2. 惠州工程职业学院, 惠州 516023
2. Huizhou Engineering Vocational College, Huizhou 516023, China
免疫抑制作用是由病毒、霉菌毒素、农用化学物质等多种因素引起的免疫系统紊乱或对机体免疫系统产生抑制功能[1]。近些年来,一些免疫抑制性疾病在肉鸡养殖中频发,如马立克氏病、传染性法氏囊病和禽白血病[2-3],其能够抑制免疫应答,降低宿主对疫苗的敏感性,从而增加鸡群的发病率和死亡率,给家禽养殖业造成严重的经济损失。现阶段应对这些免疫抑制疾病的策略主要是通过长期的疫苗接种和适当的药物治疗。但是灭活疫苗产生的抗体不持久,而药物的过度使用也会产生诸多的食品安全问题。因此,寻找一种预防和治疗免疫抑制疾病的新方法是亟待解决的问题,其中使用中草药来增强动物的免疫力被认为是最有前景的方法。
中药具有毒性低、副作用小的优势,已然成为一种新颖的药物治疗手段。紫锥菊是一种传统的药用植物,具有免疫调节、抗氧化、抗炎、抗癌、抗病毒等作用[4-6]。免疫调节是紫锥菊重要的生物活性作用,能够增加宿主的免疫和抗感染能力。研究发现,紫锥菊乙醇提取物对流感病毒H1N1、H5N1、H7N7有良好的抑制作用[7-8]。同时,饲料中添加紫锥菊能显著促进注射蓝耳病弱病毒仔猪的生长及弱病毒的免疫效果[9]。可见紫锥菊具有一定免疫调节作用,可作为一种潜在的免疫诱导剂。环磷酰胺是一种免疫抑制剂,也是一种抗肿瘤药物,在杀灭肿瘤细胞的同时也会造成机体免疫力降低,诱发多个系统的慢性疾病[10-11]。已有研究表明,紫锥菊可以提高肉鸡的生长性能和免疫器官指数(脾、胸腺、法氏囊),并对免疫抑制鸡的免疫系统具有调节功能[12-13]。脾是机体内最大的外周淋巴器官,也是免疫反应的重要部位,但目前关于紫锥菊对免疫抑制鸡脾组织保护作用的潜在机制尚不清楚。因此,本研究利用环磷酰胺诱导的免疫抑制动物模型探讨紫锥菊对免疫抑制鸡的免疫调节功能的影响。
1 材料与方法 1.1 试验动物及处理120羽1日龄雏鸡适应性饲养7 d后,随机分为4组:对照组、环磷酰胺组、紫锥菊组、环磷酰胺+紫锥菊组(联合组),每组6个重复,每个重复30羽。对照组和紫锥菊组雏鸡连续3 d胸肌注射等量的生理盐水,环磷酰胺组和联合组雏鸡连续3 d按80 mg·kg-1体重注射生理盐水配制的环磷酰胺诱导免疫抑制模型,紫锥菊组和联合组每天饲喂含有紫锥菊全草粉末的基础日粮(含量为1%),其余各组饲喂基础日粮。试验期间雏鸡自由饮水,给予光照每天12 h,第1周室温控制在31~33 ℃,每过1周温度降1~2 ℃。每天称重并记录每只鸡的重量,连续饲喂21 d,并观察雏鸡的生长情况。试验结束时,每重复组随机选取6只鸡,称取体重,迅速采集脾并称重,并计算脾脏指数。取一部分脾用预冷PBS(磷酸盐缓冲液)清洗后放入4%的多聚甲醛中,用于形态学病理检测。其余的放入冻存管于液氮之中暂存,用于分子检测。
1.2 试验试剂和仪器TRIzol购自TaKaRa公司;Prime Script RT Master Mix、2×ChamQ SYBR qPCR Master Mix和BCA蛋白检测试剂盒购自南京诺唯赞生物科技有限公司;RIRA裂解液购自Beyotime公司;PAGE凝胶快速制备试剂盒(12.5%)购自上海雅酶生物科技有限公司;炎症抗体均购自武汉三鹰生物技术有限公司;紫锥菊全草购自安国市宏宇中药材公司,产地河北。其他试剂均为国产分析纯。
TDZ5-WS型冷冻离心机,购自湖南湘仪实验室仪器开发有限公司;5200型凝胶成像系统,购自上海医科大学仪器厂;LightCycler480型荧光定量PCR仪,购自美国Bio-Rad公司;Unique-R10型超纯水仪,购自厦门锐思捷科学仪器有限公司;DM1000型光学显微镜,购自广州德真科学仪器有限公司。
1.3 脾组织重量分析分离的脾组织用滤纸吸干表面血液,称重并记录。并计算脾脏指数:脾脏指数=脾重量(g)×100%/体重(g)。
1.4 病理切片的制备分离的脾组织在4%的多聚甲醛中固定24 h后,在梯度酒精中脱水,二甲苯透明,放入蜡机中制成石蜡块。切片机将组织蜡块切成5 μm厚的切片,经烤片、二甲苯Ⅰ、二甲苯Ⅱ、100%、90%、80%、70%、60%的梯度酒精复水(各3 min),苏木精染色,1%的盐酸酒精分色,自来水反蓝15 min,60%、70%、80%、90%的梯度酒精脱水(各3 min),伊红染色,100%酒精(2次)、二甲苯Ⅲ(5 min)、二甲苯Ⅳ(10 min),封片,光学显微镜先观察并拍照。
1.5 实时荧光定量PCR称取脾组织50 mg,提取总RNA,其方法按照TaKaRa试剂盒说明书进行。简而言之,RNA浓度用NanaDrop-2000微量核算测定仪检测。GAPDH作为看家基因。引物由Prime Express 5设计,由生工生物工程股份有限公司合成,引物序列见表 1。将RNA反转录成cDNA,按照试剂盒说明书进行。随后进行RT-qPCR反应。反应总体系为10 μL:其中cDNA 1 μL, 2× Cham Q SYBR qPCR Master Mix 5 μL,上、下游引物均为0.4 μL。反应条件:预变性95 ℃ 5 min,95 ℃ 30 s,60 ℃ 30 s;进行40次循环;检测NF-κB、IκB、IL-2、IL-4、IL-6、TNF-α、IFN-γ、TLR2、TLR4、TLR7、MyD88的mRNA水平。
|
|
表 1 引物序列 Table 1 Primer sequences |
用蛋白免疫印迹法检测鸡脾组织NF-κB、p-NF-κB、IκB、p-IκB、IL-2、IL-4、IL-6、TNF-α、IFN-γ和TLR4的蛋白表达量,以β-tubulin和GAPDH作为内参。称取鸡脾组织30 mg,加500 μL RIPA裂解液冰上匀浆、离心(12 000×g,4 ℃,15 min)取上清获得总蛋白,测定蛋白浓度后稀释至同一浓度,煮沸10 min后-80 ℃保存。采用12.5% SDS-聚丙酰胺凝胶电泳分离目的蛋白,通过转膜将目的蛋白转移到PVDF膜上,TBST洗涤3次,每次5 min,5%的脱脂牛奶封闭1 h,目的蛋白相应的一抗4 ℃摇床孵育过夜。TBST洗涤3次,然后与相应二抗摇床室温孵育1 h,后用TBST充分洗涤。ECL发光液显影,拍照并分析。
1.7 数据处理试验数据先用Excel 2019初步处理,数据均以“平均值±标准差(Mean ± SD)”表示,用SPSS统计分析软件进行差异显著性检验,使用GraphPad Prism 9.0软件进行做图。P<0.05被认为有统计学意义。
2 结果 2.1 紫锥菊对环磷酰胺致免疫功能降低肉鸡一般状况的影响如图 1A所示,注射环磷酰胺后,在14 d时,环磷酰胺组雏鸡体重和对照组相比显著降低(P<0.05),而其他组无明显差异。在21日龄时,紫锥菊组雏鸡体重和对照组相比极显著增加(P<0.001),而环磷酰胺组雏鸡生长迟缓(P<0.05),联合组雏鸡体重显著高于环磷酰胺组雏鸡(P<0.05)。在28日龄时,体重差异更为明显,紫锥菊组雏鸡体重是4个组中最高的(P<0.001),而环磷酰胺组雏鸡最低(P<0.001),联合组雏鸡体重较环磷酰胺组显著增加(P<0.001)。脾脏指数结果如图 1B所示,环磷酰胺组鸡的脾脏指数显著低于对照组和联合组(P<0.05)。这些试验结果表明,实验动物模型造模成功。
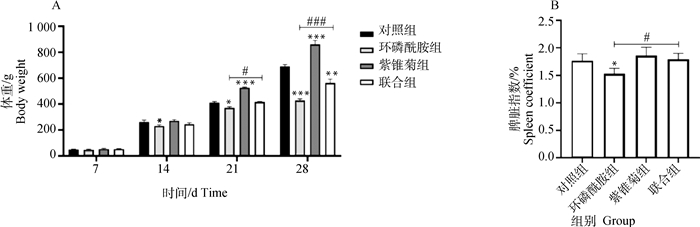
|
与对照组相比,*表示P<0.05,**表示P<0.01,***表示P<0.001。联合组与环磷酰胺组比,#表示P<0.05,##表示P<0.01,###表示P<0.001,n=6。下同 Compared with control group, * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001. The combined group vs. the cyclophosphamide group, # represents P < 0.05, ## means P < 0.01, ### indicates P < 0.001, n=6. The same as below 图 1 鸡的体重(A)及脾脏指数(B)变化 Fig. 1 The changes in body weight(A) and spleen coefficient(B) of chickens |
如图 2所示,对照组鸡脾结构正常,白髓与红髓分界线清晰,形态良好。环磷酰胺组鸡脾出现大量空泡(红色箭头表示),淋巴细胞稀疏紊乱,白髓与红髓分界线逐渐模糊,动脉周围淋巴鞘增生,淋巴细胞生成减少。紫锥菊组鸡脾结构清晰,无明显病变。联合组鸡脾淋巴细胞生成增多,间质纤维增生减轻,空泡减少。
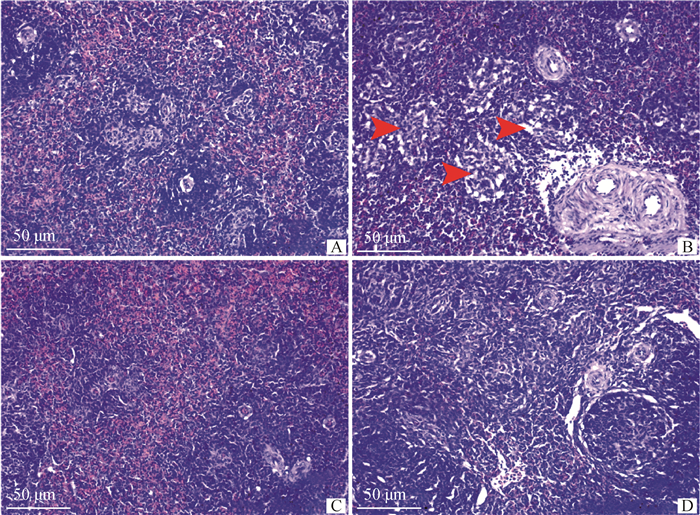
|
A~D分别为对照组、环磷酰胺组、紫锥菊组、联合组。红箭头指空泡 A-D are the control group, the cyclophosphamide treated group, the Echinacea treated group and the combined group, respectively. Red arrows point to the vacuoles degeneration 图 2 脾组织病理切片图(200×) Fig. 2 Histopathological sections of the spleen tissues (200×) |
紫锥菊对环磷酰胺致免疫功能降低肉鸡脾炎性相关基因mRNA表达的影响如图 3所示。与对照组相比,环磷酰胺处理组的NF-κB、IL-2、IL-4、IL-6、IFN-γ基因的mRNA表达量均显著降低(P<0.05、P<0.01或P<0.001),而IκB和TNF-α的mRNA表达量显著升高(P<0.01或P<0.001)。而紫锥菊组NF-κB、IL-2、IL-4、IL-6、IFN-γ的mRNA表达量均显著高于对照组(P<0.05、P<0.01或P<0.001),IκB的mRNA表达量显著低于对照组(P<0.01),TNF-α无显著变化。联合组和环磷酰胺组相比,基因NF-κB、IL-2、IL-4、IL-6、IFN-γ的表达量均显著升高(P<0.05、P<0.01或P<0.001),而IκB和TNF-α显著下降(P<0.05)。与对照组相比,环磷酰胺组TLR2、TLR4、TLR7、MyD88的mRNA水平均显著下降(P<0.05、P<0.01或P<0.001),而紫锥菊组的MyD88 mRNA的表达量显著高于对照组(P<0.001),其余基因mRNA表达量无显著差异。此外,联合组相比环磷酰胺组,TLR2、TLR4、MyD88的mRNA的表达水平显著上升(P<0.05,P<0.001),TLR7的mRNA表达量无显著差异。
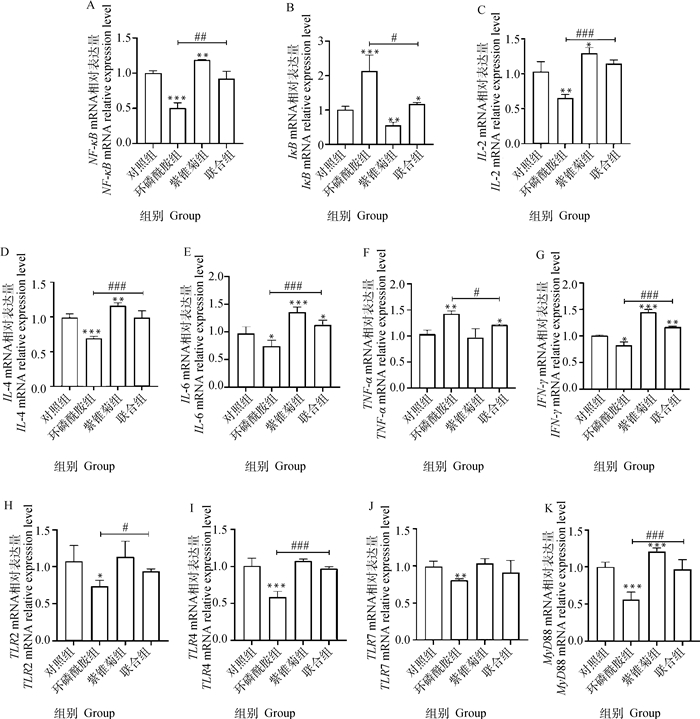
|
图 3 紫锥菊对环磷酰胺致免疫功能降低肉鸡的脾组织中炎性和免疫相关基因mRNA表达的影响 Fig. 3 Effects of Echinacea on the mRNA expression of inflammatory- and immune-related genes in spleen of immunosupressed chickens induced by cyclophosphamide |
结果如图 4,相比于对照组,p-NF-κB/NF-κB在环磷酰胺组显著降低(P<0.001),而在紫锥菊组显著升高(P<0.01),且联合组较环磷酰胺组显著增加(P<0.001)。与p-NF-κB/NF-κB相反,环磷酰胺组p-IκB /IκB显著高于对照组(P<0.01),而其他组则无显著差异。与对照组相比,环磷酰胺组炎性因子IL-2、IL-4、IL-6、TNF-α、IFN-γ的蛋白表达水平均显著降低(P<0.05或P<0.001),而上述蛋白表达量在紫锥菊组则显著增加(P<0.05或P<0.01),且联合组IL-2、IL-4、IL-6、TNF-α、IFN-γ的蛋白表达水平显著高于环磷酰胺组(P<0.05或P<0.001)。环磷酰胺组TLR4蛋白表达水平显著低于对照组,而联合组的TLR4蛋白表达水平则显著高于对照组和环磷酰胺组(P<0.01或P<0.001)。
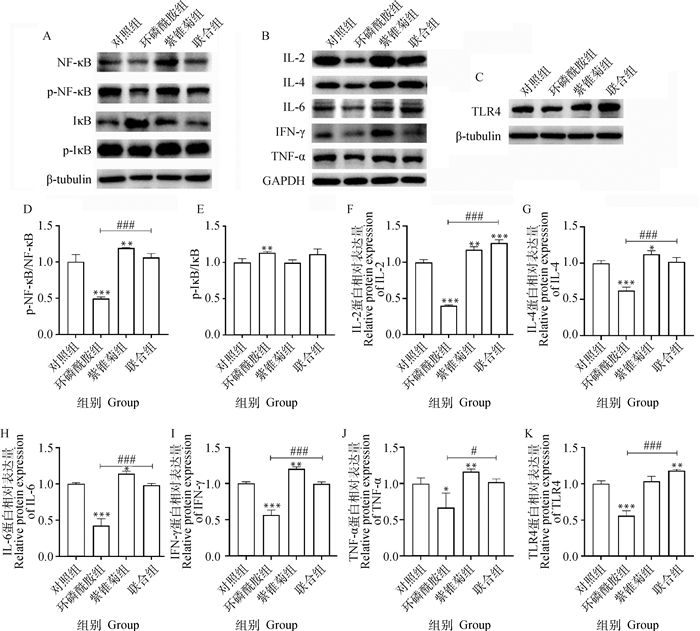
|
图 4 紫锥菊对环磷酰胺致免疫功能降低肉鸡的脾组织NF-κB/TLR4通路相关蛋白的表达影响 Fig. 4 Effects of Echinacea on expression levels of NF-κB and TLR4 pathway-related proteins in spleen of immunosuppressed chickens induced by cyclophosphamide |
环磷酰胺是一种广泛用于诱导免疫抑制动物模型的烷基化制剂,它可以抑制体液免疫和细胞免疫,导致淋巴细胞发生不可逆损伤[14-15]。因此,本研究利用环磷酰胺建立鸡免疫抑制模型,验证紫锥菊在促进雏鸡生长和免疫调节中的作用。动物的生产性能反映了饲料对机体生长发育的影响。研究表明,在猪饲料中添加紫锥菊粉末可以显著提高育肥猪的饲料转化率,且生长性能明显提高[16]。本试验结果显示,从21 ~28日龄紫锥菊组鸡的体重急剧增加且显著高于其他组,说明紫锥菊能够提高鸡的生长性能,此结果与罗俊峰等[17]的研究结果一致。体重变化可以直观地反映动物的生长状态;脾是禽类最重要的免疫器官,是各种免疫活性细胞发育、增殖分化的主要场所,免疫器官发育情况直接影响机体的免疫系统。免疫系统具有免疫监视、防御、调控的作用,当其受损时往往伴随着免疫器官的损害[18-19]。在本研究中,环磷酰胺组的脾脏指数显著低于其他组,而HE染色显示,对照组脾细胞超微结构致密,排列整齐;环磷酰胺组的脾细胞排列不规则,细胞间隙明显扩张,红、白髓结构不清晰;而联合组脾细胞的结构损伤得到缓解,病理变化减轻;紫锥菊组与对照组接近。本研究显示,紫锥菊能有效缓解环磷酰胺所致脾损伤,并通过影响免疫器官发挥其免疫调节功能。有研究显示,80 mg·kg-1的环磷酰胺作用的肉鸡脾脏指数和法氏囊指数显著低于对照组[20]。宋红卫等[21]发现,紫锥菊的添加能够显著促进免疫器官的发育,提高免疫器官指数。这与本研究结果相一致。
有研究发现,TLR家族蛋白在先天免疫中发挥重要作用[22-23]。其中TLR4在启动适应性免疫、活化抗原提呈细胞和病原体识别中起着关键作用[24-25]。TLR4的激活直接诱导核因子κB(nuclear factor kappa-B,NF-κB)进入细胞核形成磷酸化的NF-κB(p-NF-κB),进而激活免疫调节通路的级联以及下游关键转录因子[26-27]。而IκB(inhibitor of NF-κB),是NF-κB的抑制蛋白,p-IκB是其磷酸化形式,主要调节NF-κB转录[28-29]。IL-2、IL-4、IL-6、TNF-α、IFN-γ是受NF-κB调控的重要炎性因子,其对维持机体免疫功能具有重要的作用[30-31]。有研究表明,刺参糖胺聚糖、刺五加多糖、玉竹多糖和板蓝根多糖均具有较好的免疫调节功能[32-34]。紫锥菊多糖在LPS致大鼠肺损伤模型中,显著降低了白细胞、嗜酸性粒细胞、中性粒细胞、淋巴细胞和巨噬细胞的数量,减少TNF-α、IL-6和IL-1β释放,显示出较强的抗炎效果[35]。环磷酰胺作用于小鼠后,血清蛋白IgG、IgM和IgA,抗炎性因子IL-4和IL-10显著低于对照组[36]。为了阐明紫锥菊的免疫调节活性是否受TLR4/NF-κB通路的调控,本研究检测了TLR4/NF-κB通路相关的基因mRNA和蛋白的表达情况,实时荧光定量结果显示,环磷酰胺组的TLR4/NF-κB通路相关因子(除IκB外)mRNA表达水平显著低于其他各组,而联合组相关基因变化趋势与环磷酰胺组结果相反。蛋白免疫印迹结果显示,在环磷酰胺组NF-κB和IκB的磷酸化明显受阻,而添加紫锥菊后得到明显缓解,由此可见紫锥菊可激活TLR4/NF-κB信号通路,具有较强的免疫调节活性。
4 结论紫锥菊依赖于TLR4/NF-κB通路进行免疫调节,并通过该通路上调细胞因子IL-2、IL-4、IL-6、TNF-α和IFN-γ的表达来调节机体的免疫机能。本研究结果为紫锥菊在兽医临床上的应用提供理论依据和试验基础。
| [1] |
CHEN H L, LI D F, CHANG B Y, et al. Effects of Chinese herbal polysaccharides on the immunity and growth performance of young broilers[J]. Poult Sci, 2003, 82(3): 364-370. DOI:10.1093/ps/82.3.364 |
| [2] |
NAJIMUDEEN S M, HASSAN M S H, CORK S C, et al. Infectious bronchitis coronavirus infection in chickens: multiple system disease with immune suppression[J]. Pathogens, 2020, 9(10): 779. DOI:10.3390/pathogens9100779 |
| [3] |
BALAMURUGAN V, KATARIA J M. Economically important non-oncogenic immunosuppressive viral diseases of chicken--current status[J]. Vet Res Commun, 2006, 30(5): 541-566. DOI:10.1007/s11259-006-3278-4 |
| [4] |
MAO C F, SUDIRMAN S, LEE C C, et al. Echinacea purpurea ethanol extract improves male reproductive dysfunction with streptozotocin-nicotinamide-induced diabetic rats[J]. Front Vet Sci, 2021, 8: 651286. DOI:10.3389/fvets.2021.651286 |
| [5] |
SIGNER J, JONSDOTTIR H R, ALBRICH W C, et al. In vitro virucidal activity of Echinaforce(R), an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2[J]. Virol J, 2020, 17(1): 136. DOI:10.1186/s12985-020-01401-2 |
| [6] |
AARLAND R C, BAÑUELOS-HERNÁNDEZ A E, FRAGOSO-SERRANO M, et al. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts[J]. Pharm Biol, 2017, 55(1): 649-656. DOI:10.1080/13880209.2016.1265989 |
| [7] |
刘京. 中药有效成分对新型冠状病毒肺炎多靶点治疗作用的探讨[J]. 中国中西医结合杂志, 2020, 40(3): 263-268. LIU J. Discussion on multi-target treatment of active components in traditional Chinese medicine for COVID-19[J]. Chinese Journal of Integrated Traditional and Western Medicine, 2020, 40(3): 263-268. (in Chinese) |
| [8] |
KARSCH-VÖLK M, BARRETT B, KIEFER D, et al. Echinacea for preventing and treating the common cold[J]. Cochrane Database Syst Rev, 2014(2): CD000530. |
| [9] |
高仙, 龙冰雁. 紫锥菊对注射蓝耳病病毒仔猪生长性能及机体免疫水平的影响[J]. 中国饲料, 2019(24): 51-54. GAO X, LONG B Y. Effects of Echinacea purpurea on growth performance and immunity of PRRSV challenged weaned pigs[J]. China Feed, 2019(24): 51-54. (in Chinese) |
| [10] |
魏琳, 芦天怡, 王利锋, 等. 芪归补血颗粒对环磷酰胺致小鼠免疫功能降低的影响[J]. 中药材, 2021, 44(4): 966-969. WEI L, LU T Y, WANG L F, et al. Effects of Qigui buxue granules on immune function decrease in mice induced by cyclophosphamide[J]. Journal of Chinese Medicinal Materials, 2021, 44(4): 966-969. (in Chinese) |
| [11] |
LUO J, YANG C F, LUO X, et al. Chlorogenic acid attenuates cyclophosphamide-induced rat interstitial cystitis[J]. Life Sci, 2020, 254: 117590. DOI:10.1016/j.lfs.2020.117590 |
| [12] |
安妮, 于洋, 李敬双. 紫锥菊提取物对肉鸡生长性能、屠宰性能和免疫功能的影响[J]. 饲料研究, 2021, 44(7): 42-45. AN N, YU Y, LI J S. Effect of Echinacea extract on growth performance, slaughter performance and immune function of broilers[J]. Feed Research, 2021, 44(7): 42-45. (in Chinese) |
| [13] |
GURBUZ E, BALEVI T, KURTOGLU V, et al. Effects of Echinacea extract on the performance, antibody titres, and intestinal histology of layer chicks[J]. Br Poult Sci, 2010, 51(6): 805-810. DOI:10.1080/00071668.2010.528753 |
| [14] |
KIM J W, CHOI J S, SEOL D J, et al. Immunomodulatory effects of kuseonwangdogo-based mixed herbal formula extracts on a cyclophosphamide-induced immunosuppression mouse model[J]. Evid Based Complement Alternat Med, 2018, 2018: 6017412. |
| [15] |
JIE D, GAO T T, SHAN Z S, et al. Immunostimulating effect of polysaccharides isolated from Ma-Nuo-Xi decoction in cyclophosphamide-immunosuppressed mice[J]. Int J Biol Macromol, 2020, 146: 45-52. DOI:10.1016/j.ijbiomac.2019.12.042 |
| [16] |
MAASS N, BAUER J, PAULICKS B R, et al. Efficiency of Echinacea purpurea on performance and immune status in pigs[J]. J Anim Physiol Anim Nutr, 2005, 89(7-8): 244-252. DOI:10.1111/j.1439-0396.2005.00501.x |
| [17] |
罗俊峰, 董海兵, 陈洪亮, 等. 紫锥菊颗粒对肉鸡生产性能的影响[J]. 中国兽医学报, 2019, 39(7): 1385-1387. LUO J F, DONG H B, CHEN H L, et al. The effects of Echinacea granule on the performance of broilers[J]. Chinese Journal of Veterinary Science, 2019, 39(7): 1385-1387. (in Chinese) |
| [18] |
YUN L Y, WU T, LI Q, et al. Dietary supplementation with purified wheat germ glycoprotein improve immunostimulatory activity in cyclophosphamide induced Balb/c mice[J]. Int J Biol Macromol, 2018, 118: 1267-1275. DOI:10.1016/j.ijbiomac.2018.06.199 |
| [19] |
KAUFMANN S H E. Immunology's coming of age[J]. Front Immunol, 2019, 10: 684. DOI:10.3389/fimmu.2019.00684 |
| [20] |
冯焱, 赵恒寿, 刘佳斌, 等. 环磷酰胺对肉仔鸡免疫机能的影响[J]. 饲料博览, 2007, 19(19): 31-34. FENG Y, ZHAO H S, LIU J B, et al. Effects of cyclophosphamide on immunity function of broilers[J]. Feed Review, 2007, 19: 31-34. (in Chinese) |
| [21] |
宋红卫, 李秀菊, 岳立. 紫锥菊提取物对肉仔鸡免疫功能的影响[J]. 饲料博览, 2014(6): 7-10. SONG H W, LI X J, YUE L. Effects of Echinacea purpurea extract on the immune function of broilers[J]. Feed Review, 2014(6): 7-10. (in Chinese) |
| [22] |
ZHOU L D, ZHANG Q H, ZHANG Y, et al. The shiitake mushroom-derived immuno-stimulant lentinan protects against murine malaria blood-stage infection by evoking adaptive immune-responses[J]. Int Immunopharmacol, 2009, 9(4): 455-462. DOI:10.1016/j.intimp.2009.01.010 |
| [23] |
KAWAI T, AKIRA S. TLR signaling[J]. Semin Immunol, 2007, 19(1): 24-32. DOI:10.1016/j.smim.2006.12.004 |
| [24] |
CAMPOLO M, PATERNITI I, SIRACUSA R, et al. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson's diseases in vivo model[J]. Brain Behav Immun, 2019, 76: 236-247. DOI:10.1016/j.bbi.2018.12.003 |
| [25] |
ZHU W W, XU R Y, DU J Y, et al. Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw[J]. FASEB J, 2019, 33(4): 5208-5219. DOI:10.1096/fj.201801791RR |
| [26] |
FERREIRA L, NISHINO F A, FERNANDES S G, et al. Epididymal embryonic development harbors TLR4/NFKB signaling pathway as a morphogenetic player[J]. J Reprod Immunol, 2022, 149: 103456. DOI:10.1016/j.jri.2021.103456 |
| [27] |
SU Q, LI L, SUN Y H, et al. Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury[J]. Cell Physiol Biochem, 2018, 47(4): 1497-1508. DOI:10.1159/000490866 |
| [28] |
VIATOUR P, MERVILLE M P, BOURS V, et al. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation[J]. Trends Biochem Sci, 2005, 30(1): 43-52. DOI:10.1016/j.tibs.2004.11.009 |
| [29] |
LIU N, ZHANG G X, NIU Y T, et al. Anti-inflammatory and analgesic activities of indigo through regulating the IKKβ/IκB/NF-κB pathway in mice[J]. Food Funct, 2020, 11(10): 8537-8546. DOI:10.1039/C9FO02574J |
| [30] |
YANG F, LIAO J Z, YU W L, et al. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken[J]. Ecotoxicol Environ Saf, 2020, 200: 110715. DOI:10.1016/j.ecoenv.2020.110715 |
| [31] |
ZHANG R L, GUO R, LIU Q, et al. Selenium deficiency via the TLR4/TRIF/NF-κB signaling pathway leading to inflammatory injury in chicken spleen[J]. Biol Trace Elem Res, 2021, 199(2): 693-702. DOI:10.1007/s12011-020-02173-0 |
| [32] |
YANG S B, SHAN C L, MA X, et al. Immunomodulatory effect of Acanthopanax senticosus polysaccharide on immunosuppressed chickens[J]. Poult Sci, 2021, 100(2): 623-630. DOI:10.1016/j.psj.2020.11.059 |
| [33] |
WANG H, XU L, YU M M, et al. Glycosaminoglycan from Apostichopus japonicus induces immunomodulatory activity in cyclophosphamide-treated mice and in macrophages[J]. Int J Biol Macromol, 2019, 130: 229-237. DOI:10.1016/j.ijbiomac.2019.02.093 |
| [34] |
鲁振国, 李美娣, 武力, 等. 玉竹多糖和板蓝根多糖对环磷酰胺致雏鸡免疫抑制的调节作用研究[J]. 中兽医医药杂志, 2021, 40(1): 74-77. LU Z G, LI M D, WU L, et al. Study on the regulatory effect of Polygonatum odoratum polysaccharide and Isatis indigotica polysaccharide on the immunosuppression of chickens induced by cyclophosphamide[J]. Journal of Traditional Chinese Veterinary Medicine, 2021, 40(1): 74-77. (in Chinese) |
| [35] |
ZHANG H H, LANG W Y, WANG S Y, et al. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway[J]. Int Immunopharmacol, 2020, 88: 106974. DOI:10.1016/j.intimp.2020.106974 |
| [36] |
RAICEVIC S, CUBRILO D, ARSENIJEVIC S, et al. Oxidative stress in fetal distress: potential prospects for diagnosis[J]. Oxidat Med Cell Long, 2010, 3(3): 214-218. DOI:10.4161/oxim.3.3.12070 |
(编辑 范子娟)



