2. 临沂市农业科学院, 临沂 276003
2. Academy of Agricultural Sciences, Linyi 276003, China
在畜牧养殖中,断奶是幼畜生长发育过程必须要经历的阶段。近年来,在资源与环境的限制下,草食畜牧业的发展趋向规模化、集约化的舍饲养殖。饲养周期长、成本高和利润低成为阻碍草食动物养殖业发展的不利因素。在肉羊集约化养殖中,提高生产效益的关键之一是提高母畜繁殖率,在产多胎的基础上缩短繁殖周期,达到一年两产或两年三产[1],而这其中主要的技术策略是实施羔羊早期断奶。我国羔羊断奶多采用传统断奶方式,即羔羊出生后随母羊生活在一起,母乳喂养至2~4月龄自然断奶。传统断奶方式导致母羊配种周期长、繁殖利用率降低。早期断奶(30 d左右甚至更早)相对于常规断奶(45~60 d)可以实现母羊利用最大化,并且有利于羔羊的快速生长发育和提前出栏[2]。实施早期断奶意味着对羔羊母性护理的提前剥夺。
哺乳动物的子代早期对母性依恋程度高,母子互动极大地影响子代行为的发展。来自人类流行病学研究和广泛的动物试验数据表明,生命早期应激(early life stress,ELS)影响个体日后对压力的行为反应和情绪。ELS是与精神疾病发展相关的常见风险因素,如焦虑和抑郁[3-4]。发育时期机体对外界压力更为敏感,儿童时期经历应激事件会增加精神疾病和胃肠道疾病(如肠易激综合征)的患病率[5-6]。经历过ELS的动物表现出持久的行为和生理变化,包括应激反应增强、焦虑和抑郁样行为、下丘脑-垂体-肾上腺轴(hypothalamic-pituitary-adrenal, HPA)亢进和异常的神经化学变化[7]。
焦虑样行为会对动物的健康、福利和生产力产生不利影响。动物表现出更高的警惕性(警惕状态的持续时间更长,表现出频繁的战斗或逃跑反应)[8],血浆皮质醇和儿茶酚胺浓度升高[9],采食量下降[10],最终导致生产和繁殖性能降低[11]。因此,本文就近几年国内外有关生命早期应激(早期母幼分离)对子代异常行为的影响以及肠道菌群在改善子代异常行为中的作用进行综述,为提高早期断奶幼畜行为福利和促进其生产效益提供方法依据。
1 母幼分离引发子代行为异常及其调控途径 1.1 母幼分离引发仔猪行为异常表现目前,早期断奶普遍用于提高养猪业经济效益。仔猪断奶日龄由5~7周龄转变为3~4周龄[12]。中国首部农场动物福利团体标准《农场动物福利要求:猪》(T/CAS 235—2014)明确规定,仔猪平均断奶日龄不宜早于28日龄。但目前很多规模化猪场为追求经济效益,将仔猪的断奶时间提前至14~21日龄。这一过程中仔猪面临过早地与母猪分离,同时进行转群、运输、混群等操作,伴随着饮食的改变、频繁的人员管理,造成仔猪严重的心理和生理应激,极大地降低生产过程中仔猪的福利水平[13]。断奶应激会造成许多负面影响,包括损坏机体胃肠道健康、免疫系统以及激活下丘脑-垂体-肾上腺轴[14-15]。此外,断奶会导致仔猪激烈的打斗[16]、应激相关激素水平升高[17]。研究发现,断奶应激降低仔猪采食、休息、玩耍行为,而探究、独处、哀叫、抱团、攻击和恐人行为显著升高,部分行为可持续3~5 d[18]。
通过饲养管理手段改善断奶仔猪福利相关问题已被广泛研究。早期社会化是指动物在未断奶或生命早期阶段与不熟悉的个体相互认识的过程[19]。早期的社会化使仔猪迅速建立新的等级秩序,减少断奶后仔猪之间的攻击行为[19-20]。除了早期社会接触外,环境富集也是减缓仔猪断奶应激的有效方法。研究发现,环境富集能提高断奶前仔猪的探究行为,减弱断奶后仔猪的攻击行为[21];同时仔猪断奶后皮肤损伤程度低,应激相关标志物(唾液中皮质醇和嗜铬粒蛋白A水平)未见明显升高[21]。也有相关报道研究了断奶转群方式对仔猪行为的影响,与早期断奶转群的仔猪相比,早期断奶不转群仔猪的应激反应相对较低,可减少对仔猪的不利影响[18]。上述研究为通过饲养管理方式(早期社会化、环境富集以及断奶不转群等)减缓仔猪的早期断奶应激,以期提高断奶仔猪的福利水平。
1.2 母幼分离引发羔羊行为异常表现羔羊断奶时面临亲子纽带破裂,同时饲料成分和物理形态发生改变,此外还存在社群重组以及外部环境变化,这些因素对羔羊造成心理、生理和免疫应激[22]。断奶后,羊羔寻找母羊的行为频率增加,比如发声、步行和踱步[23-25],而采食、卧息行为和反刍时间减少[26]。由于在生命的早期阶段,母幼依恋程度更强,早期断奶的羔羊表现出更大程度的痛苦[27-28]。不同性别羔羊断奶时行为反应不同,Freitas-de-Melo和Ungerfeld[29]比较分析了不同性别羔羊之间在断奶应激时的差异以及羔羊性别对母羊行为生理的影响。研究发现,断奶当天,哺乳公羔的母羊比哺乳母羔的母羊踱步次数多;公羔采食和反刍的频次多于母羔。断奶后,母羔血清中白蛋白含量显著增加,而公羔无此变化,说明断奶应激时,母羔比公羔反应更强烈,哺乳公羔的母羊比哺乳母羔的母羊反应更强烈[29]。出现上述结果的主要原因在于母羔对母羊的依恋程度更强,对应激的敏感度比公羔高,公羔比母羔生长速度快,在营养需求上更独立于母羊。除了行为上的变化外,生理内分泌也波动明显。Bergamasco等[30]研究发现,断奶应激增加了15日龄的萨能奶山羊羔羊血浆中肾上腺素、去甲肾上腺素和皮质醇的含量,降低了多巴胺含量,而日龄较大的羔羊(30日龄、45日龄、60日龄和75日龄)断奶前后血浆中上述物质没有显著变化。早期断奶会导致羔羊血浆中皮质醇的含量增加[31]。
1.3 母幼分离引发犊牛行为异常表现犊牛传统的断奶时间在6~7月龄[32]。目前,在集约化生产模式下犊牛较早与母牛分离(45~120日龄不等)[33],甚至在母牛最佳繁殖期,采用更早的断奶日龄,即30~45日龄断奶[34]。虽然早期断奶有利于提高母牛的繁殖性能[35],但早期断奶引发犊牛诸多负面影响。断奶时犊牛面临即刻发生的诸多转变,例如失去母亲[36]、食物和环境的变化、与人类的接触以及更频繁的管理[37]。此外,早期断奶也影响犊牛学习行为的发展。在某些学习行为发生之前因断奶致使母子关系已断开,而这些本应习得的行为活动对犊牛的生存是至关重要的[38]。断奶后,犊牛表现出痛苦,比如发出叫声、踱步增加、卧息时间减少[39],甚至出现刻板行为[40]。早期断奶犊牛发声频次增加,采食频次降低,且接近料槽而不取食的频率增加,交叉吮吸行为增加[41]。除行为外,还会引发生理上的变化,例如早期断奶引发犊牛呼吸频率增加,血液中纤维蛋白原和皮质醇水平增加[41]。
目前,主要通过饲养管理的方式改善犊牛的断奶应激。断奶前是犊牛大脑发育的敏感时期,会影响其后期行为的机动性[42]。断奶前群饲的方式已作为提高犊牛适应环境变化能力的一种手段。断奶前环境富集可提高断奶后犊牛的探究和嗅闻行为,且对新奇事物的探索行为增加[43]。除饲养管理措施外,在断奶前为犊牛提供浓缩饲料有助于减少犊牛接近料槽但不采食这一现象[44]。
1.4 母幼分离引发啮齿类动物行为异常表现早期生命经历应激事件会影响成年后的行为,并与精神疾病的发生有关,如重度抑郁症、创伤后应激障碍和焦虑症[45-46]。相关试验和临床研究表明,儿童时期中枢神经系统发育不成熟,使其对压力特别敏感,生命早期应激会对子代的脑部发育产生不利影响[47]。小鼠上,母幼分离(maternal separation, MS)是早期应激的代表性动物模型,该模型能够导致焦虑行为的增加和下丘脑-垂体-肾上腺轴的异常[46]。MS增加子代在行为学测试(如旷场实验、明暗箱实验)中的焦虑样行为(anxiety-like be-haviors)[48-49]。过早断奶会对子代造成长期影响,使其在成年期出现皮质酮分泌增加并表现出焦虑和应激反应[50]。此外,研究发现,MS已被证明会影响大脑中血清素水平、多巴能神经系统和HPA轴的发育[51-52]。
长时间的母幼分离使子代学习和记忆能力受损,改变HPA轴对压力的反应[53]。ELS动物在成年时期再次暴露于应激时表现出对应激的敏感性加强[54]。ELS动物表现出成年焦虑行为,并且当面对新的应激源(束缚应激)时皮质酮分泌增加[55]。母幼分离并没有明显改变雄性小鼠的行为,但增加了二次应激后抑郁样行为的风险[56]。研究发现,早期生活中的不良事件会增加小鼠青春期和成年期长期情绪变化的风险,而这些情感变化在女性上尤其强烈[45]。
1.5 母幼分离对子代TRP-KYN代谢途径的影响目前关于抑郁症的发病机制存在多种假说,其中较为公认的有细胞因子假说、下丘脑-垂体-肾上腺皮质轴假说、单胺能假说、神经可塑性假说等。近年来,色氨酸-犬尿氨酸(tryptophan-kynurenine, TRP-KYN)代谢异常逐渐受到重视。色氨酸(tryptophan,TRP)是神经递质5-羟色胺(5-hydroxytryptamine,5-HT)的合成原料,能够在色氨酸羟化酶(tryptophan hydroxylase,TPH)的作用下合成5-HT;而在吲哚胺2, 3-双加氧酶(indoleamine-2, 3-dioxygenase,Ido)的作用下,则发生TRP-KYN代谢形成犬尿氨酸(kynurenine,KYN)等活性产物。当TRP-KYN代谢发生异常,5-HT的合成原料TRP耗竭,造成5-HT合成降低,与此同时KYN进一步代谢生成具有神经毒性作用的喹啉酸,损伤神经元并引发抑郁症[57]。在正常生理条件下,Ido活性较低,然而当身体处于应激或炎症状态时,促炎性细胞因子如白细胞介素-1β(interleukin-1β,IL-1β)、肿瘤坏死因子α(tumor necrosis factor α,TNF-α)等诱导Ido激活,增加KYN的合成,减少5-HT的合成[58]。研究发现,抑郁症期间色氨酸代谢途径失衡,5-HT的合成减少[45, 59]。
行为异常与小胶质细胞激活和情绪控制相关的大脑区域TRP-KYN代谢紊乱有关[45]。母幼分离导致后代小胶质细胞发育异常(细胞突起回缩、细胞体增大),发生促炎症反应[56, 60],引起大脑中促炎性细胞因子分泌增加[61],最终导致5-HT神经递质的合成减少[59]。在抑郁样大鼠中,促炎性细胞因子升高,同时KYN/TRP比值增加[62]。在SD大鼠上的研究表明,海马体中Ido-1、Ido-2表达量和KYN/TRP比值在母幼分离子代中均有升高[63]。此外,有关Wistar大鼠的研究发现,下丘脑血清素在母性剥夺和母幼分离的子代中均显著降低[64]。
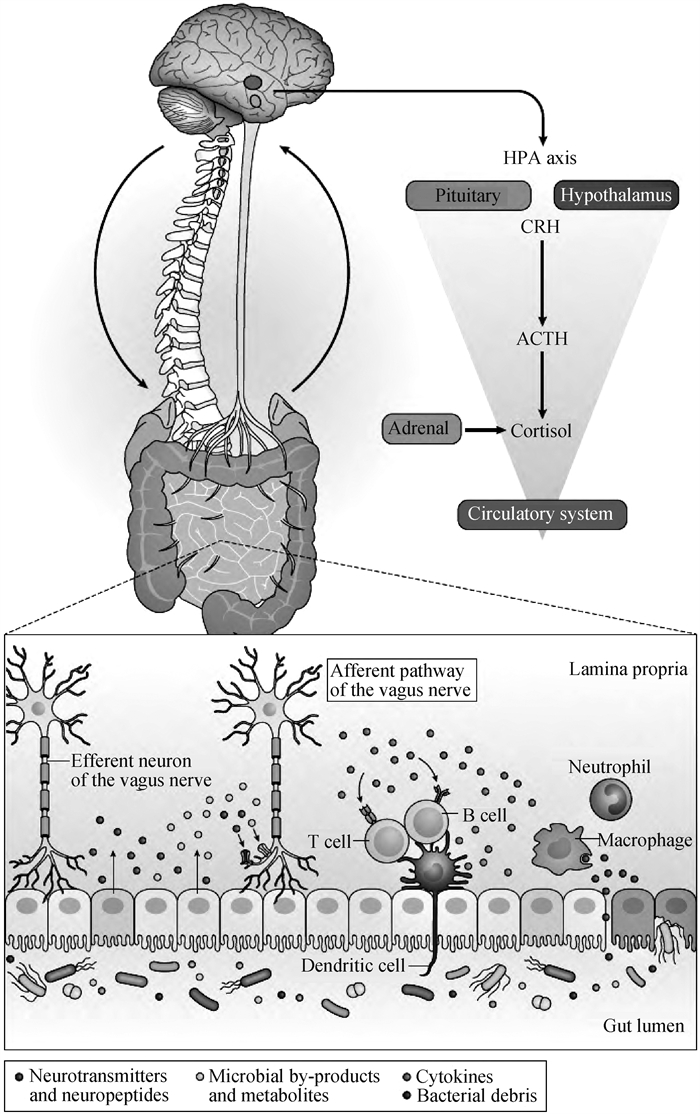
2 肠道菌群通过微生物-肠道-脑轴调控宿主行为情绪肠道菌群是指在肠道内定植并参与宿主多种生理活动的微生物群落[65]。其参与宿主的各种基本生命活动,包括营养代谢、维持肠道黏膜屏障、免疫调节、改变大脑发育、调控行为和情绪、调节神经内分泌和神经免疫系统等[66-69]。肠道菌群的组成受到多种因素的影响,包括营养、生活方式、激素变化、免疫、健康状况、药物应用以及情绪(如压力、抑郁、自闭症)等[70-71]。研究表明,肠道微生物能够影响中枢神经系统相关疾病,例如自闭症、抑郁症、帕金森病和阿尔茨海默病[72]。微生物-肠道-脑轴在调控宿主行为和中枢神经系统方面发挥重要作用[73-74]。它是胃肠道与中枢神经系统之间双向交流的复杂系统,主要包括迷走神经、免疫介质、神经内分泌通路和肠道菌群衍生的代谢通路(图 1)[68]。
哺乳动物胃肠道存在复杂多样的微生物群落,影响宿主的健康。肠道微生物群落的发展从出生后不久开始(主要来源于母亲和环境),并随着时间的推移逐渐稳定下来[75-76]。越来越多的研究表明,肠道菌群调控宿主行为[77-80],主要方式是通过调节肠道和大脑神经递质的产生[81-82]、影响大脑发育和髓鞘形成[83-84]。有研究发现,无菌小鼠的杏仁核和海马存在形态上的损伤[85]。此外,在动物上的大量研究表明,肠道微生物可通过其代谢产物,直接或间接影响大脑稳态,调节宿主焦虑行为和神经系统相关疾病[86-87]。研究发现,抑郁症患者存在肠道菌群紊乱现象,如拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes)和放线菌门(Actinobacteria)丰度减少[62, 88],另枝菌(Alistipes spp.)丰度增加[89],并伴有肠易激综合征[90-91]。在大鼠上研究发现,生命早期母幼分离会引起肠道菌群多样性的长期变化[92-93]。抑郁症患者体内的微生物群移植到服用抗生素的大鼠体内后,大鼠表现出抑郁样行为[62]。将抑郁症患者的粪便移植到小鼠体内可产生抑郁样行为[88, 94],受试小鼠表现出快乐缺失、焦虑样行为增加和色氨酸代谢障碍,海马中干扰素γ(interferon-γ,IFN-γ)和TNF-α水平显著升高[95]。Zheng等[88]通过采集抑郁症患者(试验组)和健康人(对照组)粪便中微生物,移植到无菌小鼠体内,发现移植试验组小鼠表现出更加明显的抑郁症状,肠道微生物组成与移植对照组小鼠不同。上述试验结果证明,肠道菌群组成的变化可以影响中枢神经系统,导致抑郁。
肠道菌群能够调控神经递质的产生。有研究表明,婴儿双歧杆菌(Bifidobacterium infantis)能够增加色氨酸的可利用程度,提高大脑中血清素水平[96-97];链球菌(Streptococcus)、埃希氏杆菌(Escherichia)和肠球菌(Enterococcus)也能够产生血清素;埃希氏杆菌(Escherichia)、芽孢杆菌(Bacillus)和酵母菌(Saccharomyces)能产生去甲肾上腺素,芽孢杆菌(Bacillus)和沙雷氏菌(Serratia)产生多巴胺[98-99]。说明肠道菌群通过影响神经递质的产生,调控宿主行为。然而,肠道微生物与行为调控之间的分子机制在很大程度上仍然未知,如何影响神经递质的产生,还需大量深入的研究。
肠道微生物与机体色氨酸代谢相关。生命早期肠道微生物群的定植以及在整个生命周期中其组成和多样性的变化影响色氨酸的可利用性,并对血清素信号传导通路起重要作用[100]。肠道菌群对宿主行为的调节可以通过肠-脑轴影响色氨酸代谢和血清素信号通路实现[101]。肠道微生物代谢产物,如短链脂肪酸,可以促进色氨酸合成血清素,阻止色氨酸转化为犬尿氨酸途径[102]。由此可知,肠道微生物及其代谢产物(如短链脂肪酸)调控色氨酸的代谢途径,进而影响宿主行为情绪。
3 哺乳动物行为异常可通过改善肠道菌群结构来治疗益生菌,包括细菌和酵母,是活的微生物,当摄入量足够时,对宿主健康有益[103-104]。益生菌通过调节机体免疫系统、产生抗菌物质、增加肠上皮细胞屏障功能和肠黏膜黏附力, 对宿主发挥有益作用[105]。研究表明,益生菌可通过肠道-微生物-脑轴改善神经和精神疾病,调控机体代谢状态、炎症反应和氧化应激[106-107]。益生菌能够缓解抑郁症模型动物的抑郁样行为[108],改善抑郁症患者的情绪[109]。乳酸菌(Lactobacillus)和双歧杆菌(Bifidobacterium)被证明能够有效治疗精神疾病[110],缓解啮齿类动物因应激引起的行为变化[111-112],说明肠道微生物能够影响中枢神经系统应激反应途径。研究表明,通过特定的活菌制剂接种可改善母幼分离引发的行为和生理上的缺陷[97, 113-116]。啮齿动物生命早期由母性剥夺引起的子代抑郁样行为以及免疫系统和单胺能系统的改变,通过长期服用婴儿双歧杆菌(Bifidobacterium infantis)可有效缓解抑郁样行为,且免疫反应正常化,脑干中去甲肾上腺素(norepinephrine,NA)浓度恢复[97],说明微生物群可能通过多种方式调控中枢神经系统。给予长双歧杆菌NCC3001(Bifidobacterium longum NCC3001)可显著改善肠易激综合征患者的抑郁症[117]。暴露于慢性束缚应激的小鼠表现出抑郁样行为,神经炎症标志物增加;而长时间(21 d)摄入青春双歧杆菌(Bifidobacterium adolescentis)能够逆转上述变化[118],表明双歧杆菌的抗抑郁作用与减少炎症细胞因子和重塑肠道微生物群有关。Yang等[108]研究发现,遭受慢性社交挫败的小鼠恢复力可能与肠道中双歧杆菌有关,双歧杆菌(Bifidobacterium)灌服可提高应激小鼠的恢复力。长双歧杆菌1714(Bifidobacterium longum 1714)和短双歧杆菌1205(Bifidobacterium breve 1205)能够减缓BALB/c小鼠焦虑行为[111]。
肠道微生物群有助于恢复机体的稳态,例如乳杆菌属(Lactobacillus spp.)能够使经历早期生命应激的小鼠糖皮质激素水平正常化[113],鼠李糖乳杆菌(Lactobacillus rhamnosus)可以减轻BALB/c小鼠的焦虑样行为[119]。接种瑞士乳杆菌(Lactobacillus helveticus)能够减缓啮齿类动物的焦虑样行为[107, 120]。植物乳杆菌(Lactobacillus plantarum PS128)对MS小鼠具有抗抑郁样作用[115]。长期应激导致小鼠肠道菌群组成发生明显改变,乳酸杆菌(Lactobacillus)减少,犬尿氨酸水平升高;而乳酸杆菌水平的恢复能够改善代谢和行为异常[121]。接种副干酪乳杆菌(Lactobacillus paracasei PS23)缓解早期母幼分离小鼠焦虑或者抑郁样行为,降低血浆中的皮质醇含量[122]。通过移植慢性不可预测温和刺激小鼠模型的肠道菌群,受试小鼠同样表现出抑郁样行为,受试小鼠的脂肪酸代谢发生了改变,导致大脑中内源性大麻素(endocannabinoid,eCB)系统的活性受损;乳酸杆菌(Lactobacilli)的补给增加了受试小鼠脑部eCB水平,减轻抑郁样行为[123]。Sun等[124]研究发现,植物乳杆菌WLPL04(Lactobacillus plantarum WLPL04)可以缓解焦虑样行为。
4 展望与小结生命阶段早期应激对子代行为产生长期的影响,表现为焦虑加剧和应激反应增强。肠道微生物的组成不仅影响肠道的健康,还可以改变大脑发育、调控情绪和行为,调节神经内分泌和神经免疫系统。特定的活菌制剂接种可通过神经内分泌、神经炎症等调控途径改善母幼分离引发的行为和生理上的缺陷。瞄准生命发育早期的“窗口期”调节肠道微生物组成与结构对于改善早期断奶应激至关重要。肠道菌群的组成和水平通过何种途径影响宿主行为,以及大脑控制行为的区域与肠道菌群双向对话机制如何尚不清晰。此外,本文将为提高早期断奶幼畜行为福利水平以及促进健康养殖,提供参考依据和新的方法思路。
| [1] |
刁其玉, 屠焰, 杨丹. 羔羊代乳品的研制与应用效果研究[J]. 中国草食动物, 2002, 22(4): 9-12. DIAO Q Y, TU Y, YANG D. A study of milk replacer for kid goats[J]. China Herbivore Science, 2002, 22(4): 9-12. DOI:10.3969/j.issn.2095-3887.2002.04.003 (in Chinese) |
| [2] |
马志远, 李飞, 李发弟, 等. 早期断奶对湖羊羔羊生长性能及胃肠道发育的影响[J]. 动物营养学报, 2015, 27(5): 1385-1393. MA Z Y, LI F, LI F D, et al. Effect of early weaning on performance and gastrointestinal tract development of Hu lambs[J]. Chinese Journal of Animal Nutrition, 2015, 27(5): 1385-1393. DOI:10.3969/j.issn.1006-267x.2015.05.008 (in Chinese) |
| [3] |
SYED S A, NEMEROFF C B. Early life stress, mood, and anxiety disorders[J]. Chronic Stress (Thousand Oaks), 2017, 1: 2470547017694461. |
| [4] |
PEÑA C J, KRONMAN H G, WALKER D M, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2[J]. Science, 2017, 356(6343): 1185-1188. DOI:10.1126/science.aan4491 |
| [5] |
NEMEROFF C B. Paradise lost: The neurobiological and clinical consequences of child abuse and neglect[J]. Neuron, 2016, 89(5): 892-909. DOI:10.1016/j.neuron.2016.01.019 |
| [6] |
O'MAHONY S M, CLARKE G, DINAN T G, et al. Irritable bowel syndrome and stress-related psychiatric co-morbidities: Focus on early life stress[J]. Handb Exp Pharmacol, 2017, 239: 219-246. |
| [7] |
CRYAN J F, HOLMES A. The ascent of mouse: Advances in modelling human depression and anxiety[J]. Nat Rev Drug Discov, 2005, 4(9): 775-790. DOI:10.1038/nrd1825 |
| [8] |
GOMA A A, PEARCE G P, UDDIN J, et al. A forced lateralisation test for dairy cows and its relation to their behaviour[J]. Appl Anim Behav Sci, 2018, 207: 8-19. DOI:10.1016/j.applanim.2018.06.008 |
| [9] |
BRISTOW D J, HOLMES D S. Cortisol levels and anxiety-related behaviors in cattle[J]. Physiol Behav, 2017, 90(4): 626-628. |
| [10] |
OSEI-AMPONSAH R, DUNSHEA F R, LEURY B J, et al. Heat stress impacts on lactating cows grazing australian summer pastures on an automatic robotic dairy[J]. Animals, 2020, 10(5): 869. DOI:10.3390/ani10050869 |
| [11] |
BERNABUCCI U, LACETERA N, BAUMGARD L H, et al. Metabolic and hormonal acclimation to heat stress in domesticated ruminants[J]. Animal, 2010, 4(7): 1167-1183. DOI:10.1017/S175173111000090X |
| [12] |
WIDOWSKI T M, TORREY S, BENCH C J, et al. Development of ingestive behaviour and the relationship to belly nosing in early-weaned piglets[J]. Appl Anim Behav Sci, 2008, 110(1-2): 109-127. DOI:10.1016/j.applanim.2007.04.010 |
| [13] |
CAMPBELL J M, CRENSHAW J D, POLO J. The biological stress of early weaned piglets[J]. J Anim Sci Biotechnol, 2013, 4(1): 19. DOI:10.1186/2049-1891-4-19 |
| [14] |
GABLER N K, HELM E T, DE MILLE C. 57 Impact of weaning stress, disease, and diet on pig performance, intestinal function and integrity[J]. J Anim Sci, 2019, 97(Supplement2): 31-32. |
| [15] |
YU C H, CHEN C Y, CHANG C C. The immediate effects of weaning stress on the hypothalamus-pituitary-adrenal alteration of newly weaned piglets[J]. J Anim Physiol Anim Nutr, 2019, 103(4): 1218-1223. DOI:10.1111/jpn.13104 |
| [16] |
PEDEN R S E, TURNER S P, BOYLE L A, et al. The translation of animal welfare research into practice: The case of mixing aggression between pigs[J]. Appl Anim Behav Sci, 2018, 204: 1-9. DOI:10.1016/j.applanim.2018.03.003 |
| [17] |
HELD S, MENDL M T. Behaviour of the young weaner pig[M]. New York: CABI Publishing, 2001.
|
| [18] |
杨志维, 李智星, 黄涛, 等. 早期断奶转群对仔猪行为、生长性能和血液指标的影响[J]. 中国畜牧兽医, 2022, 49(3): 904-912. YANG Z W, LI Z X, HUANG T, et al. Effects of early weaning and transfer on behavior, growth performance and blood indexes of piglets[J]. China Animal Husbandry & Veterinary Medicine, 2022, 49(3): 904-912. DOI:10.16431/j.cnki.1671-7236.2022.03.012 (in Chinese) |
| [19] |
SALAZAR L C, KO H L, YANG C H, et al. Early socialisation as a strategy to increase piglets' social skills in intensive farming conditions[J]. Appl Anim Behav Sci, 2018, 206: 25-31. DOI:10.1016/j.applanim.2018.05.033 |
| [20] |
WELLER J E, CAMERLINK I, TURNER S P, et al. Socialisation and its effect on play behaviour and aggression in the domestic pig (Sus scrofa)[J]. Sci Rep, 2019, 9(1): 4180. DOI:10.1038/s41598-019-40980-1 |
| [21] |
KO H L, CHONG Q, ESCRIBANO D, et al. Pre-weaning socialization and environmental enrichment affect life-long response to regrouping in commercially-reared pigs[J]. Appl Anim Behav Sci, 2020, 229: 105044. DOI:10.1016/j.applanim.2020.105044 |
| [22] |
O'LOUGHLIN A, MCGEE M, DOYLE S, et al. Biomarker responses to weaning stress in beef calves[J]. Res Vet Sci, 2014, 97(2): 458-463. DOI:10.1016/j.rvsc.2014.06.003 |
| [23] |
SCHICHOWSKI C, MOORS E, GAULY M. Effects of weaning lambs in two stages or by abrupt separation on their behavior and growth rate[J]. J Anim Sci, 2008, 86(1): 220-225. DOI:10.2527/jas.2007-0198 |
| [24] |
DAMIÁN J P, HÖTZEL M J, BANCHERO G, et al. Behavioural response of grazing lambs to changes associated with feeding and separation from their mothers at weaning[J]. Res Vet Sci, 2013, 95(3): 913-918. DOI:10.1016/j.rvsc.2013.08.001 |
| [25] |
FREITAS-DE-MELO A, UNGERFELD R, HÖTZEL M J, et al. Low pasture allowance until late gestation in ewes: Behavioural and physiological changes in ewes and lambs from lambing to weaning[J]. Animal, 2017, 11(2): 285-294. DOI:10.1017/S1751731116001427 |
| [26] |
FREITAS-DE-MELO A, BANCHERO G, HÖTZEL M J, et al. Progesterone administration reduces the behavioural and physiological responses of ewes to abrupt weaning of lambs[J]. Animal, 2013, 7(8): 1367-1373. DOI:10.1017/S1751731113000621 |
| [27] |
NAPOLITANO F, DE ROSA G, SEVI A. Welfare implications of artificial rearing and early weaning in sheep[J]. Appl Anim Behav Sci, 2008, 110(1-2): 58-72. DOI:10.1016/j.applanim.2007.03.020 |
| [28] |
NOROUZIAN M A. Effect of weaning method on lamb behaviour and weight gain[J]. Small Ruminant Res, 2015, 133: 17-20. DOI:10.1016/j.smallrumres.2015.10.028 |
| [29] |
FREITAS-DE-MELO A, UNGERFELD R. The sex of the offspring affects the lamb and ewe responses to abrupt weaning[J]. Appl Anim Behav Sci, 2020, 229: 105008. DOI:10.1016/j.applanim.2020.105008 |
| [30] |
BERGAMASCO L, MACCHI E, FACELLO C, et al. Effects of brief maternal separation in kids on neurohormonal and electroencephalographic parameters[J]. Appl Anim Behav Sci, 2005, 93(1-2): 39-52. DOI:10.1016/j.applanim.2004.12.002 |
| [31] |
PASCUAL-ALONSO M, LA LAMA G C M D, AGUAYO-ULLOA L, et al. Effect of postweaning handling strategies on welfare and productive traits in lambs[J]. J Appl Anim Welf Sci, 2015, 18(1): 42-56. DOI:10.1080/10888705.2014.941107 |
| [32] |
RASBY R. Early weaning beef calves[J]. Vet Clin North Am: Food Anim Pract, 2007, 23(1): 29-40. DOI:10.1016/j.cvfa.2007.01.002 |
| [33] |
DE OLIVEIRA T E, BARCELLOS J O J, WHITTIER J C, et al. Risks associated to different methods of increasing pregnancy rate of cows in cow-calf systems[J]. Rev Bras Zootec, 2018, 47: e20180051. |
| [34] |
GONZALEZ D D, VITTONE J C, LADO M, et al. Detection of antibodies against bovine herpes virus 1, bovine viral diarrhea virus and bovine respiratory syncytial virus in early and ultra-early weaned beef calves[J]. Am J Anim Vet Sci, 2013, 8(4): 210-219. DOI:10.3844/ajavsp.2013.210.219 |
| [35] |
ORIHUELA A, GALINA C S. Effects of separation of cows and calves on reproductive performance and animal welfare in tropical beef cattle[J]. Animals, 2019, 9(5): 223. DOI:10.3390/ani9050223 |
| [36] |
PÉREZ L I, ORIHUELA A, GALINA C S, et al. Effect of different periods of maternal deprivation on behavioral and cortisol responses at weaning and subsequent growth rate in zebu (Bos indicus) type cattle[J]. Livest Sci, 2017, 197: 17-21. DOI:10.1016/j.livsci.2016.12.006 |
| [37] |
LYNCH E, MCGEE M, EARLEY B. Weaning management of beef calves with implications for animal health and welfare[J]. J Appl Anim Res, 2019, 47(1): 167-175. DOI:10.1080/09712119.2019.1594825 |
| [38] |
ENRÍQUEZ D, HÖTZEL M J, UNGERFELD R. Minimising the stress of weaning of beef calves: a review[J]. Acta Vet Scand, 2011, 53(1): 28. DOI:10.1186/1751-0147-53-28 |
| [39] |
LOBERG J M, HERNANDEZ C E, THIERFELDER T, et al. Weaning and separation in two steps-a way to decrease stress in dairy calves suckled by foster cows[J]. Appl Anim Behav Sci, 2008, 111(3-4): 222-234. DOI:10.1016/j.applanim.2007.06.011 |
| [40] |
LATHAM N R, MASON G J. Maternal deprivation and the development of stereotypic behaviour[J]. Appl Anim Behav Sci, 2008, 110(1-2): 84-108. DOI:10.1016/j.applanim.2007.03.026 |
| [41] |
DE SOUZA TEIXEIRA O, DA ROCHA M K, ALFORMA A M P, et al. Behavioural and physiological responses of male and female beef cattle to weaning at 30, 75 or 180 days of age[J]. Appl Anim Behav Sci, 2021, 240: 105339. DOI:10.1016/j.applanim.2021.105339 |
| [42] |
MEAGHER R K, DAROS R R, COSTA J H C, et al. Effects of degree and timing of social housing on reversal learning and response to novel objects in dairy calves[J]. PLoS One, 2015, 10(8): e0132828. DOI:10.1371/journal.pone.0132828 |
| [43] |
ZHANG C Y, JUNIPER D T, MEAGHER R K. Effects of physical enrichment items and social housing on calves ' growth, behaviour and response to novelty[J]. Appl Anim Behav Sci, 2021, 237: 105295. DOI:10.1016/j.applanim.2021.105295 |
| [44] |
CARVALHO V V, PAULINO M F, DETMANN E, et al. A meta-analysis of the effects of creep-feeding supplementation on performance and nutritional characteristics by beef calves grazing on tropical pastures[J]. Livest Sci, 2019, 227: 175-182. DOI:10.1016/j.livsci.2019.07.009 |
| [45] |
GRACIA-RUBIO I, MOSCOSO-CASTRO M, POZO O J, et al. Maternal separation induces neuroinflammation and long-lasting emotional alterations in mice[J]. Prog Neuro-Psychopharmacol Biol Psychiatry, 2016, 65: 104-117. DOI:10.1016/j.pnpbp.2015.09.003 |
| [46] |
KIM E G, CHANG W, SHIN S Y, et al. Maternal separation in mice leads to anxiety-like/aggressive behavior and increases immunoreactivity for glutamic acid decarboxylase and parvalbumin in the adolescence ventral hippocampus[J]. Korean J Physiol Pharmacol, 2023, 27(1): 113-125. |
| [47] |
DE AZEREDO L A, WEARICK-SILVA L E, VIOLA T W, et al. Maternal separation induces hippocampal changes in cadherin-1 (CDH-1) mRNA and recognition memory impairment in adolescent mice[J]. Neurobiol Learn Mem, 2017, 141: 157-167. DOI:10.1016/j.nlm.2017.04.0061016/j.pnpbp.2015.09.003 |
| [48] |
MALCON L M C, WEARICK-SILVA L E, ZAPARTE A, et al. Maternal separation induces long-term oxidative stress alterations and increases anxiety-like behavior of male Balb/cJ mice[J]. Exp Brain Res, 2020, 238(9): 2097-2107. DOI:10.1007/s00221-020-05859-y |
| [49] |
QIN X, LIU X X, WANG Y, et al. Early life stress induces anxiety-like behavior during adulthood through dysregulation of neuronal plasticity in the basolateral amygdala[J]. Life Sci, 2021, 285: 119959. DOI:10.1016/j.lfs.2021.119959 |
| [50] |
KIKUSUI T, KANBARA N, OZAKI M, et al. Early weaning increases anxiety via brain-derived neurotrophic factor signaling in the mouse prefrontal cortex[J]. Sci Rep, 2019, 9(1): 3991. DOI:10.1038/s41598-019-40530-9 |
| [51] |
BRAVO J A, DINAN T G, CRYAN J F. Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain[J]. Front Mol Neurosci, 2014, 7: 24. |
| [52] |
RENTESI G, ANTONIOU K, MARSELOS M, et al. Long-term consequences of early maternal deprivation in serotonergic activity and HPA function in adult rat[J]. Neurosci Lett, 2010, 480(1): 7-11. DOI:10.1016/j.neulet.2010.04.054 |
| [53] |
MAGHAMI S, ZARDOOZ H, KHODAGHOLI F, et al. Maternal separation blunted spatial memory formation independent of peripheral and hippocampal insulin content in young adult male rats[J]. PLoS One, 2018, 13(10): e0204731. DOI:10.1371/journal.pone.0204731 |
| [54] |
ZHOU L, WU Z T, WANG G H, et al. Long-term maternal separation potentiates depressive-like behaviours and neuroinflammation in adult male C57/BL6J mice[J]. Pharmacol Biochem Behav, 2020, 196: 172953. DOI:10.1016/j.pbb.2020.172953 |
| [55] |
MACHADO T D, MOLLE R D, LAUREANO D P, et al. Early life stress is associated with anxiety, increased stress responsivity and preference for "comfort foods" in adult female rats[J]. Stress, 2013, 16(5): 549-556. DOI:10.3109/10253890.2013.816841 |
| [56] |
HAN Y, ZHANG L J, WANG Q Z, et al. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation[J]. Psychoneuroendocrinology, 2019, 107: 37-45. |
| [57] |
HESTAD K A, ENGEDAL K, WHIST J E, et al. The relationships among tryptophan, kynurenine, indoleamine 2, 3-dioxygenase, depression, and neuropsychological performance[J]. Front Psychol, 2017, 8: 1561. DOI:10.3389/fpsyg.2017.01561 |
| [58] |
HUNT C, MACEDO E, CORDEIRO T M E, et al. Effect of immune activation on the kynurenine pathway and depression symptoms-A systematic review and meta-analysis[J]. Neurosci Biobehav Rev, 2020, 118: 514-523. DOI:10.1016/j.neubiorev.2020.08.010 |
| [59] |
CHRISTMAS D M, POTOKAR J P, DAVIES S J C. A biological pathway linking inflammation and depression: Activation of indoleamine 2, 3-dioxygenase[J]. Neuropsychiatr Dis Treat, 2011, 7: 431-439. |
| [60] |
GONG Y, TONG L J, YANG R R, et al. Dynamic changes in hippocampal microglia contribute to depressive-like behavior induced by early social isolation[J]. Neuropharmacology, 2018, 135: 223-233. DOI:10.1016/j.neuropharm.2018.03.023 |
| [61] |
ROQUE A, OCHOA-ZARZOSA A, TORNER L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels[J]. Brain Behav Immun, 2016, 55: 39-48. DOI:10.1016/j.bbi.2015.09.017 |
| [62] |
KELLY J R, BORRE Y, O'BRIEN C, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat[J]. J Psychiatr Res, 2016, 82: 109-118. DOI:10.1016/j.jpsychires.2016.07.019 |
| [63] |
PARK H J, KIM S A, KANG W S, et al. Early-life stress modulates gut microbiota and peripheral and central inflammation in a sex-dependent manner[J]. Int J Mol Sci, 2021, 22(4): 1899. DOI:10.3390/ijms22041899 |
| [64] |
DE LIMA R M S, DOS SANTOS BENTO L V, DI MARCELLO VALLADÃO LUGON M, et al. Early life stress and the programming of eating behavior and anxiety: Sex-specific relationships with serotonergic activity and hypothalamic neuropeptides[J]. Behav Brain Res, 2020, 379: 112399. DOI:10.1016/j.bbr.2019.112399 |
| [65] |
MOSZAK M, SZULIŃSKA M, BOGDAŃSKI P. You are what you eat-the relationship between diet, microbiota, and metabolic disorders-a review[J]. Nutrients, 2020, 12(4): 1096. DOI:10.3390/nu12041096 |
| [66] |
CLARKE G, GRENHAM S, SCULLY P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner[J]. Mol Psychiatry, 2013, 18(6): 666-673. DOI:10.1038/mp.2012.77 |
| [67] |
EL AIDY S, RAMSTEIJN A S, DINI-ANDREOTE F, et al. Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress[J]. Front Cell Neurosci, 2017, 11: 222. |
| [68] |
MORAIS L H, SCHREIBER IV H L, MAZMANIAN S K. The gut microbiota-brain axis in behaviour and brain disorders[J]. Nat Rev Microbiol, 2021, 19(4): 241-255. DOI:10.1038/s41579-020-00460-0 |
| [69] |
RINNINELLA E, RAOUL P, CINTONI M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases[J]. Microorganisms, 2019, 7(1): 14. DOI:10.3390/microorganisms7010014 |
| [70] |
KUNDU P, BLACHER E, ELINAV E, et al. Our gut microbiome: the evolving inner self[J]. Cell, 2017, 171(7): 1481-1493. DOI:10.1016/j.cell.2017.11.024 |
| [71] |
WANG X W, LIU Y Y. Comparative study of classifiers for human microbiome data[J]. Med Microecol, 2020, 4: 100013. DOI:10.1016/j.medmic.2020.100013 |
| [72] |
ZHU F Y, TU H J, CHEN T T. The microbiota-gut-brain axis in depression: the potential pathophysiological mechanisms and microbiota combined antidepression effect[J]. Nutrients, 2022, 14(10): 2081. DOI:10.3390/nu14102081 |
| [73] |
DESBONNET L, CLARKE G, TRAPLIN A, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour[J]. Brain Behav Immun, 2015, 48: 165-173. DOI:10.1016/j.bbi.2015.04.004 |
| [74] |
FOURIE N H, WANG D, ABEY S K, et al. Structural and functional alterations in the colonic microbiome of the rat in a model of stress induced irritable bowel syndrome[J]. Gut Microbes, 2017, 8(1): 33-45. DOI:10.1080/19490976.2016.1273999 |
| [75] |
SCHOKKER D, ZHANG J, VASTENHOUW S A, et al. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs[J]. PLoS One, 2015, 10(2): e0116523. DOI:10.1371/journal.pone.0116523 |
| [76] |
TIMMERMAN H M, RUTTEN N B M M, BOEKHORST J, et al. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures[J]. Sci Rep, 2017, 7(1): 8327. DOI:10.1038/s41598-017-08268-4 |
| [77] |
BORRE Y E, MOLONEY R D, CLARKE G, et al. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential[J]. Adv Exp Med Biol, 2014, 817: 373-403. |
| [78] |
CRUMEYROLLE-ARIAS M, JAGLIN M, BRUNEAU A, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats[J]. Psychoneuroendocrinology, 2014, 42: 207-217. DOI:10.1016/j.psyneuen.2014.01.014 |
| [79] |
VUONG H E, YANO J M, FUNG T C, et al. The microbiome and host behavior[J]. Annu Rev Neurosci, 2017, 40: 21-49. DOI:10.1146/annurev-neuro-072116-031347 |
| [80] |
HERTLI S, ZIMMERMANN P. Molecular interactions between the intestinal microbiota and the host[J]. Mol Microbiol, 2022, 117(6): 1297-1307. DOI:10.1111/mmi.14905 |
| [81] |
NEEDHAM B D, KADDURAH-DAOUK R, MAZMANIAN S K. Gut microbial molecules in behavioural and neurodegenerative conditions[J]. Nat Rev Neurosci, 2020, 21(12): 717-731. |
| [82] |
HUANG F, WU X J. Brain neurotransmitter modulation by gut microbiota in anxiety and depression[J]. Front Cell Dev Biol, 2021, 9: 649103. DOI:10.3389/fcell.2021.649103 |
| [83] |
GACIAS M, GASPARI S, SANTOS P M G, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior[J]. eLife, 2016, 5: e13442. DOI:10.7554/eLife.13442 |
| [84] |
HOBAN A E, STILLING R M, RYAN F J, et al. Regulation of prefrontal cortex myelination by the microbiota[J]. Transl Psychiatry, 2016, 6(4): e774. DOI:10.1038/tp.2016.42 |
| [85] |
LUCZYNSKI P, WHELAN S O, O'SULLIVAN C, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus[J]. Eur J Neurosci, 2016, 44(9): 2654-2666. DOI:10.1111/ejn.13291 |
| [86] |
BERCIK P, DENOU E, COLLINS J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice[J]. Gastroenterology, 2011, 141(2): 599-609. DOI:10.1053/j.gastro.2011.04.052 |
| [87] |
SAMPSON T R, DEBELIUS J W, THRON T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson 's disease[J]. Cell, 2016, 167(6): 1469-1480. DOI:10.1016/j.cell.2016.11.018 |
| [88] |
ZHENG P, ZENG B, ZHOU C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host 's metabolism[J]. Mol Psychiatry, 2016, 21(6): 786-796. DOI:10.1038/mp.2016.44 |
| [89] |
JIANG H Y, LING Z X, ZHANG Y H, et al. Altered fecal microbiota composition in patients with major depressive disorder[J]. Brain Behav Immun, 2015, 48: 186-194. DOI:10.1016/j.bbi.2015.03.016 |
| [90] |
CHEUNG S G, GOLDENTHAL A R, UHLEMANN A C, et al. Systematic review of gut microbiota and major depression[J]. Front Psychiatry, 2019, 10: 34. |
| [91] |
AZIZ M N M, KUMAR J, NAWAWI K N M, et al. Irritable bowel syndrome, depression, and neurodegeneration: A bidirectional communication from gut to brain[J]. Nutrients, 2021, 13(9): 3061. DOI:10.3390/nu13093061 |
| [92] |
GARCÍA-RÓDENAS C L, BERGONZELLI G E, NUTTEN S, et al. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats[J]. J Pediatr Gastroenterol Nutr, 2006, 43(1): 16-24. DOI:10.1097/01.mpg.0000226376.95623.9f |
| [93] |
O'MAHONY S M, MARCHESI J R, SCULLY P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses[J]. Biol Psychiatry, 2009, 65(3): 263-267. DOI:10.1016/j.biopsych.2008.06.026 |
| [94] |
STILLING R M, DINAN T G, CRYAN J F. Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis[J]. Genes Brain Behav, 2014, 13(1): 69-86. DOI:10.1111/gbb.12109 |
| [95] |
LI N N, WANG Q, WANG Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis[J]. Stress, 2019, 22(5): 592-602. DOI:10.1080/10253890.2019.1617267 |
| [96] |
DESBONNET L, GARRETT L, CLARKE G, et al. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat[J]. J Psychiatr Res, 2008, 43(2): 164-174. DOI:10.1016/j.jpsychires.2008.03.009 |
| [97] |
DESBONNET L, GARRETT L, CLARKE G, et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression[J]. Neuroscience, 2010, 170(4): 1179-1188. DOI:10.1016/j.neuroscience.2010.08.005 |
| [98] |
ÖZOGUL F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen[J]. Int J Food Sci Technol, 2011, 46(3): 478-484. DOI:10.1111/j.1365-2621.2010.02511.x |
| [99] |
HOLZER P, FARZI A. Neuropeptides and the microbiota-gut- brain axis[J]. Adv Exp Med Biol, 2014, 817: 195-219. |
| [100] |
CARLESSI A S, BORBA L A, ZUGNO A I, et al. Gut microbiota-brain axis in depression: The role of neuroinflammation[J]. Eur J Neurosci, 2021, 53(1): 222-235. DOI:10.1111/ejn.14631 |
| [101] |
O'MAHONY S M, CLARKE G, BORRE Y E, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis[J]. Behav Brain Res, 2015, 277: 32-48. DOI:10.1016/j.bbr.2014.07.027 |
| [102] |
REIGSTAD C S, SALMONSON C E, RAINEY J F, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells[J]. FASEB J, 2015, 29(4): 1395-1403. DOI:10.1096/fj.14-259598 |
| [103] |
KIM S K, GUEVARRA R B, KIM Y T, et al. Role of probiotics in human gut microbiome-associated diseases[J]. J Microbiol Biotechnol, 2019, 29(9): 1335-1340. DOI:10.4014/jmb.1906.06064 |
| [104] |
PUEBLA-BARRAGAN S, REID G. Probiotics in cosmetic and personal care products: trends and challenges[J]. Molecules, 2021, 26(5): 1249. DOI:10.3390/molecules26051249 |
| [105] |
BERMUDEZ-BRITO M, PLAZA-DÍAZ J, MUÑOZ-QUEZADA S, et al. Probiotic mechanisms of action[J]. Ann Nutr Metab, 2012, 61(2): 160-174. DOI:10.1159/000342079 |
| [106] |
RÉUS G Z, FRIES G R, STERTZ L, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders[J]. Neuroscience, 2015, 300: 141-154. DOI:10.1016/j.neuroscience.2015.05.018 |
| [107] |
MESSAOUDI M, LALONDE R, VIOLLE N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects[J]. Br J Nutr, 2011, 105(5): 755-764. DOI:10.1017/S0007114510004319 |
| [108] |
YANG C, FUJITA Y, REN Q, et al. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice[J]. Sci Rep, 2017, 7: 45942. DOI:10.1038/srep45942 |
| [109] |
NG Q X, PETERS C, HO C Y X, et al. A meta-analysis of the use of probiotics to alleviate depressive symptoms[J]. J Affect Disord, 2018, 228: 13-19. DOI:10.1016/j.jad.2017.11.063 |
| [110] |
KIM N, YUN M, OH Y J, et al. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics[J]. J Microbiol, 2018, 56(3): 172-182. DOI:10.1007/s12275-018-8032-4 |
| [111] |
SAVIGNAC H M, KIELY B, DINAN T G, et al. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice[J]. Neurogastroenterol Motil, 2014, 26(11): 1615-1627. DOI:10.1111/nmo.12427 |
| [112] |
BERCIK P, PARK A J, SINCLAIR D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication[J]. Neurogastroenterol Motil, 2011, 23(12): 1132-1139. DOI:10.1111/j.1365-2982.2011.01796.x |
| [113] |
GAREAU M G, JURY J, MACQUEEN G, et al. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation[J]. Gut, 2007, 56(11): 1522-1528. DOI:10.1136/gut.2006.117176 |
| [114] |
COWAN C S M, CALLAGHAN B L, RICHARDSON R. The effects of a probiotic formulation (Lactobacillus rhamnosus and L.helveticus) on developmental trajectories of emotional learning in stressed infant rats[J]. Transl Psychiatry, 2016, 6(5): e823. DOI:10.1038/tp.2016.94 |
| [115] |
LIU Y W, LIU W H, WU C C, et al. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice[J]. Brain Res, 2016, 1631: 1-12. DOI:10.1016/j.brainres.2015.11.018 |
| [116] |
MOYA-PÉREZ A, PEREZ-VILLALBA A, BENÍTEZ-PÁEZ A, et al. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice[J]. Brain Behav Immun, 2017, 65: 43-56. DOI:10.1016/j.bbi.2017.05.011 |
| [117] |
PINTO-SANCHEZ M I, HALL G B, GHAJAR K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome[J]. Gastroenterology, 2017, 153(2): 448-459. DOI:10.1053/j.gastro.2017.05.003 |
| [118] |
GUO Y, XIE J P, DENG K, et al. Prophylactic effects of Bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: A role of the gut microbiota-inflammation axis[J]. Front Behav Neurosci, 2019, 13: 126. |
| [119] |
BRAVO J A, FORSYTHE P, CHEW M V, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve[J]. Proc Natl Acad Sci U S A, 2011, 108(38): 16050-16055. DOI:10.1073/pnas.1102999108 |
| [120] |
OHLAND C L, KISH L, BELL H, et al. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome[J]. Psychoneuroendocrinology, 2013, 38(9): 1738-1747. DOI:10.1016/j.psyneuen.2013.02.008 |
| [121] |
MARIN I A, GOERTZ J E, REN T T, et al. Microbiota alteration is associated with the development of stress-induced despair behavior[J]. Sci Rep, 2017, 7: 43859. DOI:10.1038/srep43859 |
| [122] |
LIAO J F, HSU C C, CHOU G T, et al. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model[J]. Benef Microbes, 2019, 10(4): 425-436. DOI:10.3920/BM2018.0077 |
| [123] |
CHEVALIER G, SIOPI E, GUENIN-MACÉ L, et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system[J]. Nat Commun, 2020, 11(1): 6363. DOI:10.1038/s41467-020-19931-2 |
| [124] |
SUN X, ZHANG H F, MA C L, et al. Alleviation of anxiety/depressive-like behaviors and improvement of cognitive functions by Lactobacillus plantarum WLPL04 in chronically stressed mice[J]. Can J Infect Dis Med Microbiol, 2021, 2021: 6613903. |
(编辑 范子娟)