2. 湖南农业大学 畜禽保健湖南省工程研究中心, 长沙 410128
2. Hunan Engineering Research Center of Livestock and Poultry Health Care, Hunan Agricultural University, Changsha 410128, China
霉菌毒素是霉菌在生长繁殖过程中产生的有毒次级代谢产物,会对人和动物产生急性或慢性毒性。桔青霉素(citrinin, CTN)是由青霉属、曲霉属和红曲霉属等菌株产生的,广泛存在于各类农产品中[1],对肝、肾、胃肠道、免疫和生殖系统具有潜在的毒性作用[2-4]。肠道是营养物质消化吸收的重要器官,桔青霉素污染的食物摄入机体时,胃肠道首先对其进行吸收,肠道健康严重受损[5]。研究表明,桔青霉素的摄入能兴奋试验动物小肠平滑肌,诱导出血性肠炎及腹泻甚至导致动物死亡[6-7]。桔青霉素主要通过诱导氧化应激及细胞凋亡发挥其毒性作用[8]。有研究表明,桔青霉素可通过诱导内质网应激(endoplasmic reticulum stress, ERS)介导的细胞凋亡对人肠道HCT116细胞产生毒性作用,引发肠道损伤[9]。
内质网是一种多功能细胞器,参与蛋白质合成,脂质代谢及维持Ca2+稳态。有研究通过体外培养猪肠上皮细胞,并在体内对断奶仔猪的空肠组织进行检测,发现肠道屏障功能障碍的发生与内质网应激密切相关[10]。张同坤等[11]的研究证实,内质网应激在呕吐毒素诱导的肠细胞屏障功能障碍中发挥着重要作用。然而,桔青霉素是否通过诱导内质网应激损害肠屏障功能还有待研究。因此,本研究通过饲喂小鼠不同浓度桔青霉素,探讨其对肠屏障功能的影响,并给小鼠注射内质网应激抑制剂,探究内质网应激在桔青霉素诱导肠屏障功能障碍中的作用,为寻找桔青霉素的毒性作用靶点提供理论参考。
1 材料与方法 1.1 试验动物本试验所用SPF级昆明小鼠(雄性)购自湖南省长沙市斯莱克景达实验动物有限公司,体重(27.8± 1.5)g,试验期间小鼠自由采食和饮水。适应性饲喂1周后将24只小鼠随机分为4组(n=6):即空白组(5%乙醇)、低CTN组(1.25 mg·kg-1)、中CTN组(5 mg·kg-1)、高CTN组(20 mg·kg-1),分别给小鼠灌胃不同剂量桔青霉素,14 d后采集小鼠血清及空肠进行相关检测。
将32只SPF级昆明雄鼠随机分为4组,分别为对照组(5%乙醇)、桔青霉素中剂量(5 mg·kg-1)组、内质网应激抑制剂4-PBA(240 mg·kg-1)组和桔青霉素+4-PBA组。对照组给小鼠灌胃5%乙醇;桔青霉素中剂量组给小鼠灌胃5 mg·kg-1桔青霉素;4-PBA组小鼠经腹腔注射4-PBA预处理后,用5%乙醇对小鼠进行灌胃;4-PBA+桔青霉素组小鼠经腹腔注射4-PBA预处理后,用5 mg·kg-1桔青霉素对小鼠进行灌胃;试验持续14 d。
1.2 主要试剂桔青霉素(普瑞邦,中国);4-PBA(MedChemExpress,中国);MDA、SOD、CAT、T-AOC、BCA检测试剂盒(南京建成,中国);TUNEL、DHE、DAPI、HE染液(赛维尔,中国);DAO、D-LA、ET的ELISA试剂盒,二抗(艾方,中国);TRIzol、反转录试剂盒、荧光染料SYBR Green(艾科瑞,中国);增强化学发光试剂(ECL)(翌圣,中国);Bax、Bcl-2、GRP78、CHOP、IRE1α、ATF6、β-actin一抗(Proteintech,中国)。
1.3 HE染色观察空肠病变新鲜空肠组织用4%多聚甲醛固定,经脱水透明、包埋、切片后用苏木素和伊红染色,脱水封片后显微镜下观察组织形态。
1.4 TUNEL法检测细胞凋亡在HE切片的基础上,用TUNEL试剂盒对切片进行染色,将TUNEL阳性细胞染成绿色,以此评估细胞凋亡率。
1.5 检测空肠中ROS含量新鲜空肠组织经液氮速冻后进行包埋及切片处理,滴加ROS染液DHE于37 ℃避光孵育30 min,后用DAPI染液避光孵育10 min,经抗荧光淬灭封片剂封片后置于荧光显微镜下观察。
1.6 ELISA检测血清中DAO、D-LA、ET水平小鼠经眼球采血收集血清,按照ELISA试剂盒说明书检测血清中DAO、D-LA、ET水平。
1.7 检测空肠氧化指标根据南京建成生物工程公司检测试剂盒说明书,称取0.1 g空肠组织,用预冷的生理盐水按1 ∶9的比例稀释,经匀浆并离心后取上清进行CAT、T-SOD、T-AOC、MDA的检测。
1.8 实时荧光定量PCR(qRT-PCR)检测空肠紧密连接相关基因表达0.1 g空肠组织加至预冷的TRIzol中以提取RNA,按照反转录试剂盒的说明,将RNA反转为cDNA,cDNA与上、下游引物及荧光染料SYBR green充分混合后于PCR仪进行扩增,以GAPDH为内参,采用2-△△Ct法对紧密连接相关基因的相对表达量进行计算。试验所涉及的相关引物信息如表 1所示。
|
|
表 1 相关引物信息 Table 1 The relative primers information |
用RIPA裂解液按照RIPA ∶PMSF=100 ∶1的比例提取空肠组织中的总蛋白,经BCA试剂盒进行蛋白定量。每组等量的蛋白上样后进行电泳并转移至PVDF膜,将膜用5%脱脂牛奶封闭后转入TBST中洗3次,一抗4 ℃过夜,TBST洗涤3次后二抗室温孵育1 h,TBST洗涤10 min×3次完成后,配置ECL显影液进行显影及图像分析。
1.10 数据处理及统计学分析经3次独立重复试验后,用GraphPad Prism 9对试验数据进行作图并用SPSS 23.0对试验数据进行统计学分析,试验结果以“平均值±标准误差(X±SEM)”表示,P<0.05表示差异显著,P<0.01表示差异极显著。
2 结果 2.1 桔青霉素对小鼠空肠组织病理损伤的影响HE染色观察桔青霉素对小鼠空肠组织的病理损伤,结果如图 1所示,空白组空肠黏膜完整,绒毛排列整齐,肠上皮细胞形态清晰,排列紧密,大小均匀,无明显病理变化;桔青霉素低、中、高剂量组肠绒毛明显萎缩、脱落,基底部肠上皮细胞排列紊乱,染色加深,肠上皮细胞挤压明显。
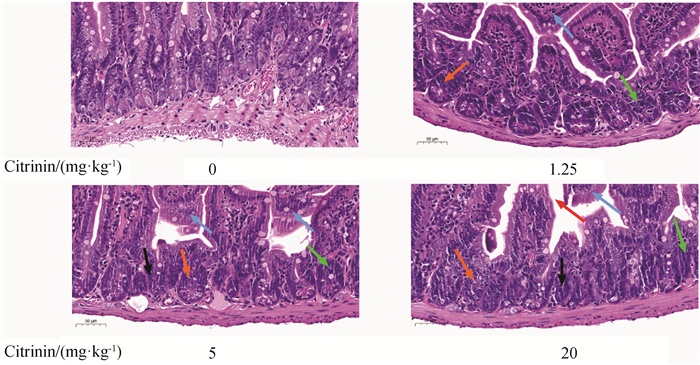
|
蓝色箭头:肠绒毛萎缩、脱落;橙色箭头:基底部肠上皮细胞排列紊乱;绿色箭头:基底部肠上皮细胞染色加深;红色箭头: 肠绒毛间隙增大。黑色箭头:肠上皮细胞挤压明显。比例尺=50 μm。下同 Blue arrows: Atrophy and shedding of intestinal villi; Orange arrows: Disorganization in basal portions of intestinal epithelial cells; Green arrows: Deeply stained in basal portions of intestinal epithelial cells; Red arrows: Increased gaps of intestinal villi; Black arrows: Obvious extrusion of intestinal epithelial cells. Scale bar=50 μm. The same below 图 1 桔青霉素对小鼠空肠损伤的组织学观察(20×)(扫描文章首页OSID码可查看彩图) Fig. 1 Histological observation of citrinin induced jejunum injury in mice(20×)(Scan the OSID code on the homepage of the article to view the color map) |
进一步探讨桔青霉素对小鼠空肠屏障功能的影响,结果如图 2所示,与空白组相比,经低、中、高剂量桔青霉素处理后,小鼠血清中D-LA和ET的含量显著升高(P<0.05或P<0.01),中、高剂量桔青霉素显著增加了小鼠血清DAO(P<0.05)。
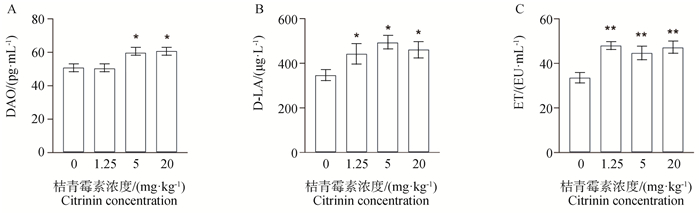
|
与对照组相比,*. P<0.05表示差异显著;**. P<0.01表示差异极显著。下同 Compared with the control group.*. P<0.05 means the difference is significant; **. P<0.01 means the difference is extremely significant. The same below 图 2 桔青霉素对小鼠血清DAO(A)、D-LA(B)、ET(C)的影响 Fig. 2 Effects of citrinin on the content of DAO(A), D-LA(B), ET(C) in mice serum |
用不同剂量桔青霉素对小鼠进行灌胃处理,取空肠组织检测抗氧化酶活性,结果如图 3所示,与空白组相比,桔青霉素中剂量组CAT活性显著降低(P<0.05),桔青霉素高剂量组总SOD活性显著降低(P<0.05),且经低、中、高剂量桔青霉素处理后,氧化产物ROS的生成极显著增多(P<0.01)。
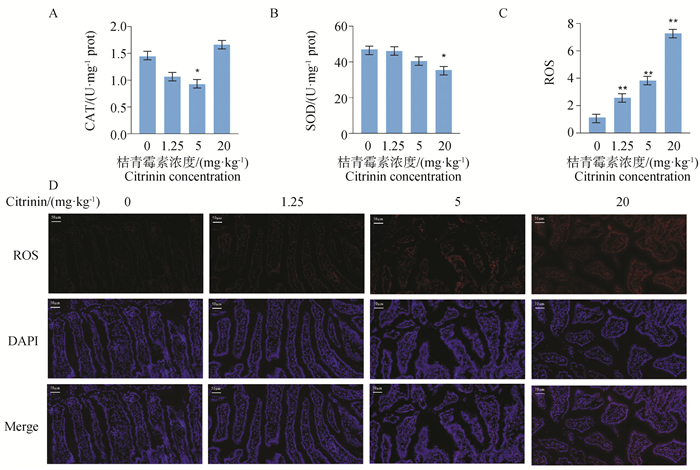
|
A. 空肠中CAT的活性;B. 空肠中SOD的活性;C. ROS的定量分析;D. 空肠中ROS的荧光图。比例尺=50 μm A. The activity of CAT in mice jejunum; B. The activity of SOD in mice jejunum; C. Quantitative analysis of ROS; D. The fluorescence images of ROS in mice jejunum. Bar=50 μm 图 3 桔青霉素对小鼠空肠氧化损伤的影响 Fig. 3 Effects of citrinin on oxidative damage in mice jejunum |
小鼠经内质网应激抑制剂4-PBA预处理后,采用Western blot检测内质网应激相关蛋白的表达。结果如图 4所示,与空白组相比,经5 mg·kg-1桔青霉素处理后,小鼠空肠组织内质网应激相关蛋白肌醇激酶(inosital-requiring enzyme-1,IRE1)、活化转录因子6(activating transcription factor 6,ATF6)、葡萄糖调节蛋白(glucose regulating protein78,GRP78/Bip)和转录因子C/EBP(C/EBP-homologous protein, CHOP)表达显著增强(P<0.01),4-PBA单独处理组内质网应激相关蛋白的表达无明显变化。与单独桔青霉素组相比,4-PBA+ CTN组内质网应激相关蛋白的表达减弱(P<0.05)。
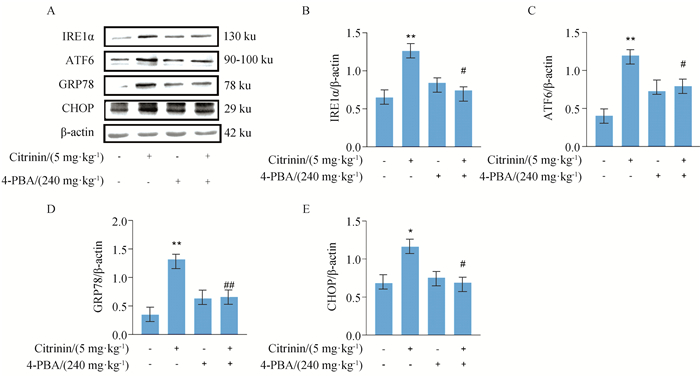
|
A.相关蛋白的蛋白免疫印迹图;B.IRE1蛋白表达的灰度值分析;C.ATF6蛋白表达的灰度值分析;D.GRP78蛋白表达的灰度值分析;E.CHOP蛋白表达的灰度值分析。与对照组相比,*、**表示差异显著(P<0.05)或极显著(P<0.01);与桔青霉素组相比,#、##表示差异显著(P<0.05)或极显著(P<0.01)。下同 A. Western blot of related proteins; B. Gray value analysis of IRE1 protein; C. Gray value analysis of ATF6 protein; D. Gray value analysis of GRP78 protein; E. Gray value analysis of CHOP protein. Compared with the control group, *, ** mean the difference is significant(P<0.05) or extremely significant (P<0.01); Compared with the citrinin group, #, ## mean the difference is significant(P<0.05) or extremely significant (P<0.01). The same as below 图 4 4-PBA对桔青霉素诱导小鼠空肠内质网应激相关蛋白的影响 Fig. 4 Effects of 4-PBA on expression of ERS-related protein induced by citrinin in mice jejunum |
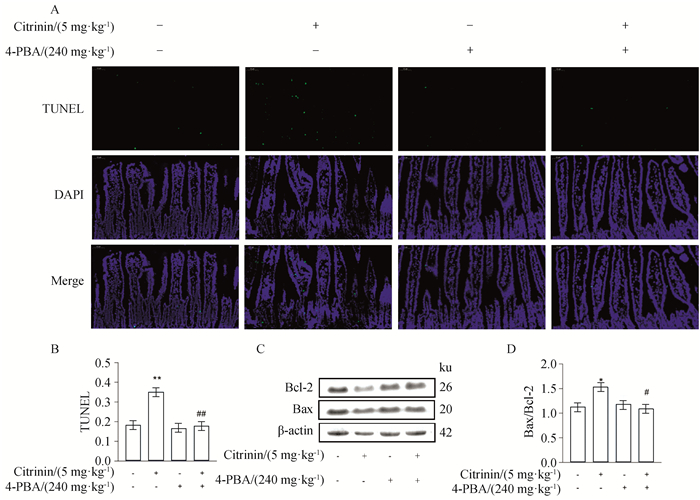
给小鼠腹腔注射内质网应激抑制剂4-PBA,TUNEL法检测细胞凋亡率。结果如图 5A~B所示,与空白组相比,经5 mg·kg-1g桔青霉素处理后,小鼠空肠组织凋亡细胞极显著增多(P<0.01),4-PBA单独处理组凋亡细胞无明显变化。与单独桔青霉素组相比,4-PBA+CTN组凋亡细胞极显著减少(P<0.01)。此外,对细胞凋亡相关蛋白Bax、Bcl-2进行检测,结果如图 5C~D所示,与空白组相比,经5 mg·kg-1桔青霉素处理后,Bax/Bcl-2显著升高(P<0.05),4-PBA单独处理组Bax/Bcl-2无明显变化。与单独桔青霉素组相比,4-PBA+CTN组Bax/Bcl-2显著降低(P<0.05)。
|
A. TUNEL检测细胞凋亡率;B. 细胞凋亡率的定量分析;C. Bax、Bcl-2的蛋白免疫印迹图;D. Bax/Bcl-2的比值。比例尺=50 μm A. Apoptosis rate detected by TUNEL; B. Quantitative analysis of apoptosis rate; C. Western blot of Bax and Bcl-2; D. The ratio of Bax/Bcl-2. Bar=50 μm 图 5 4-PBA对桔青霉素诱导小鼠空肠细胞凋亡的影响 Fig. 5 Effects of 4-PBA on cell apoptosis induced by citrinin in mice jejunum |
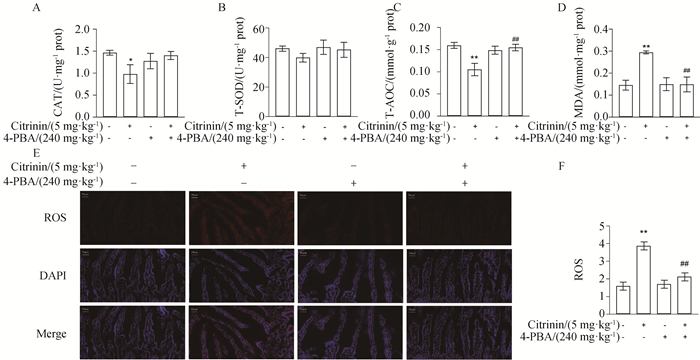
小鼠经内质网应激抑制剂4-PBA预处理后,检测其空肠组织中抗氧化酶CAT、T-SOD、T-AOC的活性,氧化产物MDA含量及ROS的释放。结果如图 6所示,与空白组相比,经5 mg·kg-1桔青霉素处理后,小鼠空肠组织CAT、T-AOC活性显著降低(P<0.05或P<0.01),总SOD活性有下降的趋势,MDA含量及ROS的释放极显著增多(P<0.01),4-PBA单独处理组抗氧化酶、MDA含量及ROS释放无明显变化。与单独桔青霉素组相比,4-PBA+CTN组CAT、T-SOD活性有升高的趋势,T-AOC活性极显著升高(P<0.01),MDA含量及ROS的释放极显著减少(P<0.01)。
|
A. 空肠中CAT的活性;B. 空肠中T-SOD的活性;C. 空肠中T-AOC的活性;D. 空肠中MDA的含量;E. 空肠中ROS的荧光图;F. ROS的定量分析。比例尺=50 μm A. The activity of CAT in mice jejunum; B. The activity of T-SOD in mice jejunum; C. The activity of T-AOC in mice jejunum; D. The content of MDA in mice jejunum; E. The fluorescence images of ROS in mice jejunum; F. Quantitative analysis of ROS. Bar=50 μm 图 6 4-PBA对桔青霉素诱导小鼠空肠氧化损伤的影响 Fig. 6 Effects of 4-PBA on oxidative damage induced by citrinin in mice jejunum |
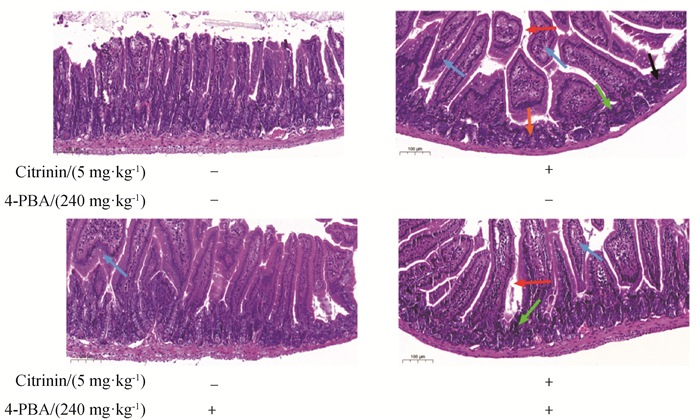
预先腹腔注射4-PBA至小鼠体内,观察小鼠空肠组织的病理损伤。HE结果如图 7所示,与空白组相比,经5 mg·kg-1桔青霉素处理后,肠黏膜完整性受到破坏,大量肠绒毛断裂、脱落,基底部肠上皮细胞染色加深,排列疏松、紊乱,4-PBA单独处理组肠黏膜较为完整,肠绒毛排列紧密,偶见肠绒毛断裂,基底部肠上皮细胞未见染色加深。与单独桔青霉素组相比,4-PBA+CTN组肠绒毛排列较CTN组稍紧密,少见肠绒毛脱落。
|
图 7 4-PBA对桔青霉素诱导小鼠空肠组织损伤的影响(20×) Fig. 7 Effects of 4-PBA on histological damage induced by citrinin in mice jejunum(20×) |
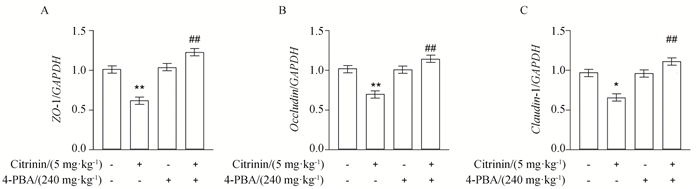
经腹腔注射4-PBA后,检测小鼠空肠中紧密连接蛋白ZO-1、Occludin、Claudin-1的mRNA表达,结果如图 8所示,与空白组相比,经5 mg·kg-1桔青霉素处理后,小鼠空肠组织ZO-1、Occludin、Claudin-1的mRNA表达显著减少(P<0.01或P<0.05),4-PBA单独处理组ZO-1、Occludin、Claudin-1的mRNA表达无明显变化。与单独桔青霉素组相比,4-PBA+CTN组ZO-1、Occludin、Claudin-1的mRNA表达得到恢复(P<0.01)。
|
图 8 4-PBA对桔青霉素诱导小鼠空肠物理屏障的影响 Fig. 8 Effects of 4-PBA on physical barriers induced by citrinin in mice jejunum |
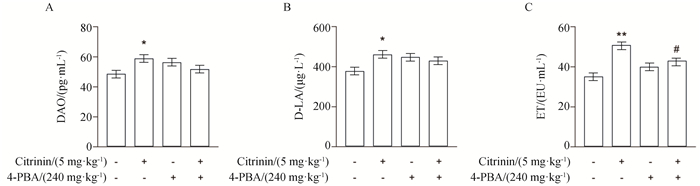
小鼠经4-PBA预处理后,检测其血清中DAO、D-LA、ET的含量。结果如图 9所示,与空白组相比,经5 mg·kg-1桔青霉素处理后,小鼠血清中DAO、D-LA、ET的含量显著升高(P<0.05或P<0.01),4-PBA单独处理组DAO、D-LA、ET的含量无明显变化。与单独桔青霉素组相比,4-PBA+CTN组ET的含量显著降低(P<0.05),DAO、D-LA的含量有降低的趋势。
|
图 9 4-PBA对桔青霉素诱导小鼠空肠化学屏障的影响 Fig. 9 Effects of 4-PBA on chemical barriers induced by citrinin in mice jejunum |
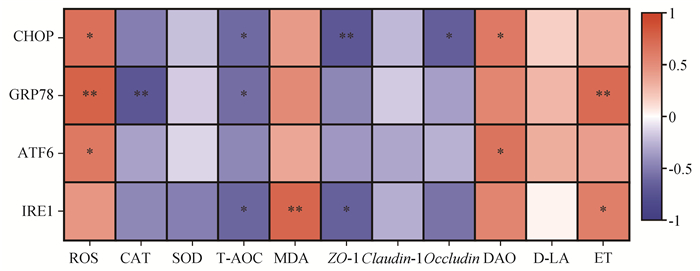
前面的研究已经证实,桔青霉素可以激活内质网应激信号通路,诱导肠道发生氧化应激,损害肠道屏障功能。为进一步证实内质网应激信号通路在桔青霉素诱导肠道屏障功能障碍及氧化应激中的作用,对内质网应激相关蛋白和肠道屏障功能相关指标、相关抗氧化酶及氧化产物进行Spearman相关性分析。结果如图 10所示,内质网应激信号通路中CHOP与ROS及DAO呈显著正相关(P<0.05),与T-AOC和Occludin呈显著负相关(P<0.05),与ZO-1呈极显著负相关(P<0.01);内质网应激信号通路中GRP78与ET、ROS呈极显著正相关(P<0.01),与CAT呈极显著负相关(P<0.01),与T-AOC呈显著负相关(P<0.05);内质网应激信号通路中ATF6与ROS和DAO呈显著正相关(P<0.05);内质网应激信号通路中IRE1与ZO-1及T-AOC呈显著负相关(P<0.05),与ET呈显著正相关(P<0.05),与MDA呈极显著正相关(P<0.01)。以上结果说明内质网应激信号通路与肠道氧化应激、肠道屏障功能障碍有显著相关性,进一步证实桔青霉素通过激活内质网应激介导的细胞凋亡,降低抗氧化酶活性,促进ROS及MDA的积累,从而使小鼠肠道屏障功能发生障碍。
|
图 10 内质网应激与肠道损伤的相关性分析 Fig. 10 Correlation analysis in endoplasmic reticulum stress and intestinal injury |
桔青霉素分布范围广,毒性大,其广泛存在于各类蔬菜、饲料及谷物中,对人和动物造成极大威胁。胃肠道是保护机体免受霉菌毒素及其他环境污染物损害的重要屏障,且有害物质在进入机体前首先要经胃肠道,因此,肠道中的霉菌毒素含量往往高于其他组织,表现为肠道发生氧化应激,其屏障功能受损及结构完整性被破坏[12-13]。本研究中,给小鼠灌胃不同剂量桔青霉素后,空肠绒毛萎缩断裂,肠上皮细胞受损。高亿清等[12]的研究显示,给小鼠饲喂霉变玉米,其空肠绒毛脱落,上皮细胞形态结构受损,这本试验结果一致。
研究证实,霉菌毒素可破坏肠上皮的形态结构完整性从而诱导肠道的屏障功能受损[14]。当肠道化学屏障功能受损时,肠道通透性增加,DAO、D-LA、ET释放入血,血液中DAO、D-LA、ET含量升高,检测血清中DAO、D-LA、ET的含量可用于反映肠屏障功能。与此同时,紧密连接蛋白ZO-1、Occludin、Claudin-1被认为与肠道通透性密切相关,广泛用于评价肠道物理屏障[15-16]。娄文芳等[17]通过在蛋鸡饲粮中添加DON和T-2毒素,发现DON和T-2毒素能使蛋鸡血清中ET、DAO含量增多,肠道屏障被破坏。Liu等[18]研究发现,黄曲霉毒素B1处理的小鼠肠道紧密连接蛋白ZO-1、Occludin、Claudin-1的表达显著降低。这些研究为本研究中桔青霉素显著提高小鼠血清中DAO、D-LA、ET含量,降低ZO-1、Occludin、Claudin-1的mRNA表达,引起肠道屏障功能发生障碍的结果提供了佐证。
据报道,在霉菌毒素致肠道损伤的过程中,氧化应激可引起肠上皮细胞损伤和屏障功能障碍[19]。有研究表明,DON能够诱导仔猪肠道发生氧化应激,损害小肠屏障功能,最终对仔猪健康造成严重威胁[20]。类似地,本研究发现桔青霉素通过降低小鼠空肠组织抗氧化酶总SOD和CAT活性,增加氧化产物ROS、MDA的生成促进氧化应激,从而使空肠屏障功能发生障碍。
目前,内质网已被证实是参与维持肠道健康的重要细胞器[21]。当机体受到毒素、氧化应激等因素刺激时,蛋白质加工受阻,内质网稳态被打破,从而诱导了内质网应激,同时,持续的内质网应激将最终激活细胞凋亡[22-23]。内质网应激介导的细胞凋亡主要受肌醇需要酶1α(inositol-requiring enzyme 1α,IRE1α)、双链RNA依赖的蛋白激酶样内质网激酶(PKR-like ER-resident kinase,PERK)和活化转录因子6α(activating transcription factor-6,ATF6α)这3个蛋白调控,内质网发生应激时,IRE1α、PERK、ATF6α与GRP78解离而被激活,共同促进其下游凋亡因子C/EBP同源蛋白(C/EBP homology protein,CHOP)的表达,CHOP表达的上调进一步调控相关凋亡蛋白Bax及Bcl-2的表达,并同时激活Caspase-12,诱发caspase级联反应,最终导致细胞凋亡[24-26]。研究表明,桔青霉素可激活内质网应激,促进细胞凋亡从而损伤人肠道上皮细胞[9]。为进一步探讨内质网应激在桔青霉素诱导小鼠空肠损伤中的作用,本研究添加内质网应激抑制剂4-PBA预处理小鼠,结果显示内质网应激相关蛋白IRE1α、GRP78、CHOP、ATF6、细胞凋亡相关蛋白Bax/Bcl-2的表达受到抑制,细胞凋亡率减少,说明桔青霉素通过激活内质网应激信号通路促进空肠凋亡,从而诱导空肠损伤。此外,有研究表明,内质网应激能诱导氧化应激,内质网应激抑制剂4-PBA可以提高糖尿病大鼠的抗氧化酶活性,降低脂质过氧化水平[27]。这也与本试验结果相符:与桔青霉素组相比,用4-PBA预处理小鼠后可减轻空肠组织的氧化应激水平,对桔青霉素诱导的空肠损伤产生保护作用。Cui等[28]通过研究发现,IPEC-J2细胞经热应激处理后,内质网应激信号通路被激活,肠道上皮细胞屏障功能受损,提示肠道屏障功能受内质网应激信号通路调控。本研究通过Spearman相关性分析发现,内质网应激信号通路与氧化应激、肠道屏障功能相关指标存在显著相关性,4-PBA预处理可抑制桔青霉素诱导的内质网应激信号通路的激活,缓解氧化应激和细胞凋亡,保护肠道屏障功能免受桔青霉素的损害。综上所述,这些结果表明内质网应激信号通路在桔青霉素诱导的肠道损伤中起重要调控作用。
4 结论本研究证实,桔青霉素对小鼠空肠有损伤作用,随着桔青霉素剂量的升高,小鼠空肠绒毛断裂,肠上皮细胞损伤明显,血清中DAO、D-LA、ET的含量升高,致其肠道屏障受损,空肠抗氧化酶SOD、CAT活性降低,ROS生成增多,说明桔青霉素可诱导小鼠空肠屏障功能障碍且诱导氧化应激发生。该损伤受内质网应激通路调控,添加4-PBA预处理后,小鼠空肠细胞凋亡率减少,相关抗氧化酶活性及紧密连接蛋白的表达增强,血清中DAO、D-LA、ET的含量减少,进一步证实桔青霉素能够激活内质网应激介导的氧化应激和细胞凋亡,促进空肠结构损伤及肠道屏障功能障碍。
| [1] |
毛妍, 杨梦然, 梁曾恩妮, 等. 桔青霉素的研究现状[J]. 动物医学进展, 2021, 42(11): 121-124. MAO Y, YANG M R, LIANG Z E N, et al. Current status of citrinin research[J]. Progress in Veterinary Medicine, 2021, 42(11): 121-124. DOI:10.16437/j.cnki.1007-5038.2021.11.023 (in Chinese) |
| [2] |
DE OLⅣEIRA FILHO J, ISLAM M T, ALI E S, et al. A comprehensive review on biological properties of citrinin[J]. Food Chem Toxicol, 2017, 110: 130-141. DOI:10.1016/j.fct.2017.10.002 |
| [3] |
ISLAM M R, ROH Y S, CHO A, et al. Immune modulatory effects of the foodborne contaminant citrinin in mice[J]. Food Chem Toxicol, 2012, 50(10): 3537-3547. DOI:10.1016/j.fct.2012.06.050 |
| [4] |
QINGQING H, LINBO Y, YUNQIAN G, et al. Toxic effects of citrinin on the male reproductive system in mice[J]. Exp Toxicol Pathol, 2012, 64(5): 465-469. DOI:10.1016/j.etp.2010.10.015 |
| [5] |
LIEW W P P, MOHD-REDZWAN S. Mycotoxin: its impact on gut health and microbiota[J]. Front Cell Infect Microbiol, 2018, 8: 60. DOI:10.3389/fcimb.2018.00060 |
| [6] |
郭玉学, 金惠民, 张子玉, 等. 猪桔青霉素中毒的诊断报告[J]. 黑龙江畜牧兽医, 1990(1): 29-31. GUO Y X, JIN H M, ZHANG Z Y, et al. Diagnostic report of porcine citrinin poisoning[J]. Heilongjiang Animal Science and Veterinary Medicine, 1990(1): 29-31. (in Chinese) |
| [7] |
许建平, 孙志良, 谭超, 等. 桔青霉素对试验家兔小肠运动的影响[J]. 湖南农业大学学报: 自然科学版, 2000(6): 439-440. XU J P, SUN Z L, TAN C, et al. Effects of citrinin on the motion of experimental rabbits ' intestien[J]. Journal of Hunan Agricultural University: Natural Sciences, 2000(6): 439-440. DOI:10.3321/j.issn:1007-1032.2000.06.010 (in Chinese) |
| [8] |
GAYATHRI L, DHⅣYA R, DHANASEKARAN D, et al. Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: in vitro study in HepG2 cell[J]. Food Chem Toxicol, 2015, 83: 151-163. DOI:10.1016/j.fct.2015.06.009 |
| [9] |
SALAH A, BOUAZIZ C, PROLA A, et al. Citrinin induces apoptosis in human HCT116 colon cancer cells through endoplasmic reticulum stress[J]. J Toxicol Environ Health A, 2017, 80(23-24): 1230-1241. DOI:10.1080/15287394.2017.1359127 |
| [10] |
JIANG Q, TIAN J Q, LIU G, et al. Endoplasmic reticulum stress and unfolded protein response pathways involved in the health-promoting effects of allicin on the jejunum[J]. J Agric Food Chem, 2019, 67(21): 6019-6031. DOI:10.1021/acs.jafc.9b02180 |
| [11] |
张同坤, 贾海, 季昀, 等. 姜酮缓解3-乙酰呕吐毒素诱导的猪肠上皮细胞损伤研究[J]. 动物营养学报, 2022, 34(10): 6695-6701. ZHANG T K, JIA H, JI Y, et al. Study on zingerone alleviating injury of porcine intestinal epithelial cells induced by 3-acetyldeoxynivalenol[J]. Chinese Journal of Animal Nutrition, 2022, 34(10): 6695-6701. (in Chinese) |
| [12] |
高亿清, 陈代文, 田刚, 等. 营养性复合添加剂缓解霉变玉米饲粮对仔猪空肠黏膜结构的损伤[J]. 动物营养学报, 2015, 27(6): 1813-1822. GAO Y Q, CHEN D W, TIAN G, et al. Nutritional compound additive alleviates jejunal mucosal structure disruption of piglets challenged with feed containing corn contaminated with mycotoxins[J]. Chinese Journal of Animal Nutrition, 2015, 27(6): 1813-1822. (in Chinese) |
| [13] |
GRENIER B, APPLEGATE T J. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals[J]. Toxins (Basel), 2013, 5(2): 396-430. DOI:10.3390/toxins5020396 |
| [14] |
张曼, 霍军, 王军, 等. 霉菌毒素对肠道黏膜屏障功能影响的研究进展[J]. 中国饲料, 2021(21): 1-4. ZHANG M, HUO J, WANG J, et al. Progress on the effect of mycotoxins on intestinal mucosal barrier function[J]. China Feed, 2021(21): 1-4. (in Chinese) |
| [15] |
WANG B, FENG L, JIANG W D, et al. Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-κB, TOR and Nrf2 signaling molecules in the gills of fish: preventive role of arginine[J]. Aquat Toxicol, 2015, 158: 125-137. DOI:10.1016/j.aquatox.2014.10.025 |
| [16] |
XUE M L, JI X Q, LIANG H, et al. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer[J]. Food Funct, 2018, 9(2): 1214-1223. DOI:10.1039/C7FO01677H |
| [17] |
娄文芳, 邱文欣, 郭浩能, 等. 脱氧雪腐镰刀菌烯醇与T-2毒素对热应激蛋鸡肠道屏障功能及内质网应激和炎症反应的影响[J]. 动物营养学报, 2023, 35(4): 2296-2305. LOU W F, QIU W X, GUO H N, et al. Effects of deoxynivalenol and T-2 toxin on intestinal barrier function, endoplasmic reticulum stress and inflammatory response of laying hens under heat stress[J]. Chinese Journal of Animal Nutrition, 2023, 35(4): 2296-2305. (in Chinese) |
| [18] |
LIU S P, KANG W L, MAO X R, et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice[J]. J Pineal Res, 2022, 73(2): e12812. |
| [19] |
LONG M, CHEN X L, WANG N, et al. Proanthocyanidins protect epithelial cells from zearalenone-induced apoptosis via inhibition of endoplasmic reticulum stress-induced apoptosis pathways in mouse small intestines[J]. Molecules, 2018, 23(7): 1508. |
| [20] |
TANG M, YUAN D X, LIAO P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets[J]. Environ Pollut, 2021, 289: 117865. |
| [21] |
LUO K, CAO S S. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease[J]. Gastroenterol Res Pract, 2015, 2015: 328791. |
| [22] |
TADIC V, PRELL T, LAUTENSCHLAEGER J, et al. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis[J]. Front Cell Neurosci, 2014, 8: 147. |
| [23] |
CUI Y Y, ZHAO D M, SREEVATSAN S, et al. Mycobacterium bovis induces endoplasmic reticulum stress mediated-apoptosis by activating IRF3 in a murine macrophage cell line[J]. Front Cell Infect Microbiol, 2016, 6: 182. |
| [24] |
TIAN F, ZHAO J Z, BU S C, et al. KLF6 induces apoptosis in human lens epithelial cells through the ATF4-ATF3-CHOP axis[J]. Drug Des Devel Ther, 2020, 14: 1041-1055. |
| [25] |
CHEN Y F, GUI D K, CHEN J G, et al. Down-regulation of PERK-ATF4-CHOP pathway by astragaloside Ⅳ is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats[J]. Cell Physiol Biochem, 2014, 33(6): 1975-1987. |
| [26] |
WOO C W, KUTZLER L, KIMBALL S R, et al. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B[J]. Nat Cell Biol, 2012, 14(2): 192-200. |
| [27] |
ABDEL-GHAFFAR A, ELHOSSARY G G, MAHMOUD A M, et al. Effects of 4-phenylbutyric acid on the development of diabetic retinopathy in diabetic rats: regulation of endoplasmic reticulum stress-oxidative activation[J]. Arch Physiol Biochem, 2021, 1-11. DOI:10.1080/13813455.2021.1888302 |
| [28] |
CUI Y J, CHEN L Y, ZHOU X, et al. Heat stress induced IPEC-J2 cells barrier dysfunction through endoplasmic reticulum stress mediated apoptosis by p-eif2α/CHOP pathway[J]. J Cell Physiol, 2022, 237(2): 1389-1405. |
(编辑 范子娟)



