2. 青藏高原动物遗传资源保护与利用国家教育部重点实验室, 成都 610041;
3. 四川省阿坝州畜牧科学研究所, 阿坝 623000
2. Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization of Ministry of Education, Chengdu 610041, China;
3. Animal Husbandry Science Institute of Aba Autonomous Prefecture, Aba 623000, China
牦牛(Bos grunniens)主要分布于青藏高原及毗邻高海拔地区,有“高原之舟”的称号。现有牦牛1 400多万头,其中,90%以上分布在我国境内。牦牛是青藏高原牧民赖以生存的必要物种,在严峻的自然生长条件下,为牧民提供了维持生产、生活等的必需品,如肉、乳、毛、皮等[1]。但是,牦牛的繁殖能力偏弱、生产性能低下、生长速度缓慢。青藏高原牧区生产方式比较落后,牦牛近亲繁殖的现象严重,许多品种逐渐出现退化。因其畜群周转的速度变慢,严重影响了牦牛群体的生产力水平。长期以来,牧民的不合理选种和繁育,导致畜群平均生产力极度下降、受胎率低、繁殖力下降,畜群结构极其不合理[2]。此外,不同区域牦牛的用途存在一定差异,有些区域以乳用为主,期望群体中雌性个体为主导;有些区域以肉用为主,期望群体中雄性个体为主导。由此可见,以实际生产需求为导向,开展牦牛的性别控制意义重大,能显著提升牦牛的繁殖效率和市场价值。
性别控制技术可根据生产需求获得不同性别的后代。目前,效果最好且广泛应用的分离方法是流式细胞仪分选法[3]。流式细胞仪分离X、Y精子的本质是根据精子大小以及物理或化学性质进行的筛选[4]。由于X、Y精子的DNA含量存在差异,使之结合不同量的荧光染料Hoechst33342,当精子顺次排列通过流式细胞仪检测时,精子头部所携带的荧光染料被激发光束激发产生荧光,X精子结合的荧光染料较多,流式细胞仪为X、Y精子加上正电荷或负电荷,根据电荷的正负把X、Y精子分开[5]。Bathgate等[6]利用流式细胞仪分离羊X、Y精子,得到了羊的性控精液。Sales等[7]利用流式细胞仪分离牛X、Y精子,采用人工授精技术检测分离精子的纯度,X精子受精后母犊的产出率为83%,Y精子受精后公犊产出率为90%。随着技术的进步,利用流式细胞仪分离精子的方法已经被多个国家商业化应用。但这项技术也存在分离效率低、分离成本高、受胎率偏低等问题。利用流式细胞仪分离精子时会浪费掉大量精子[8],其中包括多个方面的因素:分离过程中约有30%的精子因为不准确的定向无法产生正常的荧光信号而被丢弃;约有15%的精子因为X、Y精子的信号峰之间可能会产生重叠,不能准确地区分而被弃;精子分离的各个步骤中也会造成15%~20%的损失[9]。这些因素都造成了分离精子效率较低,同时会在分离过程中浪费部分优质精子。此外,许多研究表明,分离精子的受胎率低于普通精液,可能原因是分离、冷冻等过程会对精子造成一定的损伤,影响精子受精的能力[9-10]。
因此,如何提高流式细胞仪分离精子的效率、减少分离精子的浪费、提高分选后精子的活力等,对推广性控精液具有重要的意义。食物色素诱惑红属于单偶氮染料,由6-羟基-5-(2-甲氧基-5-甲基-4-磺酸盐-苯偶氮)-2-萘磺酸盐组成。它以暗红色粉末或颗粒的形式存在,易溶于水,被广泛应用于食品着色。低浓度诱惑红(< 1.25 mg·L-1)对细胞无显著毒性,且无法进入细胞膜完整的细胞。本试验以流式细胞仪分选牦牛X、Y精子为基础,优化并建立高效的分选体系。通过添加诱惑红染料,与Hoechst33342共孵育精子细胞,显著提高了分选的效率。借助分子生物学技术对分选纯度进行鉴定,并结合体外受精技术,对分选后的X、Y精子分别进行受精,检测分选后的精子活力及进一步鉴定分选的纯度。本研究建立了一套高效的分选技术体系,为后期牦牛性控精液的制备及生产奠定了基础。
1 材料与方法 1.1 主要仪器与试剂主要仪器:流式细胞分选仪(MoFlo XDP,美国),CO2培养箱(Thermo,美国),PCR仪(Eppendorf,德国),荧光定量PCR仪(Bio-Rad,美国),倒置荧光显微镜(Olympus,日本),精子分析系统(AndroVision,德国)。主要试剂:DNA Taq聚合酶和DNA Marker均购自北京天根有限公司,荧光染料Hoechest33342(Sigma,美国)、诱惑红(Sigma,美国)、体外受精及胚胎培养液均购自Vitrolife公司(瑞典)。
1.2 精子细胞悬液的制备本研究采用商品化牦牛冻精,将精液冷冻细管放入42 ℃恒温水浴中解冻30 s,用PBS稀释精液,400目滤器过滤。用Falcon管预先装入精子稀释液,使精子终浓度约为1.5×108个·mL-1。加入不同量(10 μL和20 μL)染色液(5 g·L-1Hoechest33342),用移液器轻轻吹打均匀,在温度38.5 ℃、CO2浓度为5.5%、饱和湿度的CO2培养箱中孵育。
1.3 流式细胞仪分选精子本试验采用贝克曼流式细胞仪MoFlo XDP进行分选,所有分选步骤均严格遵守无菌操作。选用70 μm喷嘴,鞘液压力60 psi,分选流速控制在1×104个·s-1。将预先染色的精子样品放入进样仓,当X精子峰和Y精子峰在FL2通道的变异系数(CV值)小于2%时,开始精子分选。分别接收1×107个X、Y精子,将精子接收管取出放入38.5 ℃的CO2培养箱中备用。
1.4 单精子PCR扩增鉴定分选所得的X、Y精子在显微镜下分离,分别随机各取50个,将单个精子加入1 μL碱性裂解液(500 mmol·L-1 KOH,125 mmol·L-1 DTT)中,置于65 ℃的水浴中裂解10 min,再加入10 μL的中和液(900 mmol·L-1 Tris·HCl)混合均匀,即完成了基因组裂解及DNA提取,以雌、雄牦牛血液DNA为对照组。参考GenBank中已经公布普通牛(Bos taurus)的SRY及GAPDH基因序列,通过Premier 5.0软件设计引物,具体引物信息见表 1。引物由南京金斯瑞生物科技公司合成。PCR反应体系为15 μL:模板1.0 μL,上、下游引物各1.0 μL,Premix TaqTM DNA聚合酶7.5 μL,ddH2O 4.5 μL。PCR扩增条件:95 ℃预变性30 s;95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸40 s,30个循环;72 ℃ 5 min。采用2%琼脂糖凝胶进行PCR电泳检测。
|
|
表 1 引物序列及PCR反应条件 Table 1 Primer sequences and PCR reaction conditions |
精子的活力决定受精率,对分选的牦牛X、Y性控精子进行活力检测,与分选前的精子比较,判定分选对精子活力的影响。从液氮罐中取出未分选的牦牛性控精液,在42 ℃恒温水浴锅中解冻,将精液从冻精细管中转至1.5 mL离心管中,放置于38.5 ℃的CO2培养箱中平衡5 min。显微镜加热板开启预热,用移液器从平衡后的精液中取10 μL精液滴于载玻片,盖上盖玻片,置于精子分析系统下检测,记录数据并统计精子活力。
1.6 诱惑红孵育精子细胞精子悬浮液的制备同上,染色液Hoechest33342与5 μmol·L-1的诱惑红在38.5 ℃、CO2浓度为5.5%,饱和湿度的CO2培养箱中共孵育。1 000 r ·min-1 离心收集精子细胞,PBS洗涤3次。用稀释液进行精子稀释,采用步骤“1.3”优化的分选条件进行X、Y精子分选。分选后用PCR技术检测分选精子的纯度,精子分析系统检测分选后精子的活力。
1.7 分选后精子的体外受精牦牛卵母细胞收集及体外培养方法参考文献[2]。将成熟的COCs转移至预平衡的G-IVF清洗微滴中,清洗2~3次,然后,转移至预平衡的G-IVF受精微滴(50 μL)中培养,每个受精微滴放置20~30枚COCs。采用上游法精子获能,将分选后的X、Y精子移入盛有预平衡的精子获能液的底部,置于CO2培养箱中获能40~50 min。获能后取适量上清转移至新离心管中,1 500 r ·min-1离心5 min后弃上清,吹打混匀底部剩余的50 μL液体。将适量精子加入放置有COCs的G-IVF受精微滴中,精子浓度约为1×104个·mL-1,随后转移至CO2培养箱中。共孵育20 h后,捡出受精的卵母细胞,转移至预平衡的G-1微滴(50 μL)中培养。每个微滴放置10~20枚卵母细胞,放置于38 ℃、CO2浓度为5.5%、饱和湿度的CO2培养箱中培养,72 h后换液。培养24与168 h后分别观察并统计卵裂率和囊胚形成率。
1.8 胚胎性别鉴定收集各组囊胚期的胚胎,胚胎基因组的提取及PCR反应参照“1.4”。扩增产物用2%琼脂糖凝胶电泳检测,统计各组胚胎的性别结果。
1.9 数据统计与分析所有组别的试验至少重复3次,SPSS 19.0进行ONE-WAY ANOVA检验,用SSR法进行多重比较,P<0.05认为差异显著,P<0.01认为差异极显著。
2 结果 2.1 优化牦牛X、Y精子的分选条件检测不同染色时间、不同染色液用量对牦牛精子分选的影响。结果显示,当Hoechest33342染色液浓度为5 g·L-1、精子染色时间为40 min、染色液用量10 μL时,X、Y区域占有比例最佳,形成两个明显的峰(图 1中蓝色峰)。此外,由表 2可知,用10 μL Hoechest33342染色40 min的精子回收效率最高,精子活力与20 μL Hoechest33342染色20 min组相当,显著高于其他组(P<0.05)。
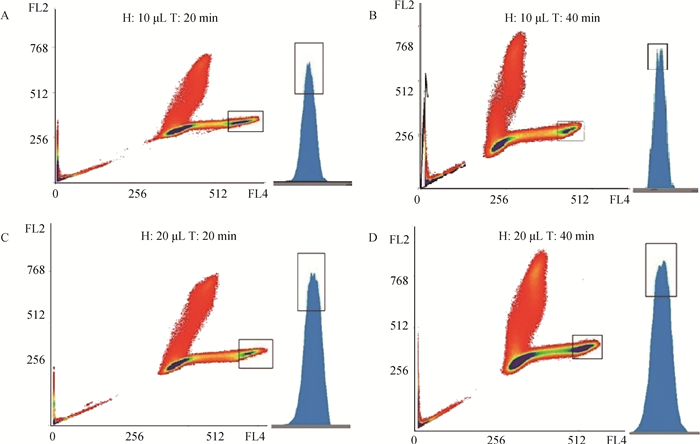
|
H为Hoechest33342,T为染色时间。FL2为Hoechest33342染色后水平角度(0度)的信号值,FL4为Hoechest33342染色后垂直角度(90度)的信号值。红色为细胞散点图,颜色越深,代表细胞数量越多;蓝色为分选峰值图,出现两个峰(即X、Y峰),两个峰越明显,分选效果越好 H represents Hoechest33342, T represents dyeing time. FL2 represents the fluorescence signal value of horizontal angle, FL4 represents the fluorescence signal value of vertical angle. The red figure is the scatter plot of cells, and the darker color means the more the number of cells; The blue figure is the sorting peak diagram, the more obvious the two peaks (X, Y peak), the better the sorting effect 图 1 染色时间与染色液用量对精子分选的影响 Fig. 1 The effects of dyeing time and dyeing solution dosage on the sperm sorting |
|
|
表 2 Hoechest33342染色液用量和染色时间对精子分选回收率及活力的影响 Table 2 The effects of Hoechest33342 dyeing solution dosage and dyeing time on sorted sperm recovery rate and motility |
对分选后的牦牛X、Y精子进行单精子裂解,提取总DNA,应用Y染色体特异基因SRY的引物检测精子的性别,电泳结果见图 2。Y精子出现294和208 bp两条特异性条带,而X精子只有唯一的208 bp条带。根据电泳结果,统计分选后精子的纯度,结果见表 3。
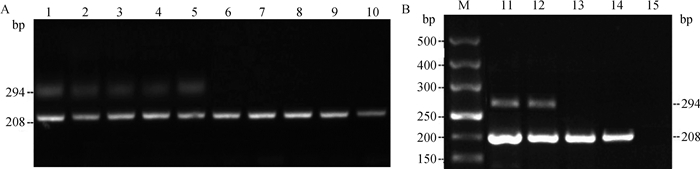
|
1~5. 分选后Y精子;6~10. 分选后X精子;M. DNA相对分子质量标准;11~12.公牦牛血液DNA;13~14.母牛血液DNA;15.阴性对照组 1-5. Sorted Y sperm of yak; 6-10. Sorted X sperm of yak; M. DNA marker; 11-12. DNA of male yak blood; 13-14. DNA of female yak blood; 15. Negative control 图 2 分选后X、Y单精子PCR扩增结果 Fig. 2 The results of sorted X and Y sperm PCR amplification |
|
|
表 3 分选后牦牛X、Y单精子PCR扩增统计结果 Table 3 The results of PCR amplification of yak X, Y sperm after sorting |
随机抽取分选后牦牛X、Y精子及未分选精子,进行活力检测(图 3)。结果显示,未添加诱惑红处理的精子,通过分选后得到的X和Y精子,其精子活力显著低于分选前的精子(P < 0.05)。添加5 μmol·L-1诱惑红与Hoechest33342共同孵育精子细胞,显著提高了精子的活力,与分选前精子活力无显著差异(P>0.05)。
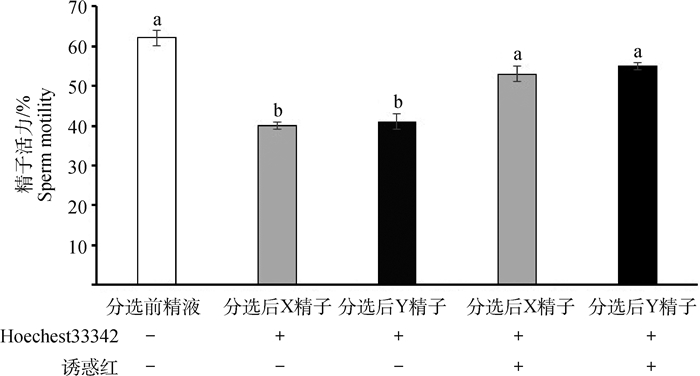
|
不同小写字母表示差异显著(P < 0.05) The different lowcase letters indicate significant different (P < 0.05) 图 3 诱惑红对分选后精子活力的影响 Fig. 3 The effects of Allura Red on sorted yak sperm motility |
根据试验设计,连续采集3次牦牛卵巢,将每次收集得到的卵母细胞随机分成3组,分别用分选后的X精子、分选后的Y精子和未分选的精子受精,记录受精率和胚胎发育结果(表 4)。结果显示,分选后的X与Y精子组的受精率及囊胚形成率略低于未分选组,但差异不显著(P>0.05)。利用已优化的PCR扩增体系对各组胚胎性别进行鉴定,结果显示,各组的胚胎性别与精子纯度基本吻合。
|
|
表 4 精子发育潜能统计结果 Table 4 The statistics results of yak sperm developmental potential |
经济动物性别的控制与畜牧业的发展有着密不可分的关系,是长期以来科研人员的研究焦点之一[11]。目前,利用性控精液进行人工授精和性控胚胎生产,是比较有效的控制方法[12]。通过此项技术,可以满足正常的畜牧生产需要,有效地提高生产效益[13]。本研究利用流式细胞仪进行牦牛X、Y精子分离,优化并建立了一套高效的分选技术体系。运用分子生物学技术,检测了分选后精子及胚胎的性别,进一步验证了分选的效率与纯度。本研究通过添加诱惑红,与Hoechest33342共孵育精子细胞,能显著提高分选后的精子活力。
染料孵育是精子分选的一个重要技术环节[14-15]。最常用的染色剂为Hochest33342,这种染料可以穿透细胞质膜且具有水溶性质,采用非嵌入方式与DNA双链上的小沟结合后,最大激发波长为350 nm[16]。然而,同一物种的不同细胞类型或不同物种的相同细胞类型对Hochest33342的耐受性存在一定差异。用16.2 mmol·L-1的Hochest33342孵育马的精子90 min,其分选后精子的活力和分选效率最佳[17]。Clulow等[18]利用Hochest3334染色马的精液,使其终浓度为55~90 μmol·L-1,显著提高了分选效果及分选后精子的抗冻能力。曾有权等[19]用5 g·L-1 Hochest3334和25 g·L-1食物色素处理猪精子约30 min,对分选后的精子进行人工授精,X精液产雌性率91.67%,Y精液产雄性率100%。在其他哺乳动物的精子分选中也发现Hoechst33342的最适浓度有一定差异,且分选后的后代动物会出现一些异常[20]。尽管有关细胞毒性或由Hoechst33342染色精子导致胚胎发育异常的报道较少,但是精子暴露在Hoechst33342中的遗传毒性效应已达成共识。本研究采用5 g·L-1 Hoechest33342染色液10 μL孵育牦牛精子细胞40 min后,其分选效果最佳。此外,本研究在精子分选前用食物色素诱惑红与Hoechest33342共孵育牦牛精子细胞。诱惑红只着色因膜破损的精子,将Hoechest33342荧光信号掩盖,导致激发光减弱,使该部分精子在分选时被废弃[3, 21]。只有膜完整的精子才进入分离程序,可以显著提高分选后有活力精子的比例。
用于评价精子分离后纯度及活力的方法较多,有出生后代的性别比例鉴定法、胚胎性别鉴定评价法、流式细胞仪重分析评价法、FISH法与PCR评价法等[22-23]。Fugger等[15]研究表明,利用流式细胞仪分选人的X、Y精子并用于体外受精,统计其后代的性别,其中X精子受精所产后代女性占92.9%,Y精子受精所产后代男性占73%。Johnson[22] 利用流式细胞仪分选猪精液,得到的性控精液用于人工受精,其所产后代母猪比例占74%,公猪比例占83%。此方法存在试验周期长,不能及时对结果优化的问题[24]。用胚胎性别鉴定来评价精子分离的纯度,此方法准确度较高,许多试验均采取这种方法来评价精子分选的纯度[25]。Monk和Handyside[26]用胚胎细胞学评价鼠的性别控制,结果显示,雌鼠准确率为91%,雄鼠准确率为100%。但因显微切割的要求较高,不宜推广使用[27]。胚胎分子生物学评价法依据对胚胎细胞中的Y染色体上特有的SRY性别基因的检测,以评价分选精子的纯度[28]。检测到特异信号的判定为雄性,未检测到特异信号的则判定为雌性[29-30]。此方法具有敏感、准确、快速等特点,是目前最常见的方法之一[31-34]。本研究中,采用单精子PCR法对分选精子进行了纯度分析,结果显示,X精子纯度率为94%,Y精子纯度率为96%。为进一步验证分选后精子的活力,将分选后的精子进行体外受精,结果显示,分选前后牦牛精子的发育潜能差异不显著,且胚胎的性别与期望值基本吻合,表明所建立的流式细胞仪分选体系可靠。
4 结论本研究通过流式细胞分选仪成功建立了高效的牦牛X、Y精子分选体系。分选后的X、Y精子纯度可达94%以上,分选后精子活力达到54%。本研究对牦牛产业的发展具有重要意义,尤其是为后期牦牛的性控精液制备及生产奠定了基础。
| [1] | FU M, CHEN Y B, XIONG X R, et al. Establishment of mammary gland model in vitro: culture and evaluation of a yak mammary epithelial cell line[J]. PLoS One, 2014, 9(12): e113669. DOI: 10.1371/journal.pone.0113669 |
| [2] | XIONG X R, LAN D L, LI J, et al. Selenium supplementation during in vitro maturation enhances meiosis and developmental capacity of yak oocytes[J]. Anim Sci J, 2018, 89(2): 298–306. DOI: 10.1111/asj.12894 |
| [3] | PICCIN J S, DOTTO G L, VIEIRA M L G, et al. Kinetics and mechanism of the food dye FD&C Red 40 adsorption onto chitosan[J]. J Chem Eng Data, 2011, 56(10): 3759–3765. DOI: 10.1021/je200388s |
| [4] | MARI G, BUCCI D, LOVE C C, et al. Effect of cushioned or single layer semen centrifugation before sex sorting on frozen stallion semen quality[J]. Theriogenology, 2015, 83(6): 953–958. DOI: 10.1016/j.theriogenology.2014.11.031 |
| [5] | FILHO M F S, GIROTTO R, ABE E K, et al. Optimizing the use of sex-sorted sperm in timed artificial insemination programs for suckled beef cows[J]. J Anim Sci, 2012, 90(6): 1816–1823. DOI: 10.2527/jas.2011-4523 |
| [6] | BATHGATE R, MACE N, HEASMAN K, et al. Birth of kids after artificial insemination with sex-sorted, frozen-thawed goat spermatozoa[J]. Reprod Domest Anim, 2013, 48(6): 893–898. DOI: 10.1111/rda.12182 |
| [7] | SALES J N S, NEVES K A L, SOUZA A H, et al. Timing of insemination and fertility in dairy and beef cattle receiving timed artificial insemination using sex-sorted sperm[J]. Theriogenology, 2011, 76(3): 427–435. DOI: 10.1016/j.theriogenology.2011.02.019 |
| [8] | GRIMES R W, IRELAND J J. Relationship of macroscopic appearance of the surface of bovine ovarian follicles, concentrations of steroids in follicular fluid, and maturation of oocytes in vitro[J]. Biol Reprod, 1986, 35(3): 725–732. DOI: 10.1095/biolreprod35.3.725 |
| [9] | HABERMANN F A, WINTER A, OLSAKER I, et al. Validation of sperm sexing in the cattle (Bos taurus) by dual colour fluorescence in situ hybridization[J]. J Anim Breed Genet, 2005, 122(S1): 22–27. DOI: 10.1111/j.1439-0388.2005.00488.x |
| [10] | SIRARD M A, BILODEAU S. Granulosa cells inhibit the resumption of meiosis in bovine oocytes in vitro[J]. Biol Reprod, 1999, 43(5): 777–783. |
| [11] | PONTES J H F, MELO STERZA F A, BASSO A C, et al. Ovum pick up, in vitro embryo production, and pregnancy rates from a large-scale commercial program using Nelore cattle (Bos indicus) donors[J]. Theriogenology, 2011, 75(9): 1640–1646. DOI: 10.1016/j.theriogenology.2010.12.026 |
| [12] | CHRENEK P, BOULANGER L, HEYMAN Y, et al. Sexing and multiple genotype analysis from a single cell of bovine embryo[J]. Theriogenology, 2001, 55(5): 1071–1081. DOI: 10.1016/S0093-691X(01)00467-8 |
| [13] | WELCH G R, JOHNSON L A. Sex preselection: laboratory validation of the sperm sex ratio of flow sorted X- and Y-sperm by sort reanalysis for DNA[J]. Theriogenology, 1999, 52(8): 1343–1352. DOI: 10.1016/S0093-691X(99)00221-6 |
| [14] | MOROTTI F, SANCHES B V, PONTES J H F, et al. Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle[J]. Theriogenology, 2014, 81(5): 696–701. DOI: 10.1016/j.theriogenology.2013.12.002 |
| [15] | FUGGER E F, BLACK S H, KEYVANFAR K, et al. Births of normal daughters after MicroSort sperm separation and intrauterine insemination, in-vitro fertilization, or intracytoplasmic sperm injection[J]. Hum Reprod, 1998, 13(9): 2367–2370. DOI: 10.1093/humrep/13.9.2367 |
| [16] | MISSIO D, FOLCHINI N P, LEIVAS F G, et al. Reduction in Percoll volume increases recovery rate of sex-sorted semen of bulls without affecting sperm quality and early embryonic development[J]. Anim Reprod Sci, 2018, 192: 146–153. DOI: 10.1016/j.anireprosci.2018.03.002 |
| [17] | DA SILVA C M B, ORTEGA-FERRUSOLA C, MORRELL J M, et al. Flow cytometric chromosomal sex sorting of stallion spermatozoa induces oxidative stress on mitochondria and genomic DNA[J]. Reprod Dom Anim, 2016, 51(1): 18–25. DOI: 10.1111/rda.12640 |
| [18] | CLULOW J R, BUSS H, EVANS G, et al. Effect of staining and freezing media on sortability of stallion spermatozoa and their post-thaw viability after sex-sorting and cryopreservation[J]. Reprod Dom Anim, 2012, 47(1): 1–7. DOI: 10.1111/j.1439-0531.2007.01010.x |
| [19] |
曾有权, 陆阳清, 杨小金, 等. 使用流式细胞仪分离精子进行仔猪性别控制的研究[J]. 畜牧兽医学报, 2012, 43(7): 1163–1169.
ZENG Y Q, LU Y Q, YANG X J, et al. Sex-preselected piglets derived from sexed sperm by flow cytometry sorting[J]. Acta Veterinaria et Zootechnica Sinica, 2012, 43(7): 1163–1169. (in Chinese) |
| [20] | GARNER D L. Hoechst 33342: the dye that enabled differentiation of living X-and Y-chromosome bearing mammalian sperm[J]. Theriogenology, 2009, 71(1): 11–21. DOI: 10.1016/j.theriogenology.2008.09.023 |
| [21] | SCHENK J L, CRAN D G, EVERETT R W, et al. Pregnancy rates in heifers and cows with cryopreserved sexed sperm: effects of sperm numbers per inseminate, sorting pressure and sperm storage before sorting[J]. Theriogenology, 2009, 71(5): 717–728. DOI: 10.1016/j.theriogenology.2008.08.016 |
| [22] | JOHNSON L A. Sex preselection in swine: altered sex ratios in offspring following surgical insemination of flow sorted X- and Y-bearing sperm[J]. Reprod Dom Anim, 1991, 26(6): 309–314. DOI: 10.1111/j.1439-0531.1991.tb01546.x |
| [23] | PONTES J H F, SILVA K C F, BASSO A C, et al. Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm[J]. Theriogenology, 2010, 74(8): 1349–1355. DOI: 10.1016/j.theriogenology.2010.06.004 |
| [24] | SPINACI M, CHLAPANIDAS T, BUCCI D, et al. Encapsulation of sex sorted boar semen: sperm membrane status and oocyte penetration parameters[J]. Theriogenology, 2013, 79(4): 575–581. DOI: 10.1016/j.theriogenology.2012.10.021 |
| [25] | KARAKAYA-BILEN E, YILMAZBAS-MECITOGLU G, KESKIN A, et al. Fertility of lactating dairy cows inseminated with sex-sorted or conventional semen after ovsynch, presynch-ovsynch and double-ovsynch protocols[J]. Reprod Domest Anim, 2019, 54(2): 309–316. DOI: 10.1111/rda.13363 |
| [26] | MONK M, HANDYSIDE A H. Sexing of pre-implantation mouse embryos by measurement of X-linked gene dosage in a single blastomere[J]. J Reprod Fertil, 1998, 82(1): 365–368. |
| [27] | LI C Y, ZHAO Y H, HAO H S, et al. Resveratrol significantly improves the fertilisation capacity of bovine sex-sorted semen by inhibiting apoptosis and lipid peroxidation[J]. Sci Rep, 2018, 8(1): 7603. DOI: 10.1038/s41598-018-25687-z |
| [28] | CRITES B R, VISHWANATH R, ARNETT A M, et al. Conception risk of beef cattle after fixed-time artificial insemination using either SexedUltraTM 4M sex-sorted semen or conventional semen[J]. Theriogenology, 2018, 118: 126–129. DOI: 10.1016/j.theriogenology.2018.05.003 |
| [29] | THOMAS J M, LOCKE J W C, BONACKER R C, et al. Evaluation of SexedULTRA 4MTM sex-sorted semen in timed artificial insemination programs for mature beef cows[J]. Theriogenology, 2019, 123: 100–107. DOI: 10.1016/j.theriogenology.2018.09.039 |
| [30] | DELL'EVA G, BOLOGNINI D, IACONO E, et al. Superovulation protocols for dairy cows bred with SexedULTRATM sex-sorted semen[J]. Reprod Domest Anim, 2019, 54(5): 756–761. DOI: 10.1111/rda.13421 |
| [31] | PELLEGRINO C A G, MOROTTI F, UNTURA R M, et al. Use of sexed sorted semen for fixed-time artificial insemination or fixed-time embryo transfer of in vitro-produced embryos in cattle[J]. Theriogenology, 2016, 86(3): 888–893. DOI: 10.1016/j.theriogenology.2016.03.010 |
| [32] | CHEBEL R C, CUNHA T. Optimization of timing of insemination of dairy heifers inseminated with sex-sorted semen[J]. J Dairy Sci, 2020, 103(6): 5591–5603. DOI: 10.3168/jds.2019-17870 |
| [33] | OSADA M, IWABUCHI H, AOKI T, et al. Economic evaluation of artificial insemination of sex-sorted semen on a Brown Swiss dairy farm-A case study[J]. Anim Sci J, 2019, 90(4): 597–603. DOI: 10.1111/asj.13156 |
| [34] | SILVA M A V, SANTOS C S, FRANÇA I G, et al. Hormonal strategy to reduce suckled beef cow handling for timed artificial insemination with sex-sorted semen[J]. Theriogenology, 2018, 114: 159–164. DOI: 10.1016/j.theriogenology.2018.03.020 |



