2. 贵州大学动物科学学院,贵阳 550025;
3. 贵州省农业科学院草业研究所,贵阳 550006
2. College of Animal Science, Guizhou University, Guiyang 550025, China;
3. Guizhou Institute of Prataculture, Guizhou Academy of Agricultural Sciences, Guiyang 550006, China
胚胎附植是哺乳动物妊娠建立的关键步骤[1]。尽管已知有许多信号分子参与胚胎附植过程,但针对胚胎附植前、后子宫角组织的生理机制变化很大程度上还未知。山羊(Capra hircus)胚胎附植属表面附植[2-3],具体过程包括胚胎从透明带孵出、定位、粘附、渗透4个具体过程,附植时胚胎不渗透到子宫基质中。研究发现,母羊排卵受精后第15天,胚胎由管状孕体延长发育为纤维状孕体,第16~17天孕体在子宫角进一步延长[4]并以子宫肉阜为粘附位点开始附植,此后孕体滋养层巨双核细胞与母体子宫内膜上皮细胞融合,在母体子宫内膜与孕体接触区域形成三核的母-胎杂交细胞,杂交细胞进一步与滋养层巨双核细胞融合形成多合胞体斑块,斑块间相互紧密连接形成子叶,最终形成子叶胎盘[5]。胚胎附植质量对后续妊娠质量具有直接影响,妊娠早期胚胎附植的任何问题都将极大地造成早期胚胎丢失或各种妊娠并发症的产生[6-7],导致妊娠失败[8],因此,胚胎的成功附植对繁殖率的提高具有重要意义。
子宫内膜和胚胎的同步发育对于成功植入都至关重要[9], 涉及一系列复杂的信号事件,包括子宫内膜容受性、免疫耐受性的建立等[10]。子宫内膜从不适应、不接受胚胎附植到接受胚胎可附植状态的改变叫做子宫内膜容受性的改变,它是激素、生长和转录因子、脂质介质和细胞因子等之间同步和相互作用的结果[11-14]。Zhang等[15]为探究萨能奶山羊妊娠早期子宫内膜容受性的形成机制,对妊娠第5和15天的子宫内膜进行转录组测序,共获得810个DEGs,对DEGs的生物信息学分析表明,妊娠早期子宫内膜的分子变化涉及许多生物学过程和途径,尤其是能量代谢和氨基酸代谢,并推测CXCL14、IGFBP3和LGALS15可能参与子宫内膜的发育。Song等[16]对处于前接受态和接受态的萨能奶山羊母体子宫内膜进行了miRNA表达分析,在前接受态和接受态分别检测到847和1 069个miRNAs,其中632个在2个阶段都有不同程度表达,hsa-miR-449a等基因和泛素介导的蛋白水解、细胞凋亡等途径可能在子宫内膜接受性形成中起重要作用。Xia等[17]对妊娠第17天体内受精和体外生产子宫内膜转录组测序,发现DEGs主要通过子宫内膜组织上细胞黏附/迁移和凋亡通路影响胚胎植入, 调控胚胎附植。
本研究对妊娠第15和30天山羊子宫角组织进行转录组测序,以期筛选山羊胚胎附植前、后子宫角生理变化的相关重要调控基因及通路,为揭示山羊胚胎附植阶段子宫角组织发育机制提供科学依据。
1 材料与方法 1.1 样品采集在贵州册亨海铭巍畜牧业开发有限公司选取体重相近((44.76 kg±3.49)kg)、健康的经产(2~4胎)努比亚母山羊16只,在同期发情、人工输精配种后的第15和30天分别随机选取3只羊,颈动脉放血处死,每只羊切下子宫角圆筒状区段后顺向为3份,分别用锡箔纸包好、标记后放入液氮中保存,立即送回实验室。第15天采样时先看子宫角整体形态,子宫轻度膨大肿胀确定为受孕羊进行采样;本试验第15天采样时第3只羊子宫整体较小,无膨大肿胀表现,判定为未孕羊舍弃,屠宰第4只受孕羊采集补齐3个重复。
1.2 RNA文库制备与子宫角组织Illumina测序从妊娠第15、30天两个时期样品中随机各选取3份子宫角组织进行RNA-Seq测序。按照制造商说明书,使用Trizol试剂(美国Invitrogen公司)从子宫角组织中提取RNA,使用NanoPhotometerⓇ分光光度计(IMPLEN, CA, USA)检测样品纯度,QubitⓇ3.0 Flurometer(Life Technologies, CA, USA)检测RNA样品浓度,安捷伦2100 RNA Nano 6000 Assay Kit(Agilent Technologies, CA, USA)检测RNA样品的完整性和浓度。RNA样品的A260/A280在1.9~2.1之间,RNA完整性(RIN)数值范围为8.6~10.0,对检测合格的mRNA选用带有Oligo(dT)的磁珠进行富集纯化。
整个转录组文库的制备和测序在Annoroad Gene Technology Corporation(中国,北京)进行。使用New England Biolabs Next、Ultra Directional RNA Library Prep Kit for Illumina(New England Biolabs,Ipswich,MA,USA),按照制造商的说明书构建整个转录组文库。使用BioAnalyzer 2100 system和定量PCR(qPCR)(Kapa Biosystems,Woburn,MA,USA)对文库进行质量控制和定量。质量合格的文库用Illumina HiSeqTM 2500(Illumina,San Diego,CA,USA)进行单个样品测序,并产生150 bp的配对末端读数。
1.3 差异表达基因及GO分析通过去除接头污染的reads、低质量的reads、含N比例大于5%的reads,clean Q30 bases rate(%)判断碱基质量,得到高质量的clean reads,再进行后续分析。使用Bowtie2(版本2.2.5)和具有默认参数的Tophat2(版本2.1.0)软件将所有样品的clean reads映射到Capra_hircus_ARS1参考基因组(https://www.ncbi.nlm.nih.gov/genome/10731?genome_assembly_id=281266, USDA ARS),以确定表达水平已鉴定的基因。使用Cufflinks软件(版本2.2.1)将来自每个样品的映射读数组装成转录物以构建、鉴定、估计TopHat比对结果的丰度,并使用FPKM(每百万fragments中比对到某一基因每千碱基长度的fragments数目)量化组装的转录物的丰度。设定|log2Fold change|≥ 1和q < 0.05的基因作为显著差异基因。
选取HISAT软件对过滤后的测序序列进行比对,并进行基因组定位分析,结合基因本体(Gene Ontology,GO)数据库对筛选的差异表达基因进行功能注释及富集分析。
1.4 荧光定量PCR验证为了验证RNA-Seq分析的可靠性,以β-actin基因为内参基因,随机选取了CCL21、ITPR3、VEGFD、TFRC、CYP19A1、STS等6个基因(3个上调,3个下调)进行qRT-PCR验证。依据GenBank中收录的山羊CCL21、ITPR3、VEGFD、TFRC、CYP19A1、STS基因序列及其mRNA序列,利用NCBI Primer-BLAST在线软件(https://www.ncbi.nlm.nih.gov/tools/primer-blast/)设计特异性荧光引物(表 1),引物由上海生工生物工程技术服务有限公司合成。
|
|
表 1 qRT-PCR引物信息 Table 1 qRT-PCR primers information |
提取D15和D30子宫角组织总RNA,随机选取6个差异表达基因进行qRT-PCR,每个基因3个生物学复孔。使用PrimeScript第一链cDNA合成试剂盒(ThermoFisher,美国)将来自6个样品的一部分(0.5 μg)DNase I处理的总RNA转化为单链cDNA。将cDNA模板用CFX 96 Real-Time PCR检测系统(Bio-Rad,Singapore)和SYBR Premix ExTaq TM(TaKaRa)扩增。qPCR试验采用20 μL的反应体系:10 μL的2×T5 Fast qPCR Mix(1×),正向和反向引物各0.8 μL(0.4 μmol·L-1),0.4 μL的50×ROX Reference Dye I(1×),1 μL的cDNA,7 μL的双蒸水。使用以下程序扩增模板:95 ℃持续15 s,60 ℃持续30 s,最后72 ℃持续20 s。
1.5 数据统计与分析根据2-△△Ct方法计算qRT-PCR所检测的DEGs在不同组织中的相对表达量,计算公式为:△△Ct=(Ct 目的基因-Ct内参基因)试验组-(Ct 目的基因-Ct内参基因)对照组,最后qRT-PCR和RNA-Seq数据结果均用“平均值±标准误”表示。
2 结果 2.1 高表达基因筛选将Illummina对D15和D30组织测序得到的结果进行数据库比对,设定FPKM≥0.5,表 2列出了表达量最高的10个基因,其中在D15时FPKM最高的3个基因分别为MGP、TMSB4X、TAGLN,在D30时FPKM最高的3个基因分别为TMSB4X、TAGLN、EEF1A1。
|
|
表 2 转录组D15、D30中表达量最高的10个基因 Table 2 The 10 highest expressed genes in the transcriptome D15 and D30 |
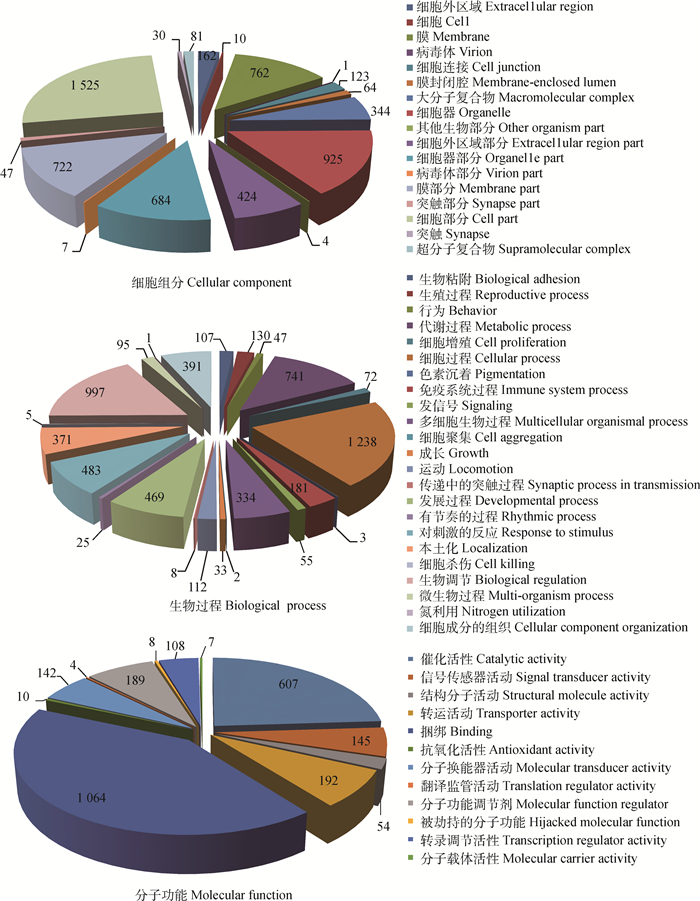
数据库比对结果设定参数:FPKM≥0.5,|log2 Fold change|≥1和q < 0.05,共筛选出2 000个差异表达基因(表 3),其中上调基因620个,下调基因1 380个,表 4列出了上调和下调的前10个高差异基因及其功能。应用DAVID v6.8对获得的2 000个差异表达基因进行GO功能聚类分析,共分为3大类52组(图 1),其中细胞组分17组(32.69%,17/52),生物过程23组(44.23%,23/52),分子功能12组(23.08%,12/52),获得细胞连接与增殖,生物粘附与调节,分子活性与转导等重要生物途径。
|
|
表 3 D15、D30组间比较差异表达基因数目 Table 3 The number of differentially expressed genes between D15 and D30 groups |
|
|
表 4 转录组D15和D30(D15 vs. D30)中的前10个上、下调基因及其功能 Table 4 Top 10 up- and down-regulated genes and their functions in the transcriptome D15 and D30(D15 vs. D30) |
|
饼状图数字表示基因富集数 The numbers in pie chart represent the numbers of genes enriched 图 1 D15和D30组差异表达基因GO分析 Fig. 1 GO analysis of differentially expressed genes between D15 vs. D30 |
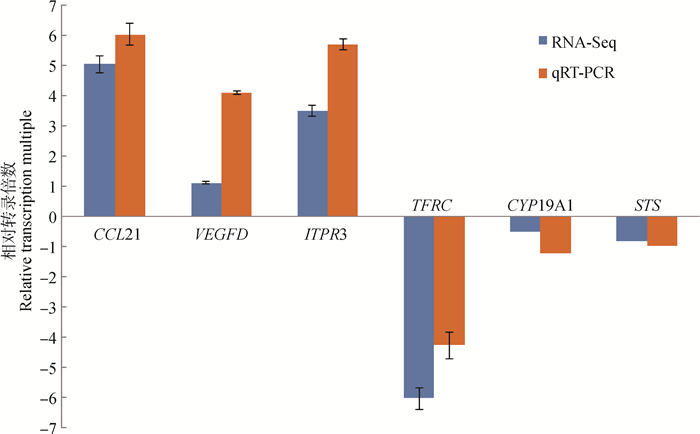
6个基因在D15和D30子宫角中表达变化趋势与RNA-Seq结果一致(图 2)。
|
图 2 差异表达基因的qRT-PCR验证 Fig. 2 qRT-PCR verification of differentially expressed genes |
胚胎附植是一个复杂的生理过程,努比亚山羊胚胎附植前后子宫角组织的变化及作用机理仍不明确。RNA-Seq是能对某一组织或器官在一定状态下获取所有转录本的技术,具有检测范围广、通量高、定量准确、重复性好等特点。本试验选用Illumina技术对胚胎与子宫建立联系前(D15)、后(D30)的子宫角组织进行测序,共筛选出7个与胚胎附植相关的基因。随机选取6个DEGs进行qRT-PCR验证,各DEGs表达趋势与转录组测序结果一致,说明RNA-Seq结果准确可靠。
MGP(matrix Gla protein)是一种维生素K依赖性细胞外基质分泌性蛋白,分子结构中有5个γ谷氨酰基残基,分子量为12 ku[18],在胚胎组织和癌细胞中富集,主要通过纤连蛋白中特定结合的位点增强纤连蛋白上的细胞附着,增加细胞-纤连蛋白的相互作用[19]。Correia等[20]研究发现,MGP表达可能与细胞迁移、分化有关,可能以某种方式参与了发育中鸡胚动脉和静脉树的重塑,可作为哺乳动物早期妊娠诊断的关键参考蛋白[21]。本试验中,MGP在D15子宫角组织中高表达,此时孕体与子宫肉阜开始粘附,MGP与纤连蛋白结合并增强纤连蛋白上细胞附着和扩散,细胞-纤连蛋白相互作用强烈,子宫角组织血管重塑活跃。
TAGLN是Transgelins家族中的3个亚型之一,分子量为22 ku,属于与肌动蛋白相关的调宁蛋白家族,分子结构特点是氨基序列的C末端调宁样重复序列和N末端存在单一CH同源性结构域。Transgelins家族作为肌动蛋白的结合蛋白,通过与肌动蛋白相互作用重构细胞骨架,定位信号分子,在多种生物学功能的调控中发挥作用[22-24]。Burmenskaya等[25]对输卵管不孕和试管婴儿成功的子宫内膜研究发现,TAGLN可作为子宫内膜两种功能状态的信息标记基因之一。TAGLN2是Transgelins家族成员之一,TAGLN2的抑制显著损害胚胎粘附和植入能力,明显降低人类滋养层细胞的侵袭和迁移能力[26],李瑞婷[27]也研究发现,TAGLN2与山羊胚胎着床有关,在胚胎粘附阶段高表达,与本试验D30较D15 TAGLN表达量升降趋势结果一致;同时,D30较D15 TAGLN表达量降低可能标志胚胎附植后与肌动蛋白作用减弱,但仍保持一定水平维持妊娠。
妊娠相关糖蛋白(PAGs)属于天冬氨酸蛋白酶基因家族,结构上氨基酸序列与组织蛋白酶D、组织蛋白酶E和胃蛋白酶的氨基酸序列有一半以上一致,都具有典型包含酶催化中心的两个双叶型三级结构[28]。PAGs是由反刍动物胎儿胎盘滋养层细胞合成分泌的一类蛋白质,在妊娠过程中具有粘附和保护胎儿的重要作用,是孕体存活的一个标志,在反刍动物妊娠早期血清、尿液[29]和奶[30]中均可检测到,可用于反刍动物的早期妊娠检测[31-33];在奶牛上已建立的bPAG可视化检测方法,对配种后第30天的奶牛通过血清进行妊娠诊断,准确率达到91.7%[34];在绵羊上,赵梦圆[21]认为PAG浓度高于42 ng·mL-1可作为绵羊早期妊娠诊断的阈值标准,Steckeler等[35]发现,在第42天时对妊娠母羊的判断准确率最高。本试验差异表达上调和下调幅度最大的前10个基因中有3个妊娠相关糖蛋白基因(PAG-3、PAG-8、PAG-12)在子宫角组织中D30相对于D15显著上调,提示从胚胎附植到发育D30期间,PAGs可能持续增强胚胎粘附牢固度来使妊娠持续、良好的进行,未发生流产等情况[36]。研究发现,PAGs表达、翻译在胚胎附植前就已经开始[37],与本试验结果PAGs在D15已经开始表达一致,同时,也提示PAGs可能在胚胎附植前的准备中发挥作用。
生长激素释放激素受体(GHRHR)属于G蛋白受体家族,具有7个跨膜结构域,通过与GHRH偶联激活腺苷酸环化酶,增加细胞内cAMP和Ca2+,促使生长激素分泌、细胞增殖,从而促进动物的生长发育[38]。在对胚胎时期鸡垂体的研究发现,GHRH mRNA的水平在第8天达到峰值,GHRHR在第12天大量表达,GH从第16天到第30天高水平表达[39]。本试验中,GHRHR在D15水平高于D30,推测在努比亚山羊胚胎发育过程中,子宫角组织GHRHR表达在D30以前就已经过了高峰期,但具体的高峰期出现和结束时间还需进一步细化确定。
IGFBP1是胰岛素样生长因子结合蛋白家族(IGFBPs)中6个同源蛋白之一,结构上可分为3个区段,N端、C端和L区,其中N端能与IGF相互作用。在对IGFBP1与孕早期大鼠子宫内膜和孕激素的研究中发现,IGFBP1可能在大鼠孕早期子宫内膜的蜕膜化、胎盘形成和胚胎生长发育中发挥作用[40],在对狒狒的研究中也发现,子宫内膜腺上皮中IGFBP1的存在可能调节着床过程中滋养层生长和渗透的自分泌和/或旁分泌[41],而本试验也发现,IGFBP1在努比亚山羊妊娠早期子宫角组织中表达,因此,推测IGFBP1在母体对胚胎附植的准备中发挥作用,促进孕早期子宫内膜蜕膜化,引导胎盘形成和胚胎生长。
综上所述,通过转录组测序数据共获得妊娠早期努比亚山羊子宫角中与胚胎附植相关的基因,为进一步认识山羊胚胎附植中关键蛋白的作用机理提供了依据,也为深入探讨山羊胚胎附植发生机理奠定了基础。
4 结论本研究筛选获得的基因MGP、TAGLN、PAG-3、PAG-8、PAG-12、GHRHR、IGFBP1可能在山羊胚胎附植中具有重要作用。
| [1] |
OJOSNEGROS S, SERIOLA A, GODEAU A L, et al. Embryo implantation in the laboratory: an update on current techniques[J]. Hum Reprod Update, 2021, 27(3): 501-530. DOI:10.1093/humupd/dmaa054 |
| [2] |
赵有璋. 羊生产学[M]. 第3版. 北京: 中国农业出版社, 2011. ZHAO Y Z. Sheep production[M]. 3rd ed. Beijing: China Agriculture Press, 2011. (in Chinese) |
| [3] |
秦鹏春. 哺乳动物胚胎学[M]. 北京: 科学出版社, 2001. QIN P C. Embryology of mammals[M]. Beijing: Science Press, 2001. (in Chinese) |
| [4] |
靳方圆. 山羊胚胎附植阶段子宫转录组测序及附植相关基因的筛选[D]. 哈尔滨: 东北农业大学, 2016. JIN F Y. Transcriptome sequencing of goat uterus at implantation and screening of implantation-related genes[D]. Harbin: Northeast Agricultural University, 2016. (in Chinese) |
| [5] |
WANGO E O, WOODING F B, HEAP R B. The role of trophoblastic binucleate cells in implantation in the goat: A morphological study[J]. J Anat, 1990, 171: 241-257. |
| [6] |
DEY S K. Fatty link to fertility[J]. Nature, 2005, 435(7038): 34-35. DOI:10.1038/435034a |
| [7] |
罗芳, 张萌, 李亚超, 等. 影响奶牛早期胚胎丢失的因素[J]. 畜牧兽医学报, 2020, 51(5): 907-913. LUO F, ZHANG M, LI Y C, et al. Factors associated with early embryo loss in dairy cows[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(5): 907-913. (in Chinese) |
| [8] |
SHEIKHANSARI G, POURMOGHADAM Z, DANAII S, et al. Etiology and management of recurrent implantation failure: a focus on intra-uterine PBMC-therapy for RIF[J]. J Reprod Immunol, 2020, 139(6): 103121. |
| [9] |
TEH W T, MCBAIN J, ROGERS P. What is the contribution of embryo-endometrial asynchrony to implantation failure?[J]. J Assist Reprod Genet, 2016, 33(11): 1419-1430. DOI:10.1007/s10815-016-0773-6 |
| [10] |
ACHACHE H, REVEL A. Endometrial receptivity markers, the journey to successful embryo implantation[J]. Hum Reprod Update, 2006, 12(6): 731-746. DOI:10.1093/humupd/dml004 |
| [11] |
张蓉, 赵乐, 杨海丽, 等. 细胞外囊泡调控哺乳动物胚胎附植研究进展[J]. 畜牧兽医学报, 2021, 52(5): 1154-1162. ZHANG R, ZHAO L, YANG H L, et al. Research progress of extracellular vesicles regulating mammalian embryo implantation[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(5): 1154-1162. (in Chinese) |
| [12] |
SAXTORPH M H, PERSSON G, HALLAGER T, et al. Are different markers of endometrial receptivity telling us different things about endometrial function?[J]. Am J Reprod Immunol, 2020, 84(6): e13323. |
| [13] |
XIA L J, MENG Q Y, XI J, et al. The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats[J]. Stem Cell Res Ther, 2019, 10(1): 244. DOI:10.1186/s13287-019-1326-6 |
| [14] |
MIRAVET-VALENCIANO J A, RINCON-BERTOLIN A, VILELLA F, et al. Understanding and improving endometrial receptivity[J]. Curr Opin Obstet Gynecol, 2015, 27(3): 187-192. DOI:10.1097/GCO.0000000000000173 |
| [15] |
ZHANG L, LIU X R, LIU J Z, et al. The developmental transcriptome landscape of receptive endometrium during embryo implantation in dairy goats[J]. Gene, 2017, 633: 82-95. DOI:10.1016/j.gene.2017.08.026 |
| [16] |
SONG Y X, AN X P, ZHANG L, et al. Identification and Profiling of microRNAs in Goat Endometrium during Embryo Implantation[J]. PLoS One, 2015, 10(4): e0122202. DOI:10.1371/journal.pone.0122202 |
| [17] |
XIA W, ZHANG X L, MIAO K, et al. Characterization and comparative analyses of transcriptomes for in vivo and in vitro produced peri-implantation conceptuses and endometria from sheep[J]. J Reprod Develop, 2016, 62(3): 279-287. DOI:10.1262/jrd.2015-064 |
| [18] |
SCHURGERS L J, UITTO J, REUTELINGSPERGER C P. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization.[J]. Trends Mol Med, 2013, 19(4): 217-226. DOI:10.1016/j.molmed.2012.12.008 |
| [19] |
NISHIMOTO S K, NISHIMOTO M. Matrix Gla protein binds to fibronectin and enhances cell attachment and spreading on fibronectin[J]. Int J Cell Biol, 2014, 2014: 807013. |
| [20] |
CORREIA E, CONCEIO N, CANCELA M L, et al. Matrix Gla Protein expression pattern in the early avian embryo[J]. Int J Dev Biol, 2016, 60(1-3): 71-76. |
| [21] |
赵梦圆. 绵羊早期妊娠诊断关键生殖激素及差异蛋白筛选[D]. 太谷: 山西农业大学, 2019. ZHAO M Y. Screening of Key Reproductive hormones and differential proteins in early pregnancy diagnosis of sheep[D]. Taigu: Shanxi Agricultural University, 2019. (in Chinese) |
| [22] |
NISHIMOTO S K, NISHIMOTO M. Matrix gla protein binds to fibronectin and enhances cell attachment and spreading on fibronectin[J]. Int J Cell Biol, 2014, 2014: 807013. |
| [23] |
VAKALOGLOU K M, MOURATIDOU M, KERAMIDIOTI A, et al. Differential expression of Drosophila transgelins throughout development[J]. Front Cell Dev Biol, 2021, 9: 648568. DOI:10.3389/fcell.2021.648568 |
| [24] |
TSUJI-TAMURA K, MORINO-KOGA S, SUZUKI S, et al. The canonical smooth muscle cell marker TAGLN is present in endothelial cells and is involved in angiogenesis[J]. J Cell Sci, 2021, 134(15): jcs254920. DOI:10.1242/jcs.254920 |
| [25] |
BURMENSKAYA O V, BOZHENKO V K, SMOLNIKOVA V Y, et al. Transcription profile analysis of the endometrium revealed molecular markers of the personalized 'window of implantation' during in vitro fertilization[J]. Gynecol Endocrinol, 2017, 33(1): 22-27. |
| [26] |
LIANG X L, JIN Y M, WANG H, et al. Transgelin 2 is required for embryo implantation by promoting actin polymerization.[J]. FASEB J, 2019, 33(4): 5667-5675. DOI:10.1096/fj.201802158RRR |
| [27] |
李瑞婷. Transgelin2(Tagln2)在山羊胚胎着床期子宫中的表达与调控[D]. 哈尔滨: 东北农业大学, 2015. LI R T. Expression and Regulation of tagln2 in goat uterus during peri-implantation period[D]. Harbin: Northeast Agricultural University, 2015. (in Chinese) |
| [28] |
XIE S C, LOW B G, NAGEL R J, et al. Identification of the major pregnancy-specific antigens of cattle and sheep as inactive members of the aspartic proteinase family[J]. Proc Natl Acad Sci U S A, 1991, 88(22): 10247-10251. DOI:10.1073/pnas.88.22.10247 |
| [29] |
金海, 赵拴平, 徐磊, 等. 肉牛妊娠初期血、尿中孕酮、妊娠相关糖蛋白、早孕因子的变化[J]. 中国牛业科学, 2018, 44(6): 8-11, 16. JIN H, ZHAO S P, XU L, et al. Changes of progesterone, pregnancy-associated glycoprotein and early pregnancy factors in blood and urine of beef cattle during early pregnancy[J]. China Cattle Science, 2018, 44(6): 8-11, 16. DOI:10.3969/j.issn.1001-9111.2018.06.003 (in Chinese) |
| [30] |
SINGH S P, NATESAN R, SHARMA N, et al. Assessment of pregnancy-associated glycoprotein profile in milk for early pregnancy diagnosis in goats[J]. Anim Biosci, 2021, 34(1): 26-35. DOI:10.5713/ajas.19.0399 |
| [31] |
HA A, MBK B, FS A, et al. Detection of twin pregnancies in ewes by pregnancy-associated glycoprotein assay and transabdominal ultrasonography- Science Direct[J]. Domest Anim Endocrinol, 2020, 72: 106399. DOI:10.1016/j.domaniend.2019.106399 |
| [32] |
DE MIRANDA E SILVA CHAVES C, DA COSTA R L D, DUARTE K M R, et al. Visual ELISA for detection of pregnancy-associated glycoproteins (PAGs) in ewe serum[J]. Theriogenology, 2017, 97: 78-82. DOI:10.1016/j.theriogenology.2017.04.026 |
| [33] |
FILHO R V O, FRANCO G A, REESE S T, et al. Using pregnancy associated glycoproteins (PAG) for pregnancy detection at day 24 of gestation in beef cattle[J]. Theriogenology, 2020, 141: 128-133. DOI:10.1016/j.theriogenology.2019.09.014 |
| [34] |
卢春霞, 刘长彬, 周平, 等. 酶联适配体可视化检测牛妊娠相关糖蛋白9(bPAG9)及其在奶牛早孕检测中的应用[J]. 农业生物技术学报, 2021, 29(7): 1407-1416. LU C X, LIU C B, ZHOU P, et al. Enzyme-linked aptamer assay for visual detection of bPAG9 and its application for pregnancy diagnosis in dairy cows (Bos taurus)[J]. Journal of Agricultural Biotechnology, 2021, 29(7): 1407-1416. (in Chinese) |
| [35] |
STECKELER P, WEBER F, ZERBE H, et al. Evaluation of a bovine visual pregnancy test for the detection of pregnancy-associated glycoproteins in sheep[J]. Reprod Dom Anim, 2019, 54(2): 280-288. DOI:10.1111/rda.13356 |
| [36] |
张昱, 王海东, 贺俊平, 等. 妊娠相关糖蛋白及其在反刍动物早期妊娠诊断中的应用[J]. 中国畜牧兽医, 2015, 42(3): 764-768. ZHANG Y, WANG H D, HE J P, et al. Research progress on pregnancy-associated glycoprotein and its application in early pregnancy diagnosis of ruminants[J]. China Animal Husbandry & Veterinary Medicine, 2015, 42(3): 764-768. (in Chinese) |
| [37] |
GARBAYO J M, SERRANO B, LOPEZ-GATIUS F. Identification of novel pregnancy-associated glycoproteins (PAG) expressed by the peri-implantation conceptus of domestic ruminants[J]. Anim Reprod Sci, 2008, 103(1-2): 120-134. DOI:10.1016/j.anireprosci.2006.12.002 |
| [38] |
GAYLINN B D, HARRISON J K, ZYSK J R, et al. Molecular cloning and expression of a human anterior pituitary receptor for growth hormone-releasing hormone[J]. Mol Endocrinol, 1993, 7(1): 77-84. |
| [39] |
WANG C Y, WANG Y, LI J, et al. Expression profiles of growth hormone-releasing hormone and growth hormone-releasing hormone receptor during chicken embryonic pituitary development[J]. Poult Sci, 2006, 85(3): 569-576. DOI:10.1093/ps/85.3.569 |
| [40] |
李尚为, 谭宗建, 韩字研, 等. IGFBP-1, IGFBP-2在围着床期大鼠子宫内膜中的表达及其与孕激素的关系[J]. 华西医科大学学报, 2002, 33(4): 592-595. LI S W, TAN Z J, HAN Z Y, et al. Expression of IGFBP-1 and IGFBP-2 in endometrium and their relationships with serum progesteron during peri-implantation in rat[J]. Journal of West China University of Medical Sciences, 2002, 33(4): 592-595. (in Chinese) |
| [41] |
FAZLEABAS A T, JAFFE R C, VERHAGE H G, et al. An insulin-like growth factor-binding protein in the baboon (Papio anubis) endometrium: synthesis, immunocytochemical localization, and hormonal regulation[J]. Endocrinology, 1989, 124(5): 2321-2329. DOI:10.1210/endo-124-5-2321 |
(编辑 郭云雁)



