牛瘤胃微生物区系及肌肉品质的影响[J]. 食品科学, 2020, 41(15): 79-87. TAN Z X, BAI X, LUO F, et al. Effects of different feeding systems on rumen microflora and muscle quality of Yaks[J]. Food Science, 2020, 41(15): 79-87. (in Chinese) |
| [6] |
李蒋伟. 日粮精粗比对育肥藏羊消化道组织形态及微生物多样性的影响[D]. 西宁: 青海大学, 2021. LI J W. Effects of dietary concentrate to roughage ratio on digestive tract morphology and microbial diversity of fattening Tibetan Sheep[D]. Xining: Qinghai University, 2021. (in Chinese) |
| [7] |
WOOD J D, RICHARDSON R I, NUTE G R, et al. Effects of fatty acids on meat quality: A review[J]. Meat Sci, 2004, 66(1): 21-32. DOI:10.1016/S0309-1740(03)00022-6 |
| [8] |
王敏. 放牧与舍饲对肉牛生产性能和肉品质影响的比较研究[D]. 长春: 吉林大学, 2020. WANG M. A comparative study on the effects of grazing and stall feeding on the performance and meat quality of beef cattle[D]. Changchun: Jilin University, 2020. (in Chinese) |
| [9] |
赵有璋. 羊生产学[M]. 3版. 北京: 中国农业出版社, 2011. ZHAO Y Z. Sheep production[M]. 3rd ed. Beijing: China Agriculture Press, 2011. (in Chinese) |
| [10] |
侯艳茹, 苏琳, 侯普馨, 等. 饲养方式对苏尼特羊肌纤维组成和肉品质的影响及其调控机理[J]. 食品科学, 2021, 42(7): 83-89. HOU Y R, SU L, HOU P X, et al. Effect of feeding regimens on muscle fiber type composition and meat quality of Sunit sheep and underlying regulatory mechanism[J]. Food Science, 2021, 42(7): 83-89. (in Chinese) |
| [11] |
王柏辉. 饲养方式对苏尼特羊胃肠道菌群、脂肪酸代谢和羊肉品质的影响及机理研究[D]. 呼和浩特: 内蒙古农业大学, 2019. WANG B H. Effects of feeding regimens on gastrointestinal microbiota, fatty acid metabolism and meat quality of Sunit sheep and its underlying mechanism[D]. Hohhot: Inner Mongolia Agricultural University, 2019. (in Chinese) |
| [12] |
吴铁梅. 不同饲养模式对绒山羊羔羊育肥性能、屠宰性能及肉品质的影响[D]. 呼和浩特: 内蒙古农业大学, 2013. WU T M. Effects of different feeding modes on fattening performance and slaughter performance and meat quality of young cashmere goats[D]. Hohhot: Inner Mongolia Agricultural University, 2013. (in Chinese) |
| [13] |
PRIOLO A, MICOL D, AGABRIEL J, et al. Effect of grass or concentrate feeding systems on lamb carcass and meat quality[J]. Meat Sci, 2002, 62(2): 179-185. |
| [14] |
PERLO F, BONATO P, TEIRA G, et al. Meat quality of lambs produced in the Mesopotamia region of Argentina finished on different diets[J]. Meat Sci, 2008, 79(3): 576-581. |
| [15] |
SAHU J, KOLEY K M, SAHU B D. Attribution of antibacterial and antioxidant activity of Cassia tora extract toward its growth promoting effect in broiler birds[J]. Vet World, 2017, 10(2): 221-226. |
| [16] |
武晓东, 赵俊星, 刘文忠, 等. 日粮中胡麻饼代替豆粕对绵羊生产性能、肉品质、脂肪酸含量及血液生化指标的影响[J]. 畜牧兽医学报, 2017, 48(7): 1260-1270. WU X D, ZHAO J X, LIU W Z, et al. Effect of replacement of soybean meal by oil cake of flax seed in diet on growth performance, meat quality, fatty acid content and blood biochemical indicators of sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(7): 1260-1270. (in Chinese) |
| [17] |
荷花. 饲养模式对绒山羊成年母羊育肥和屠宰性能的影响[D]. 呼和浩特: 内蒙古农业大学, 2013. HE H. Effects of feeding mode on fattening performance and slaughter performance of adult female Cashmere goats[D]. Hohhot: Inner Mongolia Agricultural University, 2013. (in Chinese) |
| [18] |
和世春, 程月, 李清, 等. 不同饲养方式对江城牛生长性能和屠宰性能的影响[J]. 江苏农业科学, 2020, 48(15): 226-229. HE S C, CHENG Y, LI Q, et al. Effects of different feeding methods on growth performance and slaughter performance of Jiangcheng cattle[J]. Jiangsu Agricultural Sciences, 2020, 48(15): 226-229. (in Chinese) |
| [19] |
陈中卫, 王瑞秀, 刘强, 等. 低聚壳聚糖替代抗生素对樱桃谷肉鸭生长性能、屠宰性能、肠道屏障功能和肌肉品质的影响[J]. 畜牧兽医学报, 2021, 52(7): 1927-1941. CHEN Z W, WANG R X, LIU Q, et al. Effects of chitosan oligosaccharide supplementation as an alternative to antibiotic on the growth performance, slaughter performance, intestinal barrier function and meat quality of Cherry Valley ducks[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(7): 1927-1941. (in Chinese) |
| [20] |
侯艳茹, 马晓冰, 苏琳, 等. 饲养方式对宰后苏尼特羊肉一磷酸腺苷激活的蛋白激酶活力及糖酵解的影响[J]. 食品科学, 2018, 39(11): 15-20. HOU Y R, MA X B, SU L, et al. Effect of different feeding methods on AMP-activated protein kinase activity and glycolysis of postmortem Sunit Sheep[J]. Food Science, 2018, 39(11): 15-20. (in Chinese) |
| [21] |
杨昌福, 柏雪, 高彦华, 等. 全放牧与舍饲育肥对牦牛肉品质及安全性的影响[J]. 畜牧与兽医, 2019, 51(1): 23-28. YANG C F, BAI X, GAO Y H, et al. Effects of free-grazing and house-feeding on meat quality and safety in domesticated yaks[J]. Animal Husbandry & Veterinary Medicine, 2019, 51(1): 23-28. (in Chinese) |
| [22] |
胡猛, 张文举, 尹君亮, 等. 育成荷斯坦奶公牛与其他3个品种牛的肉品质比较研究[J]. 中国畜牧兽医, 2013, 40(3): 95-99. HU M, ZHANG W J, YIN J L, et al. Study on beef quality of growing holstein dairy bull compared with other three breeds[J]. China Animal Husbandry & Veterinary Medicine, 2013, 40(3): 95-99. (in Chinese) |
| [23] |
罗玉龙. 放牧与舍饲条件下苏尼特羊肉风味差异及形成机制研究[D]. 呼和浩特: 内蒙古农业大学, 2019. LUO Y L. Difference of meat flavor in Sunit lamb (concentrated vs. grazing) and primary formation mechanism[D]. Hohhot: Inner Mongolia Agricultural University, 2019. (in Chinese) |
| [24] |
MARTÍNEZ-CEREZO S, SAÑUDO C, MEDEL I, et al. Breed, slaughter weight and ageing time effects on sensory characteristics of lamb[J]. Meat Sci, 2005, 69(3): 571-578. |
| [25] |
韦肖, 张建童, 龙唐晖, 等. 日粮能量水平对湖羊瘤胃发酵特性和微生物组成的影响[J]. 畜牧兽医学报, 2022, 53(9): 3042-3051. WEI X, ZHANG J T, LONG T H, et al. Effects of dietary energy level on rumen fermentation characteristics and microbial composition of Hu Sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(9): 3042-3051. (in Chinese) |
| [26] |
张晨光. 放牧和舍饲对藏山羊生长发育、肉品质及胃肠道细菌区系的影响[D]. 咸阳: 西北农林科技大学, 2020. ZHANG C G. Growth and development, meat quality, gastrointestinal bacteria of grazing and drylot raised Tibetan goat[D]. Xianyang: Northwest A&F University, 2020. (in Chinese) |
| [27] |
SHABAT S K B, SASSON G, ORON-FAIGENBOIM A, et al. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants[J]. ISME J, 2016, 10(12): 2958-2972. |
| [28] |
CLEMMONS B A, VOY B H, MYER P R. Altering the gut microbiome of cattle: Considerations of host-microbiome interactions for persistent microbiome manipulation[J]. Microb Ecol, 2019, 77(2): 523-536. |
| [29] |
BRULC J M, ANTONOPOULOS D A, MILLER M E B, et al. Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases[J]. Proc Natl Acad Sci U S A, 2009, 106(6): 1948-1953. |
| [30] |
FU Z L, XU X F, ZHANG J, et al. Effect of different feeding methods on rumen microbes in growing Chinese Tan sheep[J]. R Bras Zootec, 2020, 49: e20190258. |
| [31] |
CUI X X, WANG Z F, YAN T H, et al. Rumen bacterial diversity of Tibetan sheep (Ovis aries) associated with different forage types on the Qinghai-Tibetan Plateau[J]. Can J Microbiol, 2019, 65(12): 859-869. |
| [32] |
PITTA D W, INDUGU N, KUMAR S, et al. Metagenomic assessment of the functional potential of the rumen microbiome in Holstein dairy cows[J]. Anaerobe, 2016, 38: 50-60. |
| [33] |
WANG Y Y, CAO P H, WANG L, et al. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep[J]. Appl Microbiol Biotechnol, 2017, 101(9): 3717-3728. |
| [34] |
AVGUSTIN G, WALLACE R J, FLINT H J. Phenotypic diversity among ruminal isolates of Prevotella ruminicola: Proposal of Prevotella brevis sp. nov., Prevotella bryantii sp. nov., and Prevotella albensis sp.nov. and redefinition of Prevotella ruminicola[J]. Int J Syst Bacteriol, 1997, 47(2): 284-288. |
| [35] |
PURUSHE J, FOUTS D E, MORRISON M, et al. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: insights into their environmental niche[J]. Microb Ecol, 2010, 60(4): 721-729. |
| [36] |
COE M L, NAGARAJA T G, SUN Y D, et al. Effect of virginiamycin on ruminal fermentation in cattle during adaptation to a high concentrate diet and during an induced acidosis[J]. J Anim Sci, 1999, 77(8): 2259-2268. |
| [37] |
ZHOU L Y, XIAO X H, ZHANG Q, et al. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota[J]. Front Endocrinol, 2018, 9: 516. |
| [38] |
WANG B H, LUO Y L, WANG Y, et al. Rumen bacteria and meat fatty acid composition of Sunit sheep reared under different feeding regimens in China[J]. J Sci Food Agric, 2021, 101(3): 1100-1110. |
| [39] |
张双双, 杨承剑, 梁明振, 等. 瘤胃溶纤维丁酸弧菌调控共轭亚油酸合成的研究进展[J]. 畜牧与兽医, 2014, 46(2): 105-108. ZHANG S S, YANG C J, LIANG M Z, et al. Research progress on regulation of conjugated linoleic acid synthesis by rumen fibrinobutyribrio[J]. Animal Husbandry & Veterinary Medicine, 2014, 46(2): 105-108. (in Chinese) |
(编辑 范子娟)



