2. 四川省巴中市巴州区农业农村局, 巴中 636000;
3. 四川省巴中市巴州区畜牧站, 巴中 636000;
4. 西南大学食品科学学院, 重庆 400715;
5. 川渝共建特色食品重庆市重点实验室, 重庆 400715
2. Agricultural and Rural Bureau of Bazhou District, Bazhong City, Sichuan Province, Bazhong 636000, China;
3. Bazhou Animal Husbandry Station, Bazhong City, Sichuan Province, Bazhong 636000, China;
4. College of Food Science, Southwest University, Chongqing 400715, China;
5. Chongqing Key Laboratory of Special Foods Jointly Built by Sichuan and Chongqing, Chongqing 400715, China
近年来,肉兔作为畜产品、宠物和科研实验动物的重要来源,出栏量逐年提高,对运输的需求也相应加大。道路运输是畜禽常见的一种应激源,粗暴装笼、混群、断水断食、不良气味、噪音、拥挤和远途等恶劣的运输条件给家畜带来了诸多应激[1]。在运输途中,肉兔血液中皮质酮、谷丙转氨酶、谷草转氨酶、葡萄糖、红细胞压积、肌酸激酶、乳酸、乳酸脱氢酶、总蛋白等的浓度随之发生变化[2-4]。运输还提高了肌肉的滴水损失[2],影响肌肉的构形参数与肉色,但不影响pH、吸水力和剪切力[5]。另外,道路运输应激还提高了家畜的站立、排尿和排粪等行为的频次和持续时间[6-8],降低了兴奋性,使家畜表现出抑郁样行为[9]。一般来说,家畜被运输到屠宰场后并不直接屠宰,而是在屠宰场入栏静养一段时间,这样有助于家畜从应激中恢复[10]。屠宰前禁食与肉质、胴体重和肉类安全性有关[11],是目前动物生产中常用的措施。运输后禁食可提高肉色、pH和系水力[12]。研究表明,屠宰前48 h内禁食能最大限度地减少不良微生物的生长[13]。然而,有学者认为屠宰前的禁食和非禁食与胴体微生物感染无关,胴体污染可能是由不同的肉类包装工厂的工艺和操作不同所导致的[14-15]。屠宰前禁食增加了DFD肉的发生率,降低了胴体质量[16]。同时,运输前禁食会使动物饥饿,沮丧、疲劳和兴奋增加[17],家畜装载时抗拒和喊叫更加明显[18],而在屠宰场入栏期间提供饮食可以一定程度上缓解家畜的应激[19]。所以,探索合理的运输后处理方式,寻找缓解肉兔运输后应激的措施是非常必要的。本试验从肉兔血清生化指标、肉品质、应激基因表达和行为等方面探究运输后禁食、非禁食对肉兔应激的影响,希望能提高肉兔的福利水平,减少运输应激造成的经济损失。
1 材料与方法 1.1 试验设计与试验动物将80只体重(2.5±0.5) kg的伊拉公兔随机分为8个组:对照组(不运输)、运输后0 h组(运输后与对照组一起立即屠宰)、禁食6 h组、禁食24 h组、非禁食6 h组、非禁食24 h组、禁食行为观察组(不屠宰)和非禁食行为观察组(不屠宰),每组10只肉兔。预试期为7 d。运输笼的大小为750 mm × 550 mm × 270 mm,装载密度为10只·笼-1。在试验当日6:00,将肉兔经货车以80 km·h-1的平均时速运输2 h后返回兔场后,并被转移至兔笼中。禁食组不采食饲料,非禁食组自由采食,但均可自由饮水。除行为观察组外,在运输后0、6、24 h将其余各组的肉兔分批屠宰。使用海康威视1200万高清星光摄像头摄像记录2个行为观察组运输后36 h内的行为变化。
1.2 饲养管理将兔舍定期清扫并严格消毒,兔舍采用自然光照、定时通风,室内温度为(22±2)℃。每个兔笼装两只肉兔。
1.3 测定指标与方法1.3.1 血清生化指标的测定 将肉兔颈部脱臼处死后倒挂,用10 mL离心管收集血液。血液在室温静置30 min后,3 500 r·min-1离心10 min后采集血清,在-80 ℃冰箱保存。血清丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)、乳酸脱氢酶(LDH)、肌酸激酶(CK)、总蛋白(TP)、葡萄糖(Glu)、甘油三酯(TG)等的含量在Olympus AU400全自动生化分析仪进行检测。
1.3.2 血清激素水平测定 用双抗体夹心法原理测定血清中皮质醇的含量,试剂盒来自武汉华美生物工程有限公司。
1.3.3 肉品质测定 将肉兔屠宰后取背最长肌,装在自封袋中,保存在4 ℃冰箱中,于24 h后测定肌肉的pH、肉色、失水率、蒸煮损失率和剪切力。使用pH-STAR直测仪测定pH,使用UltraScan PRO测色仪测定肉色,用GR-150 Warner-Bratzler剪切力仪测定剪切力,指标的测定方法参照NY/T 1333—2007《畜禽肉质的测定》[20]。
1.3.4 实时荧光定量PCR 在采样时,用锡箔纸包裹肝组织样品后,快速投入液氮中冷冻,随后转移至-80 ℃冰箱。通过GenBank查找并设计JNK、Caspase-3基因的引物序列(表 1),由华大基因科技服务有限公司合成。RNA提取试剂盒购自北京天根生化科技有限公司,反转录试剂盒购自美国ABM生物科技有限公司,荧光定量试剂盒购自TaKaRa公司。25 μL的PCR反应体系为Hotstart Fluo-PCR mix 12 μL、上游和下游引物(25 μmol·L-1) 各1 μL、cDNA 1 μL和ddH2O 10 μL。用BIO-RAD CFXTM实时荧光定量PCR仪测定基因表达水平,仪器设置的反应条件为:94 ℃预变性4 min;94 ℃变性3 s,60 ℃退火30 s,72 ℃延伸30 s,共35个循环。每个样品设3个技术重复。
|
|
表 1 引物序列 Table 1 Primer sequences |
1.3.5 兔行为学观测 用摄像头全程记录行为观察组肉兔在运输后的行为,行为定义参考Prebble等[21]的方法。为掌握肉兔运输后36 h内的行为变化,于每天7:00、13:00、18:00和24:00进行观察,每次持续30 min,记录并计算观察期内各行为发生累计时间或频次占总观察时间或频次的百分率。
1.4 数据处理和统计分析所有数据用SPSS22.0统计软件进行处理,其中行为学数据用独立样本t检验方法进行分析,其他试验数据用单因素ANOVA法进行分析,分析结果表示为“平均值±标准差”,后用Duncan法进行多重比较。P<0.05表示差异显著,P<0.01表示差异极显著。
2 结果 2.1 运输后禁食与非禁食对肉兔应激水平的影响如表 2所示,运输后0 h组兔血清TP浓度相对于对照组显著降低(P<0.05),而血清皮质醇浓度相对于对照组显著升高(P<0.05)。禁食6 h组兔血清LDH水平(P<0.01)和禁食24 h组兔血清CK水平(P<0.05)显著高于对照组,禁食24 h组兔血清Glu水平显著低于对照组(P<0.05)。与禁食组相比,非禁食6 h组兔的血清LDH水平(P<0.01)和非禁食24 h组兔血清CK水平(P<0.05)显著降低,而非禁食24 h组血清Glu浓度显著升高(P<0.05)。
|
|
表 2 运输后禁食与非禁食方式对肉兔血清生化指标及激素的影响 Table 2 Effects of fasting and non-fasting on blood biochemical parameters and stress hormones in rabbits after transportation |
表 3所示,和对照组相比,运输后0 h背最长肌pH显著上升(P<0.05),肌肉L*值显著降低(P<0.05),a*值极显著下降(P<0.01),b*值则无显著变化(P>0.05)。运输后6 h和运输后0 h相比,pH、b*值和剪切力显著下降(P<0.05),禁食6 h组和非禁食6 h组之间的pH、L*、a*和b*值差异不显著(P>0.05),但非禁食24 h组的L*、a*和b*值显著高于禁食24 h组(P<0.05)。各处理组之间背最长肌失水率和蒸煮损失率的差异并不显著(P>0.05)。背最长肌的剪切力随着时间的增长而依次显著降低(P<0.05),但禁食组与非禁食组间无显著差异(P>0.05)。
|
|
表 3 运输及运输后禁食与非禁食方式对兔肌肉肉品质的影响 Table 3 Effects of fasting and non fasting on meat quality of rabbit muscle after transportation |
如图 1所示,运输后0 h组JNK和Caspase-3的mRNA表达量显著高于对照组(P<0.05)。非禁食组JNK的mRNA表达量显著低于禁食组(P<0.05),但禁食与非禁食组间的Caspase-3 mRNA的表达水平并无显著差异(P>0.05)。
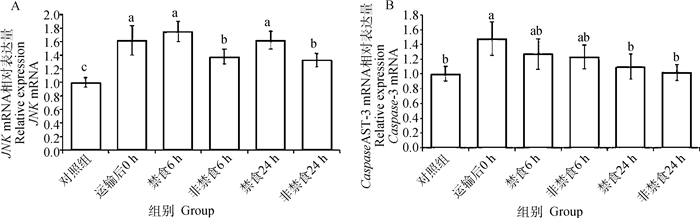
|
柱上不同小写字母表示差异显著(P<0.05) Column with different lowercase letters mean significant differences among treatments (P < 0.05) 图 1 非禁食和禁食对运输后肉兔肝JNK和Caspase-3 mRNA表达水平的影响 Fig. 1 Effects of non-fasting and fasting on the expression of JNK and Caspase-3 mRNA in liver of rabbits after transportation |
由表 4和表 5可知,非禁食组肉兔进食频次极显著高于禁食组、食粪行为频次极显著低于禁食组(P<0.01),非禁食组进食行为和饮水行为的持续时间显著高于禁食组(P<0.05),而食粪行为的持续时间显著低于禁食组(P<0.05)。
|
|
表 4 非禁食和禁食对运输后肉兔行为频次的影响 Table 4 Effects of non-fasting and fasting on behavior frequency of rabbits after transportation |
|
|
表 5 非禁食和禁食对运输后肉兔行为时间的影响 Table 5 Effects of non-fasting and fasting on behavior duration of rabbits after transportation |
运输过程中产生的系列应激使动物体内的稳态和代谢遭到破坏,导致血液中ALT、AST、CK、皮质醇、Glu、TP、乳酸等的浓度产生波动[22]。公路运输常会导致肾上腺皮质活动增加,血液中皮质醇的增加是衡量家畜心理压力的一个指标,是高度可变的[23]。Kannan等[10]发现,绵羊血清皮质醇在运输后0 h上升,静养一段时间后又显著下降,与本试验结果一致。运输过程中的捕获、装载和应激造成家畜肌肉融合不良、缺氧、疲劳,导致了肌膜通透性增加,细胞中AST、ALT、CK和LDH流失[24],而本试验中肉兔血清ALT、AST和CK在运输前后并没有显著的变化,可能是由于运输前未禁食,营养相对充足,肉兔没有饥饿感,抗拒较少的原因。在本试验中,非禁食24 h组肉兔血清CK浓度、禁食6 h组血清的LDH浓度显著低于禁食组,而非禁食24 h组血清Glu显著高于禁食组。CK催化高能磷酸键从ATP转移到肌酸上的可逆反应,可以在能量匮乏期间维持适当的ATP/ADP比例以提供短期活动的能量[25]。LDH作为糖酵解的关键酶会在运动后上升,也与细胞损伤有关[26]。葡萄糖参与能量代谢,也可以作为量化应激的有效指标。糖异生与糖皮质激素的增加有关,在应激条件下糖原分解增加,血液中葡萄糖的浓度也随之增加[27]。本研究,肉兔血清CK、LDH和葡萄糖的数据表明,非禁食显著改善了肉兔运输后的能量匮乏与机体损伤。Parker等[28]发现,家畜在运输过程中脱水时,运输后血液TP的浓度会增加。但本试验中运输却导致TP浓度显著下降,可能是由于试验当天温度(15~20 ℃)适宜,与de la Fuente等[29]描述的运输中高温脱水导致的血液中TP上升的状况不同,血清TP水平随着血清皮质醇水平的降低逐渐升高。本试验中,血清CK、LDH的变化趋势与血清皮质醇相一致,TP的变化趋势却与血清皮质醇相反,对于它们之间的联系还有待研究。
3.2 运输后非禁食对肌肉肉品质的影响屠宰前的应激会影响兔肉的品质[5]。肌肉pH是衡量疲劳的有效指标,也与肉的嫩度、色泽密切相关。肌肉的糖原浓度随着运输应激的增加而不断降低[30],屠宰后24 h肌肉pH取决于肌肉最初糖原的含量,肌糖原含量越低,pH越高[31]。本试验中肉兔在运输应激下,肌糖原快速消耗导致肌肉pH显著升高(P<0.05)。Brown等[32]发现,长途运输后肌肉的pH增高,并在屠宰前仍保持较高的水平,与本试验中运输后6、24 h后肌肉pH仍较高的结果一致。然而,本研究中运输后禁食、非禁食对肌肉pH影响并不显著。有研究发现,持水力依赖于肌肉的pHu(屠宰后24 h的pH)[33],当5.2<pHu<5.7,滴水损失随着pHu的减少而增加[34],但本试验中失水率和蒸煮损失率并没有随pHu的显著升高而降低,而是保持不变,其原因有待于进一步研究。肉色是肉质评定中的重要指标。Hussnain等[35]的研究表明,运输应激降低了肉的亮度和色饱和度,使肉色变暗,在高pH条件下,氧合肌红蛋白会被转化为暗红色的还原肌红蛋白,肌肉结构反射性变弱。María等[5]发现,运输应激会降低a*和C*值(C=(a*2+b*2)1/2表示色度,C值越大则颜色越鲜艳),Lambertini等[36]研究表明,长途运输会降低兔肉L*值,pH是导致肉色变化的重要原因。不同于María等[5]和Lambertini等[36]的分析,本试验中肌肉L*、a*和b*值与pH间无显著相关关系,非禁食显著提高了肉兔的肌肉肉色,这表明运输后充足的营养有相当的重要性。在本研究中,肌肉的剪切力不受运输、禁食和非禁食等因素的影响,随着时间的推移而不断降低,这与前人的研究结果一致,即屠宰前的静养时间延长可以降低肉质的剪切力[37-38]。Trocino等[39]发现,延长屠宰前的静养时间可以提高兔肉的pH、肉色和嫩度,这与本研究中运输后0 ~24 h肌肉品质的变化基本一致。另外,本研究表明,运输后非禁食的方式显著改善了肉色,比运输后禁食的措施更好。
3.3 运输后非禁食对肝JNK和Caspase-3 mRNA表达的影响在运输应激造成机体损伤的过程中,应激介导的细胞凋亡通路发挥了重要的作用。c-Jun N末端激酶(JNK)是MAPK家族的应激激活蛋白激酶,响应多种应激信号,与多种细胞事件有关[40],通过不同的下游效应物介导自噬与凋亡[41]。Caspase-3是JNK下游重要的效应因子,触发细胞调亡[42]。本试验中运输后0 h肉兔肝组织Caspase-3的mRNA表达量显著高于对照组(P<0.05),在6、24 h后Caspase-3 mRNA相对表达量逐渐下降,而禁食与非禁食组间没有显著差异。Caspase-3 mRNA的表达与血清皮质醇的浓度变化相一致,表明运输应激激增导致细胞凋亡,应激的缓解会降低细胞凋亡。但是,JNK的mRNA相对表达量与Caspase-3和皮质醇的mRNA表达量变化趋势并不一致,说明JNK通路还可能介导了其他细胞活动如自噬的发生。自噬是循环利用细胞质内容物使机体适应饥饿的一种机制,在细胞稳态中发挥重要的作用。自噬主要由mTOR通路调节[43],mTOR可以激活MAPK8/9[44],MAPK8/JNK1通过磷酸化BCL2,破坏了BCL2与BECN1的相互作用(BECN1与自噬相关),并启动BECN1依赖的自噬[45]。本试验中非禁食组肝组织中JNK mRNA的相对表达量显著低于禁食组,表明非禁食比长时间禁食更能有效减少机体由于细胞自噬和凋亡造成的损伤。
3.4 运输后非禁食对肉兔行为的影响行为是机体最重要的适应机制之一,是动物适应应激的身体防御,有助于减轻应激,也有可能因应激过强而导致机制性死亡。应激与行为紧密相连,刻板行为比如啃咬、笼子里踱步、过度修饰通常表明动物的无聊与应激。根据动物不正常行为的描述[46-47],家兔过度的社交、啃咬笼具和闻嗅行为都属于刻板行为。本试验中禁食组和非禁食组肉兔的社交、啃咬笼具和闻嗅行为并无显著差异,表明非禁食并不能显著改善肉兔的刻板行为,这与表 3中血清皮质醇的结果相互印证,说明非禁食并没有通过降低应激的方式改变刻板行为。禁食组食粪行为显著提升,食粪行为是中小型食草动物在长期的适应性进化过程中为了满足低营养条件下代谢需要的生理机制[48],正常的食粪行为能提高肉兔对蛋白质的消化吸收效率[49],减少肠道菌群及其所含的特殊蛋白质的流失[50],禁止食粪抑制肝脂质的合成,不利于家兔生长发育[51]。本试验中禁食组食粪行为比非禁食组多的原因一方面是对进食不足的替代,与表 3中禁食组和非禁食组血清TG浓度变化的结果相印证,即禁食组肉兔通过食粪补充血液中脂质合成;另一方面是禁食组肉兔饥饿的表现,与禁食组JNK介导细胞自噬通路的显著激活结果一致。非禁食则避免了饥饿,有利于提升肉兔的福利水平。
4 结论和禁食相比,运输后24 h非禁食降低了肉兔的应激水平,提高了肌肉品质,并一定程度改善肉兔的福利水平。
| [1] |
MINKA N S, AYO J O. Physiological responses of food animals to road transportation stress[J]. Afr J Biotechnol, 2009, 8(25): 7228-7420. |
| [2] |
MAZZONE G, VIGNOLA G, GIAMMARCO M, et al. Effects of loading methods on rabbit welfare and meat quality[J]. Meat Sci, 2010, 85(1): 33-39. DOI:10.1016/j.meatsci.2009.11.019 |
| [3] |
NAKYINSIGE K, SAZILI A Q, AGHWAN Z A, et al. Changes in blood constituents of rabbits subjected to transportation under hot, humid tropical conditions[J]. Asian-Australas J Anim Sci, 2013, 26(6): 874-878. DOI:10.5713/ajas.2012.12652 |
| [4] |
FAZIO F, CASELLA S, GIUDICE E, et al. Evaluation of secondary stress biomarkers during road transport in rabbit[J]. Livest Sci, 2015, 173: 106-110. DOI:10.1016/j.livsci.2015.01.006 |
| [5] |
MARÍA G A, BUIL T, LISTE G, et al. Effects of transport time and season on aspects of rabbit meat quality[J]. Meat Sci, 2006, 72(4): 773-777. DOI:10.1016/j.meatsci.2005.10.012 |
| [6] |
MINKA N S, AYO J O. Assessment of the stresses imposed on adult ostriches (Struthio camelus) during handling, loading, transportation and unloading[J]. Vet Rec, 2008, 162(26): 846-851. DOI:10.1136/vr.162.26.846 |
| [7] |
CHANDRA B S, DAS N. The handling and short-haul road transportation of spent buffaloes in relation to bruising and animal welfare[J]. Trop Anim Health Prod, 2001, 33(2): 155-163. DOI:10.1023/A:1005242815540 |
| [8] |
EICHER S D. Transportation of cattle in the dairy industry: current research and future directions[J]. J Dairy Sci, 2001, 84 Suppl: E19-E23. |
| [9] |
AYO J O, MINKA N S, MAMMAN M. Excitability scores of goats administered ascorbic acid and transported during hot-dry conditions[J]. J Vet Sci, 2006, 7(2): 127-131. DOI:10.4142/jvs.2006.7.2.127 |
| [10] |
KANNAN G, TERRILL T H, KOUAKOU B, et al. Transportation of goats: effects on physiological stress responses and live weight loss[J]. J Anim Sci, 2000, 78(6): 1450-1457. DOI:10.2527/2000.7861450x |
| [11] |
ZOTTE A D. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality[J]. Livest Prod Sci, 2002, 75(1): 11-32. DOI:10.1016/S0301-6226(01)00308-6 |
| [12] |
DRIESSEN B, FRESON L, BUYSE J. Fasting finisher pigs before slaughter influences pork safety, pork quality and animal welfare[J]. Animals, 2020, 10(12): 2206. DOI:10.3390/ani10122206 |
| [13] |
POINTON A, KIERMEIER A, FEGAN N. Review of the impact of pre-slaughter feed curfews of cattle, sheep and goats on food safety and carcase hygiene in Australia[J]. Food Control, 2012, 26(2): 313-321. DOI:10.1016/j.foodcont.2012.01.034 |
| [14] |
ZWEIFEL C, STEPHAN R. Microbiological monitoring of sheep carcass contamination in three Swiss abattoirs[J]. J Food Prot, 2003, 66(6): 946-952. DOI:10.4315/0362-028X-66.6.946 |
| [15] |
VERGARA H, CÓZAR A, RODRÍGUEZ A I, et al. Effect of space allowance during transport and fasting or non-fasting during lairage on carcass contamination and meat traits in Merino lamb[J]. Span J Agric Res, 2017, 15(2): e0503. DOI:10.5424/sjar/2017152-10227 |
| [16] |
JONES S D M, ROMPALA R E, HAWORTH C R. Effects of fasting and water restriction on carcass shrink and pork quality[J]. Can J Anim Sci, 1985, 65(3): 613-618. DOI:10.4141/cjas85-072 |
| [17] |
LEWIS N J. Frustration of goal-directed behaviour in swine[J]. Appl Anim Behav Sci, 1999, 64(1): 19-29. DOI:10.1016/S0168-1591(99)00025-8 |
| [18] |
COSTA F A D, DEVILLERS N, DA COSTA M J R P, et al. Effects of applying preslaughter feed withdrawal at the abattoir on behaviour, blood parameters and meat quality in pigs[J]. Meat Sci, 2016, 119: 89-94. DOI:10.1016/j.meatsci.2016.03.033 |
| [19] |
COZAR A, RODRIGUEZ A I, GARIJO P, et al. Effect of space allowance during transport and fasting or non-fasting during lairage on welfare indicators in Merino lambs[J]. Span J Agric Res, 2016, 14(1): e0501. DOI:10.5424/sjar/2016141-8313 |
| [20] |
中华人民共和国农业部. NY/T 1333-2007畜禽肉质的测定[S]. 2007. Ministry of Agriculture of the PRC. NY/T 1333-2007 Determination of livestock and poultry meat quality[S]. 2007. (in Chinese) |
| [21] |
PREBBLE J L, LANGFORD F M, SHAW D J, et al. The effect of four different feeding regimes on rabbit behaviour[J]. Appl Anim Behav Sci, 2015, 169: 86-92. DOI:10.1016/j.applanim.2015.05.003 |
| [22] |
LÓPEZ-OLVERA J R, MARCO I, MONTANÉ J, et al. Transport stress in Southern chamois (Rupicapra pyrenaica) and its modulation by acepromazine[J]. Vet J, 2006, 172(2): 347-355. DOI:10.1016/j.tvjl.2005.06.007 |
| [23] |
GRANDIN T. Assessment of stress during handling and transport[J]. J Anim Sci, 1997, 75(1): 249-257. DOI:10.2527/1997.751249x |
| [24] |
TADICH N, GALLO C, BUSTAMANTE H, et al. Effects of transport and lairage time on some blood constituents of Friesian-cross steers in Chile[J]. Livest Prod Sci, 2005, 93(3): 223-233. DOI:10.1016/j.livprodsci.2004.10.004 |
| [25] |
MIR N A, ASHUTOSH A, SHERGOJRY S A, et al. Effect of induced transportation stress in goats supplemented with vitamin C and jaggery during hot dry season[J]. Biol Rhythm Res, 2019, 50(3): 389-399. DOI:10.1080/09291016.2018.1452591 |
| [26] |
BARRANCO T, TVARIJONAVICIUTE A, TECLES F, et al. Changes in creatine kinase, lactate dehydrogenase and aspartate aminotransferase in saliva samples after an intense exercise: a pilot study[J]. J Sports Med Phys Fitness, 2018, 58(6): 910-916. |
| [27] |
SHAW F D, TUME R K. The assessment of pre-slaughter and slaughter treatments of livestock by measurement of plasma constituents—A review of recent work[J]. Meat Sci, 1992, 32(3): 311-329. DOI:10.1016/0309-1740(92)90095-L |
| [28] |
PARKER A J, HAMLIN G P, COLEMAN C J, et al. Quantitative analysis of acid-base balance in Bos indicus steers subjected to transportation of long duration[J]. J Anim Sci, 2003, 81(6): 1434-1439. DOI:10.2527/2003.8161434x |
| [29] |
DE LA FUENTE J, SALAZAR M I, IBÁÑEZ M, et al. Effects of season and stocking density during transport on live weight and biochemical measurements of stress, dehydration and injury of rabbits at time of slaughter[J]. Anim Sci, 2016, 78(2): 285-292. |
| [30] |
ZHANG L, YUE H Y, ZHANG H J, et al. Transport stress in broilers: Ⅰ.Blood metabolism, glycolytic potential, and meat quality[J]. Poult Sci, 2009, 88(10): 2033-2041. DOI:10.3382/ps.2009-00128 |
| [31] |
ZHENG A J, LIN S M, PIRZADO S A, et al. Stress associated with simulated transport, changes serum biochemistry, postmortem muscle metabolism, and meat quality of broilers[J]. Animals, 2020, 10(8): 1442. DOI:10.3390/ani10081442 |
| [32] |
BROWN S N, KNOWLES T G, EDWARDS J E, et al. Behavioural and physiological responses of pigs to being transported for up to 24 hours followed by six hours recovery in lairage[J]. Vet Rec, 1999, 145(15): 421-426. DOI:10.1136/vr.145.15.421 |
| [33] |
JOSELL Å, VON SETH G, TORNBERG E. Sensory quality and the incidence of PSE of pork in relation to crossbreed and RN phenotype[J]. Meat Sci, 2003, 65(1): 651-660. DOI:10.1016/S0309-1740(02)00268-1 |
| [34] |
HAMOEN J R, VOLLEBREGT H M, VAN DER SMAN R G M. Prediction of the time evolution of pH in meat[J]. Food Chem, 2013, 141(3): 2363-2372. DOI:10.1016/j.foodchem.2013.04.127 |
| [35] |
HUSSNAIN F, MAHMUD A, MEHMOOD S, et al. Meat quality and cooking characteristics in broilers influenced by winter transportation distance and crate density[J]. J Poult Sci, 2020, 57(2): 175-182. DOI:10.2141/jpsa.0190014 |
| [36] |
LAMBERTINI L, VIGNOLA G, BADIANI A, et al. The effect of journey time and stocking density during transport on carcass and meat quality in rabbits[J]. Meat Sci, 2006, 72(4): 641-646. DOI:10.1016/j.meatsci.2005.09.012 |
| [37] |
KADIM I T, MAHGOUB O, AL-MARZOOQI W, et al. Effects of transportation during the hot season and low voltage electrical stimulation on histochemical and meat quality characteristics of sheep longissimus muscle[J]. Livest Sci, 2009, 126(1-3): 154-161. DOI:10.1016/j.livsci.2009.06.014 |
| [38] |
EKIZ B, EKIZ E E, KOCAK O, et al. Effect of pre-slaughter management regarding transportation and time in lairage on certain stress parameters, carcass and meat quality characteristics in Kivircik lambs[J]. Meat Sci, 2012, 90(4): 967-976. DOI:10.1016/j.meatsci.2011.11.042 |
| [39] |
TROCINO A, ZOMEÑO C, BIROLO M, et al. Impact of pre-slaughter transport conditions on stress response, carcass traits, and meat quality in growing rabbits[J]. Meat Sci, 2018, 146: 68-74. DOI:10.1016/j.meatsci.2018.07.035 |
| [40] |
KYRIAKIS J M, BANERJEE P, NIKOLAKAKI E, et al. The stress-activated protein kinase subfamily of c-jun kinases[J]. Nature, 1994, 369(6476): 156-160. DOI:10.1038/369156a0 |
| [41] |
SUI X B, KONG N, YE L, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents[J]. Cancer Lett, 2014, 344(2): 174-179. DOI:10.1016/j.canlet.2013.11.019 |
| [42] |
TANG R X, KONG F Y, FAN B F, et al. HBx activates FasL and mediates HepG2 cell apoptosis through MLK3-MKK7-JNKs signal module[J]. World J Gastroenterol, 2012, 18(13): 1485-1495. DOI:10.3748/wjg.v18.i13.1485 |
| [43] |
KLIONSKY D J, CREGG J M, DUNN W A Jr, et al. A unified nomenclature for yeast autophagy-related genes[J]. Dev Cell, 2003, 5(4): 539-545. DOI:10.1016/S1534-5807(03)00296-X |
| [44] |
LEE J H, BUDANOV A V, PARK E J, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies[J]. Science, 2010, 327(5970): 1223-1228. DOI:10.1126/science.1182228 |
| [45] |
BARUTCU S A, GIRNIUS N, VERNIA S, et al. Role of the MAPK/cJun NH2-terminal kinase signaling pathway in starvation-induced autophagy[J]. Autophagy, 2018, 14(9): 1586-1595. DOI:10.1080/15548627.2018.1466013 |
| [46] |
BLOKHUIS H. Stereotypic animal behavior: fundamentals and applications to welfare: 1993.Ed: A. B. Lawrence and J. Rushen.Commonwealth Agricultural Bureau International, Wallingford, Oxon, OX10 8DE, U.K. 212 pp, many refs.index hardback.£37.50 ($71.25).ISBN 0 85198 824 5[J]. Livest Prod Sci, 1994, 40(3): 359-360. DOI:10.1016/0301-6226(94)90115-5 |
| [47] |
GUNN D, MORTON D B. Inventory of the behaviour of New Zealand White rabbits in laboratory cages[J]. Appl Anim Behav Sci, 1995, 45(3-4): 277-292. DOI:10.1016/0168-1591(95)00627-5 |
| [48] |
TRAVERS S K, ELDRIDGE D J, DORROUGH J, et al. Introduced and native herbivores have different effects on plant composition in low productivity ecosystems[J]. Appl Veg Sci, 2018, 21(1): 45-54. DOI:10.1111/avsc.12334 |
| [49] |
刘海纯, 杨冬梅, 龙灿, 等. 限制食粪对东方田鼠食物消化和体重生长的影响[J]. 兽类学报, 2016, 36(1): 64-71. LIU H C, YANG D M, LONG C, et al. Effects of caecotrophy prevention on food digestion and growth in Microtus fortis[J]. Acta Theriologica Sinica, 2016, 36(1): 64-71. (in Chinese) |
| [50] |
GIDENNE T, LAPANOUSE A. Impact of caecotrophy on rate of passage, intake and faecal excretion pattern in the growing rabbit[J]. World Rabbit Sci, 2010, 12(2): 81-94. |
| [51] |
WANG Y D, XU H F, SUN G R, et al. Transcriptome analysis of the effects of fasting caecotrophy on hepatic lipid metabolism in new Zealand rabbits[J]. Animals, 2019, 9(9): 648. DOI:10.3390/ani9090648 |
(编辑 范子娟)



