2. 青岛博隆基因工程公司,青岛 266041
2. Qingdao Bolong Genetic Engineering Co., Ltd., Qingdao 266041, China
禽腺病毒-Ⅰ群(fowl adenoviruses,FAdVs) 感染与多种疾病有关,如肌胃糜烂、心包积液综合征(hydropericardium syndrome,HPS) 和包涵体肝炎(inclusion body hepatitis,IBH)[1]。根据病毒的分子结构特征,FAdVs可分为5个种,分别为A、B、C、D和E;根据血清交叉中和反应,可分为12个血清型(FAdVs 1~7、8a、8b、9~11)。其中,肌胃糜烂主要与FAdV-1相关,尽管也有零星报道临床肌胃损伤病例中分离到FAdV-8a和FAdV-8b[2-4];HPS主要由FAdV-4引起;IBH与FAdV-2、-8a、-8b和-11相关[5]。近年来,FAdVs感染呈现全球增长趋势,给肉鸡养殖业造成严重的经济损失[5]。
2007-2017年的调查表明,我国主要流行FAdV-4、-8a、-8b和-11[6],且目前主要流行的血清型FAdVs与IBH和HPS相关。其中,2007—2014年,FAdV-11为主要流行血清型,引起IBH[7];从2014年7月以来,FAdV-4引起的HPS大量发生[8];2017年,临床分离的FAdVs以FAdV-8b为主[7]。目前,我国FAdVs的致病性研究主要集中到FAdV-4 [9],其他血清型FAdVs的致病性多见于国外报道,且报道结果并不一致,如Steer等[10]报道FAdV-8b感染SPF鸡引起IBH,其死亡率为7%,但Matos等[11]报道FAdV-8b感染SPF肉鸡和蛋鸡的死亡率分别为100%和20%。这表明同一血清型FAdVs不同毒株对SPF鸡的致病性并不完全一致[5]。
垂直传播被认为是FAdVs的重要生物学特性,但并不是所有血清型FAdVs均可引起鸡胚发生明显损伤,如FAdV-2和FAdV-11感染11日龄SPF鸡胚不发生死亡且剖检无明显病变,但也有报道FAdV-11具有较高的鸡胚致死率[12],FAdV-4和FAdV-8对鸡胚的致死率为100%[13-14]。这也表明,同一血清型FAdVs不同毒株对SPF鸡胚的致病性并不完全一致。
鉴于同一血清型FAdVs不同毒株对SPF鸡胚和鸡的致病性存在差异,且我国主要流行的血清型FAdV-4、-8a、-8b和-11的致病性未见详细研究报道,故本研究拟对这4种血清型FAdVs感染鸡胚和鸡的比较病理学进行研究,以期为FAdVs发病机制的研究提供基础,为FAdVs感染的防控措施的制定提供依据。
1 材料与方法 1.1 毒株FAdV毒株KU981149(FAdV-4)、MF573907(FAdV-8a)、MF573909(FAdV-8b)和MF573929(FAdV-11)由本实验室分离、鉴定和保存。
1.2 主要试剂与实验动物病毒基因组DNA提取试剂盒、小量胶回收试剂盒、多功能DNA纯化回收试剂盒、克隆载体pMD19-T、DL2000 DNA Marker和感受态细胞DH5α均购自TaKaRa公司、DMEM/F-12 1∶1营养液购自Hyclone公司、胎牛血清购自Gibco公司。
12日龄SPF鸡胚和10日龄SPF鸡均购自北京梅里亚维通实验动物技术有限公司。
1.3 4种血清型毒株组织半数感染量测定取4瓶(25 cm2)LMH细胞,待细胞生长到约占瓶底面积的90%时,弃掉培养液,用PBS冲洗3次后,加入0.25%胰酶500 μL室温消化3 min,消化完成后,加入一定量含10%胎牛血清、1%青链霉素双抗的DMEM/F-12反复吹打,使细胞悬液中细胞量为(2~3)×105个·mL-1。取96孔细胞培养板,每孔加入细胞悬液100 μL,置于二氧化碳培养箱中37 ℃培养24 h。培养完成后倒出培养液,每孔加入用含2%胎牛血清、1%青链霉素双抗的DMEM/F-12 10倍比稀释的细胞毒100 μL,每个稀释度接种8孔,留阴性对照(加入不含细胞毒的DMEM/F-12)两排,置于二氧化碳培养箱中37 ℃,培养7 d。记录病变孔数,按Reed-Muench法计算组织半数感染量(the 50% tissue culture infective dose,TCID50) [15]。
1.4 4种血清型毒株感染SPF鸡胚12日龄SPF鸡胚150枚,随机分为5组,每组30枚,分别为FAdV-4组、FAdV-8a组、FAdV-8b组、FAdV-11组和对照组。各感染组鸡胚经绒毛尿囊膜途径接种105TCID50 300 μL病毒液,对照组接种等量的DMEM/F-12。将鸡胚置于孵化器中孵化,每天检查鸡胚发育情况,弃去接种后24 h内死亡鸡胚,接种后7 d统计各组鸡胚死亡率,测定存活鸡胚质量,观察胚体变化。
1.5 4种血清型毒株感染SPF鸡150只10日龄SPF鸡随机分为5组,每组30只,分别经腿部肌肉途径感染300 μL 102TCID50 FAdV-4、600 μL 105TCID50FAdV-8a、600 μL 105TCID50 FAdV-8b、600 μL 105TCID50 FAdV-11和600 μL DMEM/F-12。每天观察SPF鸡状态,于感染后第5天统计各组死亡率,并每组随机处死7只SPF鸡进行病理剖检,记录各组病变并进行评分,无明显病变为0,轻度损伤为1~2,中度损伤为3,严重损伤为4~5,各组SPF鸡各器官评分进行相加。取重要组织和主要免疫器官用10%福尔马林固定,进行HE染色和病理组织学观察。
1.6 4种血清型毒株感染SPF鸡主要脏器病毒载量测定应用DNA提取试剂盒根据操作步骤提取各血清型FAdVs病毒DNA,根据Meulemans等[16]报道的FAdVs Hexon基因扩增引物进行PCR扩增,扩增产物连接pMD19-T载体16 ℃过夜,后转入DH5α感受态细胞中。经测序验证后,正确的样品大量摇菌培养后提取质粒,紫外分光光度计测定质粒浓度,并按照公式计算DNA拷贝数。将质粒样品稀释,用ddH2O将质粒样品依次稀释成1×1010~ 1×103 copies·μL-1,将8个样品梯度作为标准品模板进行荧光定量PCR,得到相应的Ct值。以Ct值作为纵坐标,相应模板浓度的对数作为横坐标,制作标准曲线。同时制作熔解曲线,以检验荧光定量PCR程序的准确性。同时制作熔解曲线,以检验荧光定量PCR程序的准确性。
130只10日龄SPF鸡随机分为5组,FAdV-4组50只,其余各组每组20只,分别经腿部肌肉途径感染300 μL 102TCID50 FAdV-4、600 μL 105TCID50 FAdV-8a、600 μL 105TCID50 FAdV-8b、600 μL 105TCID50FAdV-11和600 μL DMEM/F-12。于感染后第3、5、7天每组随机处死5只,采集心、肝、肾、胰、脾和法氏囊保存于-70 ℃。提取各组织总核酸进行荧光定量PCR测定,反应条件95 ℃ 5 min;35个循环,95 ℃ 10 s,55 ℃ 15 s和72 ℃ 20 s;参照试验建立的标准曲线对检测结果进行比较分析。
1.7 统计分析数据采用SPSS 21.0软件进行分析,多组差异比较采用单因素方差分析,以P < 0.05为差异显著,P < 0.01为差异极显著。
2 结果 2.1 4种血清型毒株TCID50LMH细胞在96孔细胞培养板中经病毒接种后观察7 d,记录出现细胞病变的孔数。根据细胞病变的孔数,根据Reed-Muench法计算,FAdV-4、FAdV-8a、FAdV-8b和FAdV-11的TCID50分别为1×10-8.51·100 μL-1、1×10-7.91·100 μL-1、1×10-5.11·100 μL-1和1×10-7.33·100 μL-1。
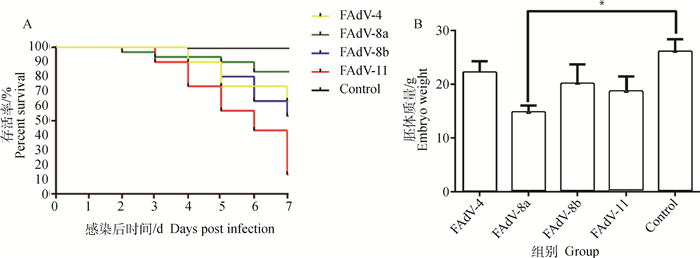
2.2 4种血清型毒株感染SPF鸡胚的研究结果4种血清毒株感染鸡胚后,FAdV-8a感染组鸡胚最早开始死亡;感染后7 d FAdV-8a感染组死亡率达到16.67%,FAdV-11感染组达到86.67%,FAdV-4感染组为40%,FAdV-8b感染组为46.67%,对照组无死亡(图 1A)。鸡胚感染后7 d,各感染组存活鸡胚的质量均低于对照组,其中,FAdV-4感染组质量减少最少,FAdV-8a感染组质量显著低于对照组(P < 0.05)(图 1B)。
|
A.鸡胚存活率;B.鸡胚胚体质量(*. P < 0.05) A. Survival rate of embryo; B. Embryo body weights (*. P < 0.05) 图 1 FAdV-4、-8a、-8b和-11感染组鸡胚感染后7 d的存活率和鸡胚胚体质量 Fig. 1 Survival rate and body weights of SPF embryonated chicken eggs infected with different FAdV serotypes at 7 dpi |
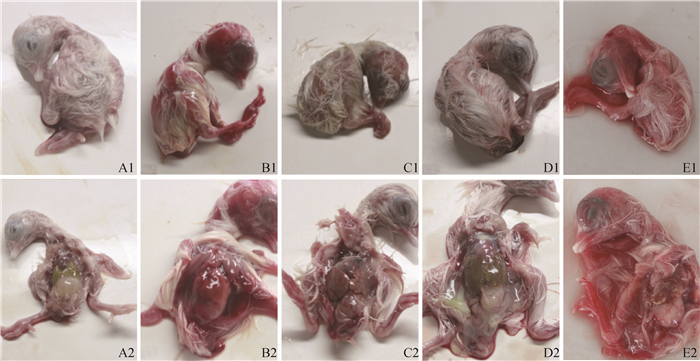
除胚体大小外,各感染组鸡胚出现相似的变化,均出现胚体出血、蜷缩;胚肝出血、坏死、胆汁淤积,对照组鸡胚无明显变化(图 2)。
|
A1、A2.对照组;B1、B2.FAdV-4感染组;C1、C2.FAdV-8a感染组;D1、D2.FAdV-8b感染组;E1、E2.FAdV-11感染组 A1, A2. Mock infection; B1, B2. FAdV-4 infection; C1, C2. FAdV-8a infection; D1, D2. FAdV-8b infection; E1, E2. FAdV-11 infection 图 2 FAdV-4、-8a、-8b和-11感染组鸡胚眼观病变 Fig. 2 Gross lesions of SPF embryonated chicken eggs infected with different FAdV serotypes |
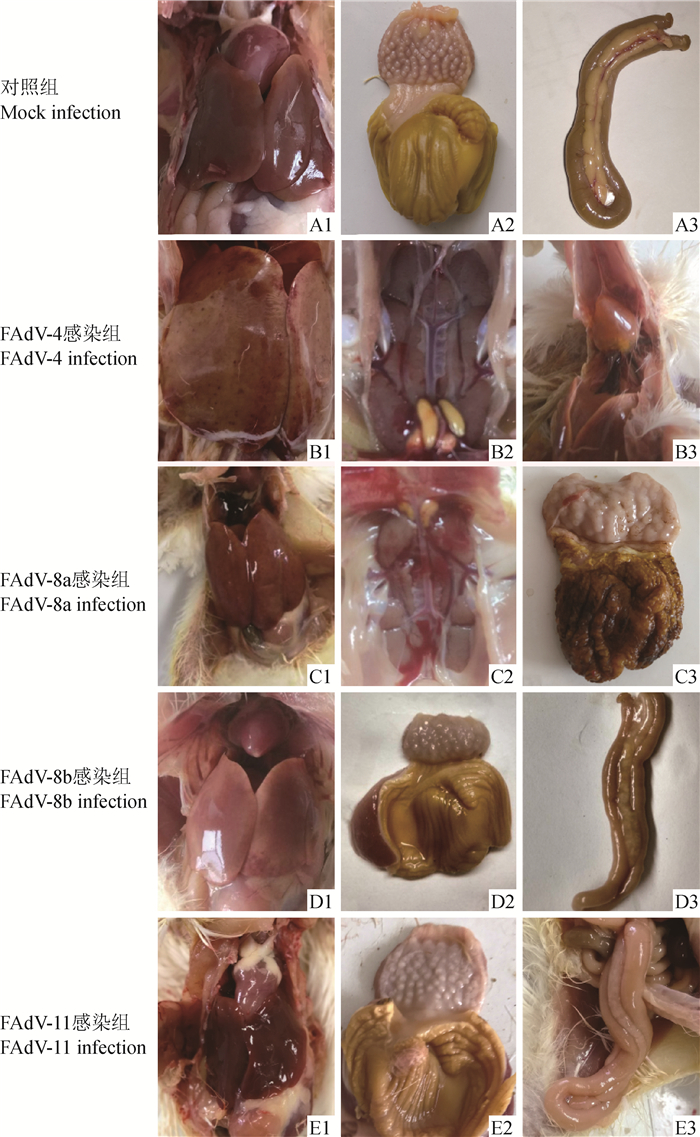
FAdV-4感染组SPF鸡在感染后2 d出现被毛蓬乱,FAdV-8b感染组SPF鸡在感染后4 d出现精神沉郁现象。感染后5 d,FAdV-4感染组SPF鸡死亡率达53.3%,而其他组均无死亡。剖检可见,除FAdV-11感染组外,其他病毒感染SPF鸡后均出现了肝脂肪变性、肿胀和出血,且FAdV-4感染组最明显;FAdV-4感染组和FAdV-8a感染组SPF鸡明显出现肾肿大、颜色苍白;FAdV-8b感染组和FAdV-11感染组出现胰腺坏死。此外,FAdV-4感染组、FAdV-8a感染组和FAdV-8b感染组分别出现明显的心包积液、肌胃糜烂和腺胃肿胀现象;对照组SPF鸡各器官均无明显病变(图 3)。
|
A1、A2、A3.对照组肝、肌胃腺胃和胰;B1、B2、B3. FAdV-4感染组肝、肾和心;C1、C2、C3. FAdV-8a感染组肝、肾和肌胃腺胃;D1、D2、D3. FAdV-8b感染组肝、肌胃腺胃和胰;E1、E2、E3. FAdV-11感染组肝、肌胃腺胃和胰 Liver (A1), gizzard and proventriculus (A2) and pancreas (A3) of the Mock infection chickens; Liver (B1), kidney (B2) and heart (B3) of the FAdV-4 infected chickens; Liver (C1), kidney (C2) and gizzard and proventriculus (C3) of the FAdV-8a infected chickens; Liver (D1), gizzard and proventriculus (D2) and pancreas (D3) of the FAdV-8b infected chickens; Liver (E1), gizzard and proventriculus (E2) and pancreas (E3) of the FAdV-11 infected chickens 图 3 FAdV-4, -8a, -8b和-11感染组SPF鸡主要器官眼观病变 Fig. 3 Gross characteristic lesions of organs in SPF chickens infected with different FAdV serotypes |
对各组SPF鸡器官病变进行评分,FAdV-4感染组SPF鸡心、肝和肾损伤较明显,FAdV-11感染组肝和胰腺出现明显损伤,FAdV-8b感染组胰腺、腺胃出现明显损伤;FAdV-8a感染组和FAdV-8b感染组肝和肾损伤评分相似(表 1)。
|
|
表 1 FAdV-4、-8a、-8b和-11感染组SPF鸡主要器官损伤评价分数和 Table 1 Total Lesion scores of lesions in organs of chickens infected with FAdV-4, -8a, -8b and -11 |
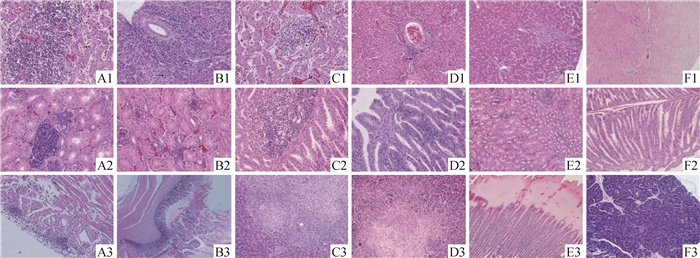
组织学观察发现各感染组SPF鸡肝出现相似变化,肝淤血、肝细胞变性和坏死,变性的肝细胞内出现核内包涵体,坏死区域出现大量炎性细胞浸润,浸润的细胞主要为淋巴细胞和巨噬细胞;此外,FAdV-11感染组血管周围出现炎性细胞浸润,FAdV-8a感染组部分胆管周围出现炎性细胞浸润,对照组肝组织无明显病变(图 4 A1、B1、C1、D1和E1)。
|
A1、A2、A3.FAdV-4感染组肝、肾和心;B1、B2、B3. FAdV-8a感染组肝、肾和肌胃;C1、C2、C3.FAdV-8b感染组肝、腺胃和胰;D1、D2、D3. FAdV-11感染组肝、腺胃和胰;E1、E2、E3.对照组肝、肾和肌胃;F1、F2、F3.对照组心、腺胃和胰。放大倍数:A1~2、B1~2、C1~2、D2均为400×; A3、B3、C3、D1、D3、E1~3、F1~3均为200× Liver (A1), kidney (A2) and heart (A3) of chicken infected with FAdV-4; Liver (B1), kidney (B2) and Ventriculus (B3) of chicken infected with FAdV-8a; Liver (C1), proventriculus (C2) and pancreas (C3) of chicken infected with FAdV-8b; Liver (D1), proventriculus (D2) and pancreas (D3) of chicken infected with FAdV-11; Liver (E1), kidney(E2), ventriculus(E3), heart(F1), proventriculus (F2) and pancreas (F3) of chicken infected with DMEM/F-12. A1-2, B1-2, C1-2, D2 were taken at 400× magnification; A3, B3, C3, D1, D3, E1-3, F1-3 were taken at 200× magnification 图 4 FAdV-4、-8a、-8b和-11感染组SPF鸡主要器官病理组织学变化(HE, 200×,400×) Fig. 4 Histological characteristic lesions of tissues from SPF chickens infected with different FAdV serotypes(HE, 200×, 400×) |
FAdV-4、FAdV-8a和FAdV-8b感染组SPF鸡肾损伤相似,主要出现轻度的淤血和肾小管上皮细胞变性,肾间质有大量淋巴细胞浸润;此外,FAdV-4和FAdV-8a感染组出现肾小球炎,对照组肾组织无明显病变(图 4 A2、B2、E2)。FAdV-8b感染组SPF鸡腺胃管状腺上皮细胞坏死,间质大量炎性细胞浸润(图 4 C2);FAdV-11感染组腺胃管状腺间质有炎性细胞浸润(图 4 D2),对照组腺胃无明显病变(图 4 F2)。
FAdV-4感染组SPF鸡心外膜明显增厚、肌纤维断裂,存在水肿和大量炎性细胞浸润现象,对照组心无明显病变(图 4 A3、F1)。FAdV-8a感染组肌胃出现明显损伤,角质层破坏,固有层内混杂有细胞碎片和异嗜性粒细胞,对照组肌胃无明显损伤(图 4 B3、E3)。FAdV-8b和FAdV-11感染组SPF鸡胰腺腺泡细胞坏死,有大量吞噬细胞和淋巴细胞浸润,对照组胰腺无明显病变(图 4 C3、D3、F3)。
免疫器官中感染组SPF鸡脾白髓出现弥漫性实质细胞稀疏现象,FAdV-4感染组SPF鸡脾出现淋巴细胞局灶性增生,FAdV-8a感染组脾红髓内出现嗜伊红染物质,FAdV-8b感染组和FAdV-11感染组出现广泛的实质细胞坏死(图 5 A1、B1、C1和D1)。各感染组SPF鸡胸腺均出现明显损伤,皮质和髓质区域的淋巴细胞减少(图 5 A2、B2、C2和D2)。FAdV-8b感染组和FAdV-11感染组胸腺髓质胸腺小体增多(图 5 C2、D2),且FAdV-11感染组出现轻度淤血现象(图 5 D2)。FAdV-4感染组、FAdV-8a感染组和FAdV-11感染组滤泡髓质淋巴细胞明显缺失(图 5 A3、B3和D3)。FAdV-8b感染组法氏囊滤泡内细胞坏死,淋巴细胞局灶性增生(图 5 C3),FAdV-8b感染组和FAdV-11感染组法氏囊出现轻度充血(图 5B3和5D3)。对照组SPF鸡脾、胸腺和法氏囊无明显病变(图 5 E1、E2和E3)。
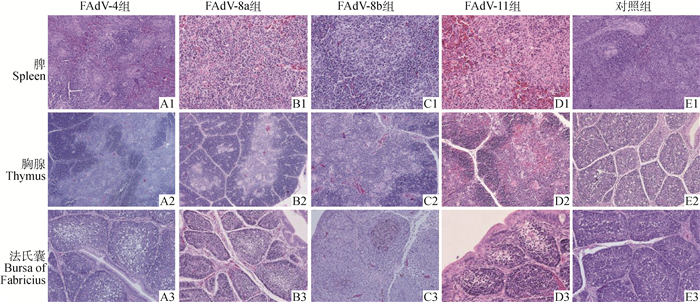
|
放大倍数:A1~3、B2~3、C2~3、D2、E1~3均为200×; B1、C1、D1、D3均为400× A1-3, B2-3, C2-3, D2, E1-3 were taken at 200× magnification; B1, C1, D1 and D3 were taken at 400× magnification 图 5 FAdV-4、-8a、-8b和-11感染组SPF鸡脾、胸腺和法氏囊病理组织学变化(HE, 200×,400×) Fig. 5 Histological lesions of immune organs of SPF chicken infected with different FAdV serotypes(HE, 200×, 400×) |
除FAdV-8a感染组外,FAdV-4感染组SPF鸡心(图 6A) 和肾(图 6C)病毒载量最高,FAdV-8b感染组和FAdV-11感染组SPF鸡胰腺(图 6D)病毒载量高于FAdV-4感染组。FAdV-4感染组SPF鸡心、肝(图 6B)和肾病毒载量在感染后第5天高于第3、7天,这与剖检时观察到的病理损伤程度相一致。FAdV-8b感染组和FAdV-11感染组SPF鸡脾(图 6E)和法氏囊(图 6F)病毒载量在感染后第3天高于FAdV-4感染组,且法氏囊病毒载量在各个时间点均高于FAdV-4感染组。FAdV-8a感染组SPF鸡各器官病毒载量均较低(图 6),这可能与该毒株的生物特性有关,有待进一步研究。
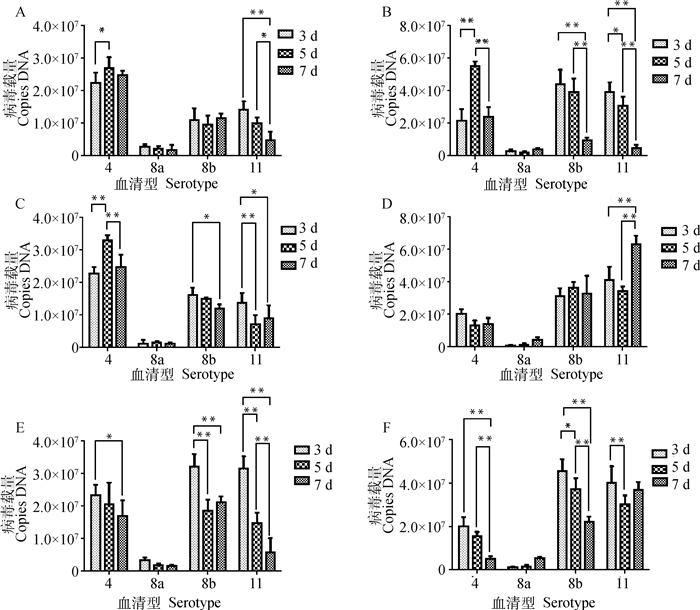
|
A.心;B.肝;C.肾;D.胰腺;E.脾;F.法氏囊。*.P < 0.05; **.P < 0.01 A. Heart; B. Liver; C. Kidney; D. Pancreas; E. Spleen; F. Bursa of Fabricius. *.P < 0.05; **.P < 0.01 图 6 FAdV-4、-8a、-8b和-11感染组SPF鸡主要器官病毒载量 Fig. 6 Viral load in the ograns of SPF chicken infected with different FAdV serotypes |
目前,FAdVs大部分血清型毒株感染鸡都会造成肝损伤或心包积液等,且临床感染在多个国家呈现增加趋势,给家禽养殖业造成巨大经济损失[5]。尤其是2014年以来,我国出现FAdV-4感染引起HPS,其死亡率高达20%~70%[17];虽然研究报道FAdV-8a、-8b和-11感染SPF鸡出现IBH,不会造成死亡[10, 18-19],但临床IBH严重程度通常由于鸡传染性法氏囊病毒或鸡传染性贫血病毒等免疫抑制性病原混合感染的影响而各异,且近年来临床病死鸡中FAdVs的分离率明显增加[7]。鉴于不同血清型FAdVs感染给我国养鸡业造成严重影响,有必要对我国主要流行血清型FAdVs对鸡的致病性进行详细研究。
FAdVs毒株可通过鸡胚肝细胞、鸡胚肾细胞、LMH细胞或鸡胚进行分离,本研究应用LMH细胞进行病毒的增殖和病毒滴度的测定,LMH细胞感染后出现明显的细胞病变。研究报道,FAdVs在鸡胚中增殖并不一定会引起明显病变[12],本研究不同血清型FAdVs感染鸡胚后均出现明显死亡,且存活鸡胚的胚体质量明显降低,其中,FAdV-11感染鸡胚死亡率达86.67%,FAdV-8a感染鸡胚死亡率最低为16.67%;FAdV-8a感染对鸡胚胚体质量影响最严重,而FAdV-4感染最轻。不同血清型FAdVs感染鸡胚后出现相似的损伤现象,与Schachner等[5]的报道一致,胚体蜷缩和出血,肝出血、坏死、胆汁淤积等现象[13]。垂直传播被认为是FAdVs感染的主要特征,这表明,与其他血清型毒株相比,种鸡感染FAdV-11、-8a后会导致鸡胚的孵化率降低或出现弱雏。
自从2014年以来,大量研究报道集中于FAdV-4引起HPS[17, 20-22]。本研究为确保FAdV-4感染后SPF鸡出现明显症状,但不会在感染后1~2 d内死亡,进行前期试验研究确定病毒感染量,感染后的死亡率达53.3%,其他血清型FAdVs感染不引起SPF鸡死亡。肝和肾为FAdVs感染损伤的主要器官,出现肝炎和肾小球肾炎[5]。4种血清型FAdVs感染SPF鸡后,FAdV-4感染鸡肝和肾损伤最明显,FAdV-11感染鸡最轻微,且损伤程度与病毒载量多少相一致。尽管FAdV-4对SPF鸡的致病性最强,但也不能忽略FAdV-8a和FAdV-11对鸡胚的致病性。综合本研究结果,认为不同血清型FAdVs对鸡胚和鸡的致病性并不一致,即对鸡胚的致病性强并不代表对鸡的致病性强。
除肝和肾的损伤外,不同血清型毒株具有其损伤的特异靶器官。FAdV-4感染后导致SPF鸡出现心包腔积液,心外膜和心肌间间隙增宽,充满炎性细胞,这被认为是HPS的重要病理特征[5, 14]。肌胃糜烂被认为是FAdV-1感染的重要特征,本研究发现FAdV-8a感染鸡出现明显鸡肌胃糜烂,且异嗜性粒细胞浸润,这与日本和埃及的两项研究报道相一致[2, 19]。腺胃炎是FAdV-8b感染SPF鸡的重要特征,这与前期实验室从临床腺胃炎病例种分离到FAdV-8b的结果相一致。FAdV-8b和FAdV-11感染鸡出现胰腺多灶状坏死,Nakamura等[23]曾报道FAdV-4感染肉鸡胰腺出现白色坏死灶,Okuda等[3]报道从临床无明显眼观病变鸡的胰腺分离到FAdV-8。胰腺、腺胃和肌胃的损伤必将影响到胰液和消化酶的分泌及消化功能,尽管这种损伤不会导致鸡的死亡,但将会影响鸡的饲料转化、生长发育,从而给养鸡业造成严重经济损失。
以前研究认为,引起HBH的几种血清型FAdVs并不会作为病原单独感染引起疾病,通常是在免疫抑制性病毒感染后继发感染而引起IBH[1]。实际上,大部分FAdVs不仅具有致病性,且具有免疫抑制的可能性。FAdV-4、-8a和-11感染后引起机体免疫器官,如脾、胸腺和法氏囊出现退行性变化[9, 12, 19]。本研究发现FAdV-8a、-8b和-11感染鸡引起的免疫器官内淋巴细胞缺失和损伤较FAdV-4更为严重,且除FAdV-8a外,脾和法氏囊的病毒载量高低与损伤程度相一致。这表明,FAdV-8a、-8b和-11感染尽管对鸡无致死性,但可导致鸡出现免疫抑制,这将会导致其他病原的继发感染,造成较大的经济损失。
本研究中与其他血清型FAdVs相比,FAdV-8a感染SPF鸡胚的死亡率最低,但对胚体质量影响最大;感染LMH细胞出现病变最早,免疫荧光染色病毒阳性信号出现最早(数据未报道),感染SPF鸡胚出现死亡最早,但各组织病毒载量最低。FAdV-8a毒株的这一特殊生物学特性有待于进一步研究。
4 结论首次对我国主要流行的4种血清型FAdVs对鸡的致病性进行比较病理学研究,结果表明,FAdVs对鸡胚的致病性强弱并不能代表其对SPF鸡的致病性;且与FAdV-4相比,虽然FAdV-8a、-8b和-11感染不会造成鸡的死亡,但对鸡胚具有较强的致死性和减轻胚体质量,造成鸡胰腺、腺胃、肌胃和免疫器官严重损伤。故FAdV-8a、-8b和-11对养鸡业的影响也应引起足够重视。
| [1] |
ABSALÓN A E, MORALES-GARZÓN A, VERA-HERNÁNDEZ P F, et al. Complete genome sequence of a non-pathogenic strain of Fowl Adenovirus serotype 11:minimal genomic differences between pathogenic and non-pathogenic viruses[J]. Virology, 2017, 501: 63-69. DOI:10.1016/j.virol.2016.11.006 |
| [2] |
MASE M, NAKAMURA K. Phylogenetic analysis of fowl adenoviruses isolated from chickens with gizzard erosion in Japan[J]. J Vet Med Sci, 2014, 76(11): 1535-1538. DOI:10.1292/jvms.14-0312 |
| [3] |
OKUDA Y, ONO M, SHIBATA I, et al. Pathogenicity of serotype 8 fowl adenovirus isolated from gizzard erosions of slaughtered broiler chickens[J]. J Vet Med Sci, 2004, 66(12): 1561-1566. DOI:10.1292/jvms.66.1561 |
| [4] |
ONO M, OKUDA Y, YAZAWA S, et al. Outbreaks of adenoviral gizzard erosion in slaughtered broiler chickens in Japan[J]. Vet Rec, 2003, 153(25): 775-779. |
| [5] |
SCHACHNER A, MATOS M, GRAFL B, et al. Fowl adenovirus-induced diseases and strategies for their control - a review on the current global situation[J]. Avian Pathol, 2018, 47(2): 111-126. DOI:10.1080/03079457.2017.1385724 |
| [6] |
LI C J, LI H Y, WANG D D, et al. Characterization of fowl adenoviruses isolated between 2007 and 2014 in China[J]. Vet Microbiol, 2016, 197: 62-67. DOI:10.1016/j.vetmic.2016.11.005 |
| [7] |
WANG J L, WANG S C, ZOU K Y, et al. Variant serotypes of fowl adenovirus isolated from commercial poultry between 2007 and 2017 in some regions of China[J]. Avian Dis, 2018, 62(2): 171-176. DOI:10.1637/11794-010618-Reg.1 |
| [8] |
LIU Y K, WAN W Y, GAO D S, et al. Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China[J]. Emerg Microbes Infec, 2016, 5(11): e117. |
| [9] |
NIU Y J, SUN Q Q, ZHANG G H, et al. Pathogenicity and immunosuppressive potential of fowl adenovirus in specific pathogen free chickens[J]. Poult Sci, 2017, 96(11): 3885-3892. DOI:10.3382/ps/pex206 |
| [10] |
STEER P A, SANDY J R, O'ROURKE D, et al. Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens[J]. Avian Pathol, 2015, 44(2): 106-113. DOI:10.1080/03079457.2015.1007919 |
| [11] |
MATOS M, GRAFL B, LIEBHART D, et al. Selected clinical chemistry analytes correlate with the pathogenesis of inclusion body hepatitis experimentally induced by fowl aviadenoviruses[J]. Avian Pathol, 2016, 45(5): 520-529. DOI:10.1080/03079457.2016.1168513 |
| [12] |
MENDELSON C, NOTHELFER H B, MONREAL G. Identification and characterization of an avian adenovirus isolated from a 'spiking mortality syndrome' field outbreak in broilers on the Delmarva Peninsula, USA[J]. Avian Pathol, 1995, 24(4): 693-706. DOI:10.1080/03079459508419108 |
| [13] |
ALEMNESH W, HAIR-BEJO M, AINI I, et al. Pathogenicity of fowl adenovirus in specific pathogen free chicken embryos[J]. J Comp Pathol, 2012, 146(2-3): 223-229. DOI:10.1016/j.jcpa.2011.05.001 |
| [14] |
MAZAHERI A, PRUSAS C, VOß M, et al. Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens[J]. Avian Pathol, 1998, 27(3): 269-276. DOI:10.1080/03079459808419335 |
| [15] |
REED L J, MUENCH H. A simple method of estimating fifty per cent endpoints[J]. Am J Epidemiol, 1938, 27(3): 493-497. DOI:10.1093/oxfordjournals.aje.a118408 |
| [16] |
MEULEMANS G, BOSCHMANS M, VAN DEN BERG T P, et al. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses[J]. Avian Pathol, 2001, 30(6): 655-660. DOI:10.1080/03079450120092143 |
| [17] |
ZHAO J, RUAN S F, GUO Y, et al. Serological and phylogenetic analysis indicating prevalence of fowl adenovirus in China[J]. Vet Rec, 2018, 182(13): 381. DOI:10.1136/vr.104517 |
| [18] |
HUANG Q H, MA X X, HUANG X Y, et al. Pathogenicity and complete genome sequence of a fowl adenovirus serotype 8b isolate from China[J]. Poult Sci, 2019, 98(2): 573-580. DOI:10.3382/ps/pey425 |
| [19] |
RADWAN M M, EL-DEEB A H, MOUSA M R, et al. First report of fowl adenovirus 8a from commercial broiler chickens in Egypt: molecular characterization and pathogenicity[J]. Poult Sci, 2019, 98(1): 97-104. DOI:10.3382/ps/pey314 |
| [20] |
ZHANG H W, JIN W J, DING K, et al. Genetic characterization of fowl adenovirus strains isolated from poultry in China[J]. Avian Dis, 2017, 61(3): 341-346. DOI:10.1637/11621-030817-RegR |
| [21] |
ZHANG T, JIN Q Y, DING P Y, et al. Molecular epidemiology of hydropericardium syndrome outbreak-associated serotype 4 fowl adenovirus isolates in central China[J]. Virol J, 2016, 13(1): 188. DOI:10.1186/s12985-016-0644-x |
| [22] |
LI P H, ZHENG P P, ZHANG T F, et al. Fowl adenovirus serotype 4:epidemiology, pathogenesis, diagnostic detection, and vaccine strategies[J]. Poult Sci, 2017, 96(8): 2630-2640. DOI:10.3382/ps/pex087 |
| [23] |
NAKAMURA K, TANAKA H, MASE M, et al. Pancreatic necrosis and ventricular erosion in adenovirus-associated hydropericardium syndrome of broilers[J]. Vet Pathol, 2002, 39(3): 403-406. DOI:10.1354/vp.39-3-403 |
(编辑 白永平)



