众所周知,线粒体是一种具有双层膜结构和拥有独立基因组以及转录系统的细胞器[1-2],可通过氧化磷酸化(oxidative phosphorylation, OXPHOS)产生三磷酸腺苷(adenosine triphosphate, ATP),以满足细胞行使功能所需能量,同时参与调节各种关键细胞事件,如活性氧的产生和封存[3]、钙稳态[4]、铁平衡[5]、细胞凋亡以及细胞衰老[6]等。线粒体是一种高度动态的细胞器,可进行连续循环的融合与分裂以改变线粒体形态、大小及位置,该生理过程被称为线粒体动力学。研究发现,线粒体可通过融合、分裂及自噬以补充、修复线粒体DNA(mitochondrial DNA, mtDNA)和相关蛋白,给机体提供能量,满足细胞能量需求并清除受损线粒体[7-8]。另外,线粒体融合与分裂相关蛋白在人、小鼠、苍蝇、植物中都高度保守,这也说明因为该机制在生物进化中具有重要作用才得以保留[9]。在生理状态下,线粒体融合和分裂互相牵制,使线粒体达到某种动态平衡。该平衡一旦被打破,即线粒体融合和分裂受阻时,将导致线粒体功能受损,最终引发多种疾病[10]。本文将对线粒体形态和动力学调节机制进行综述,分析相关基因和蛋白在融合、分裂以及氧化磷酸化过程中发挥的重要作用。强调线粒体形态和动力学的生物学意义,特别是相关基因或蛋白缺陷对细胞正常功能的影响,以期为今后线粒体的研究提供参考。
1 线粒体内稳态线粒体是一种被线粒体外膜(outer mitochondria membrane, OMM)和线粒体内膜(inner mitochondria membrane, IMM)包裹,具有膜间隙(inter- membrane space, IMS)和基质两个腔室的细胞器,为不同类型细胞行使正常功能提供能量[1-2]。在生理条件下,OMM和IMM必须在一系列持续的融合和分裂中协同工作,从而在细胞内形成一种稳态系统[11]。线粒体融合和分裂对内稳态和线粒体功能都至关重要,但每个生理过程在线粒体功能方面却扮演着不同的角色。线粒体融合可导致线粒体结构延长,ATP含量增加,以及各种线粒体活性物质被转移至新融合的线粒体中[12-13]。当细胞在应激条件下(饥饿处理或光刺激),线粒体融合将达到最大程度,以产生足够的ATP对抗应激[14]。相对于融合,线粒体分裂则会产生新的、更小的线粒体,有助于细胞分裂和有丝分裂[8]。同时,分裂不仅产生可移动的线粒体,还产生能够进行自噬的线粒体[15]。线粒体生物发生和自噬由融合或分裂高度调控,这也突出了线粒体内稳态对细胞功能的巨大影响。如表 1所示,当线粒体融合和分裂受阻时,将导致线粒体功能受损,最终可导致神经退化型疾病、心血管疾病以及代谢型疾病等多种疾病的发生[10]。因此,对线粒体内稳态的研究不仅有助于了解线粒体融合和分裂调节机制,而且能够为高原动物的逆境适应性机理以及人类健康提供指导。
|
|
表 1 与线粒体融合或分裂相关的常见疾病 Table 1 The common diseases related to mitochondria fusion or fission |
线粒体可通过融合(fusion)和分裂(fission)使其在细胞中的位置和形态结构(伸长、缩短、分叉、弯曲和肿胀)发生变化,以保持正常的生理功能。线粒体融合主要参与新线粒体的合成和受损线粒体的修复(mtDNA变异、膜电位下降),当受损线粒体与正常线粒体融合后,mtDNA将被重新整合、修复并调整膜电位至正常水平[27]。而当机体处于应激状态下(压力、疾病、饥饿等),线粒体融合将最大限度地产生ATP以支撑机体的能量需求[14]。截至目前,在哺乳动物中有3种GTPases蛋白与线粒体融合相关,其中,Mfn1和Mfn2调控OMM融合,OPA1调控IMM融合[28]。
线粒体融合包括OMM融合和IMM融合。OMM融合主要由功能相似的Mfn1和Mfn2介导(图 1)[29]。Mfn1和Mfn2都是OMM跨膜GTPases,包含氨基端GTP结合结构域、两个卷曲结构域以及一个跨膜结构域的羧基末端等几个保守区[30]。虽然Mfn1和Mfn2具有相似的功能,甚至在某些情况下能够相互替代,但只有Mfn2的突变会引起显著的生理变化,可导致神经退化型疾病,如2A型腓骨肌萎缩神经病变[12, 29]。二者在线粒体融合中发挥着不同的功能,即线粒体在缺氧情况下的伸长主要是通过Sirt1介导的Mfn1去乙酰化调控[31],而Mfn2主要通过介导线粒体和内质网接触点的形成来调控线粒体融合[32],而Mfn1和Mfn2功能存在差异的根本原因可能是因为Mfn1缺乏N端RAS结合域[33]。Koshiba等[34]研究发现,负责线粒体融合的膜蛋白需要在相邻的线粒体上介导融合,说明线粒体融合复合物需要在相邻线粒体之间才能发挥作用,即每个蛋白质的第二卷曲螺旋结构域形成同型或异型二聚体后反向平行卷曲螺旋结构,从而束缚和调节线粒体融合。因此,线粒体融合蛋白主要通过邻近线粒体上的分子二聚体作用促进融合,这在当前线粒体融合机制研究中也是一个热门领域。而IMM融合主要由OPA1介导。当OMM融合后,OPA1负责将两个IMM系统进行连接、整合,并形成一个完整的IMM系统(图 1)。在线粒体中,OPA1被蛋白水解分裂成长型和短型两个亚型,但是二者单独并没有任何生理活性,只有相互作用才可介导线粒体融合[35]。在正常生理条件下,两种亚型的相对浓度几乎相等,这是在IMM融合中发挥合适功能所必需的[36]。
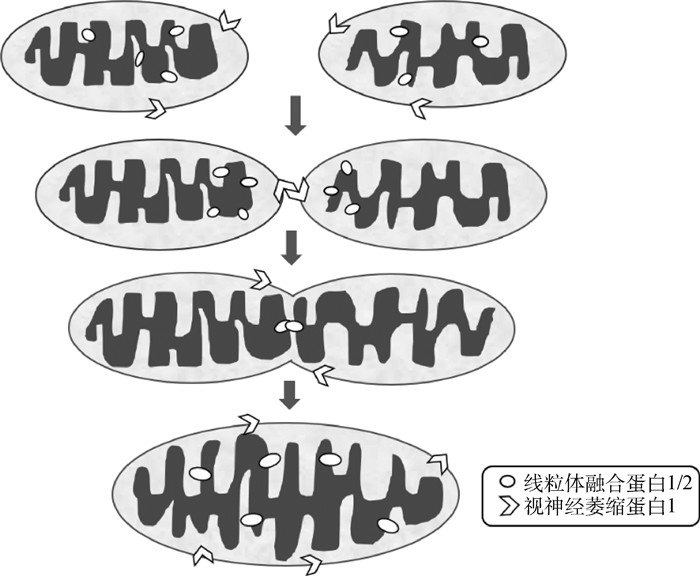
|
线粒体融合蛋白1/2(Mfn1/Mfn2)相互作用介导线粒体外膜融合,视神经萎缩蛋白1(OPA1)介导线粒体内膜融合 Mfn1/Mfn2 interaction mediates OMM fusion, OPA1 mediates IMM fusion 图 1 线粒体融合[29, 35, 45] Fig. 1 Mitochondria fusion[29, 35, 45] |
线粒体融合与细胞生理功能息息相关,融合可将线粒体蛋白质和mtDNA转移至新合成的线粒体,这有助于防止受损线粒体mtDNA的积累[37]。而线粒体融合速率会受线粒体理化特性的影响,如线粒体膜电位的改变。Little等[38]在对处于G1期的胰腺癌细胞进行高/低代谢,以改变细胞线粒体膜电位的研究中发现,随着线粒体膜电位升高,OXPHOS以及线粒体呼吸和融合速率也得到了相应的提升。Mitra等[39]在大鼠肾细胞中也证实了上述观点,在细胞从G1-S期过度时需要消耗大量的ATP以满足细胞核酸和蛋白合成,此时,线粒体融合速率得到显著加强,细胞中ATP水平远高于其他时期。但是,改变线粒体膜电位的因素有很多,比如衰老、凋亡及疾病等因素,因此,线粒体融合速率和膜电位是否存在正相关还有待于进一步的研究。另有研究发现,营养水平也将影响线粒体融合,当小鼠处于高脂膳食下,Mfn1、Mfn2表达显著下降,并伴有线粒体呼吸功能紊乱,骨骼肌ATP含量下降[40]。对肥胖小鼠进行游泳训练后发现,细胞内质网应激(endoplasmic reticulum stress, ERS)将被启动,线粒体相关细胞膜(mitochondria-associated membranes, MAM)含量增加,进而使得线粒体功能增强,骨骼肌ATP含量升高[41]。但当小鼠处于严重饥饿状态下,线粒体融合速率和线粒体网状结构将得到显著增加,使得细胞AMP水平升高,蛋白激酶A (protein kinase A, PKA)被激活。而后PKA反过来磷酸化Drp1,使得线粒体分裂速率减慢,融合速率和OXPHOS水平提升,ATP合酶高表达以维持细胞ATP供应[42]。虽然饥饿和应激可以产生更多的能量以维持细胞功能,但同时也增加了线粒体ROS产生和氧化损伤的风险[43]。Tondera等[44]使用紫外线和放线菌素D刺激小鼠成纤维细胞的试验发现,随着线粒体融合增强,ATP含量和OXPHOS水平提升,ROS水平也显著提升,线粒体损伤增加,功能减弱。由上可知,线粒体功能和融合速率呈线性关系,即当融合速率达到某种临界点时,线粒体融合速率将达到最强,但同时氧化损伤风险最大。因此,研究线粒体融合在高原哺乳动物线粒体损伤最小的情况下,得到最大的能量供应,以支撑机体抗逆境的能量需求对于高原哺乳动物的逆境适应以及选种、引种有重大意义。
2.2 线粒体分裂线粒体分裂在线粒体动力学中对线粒体融合起平衡作用。线粒体分裂主要存在两种方式[46];一种是“由内向外”,即线粒体内膜先分裂,最终导致外膜分裂;另一种是“挤压式”,即线粒体从分裂点向内积压,导致线粒体分裂。截至目前,在哺乳动物中发现与线粒体分裂相关的蛋白大都是GTPase家族蛋白,包括Drp1[47]、Fis1[48]、MFF[49]和Mid49/51[50];同时还有协助Drp1调控线粒体分裂的动力蛋白2(dynamin protein 2, Dyn2/Dnm2)[51]、吞蛋白B1(endophilin B1)和蛋白互作因子1(bax-interacting factor 1, Bif-1)[52];对线粒体分裂起重要作用的有神经节苷脂诱导分化相关蛋白1(ganglioside- induced differentiation-associated protein 1, GDAP1)[53]、死亡相关蛋白1(death-associated protein 3, DAP3)[54]和线粒体蛋白18(mitochondria protein 18, MTP18)[55]等。任何的生理活动都会消耗能量,线粒体分裂所需能量主要由GTPases水解GTP提供,但分裂过程需要内质网(endoplasmic reticulum, ER)参与,这将有助于在GTPase水解之前启动线粒体分裂。如图 2所示,线粒体分裂首先由内质网和线粒体接触部位的内质网对线粒体膜进行“标记”,使Drp1受体招募Drp1。随后Drp1在分裂位点周围将形成低聚体并进一步收缩膜GTPase活性,紧缩线粒体并导致Dyn1/Dyn2补充,使额外的GTP水解以完成分裂过程,产生两个独立的线粒体。线粒体分裂对于细胞内线粒体重塑和重排以及在有丝分裂后将健康mtDNA及其他活性物质转移到子细胞是至关重要的[56]。因此,线粒体分裂异常与很多疾病相关,比如阿尔兹海默症[57]、心肌肥大[58]等。
2.2.1 线粒体内质网接触位点的作用 研究发现,线粒体与内质网之间存在接触位点,且这些位点对于磷脂合成、Ca2+稳态以及标记分裂位点都至关重要[59]。在酵母中,接触位点又称为内质网线粒体相遇结构(endoplasmic reticulum mitochondria encounter structure, ERMES),其参与线粒体分裂[60],而在哺乳动物中,接触位点的功能主要由Mfn2调节[61]。在最近的研究中,Hirabayashi等[62]发现,酵母ERMES蛋白PDZD8(MMM1同源物)在哺乳动物神经元线粒体与内质网连接的过程中发挥着关键作用。线粒体虽然是动态的,但线粒体和内质网接触位点的位置却是保持相对恒定的,具体的调节机制还有待进一步研究[63]。有些研究者认为,ER的作用是标记线粒体收缩和分裂的起始位点[59]。Korobova等[64]证明,位于ER的 INF2诱导了ER和线粒体接触位点之间肌动蛋白的聚合,从而驱动线粒体裂变位点的收缩,这可能是因为Drp1寡聚物不能在没有受体的情况下包裹线粒体膜并诱导分裂。根据细胞类型不同,线粒体直径一般在200 nm以上,在进一步收缩膜结构之前,Drp1寡聚物识别并结合的线粒体膜直径在110~130 nm,而ER收缩位点的直径约为138~146 nm,表明ER启动的收缩先于Drp1寡聚体在线粒体膜收缩位点形成[59]。这也说明ER在线粒体收缩之前的启动收缩过程中发挥着重要的作用,同时也证明了ER是标记线粒体收缩和分裂的起始位点。
2.2.2 线粒体分裂蛋白调控机制 线粒体分裂主要受Drp1、Fis1、Dyn1/2以及MFF等相关基因和蛋白调控。Drp1主要定位于细胞质基质,但Drp1不具有与脂质结合的pleckstrin同源结构域,不能直接与线粒体膜结合,因此,其介导线粒体分裂就必须被招募至线粒体[65]。如图 2所示,线粒体蛋白必须作为Drp1受体将Drp1集结到线粒体外膜。在哺乳动物细胞中,已被鉴定的Drp1受体有Fis1、Mff以及Mid49/51[66-69]。研究发现,虽然三者都可以将Drp1招募到线粒体外膜,但Mff的结合效应最强[66]。Drp1一旦被招募到线粒体外膜则形成一个环状低聚物,并利用其GTPase活性进一步收缩线粒体,但由于收缩强度限制不能完成分裂过程[70]。Dyn2/ Dnm2已被确认为线粒体收缩和分裂蛋白[71],类似于Drp1,Dyn2/Dnm2分子可在膜周围形成环状寡聚物,随着GTP的水解进一步收缩线粒体并完成分裂[72]。在哺乳动物中,两种分裂蛋白必须协同工作,缺一不可。Lee等[73]研究发现,Dyn2/ Dnm2和Drp1两种GTPase蛋白在线粒体分裂过程中,若其中一种蛋白被耗尽,线粒体会被拉长,并显示出更精细的管状网络。线粒体分裂始于Drp1被激活,随后从细胞质基质转移至线粒体外膜,与受体结合从而启动分裂。但分裂速率取决于Drp1结合数量和磷酸化修饰水平。例如在神经元细胞中,Ca2+内流通过电压依赖性Ca2+通道导致线粒体运动的快速停止并诱导线粒体裂变,并且Ca2+通道可激活Ca2+调节依赖性蛋白激酶lalpha (Ca2+/calmodulin-dependent protein kinase Ialpha, CaMKlapha)进而刺激Drp1丝氨酸(S600)磷酸化,使得其与Fis1的亲和力增加,促进线粒体分裂[74]。在小鼠成纤维细胞研究中同样发现,在经过电离辐射同样会触发CaMKlapha并刺激Drp1(S616)磷酸化,从而加速分裂速率,但当CaMKlapha活性被抑制时,Drp1(S616)磷酸化和分裂速率均被显著抑制[75]。同时,PKA可介导小鼠成纤维细胞Drp1(S637)磷酸化使其与MFF相互作用并被募集到线粒体外膜,从而使得线粒体分裂速率加快[49]。同样,不同的营养状况也将影响细胞中线粒体分裂速率和ATP含量。Molina等[76]发现,小鼠处于高糖高脂的营养状况下时,胰岛β细胞线粒体分裂速率提高,Fis1表达下降,融合速率降低,ROS生成减少,触发该机制可能是减少细胞ATP过度消耗,对细胞正常功能起到保护作用。Liu等[40]在小鼠骨骼肌细胞中研究发现,与正常饲喂小鼠相比,高脂膳食小鼠骨骼肌中Fis1和Drp1表达上升,融合蛋白Mfn1/2表达下降。综合上述观点可知,当机体(细胞)处于高脂水平下,线粒体ATP合成速率加快,同时也伴随着ROS合成的增加,线粒体氧化损伤风险也将增加。上面说到线粒体分裂会清除受损线粒体,将健康mtDNA和活性物质传递给子代线粒体。因此为了维持线粒体正常功能,线粒体将增加分裂速率以清除受损线粒体,在传递过程中增加健康线粒体的比例,从而维持细胞能量的正常供给,满足基本活动需求。因此,在对高原哺乳动物的研究中,应着重从线粒体动力学和能量代谢层面出发,研究其调控机制或代谢途径与适应性的关系,这对于揭示高原哺乳动物对逆境、疾病或极端环境的适应有重要意义。
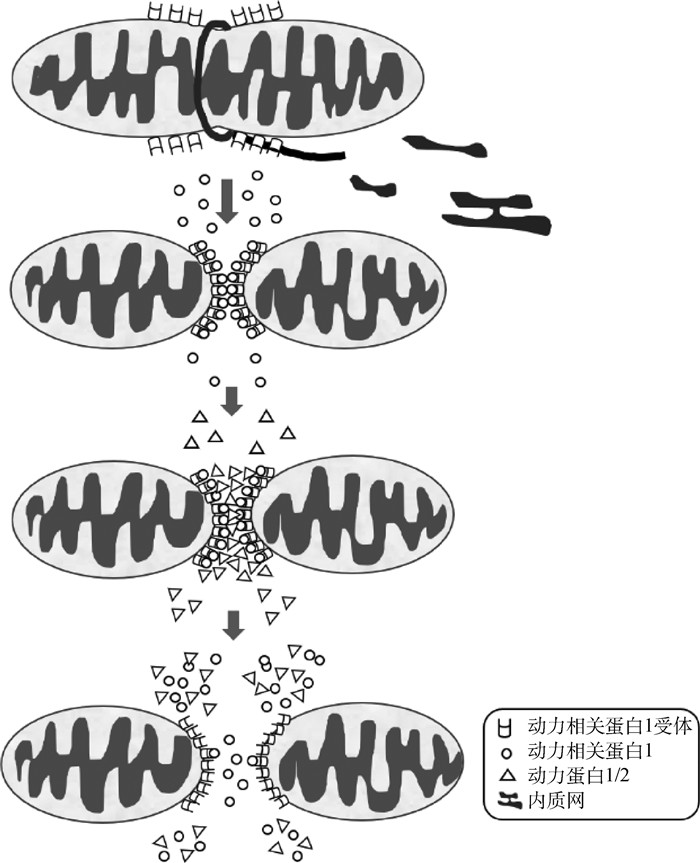
|
线粒体分裂首先由内质网和线粒体接触部位的内质网对线粒体膜进行“标记”,使得动力相关蛋白1(Drp1)受体招募Drp1。然后Drp1在分裂位点周围形成低聚体并进一步收缩膜GTPase活性,导致动力蛋白1/2(Dyn1/Dyn2)补充,使得额外的GTP水解,并完成分裂过程,产生两个独立的线粒体 First, mitochondrial membrane is "marked" by the ER at ER/mitochondria contact sites for fission, thus resulting in Drp1 recruitment by Drp1 receptors. Drp1 then forms oligomers around the fission site and further constricts the membrane through GTPase activity, leading to Dyn1/Dyn2 recruitment, additional GTP hydrolysis, and completion of the process of fission resulting in two separate mitochondrias 图 2 线粒体分裂[45, 50] Fig. 2 Mitochondria fission[45, 50] |
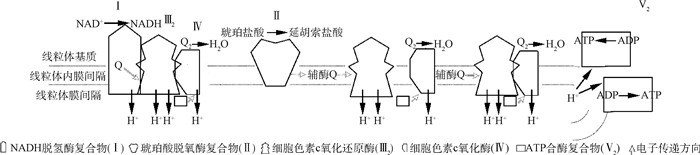
线粒体在真核细胞中最重要的作用无疑就是发生在线粒体内膜的OXPHOS。截至目前,对于线粒体OXPHOS的研究多集中在对不同复合物结构以及各个亚基之间的功能。线粒体内膜OXPHOS系统由5种复合物(复合物Ⅰ-Ⅴ)组成。在哺乳动物中,除了复合物Ⅱ外其他都是多聚体,由线粒体基因组(mtDNA)和核基因组(nDNA)编码的亚基组成。mtDNA编码的亚基大都具有疏水性,可在靠近IMM的位置翻译进而使其更容易发生移位[77]。而由nDNA编码的亚基可在细胞质中表达,进入细胞器并正确指导OXPHOS过程中的结构亚基[78]。如图 3所示,复合物Ⅰ-Ⅳ是多亚基酶协同工作,以创造一个横跨线粒体内膜的电化学质子梯度,而后在F0F1ATP合酶(复合物Ⅴ)的作用下产生ATP。
复合物Ⅰ又称还原性烟酰胺腺嘌呤二核苷酸脱氢酶(nicotinamide adenine dinucleotide H+,NADH),是呼吸链中最大的酶,由45个亚基组成,且大部分嵌在脂质双分子层,结构呈典型的L型,肩膀部分突出到线粒体基质中[79]。在细菌X射线试验中发现了复合物Ⅰ的耦合机制[80],即复合物Ⅰ将NADH底物结合到亲水基团的远端,并通过FMN和7个铁硫原子团簇一次转移2个电子给两个基团的辅酶Q,随着辅酶Q的还原可引起复合物Ⅰ构象的变化,导致4个质子通过通道跨膜移位进入线粒体膜间隙[81]。随着对哺乳动物复合物Ⅰ的深入研究发现,复合物Ⅰ由N、Q、ND1、ND2、ND4以及ND5共6个模块组成,在特定装配因子的帮助下,通过5种主要的组件聚集在一起[82]。复合物Ⅱ又称琥珀酸脱氢酶(succinate dehydrogenase, SDH),是电子进入呼吸链的第二个独立入口,由4个nDNA编码的亚基组成。2个亲水性催化亚基为SDHA/SDH1和SDHB/SDH2,2个疏水性亚基为SDHC/SDH3和SDHD/SDH4,二者共同构成了复合物Ⅱ膜锚着点,包含一个血红素b基团和两个辅酶Q结合位点[83-84]。虽然复合物Ⅱ本身不能产生质子,对质子梯度的形成不做贡献,但是它可直接氧化琥珀酸并通过3个铁硫原子团簇转移电子至辅酶Q[85]。复合物Ⅲ又称细胞色素c氧化还原酶(cytochrome coxidoreductase, COX),是一个由3个催化核心(MT-CYB、CYC1和UQCRFS1)和7个多余亚基紧密结合的对称二聚体[86],它能氧化复合物Ⅱ接受质子的辅酶Q,利用“Q-cycle”机制耦合两个质子并泵入膜间隙[87]。研究发现,从UQCRFS1上分离出的78个氨基酸线粒体靶向序列(mitochondria targeting sequence, MTS)是一个额外的亚基,在必要的时候需要被清除以保持复合物Ⅲ的结构和功能稳定性[88]。MT-CYB含有两个氧化还原电位不同的b型血红素以及两个辅酶Q结合位点,这就使得一个铁硫原子团簇可以插入UQCRFS1的C端,而后CYC1结合血红素c1基团并将电子转移至移动电子载体细胞色素c。在整个氧化还原过程中,多余的亚基并不参与催化作用,但对酶的正确组装和稳定性起到重要调节作用[89]。线粒体呼吸链的最后一种酶是复合物Ⅳ, 又称细胞色素c氧化酶(cytochrome c oxidase, CO)。它接受来自细胞色素c的电子,并将电子传递给1个氧分子,转化为2个水分子, 并偶联4个质子泵送至膜间隙[90]。在哺乳动物中,复合物Ⅳ包含MT-CO1、MT-CO2以及MT-CO3在内的13或14个亚基[91-92]。MT-CO1是最大的催化亚基,含有血红素a基团和双核血红素a3-CuB基团;MT-CO2是第二个核心亚基,并占据CuA亚基催化中心;MT-CO3是第三个核心亚基,但并不直接参与催化作用;而其余的亚基虽然不参与催化反应,但在稳定催化核心和调节活性方面发挥重要作用[93]。复合物Ⅳ是唯一含有组织特异性和参与发育调控的OXPHOS复合物,同时作为OXPHOS限速酶调控其反应速率[94-95],这也反映了对细胞色素c氧化还原酶活性进行精确调控的重要性。ATP合酶或复合物Ⅴ是转运H+的具有两个扇形结构的ATP酶或F0F1-ATP酶,由15~18个亚基组成,总质量为600 ku,利用复合物Ⅰ、Ⅲ、Ⅳ产生的质子动力将ADP合成ATP[96]。它由两个拓扑和功能不同的领域组成,由中心轴和外围轴连接的膜外部和面向矩阵的F1以及膜内部的F0[97]。在所有亚基中,只有具有F0结构域的亚基a(MT-ATP6)和A6L(MT-ATP8)是由mtDNA编码,其他所有的亚基组件均由nDNA编码[98]。当质子通过质子泵时将做旋转运动,从而为F1域中的ADP + Pi凝聚提供能量,进而产生ATP[97]。氧化磷酸化是一个极其复杂的调控过程,目前更多的研究集中在各复合物的组装,且各复合物亚基的结构也相对比较清楚,但是功能还有待挖掘。在高原哺乳动物中,若能将各复合物与各亚基的调控机制与抗寒以及抗低氧等性状联系起来,亦或研究在各种抗逆环境中各复合物亚基的网络调控、协调表达以及相互作用等,这对于高原动物的适应和生存可能是重大突破。
3.2 氧化磷酸化超配合物OXPHOS复合物之间相互作用可形成高级结构-超配合物,如复合物Ⅳ和Ⅴ可形成二聚体和寡聚物[100]。当线粒体膜提取物溶于洋地黄皂苷并经蓝原胶电泳分离后可清晰观察到复合物Ⅰ和Ⅲ的关联[101]。在小鼠心肌细胞线粒体中发现,OXPHOS复合物Ⅰ-Ⅳ存在于紧密的邻近空间中,为线粒体中超配合物的装配提供了直接证据[102]。研究发现,根据其分子大小和亚基组成,主要的超配合物包括:Ⅲ2Ⅳ1、Ⅰ1Ⅲ2、Ⅰ1Ⅲ2Ⅳ1以及Ⅰ2Ⅲ2Ⅳ1-2[103],其中超配合物Ⅰ1Ⅲ2Ⅳ1被称为是“呼吸体”,而Ⅰ2Ⅲ2Ⅳ2被认为“大型综合体”[104]。随着对超配合物研究的深入,高分辨率的冷冻电镜技术被用于揭示哺乳动物呼吸体超微结构的研究,对于单个复合物与超配合物的作用关系和结构特征已相对明确,但他们之间的具体功能机理尚不明晰,有待于进一步研究[105-107]。对于呼吸体的组装一直存在一些争议,有研究者认为,单个复合物在它们参与超配合物结合之前已经完全组装好了,如果超配合物的作用是提高电子转移的效率,这种方式将允许配合物的动态结合和解离以适应不同的能量需求[82, 108]。相反的是,Moreno-Lastres等[109]在研究复合物Ⅰ的组装中发现,在超配合物完成组装之前,不同复合物的亚基是共同组装的,除非复合物Ⅱ2型亚基和复合物Ⅳ与复合物Ⅰ前体结合,否则复合物Ⅰ则没有生物活性。此外,研究还发现,不完整的复合物在携带不同结构亚基和组装因子的细胞和组织中竟组装在一起,并参与了复合物Ⅰ和复合物Ⅲ组装的最后一步,这也从侧面说明超配合物的各个亚基是共同组装的[110]。COX7A亚基是复合物Ⅳ融入超配体结构的必要条件,被认定为超配合物组装因子[108],然而,最近的研究表明,该蛋白对Ⅲ2Ⅳ1的形成有明显作用,但对复合物Ⅳ进入呼吸体没有作用,因此,对于该蛋白在呼吸体形成中的作用机理还有待进一步确认[111-112]。OXPHOS系统中各个复合物和超配合物的组装是一个极其复杂的过程,当组装出现错误或者组装过程受到干扰时,线粒体功能将显著下降从而诱发多种疾病的发生[102]。虽然目前对复合物及超配合物的组装研究有一定的进展,但对于整个OXPHOS系统的认知有限,所以,在该生物过程中,应该重点研究每个复合物和超配合物的装配过程,以及参与基本装配和精细调节的蛋白质及其确切的分子作用和相互作用机制。这将帮助我们了解OXPHOS在动物疾病和抗逆境过程中的核心代谢机制,了解核心亚基或复合物等装配因子的突变与相关病理的关系。
4 结语与展望从19世纪初首次被提及至今,线粒体一直都是研究热点,这无非是因为其自身强大的功能-通过有氧呼吸为细胞提供源源不断的能量。目前,对于线粒体的研究更多地集中在线粒体动力学与疾病发生的关系以及氧化磷酸化各复合物的组装。本文总结了线粒体融合、分裂以及氧化磷酸化的作用机理,分析了其在细胞能量代谢和疾病调控中所发挥的作用。不管是在应激或正常情况下,融合和分裂均会互相牵制以维持某种动态平衡。目前,对线粒体动力学和机制研究成果显著,但其中仍存在一些问题:1)相邻线粒体通过分子二聚体的融合机制尚不明确;2)线粒体和内质网之间接触位点的形成机制问题;3)氧化磷酸化复合物在超配合或“呼吸体”中的相互作用机理尚不清楚等,若要解决上述问题还需要很大的努力。对于线粒体在哺乳动物中的研究,应将线粒体机理与生产实践联系起来,结合遗传、生理生化、细胞生物学以及组织形态学等手段研究哺乳动物在高/低温、低氧、高辐射等恶劣环境下线粒体动力学和能量代谢的调节机制,这将有助于揭示高原哺乳动物的适应性问题。随着现代测序技术的普及,越来越多的与线粒体融合和分裂以及能量代谢的基因和蛋白被挖掘出来,应优化试验设计,在大数据时代不应一味追求“广”,而应兼顾“深”。在适应性问题上,应研究单基因或蛋白的机制问题,研究其调控网络,这对于深入挖掘基因功能,提高动物适应能力和生产效率,增加动物产品具有重要意义。相信随着对线粒体动力学和能量代谢研究的深入,相关科学问题会一一被解开,那将是对动物和人类健康的重大贡献。
| [1] | SARASTE M. Oxidative phosphorylation at the fin de siècle[J]. Science, 1999, 283(5407): 1488–1493. DOI: 10.1126/science.283.5407.1488 |
| [2] | KRAMER P, BRESSAN P. Our (mother's) mito-chondria and our mind[J]. Perspect Psychol Sci, 2018, 13(1): 88–100. DOI: 10.1177/1745691617718356 |
| [3] | KOTIADIS V N, DUCHEN M R, OSELLAME L D. Mitochondrial quality control and communications with the nucleus are important in maintaining mito-chondrial function and cell health[J]. Biochim Biophys Acta, 2014, 1840(4): 1254–1265. DOI: 10.1016/j.bbagen.2013.10.041 |
| [4] | LUDTMANN M H R, ABRAMOV A Y. Mito-chondrial calcium imbalance in Parkinson's disease[J]. Neurosci Lett, 2018, 663: 86–90. DOI: 10.1016/j.neulet.2017.08.044 |
| [5] | TOYOKUNI S, ITO F, YAMASHITA K, et al. Iron and thiol redox signaling in cancer: an exquisite balance to escape ferroptosis[J]. Free Radic Biol Med, 2017, 108: 610–626. DOI: 10.1016/j.freeradbiomed.2017.04.024 |
| [6] | LOPEZ J, TAIT S W G. Mitochondrial apoptosis: killing cancer using the enemy within[J]. Br J Cancer, 2015, 112(6): 957–962. DOI: 10.1038/bjc.2015.85 |
| [7] | DORN Ⅱ G W, KITSIS R N. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble[J]. Circ Res, 2015, 116(1): 167–182. DOI: 10.1161/CIRCRESAHA.116.303554 |
| [8] | HORBAY R, BILYY R. Mitochondrial dynamics during cell cycling[J]. Apoptosis, 2016, 21(12): 1327–1335. DOI: 10.1007/s10495-016-1295-5 |
| [9] | NUNNARI J, WONG E D, MEEUSEN S, et al. Studying the behavior of mitochondria[J]. Methods Enzymol, 2002, 351: 381–393. |
| [10] | ARCHER S L. Mitochondrial dynamics--mito-chondrial fission and fusion in human diseases[J]. N Engl J Med, 2013, 369(23): 2236–2251. DOI: 10.1056/NEJMra1215233 |
| [11] | WAI T, LANGER T. Mitochondrial dynamics and metabolic regulation[J]. Trends Endocrinol Metab, 2016, 27(2): 105–117. DOI: 10.1016/j.tem.2015.12.001 |
| [12] | JOAQUIM M, ESCOBAR-HENRIQUES M. Role of mitofusins and mitophagy in life or death decisions[J]. Front Cell Dev Biol, 2020, 8: 572182. DOI: 10.3389/fcell.2020.572182 |
| [13] | YANG W Y. Optogenetic probing of mitochondrial damage responses[J]. Ann N Y Acad Sci, 2015, 1350(1): 48–51. DOI: 10.1111/nyas.12818 |
| [14] | RAMOS E S, LARSSON N G, MOURIER A. Bioenergetic roles of mitochondrial fusion[J]. Biochim Biophys Acta, 2016, 1857(8): 1277–1283. DOI: 10.1016/j.bbabio.2016.04.002 |
| [15] | TWIG G, ELORZA A, MOLINA A J A, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy[J]. EMBO J, 2008, 27(2): 433–446. DOI: 10.1038/sj.emboj.7601963 |
| [16] | TOBORE T O. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer's disease[J]. Neurol Sci, 2019, 40(8): 1527–1540. DOI: 10.1007/s10072-019-03863-x |
| [17] | VÖLGYI K, BADICS K, SIALANA F J, et al. Early presymptomatic changes in the proteome of mitochondria-associated membrane in the APP/PS1 mouse model of Alzheimer's disease[J]. Mol Neurobiol, 2018, 55(10): 7839–7857. DOI: 10.1007/s12035-018-0955-6 |
| [18] | NGUYEN M, WONG Y C, YSSELSTEIN D, et al. Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson's disease[J]. Trends Neurosci, 2019, 42(2): 140–149. DOI: 10.1016/j.tins.2018.11.001 |
| [19] | LIU Y, MA X P, FUJIOKA H, et al. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1[J]. Proc Natl Acad Sci U S A, 2019, 116(50): 25322–25328. DOI: 10.1073/pnas.1906565116 |
| [20] | BAYNE A N, TREMPE J F. Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond[J]. Cell Mol Life Sci, 2019, 76(23): 4589–4611. DOI: 10.1007/s00018-019-03203-4 |
| [21] | ROCHA A G, FRANCO A, KREZEL A M, et al. MFN2 agonists reverse mitochondrial defects in pre-clinical models of Charcot-Marie-Tooth disease type 2A[J]. Science, 2018, 360(6386): 336–341. DOI: 10.1126/science.aao1785 |
| [22] | LARREA D, PERA M, GONNELLI A, et al. MFN2 mutations in Charcot-Marie-Tooth disease alter mito-chondria-associated ER membrane function but do not impair bioenergetics[J]. Hum Mol Genet, 2019, 28(11): 1782–1800. DOI: 10.1093/hmg/ddz008 |
| [23] | HAM M, HAN J, OSANN K, et al. Meta-analysis of genotype-phenotype analysis of OPA1 mutations in autosomal dominant optic atrophy[J]. Mitochondrion, 2019, 46: 262–269. DOI: 10.1016/j.mito.2018.07.006 |
| [24] | JONG C J, YEUNG J, TSEUNG E, et al. Leptin-induced cardiomyocyte hypertrophy is associated with enhanced mitochondrial fission[J]. Mol Cell Biochem, 2019, 454(1-2): 33–44. DOI: 10.1007/s11010-018-3450-5 |
| [25] | DURAISAMY A J, MOHAMMAD G, KOWLURU R A. Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy[J]. Biochim Biophys Acta, 2019, 1865(6): 1617–1626. DOI: 10.1016/j.bbadis.2019.03.013 |
| [26] | LEE H J, MOON J, CHUNG I, et al. ATP synthase inhibitory factor 1 (IF1), a novel myokine, regulates glucose metabolism by AMPK and Akt dual pathways[J]. FASEB J, 2019, 33(12): 14825–14840. DOI: 10.1096/fj.201901440RR |
| [27] | GAZIEV A I, ABDULLAEV S, PODLUTSKY A. Mitochondrial function and mitochondrial DNA maintenance with advancing age[J]. Biogerontology, 2014, 15(5): 417–438. DOI: 10.1007/s10522-014-9515-2 |
| [28] | WANG G, HE Y Y, LUO Y Z. Expression of OPA1 and Mic60 genes and their association with mito-chondrial cristae morphology in Tibetan sheep[J]. Cell Tissue Res, 2019, 376(2): 273–279. DOI: 10.1007/s00441-018-2975-y |
| [29] | SLOAT S R, WHITLEY B N, ENGELHART E A, et al. Identification of a mitofusin specificity region that confers unique activities to Mfn1 and Mfn2[J]. Mol Biol Cell, 2019, 30(17): 2309–2319. DOI: 10.1091/mbc.E19-05-0291 |
| [30] | CHANDHOK G, LAZAROU M, NEUMANN B. Structure, function, and regulation of mitofusin-2 in health and disease[J]. Biol Rev Camb Philos Soc, 2018, 93(2): 933–949. DOI: 10.1111/brv.12378 |
| [31] | OANH N T K, PARK Y Y, CHO H. Mitochondria elongation is mediated through SIRT1-mediated MFN1 stabilization[J]. Cell Signal, 2017, 38: 67–75. DOI: 10.1016/j.cellsig.2017.06.019 |
| [32] | BASSO V, MARCHESAN E, PEGGION C, et al. Regulation of ER-mitochondria contacts by Parkin via Mfn2[J]. Pharmacol Res, 2018, 138: 43–56. DOI: 10.1016/j.phrs.2018.09.006 |
| [33] | CHEN K H, GUO X M, MA D L, et al. Dysregulation of HSG triggers vascular proliferative disorders[J]. Nat Cell Biol, 2004, 6(9): 872–883. DOI: 10.1038/ncb1161 |
| [34] | KOSHIBA T, DETMER S A, KAISER J T, et al. Structural basis of mitochondrial tethering by mito-fusin complexes[J]. Science, 2004, 305(5685): 858–862. DOI: 10.1126/science.1099793 |
| [35] | DEL DOTTO V, FOGAZZA M, CARELLI V, et al. Eight human OPA1 isoforms, long and short: what are they for?[J]. Biochim Biophys Acta, 2018, 1859(4): 263–269. DOI: 10.1016/j.bbabio.2018.01.005 |
| [36] | ANNESLEY S J, FISHER P R. Mitochondria in health and disease[J]. Cells, 2019, 8(7): 680. DOI: 10.3390/cells8070680 |
| [37] | TWIG G, SHIRIHAI O S. The interplay between mitochondrial dynamics and mitophagy[J]. Antioxid Redox Signal, 2011, 14(10): 1939–1951. DOI: 10.1089/ars.2010.3779 |
| [38] | LITTLE A C, KOVALENKO I, GOO L E, et al. High-content fluorescence imaging with the metabolic flux assay reveals insights into mitochondrial properties and functions[J]. Commun Biol, 2020, 3(1): 271. DOI: 10.1038/s42003-020-0988-z |
| [39] | MITRA K, WUNDER C, ROYSAM B, et al. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase[J]. Proc Natl Acad Sci U S A, 2009, 106(29): 11960–11965. DOI: 10.1073/pnas.0904875106 |
| [40] | LIU R H, JIN P P, YU L Q, et al. Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle[J]. PLoS One, 2014, 9(3): e92810. DOI: 10.1371/journal.pone.0092810 |
| [41] | ZHANG Z, CUI D, ZHANG T, et al. Swimming differentially affects T2DM-induced skeletal muscle ER stress and mitochondrial dysfunction related to MAM[J]. Diabetes Metab Syndr Obes, 2020, 13: 1417–1428. DOI: 10.2147/DMSO.S243024 |
| [42] | GOMES L C, BENEDETTO G D, SCORRANO L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability[J]. Nat Cell Biol, 2011, 13(5): 589–598. DOI: 10.1038/ncb2220 |
| [43] | LEE J Y, KAPUR M, LI M, et al. MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria[J]. J Cell Sci, 2014, 127(Pt 22): 4954–4963. |
| [44] | TONDERA D, GRANDEMANGE S, JOURDAIN A, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion[J]. EMBO J, 2009, 28(11): 1589–1600. DOI: 10.1038/emboj.2009.89 |
| [45] | FARMER T, NASLAVSKY N, CAPLAN S. Tying trafficking to fusion and fission at the mighty mito-chondria[J]. Traffic, 2018, 19(8): 569–577. DOI: 10.1111/tra.12573 |
| [46] | FUJIOKA H, TANDLER B, HOPPEL C L. Mitochondrial division in rat cardiomyocytes: an electron microscope study[J]. Anat Rec (Hoboken), 2012, 295(9): 1455–1461. DOI: 10.1002/ar.22523 |
| [47] | KAMERKAR S C, KRAUS F, SHARPE A J, et al. Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission[J]. Nat Commun, 2018, 9(1): 5239. DOI: 10.1038/s41467-018-07543-w |
| [48] | YU R, JIN S B, LENDAHL U, et al. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery[J]. EMBO J, 2019, 38(8): e99748. |
| [49] | YU R, LIU T, NING C F, et al. The phosphorylation status of Ser-637 in dynamin-related protein 1 (Drp1) does not determine Drp1 recruitment to mitochondria[J]. J Biol Chem, 2019, 294(46): 17262–17277. DOI: 10.1074/jbc.RA119.008202 |
| [50] | CHEN K H, DASGUPTA A, LIN J H, et al. Epi-genetic dysregulation of the dynamin-related protein 1 binding partners MiD49 and MiD51 increases mitotic mitochondrial fission and promotes pulmonary arterial hypertension: mechanistic and therapeutic implications[J]. Circulation, 2018, 138(3): 287–304. DOI: 10.1161/CIRCULATIONAHA.117.031258 |
| [51] | SCARPELLI P H, TESSARIN-ALMEIDA G, VIÇOSO K L, et al. Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related-genes for mitochondria fission: mitoemerald-GFP as a tool to visualize mitochondria structure[J]. J Pineal Res, 2019, 66(2): e12484. DOI: 10.1111/jpi.12484 |
| [52] | ETXEBARRIA A, TERRONES O, YAMAGUCHI H, et al. Endophilin B1/Bif-1 stimulates BAX activation independently from its capacity to produce large scale membrane morphological rearrangements[J]. J Biol Chem, 2009, 284(7): 4200–4212. DOI: 10.1074/jbc.M808050200 |
| [53] | RZEPNIKOWSKA W, KOCHA N ' SKI A. A role for the GDAP1 gene in the molecular pathogenesis of Charcot-Marie-Tooth disease[J]. Acta Neurobiol Exp (Wars), 2018, 78(1): 1–13. |
| [54] | XIAO L, XIAN H X, LEE K Y, et al. Death-associated protein 3 regulates mitochondrial-encoded protein synthesis and mitochondrial dynamics[J]. J Biol Chem, 2015, 290(41): 24961–24974. DOI: 10.1074/jbc.M115.673343 |
| [55] | AUNG L H H, LI Y Z, YU H, et al. Mitochondrial protein 18 is a positive apoptotic regulator in cardiomyocytes under oxidative stress[J]. Clin Sci (Lond), 2019, 133(9): 1067–1084. DOI: 10.1042/CS20190125 |
| [56] | PAGLIUSO A, COSSART P, STAVRU F. The ever-growing complexity of the mitochondrial fission machinery[J]. Cell Mol Life Sci, 2018, 75(3): 355–374. DOI: 10.1007/s00018-017-2603-0 |
| [57] | ZHANG L, ZHAO P H, YUE C P, et al. Sustained release of bioactive hydrogen by Pd hydride nano-particles overcomes Alzheimer's disease[J]. Biomaterials, 2019, 197: 393–404. DOI: 10.1016/j.biomaterials.2019.01.037 |
| [58] | PENNANEN C, PARRA V, LÓPEZ-CRISOSTO C, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway[J]. J Cell Sci, 2014, 127(Pt 12): 2659–2671. |
| [59] | FRIEDMAN J R, LACKNER L L, WEST M, et al. ER tubules mark sites of mitochondrial division[J]. Science, 2011, 334(6054): 358–362. DOI: 10.1126/science.1207385 |
| [60] | KORNMANN B, CURRIE E, COLLINS S R, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen[J]. Science, 2009, 325(5939): 477–481. DOI: 10.1126/science.1175088 |
| [61] | DE BRITO O M, SCORRANO L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria[J]. Nature, 2008, 456(7222): 605–610. DOI: 10.1038/nature07534 |
| [62] | HIRABAYASHI Y, KWON S K, PAEK H, et al. ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons[J]. Science, 2017, 358(6363): 623–630. DOI: 10.1126/science.aan6009 |
| [63] | FRIEDMAN J R, WEBSTER B M, MASTRONARDE D N, et al. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules[J]. J Cell Biol, 2010, 190(3): 363–375. DOI: 10.1083/jcb.200911024 |
| [64] | KOROBOVA F, RAMABHADRAN V, HIGGS H N. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2[J]. Science, 2013, 339(6118): 464–467. DOI: 10.1126/science.1228360 |
| [65] | MEARS J A, LACKNER L L, FANG S M, et al. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission[J]. Nat Struct Mol Biol, 2011, 18(1): 20–26. DOI: 10.1038/nsmb.1949 |
| [66] | LOSÓN O C, SONG Z Y, CHEN H, et al. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission[J]. Mol Biol Cell, 2013, 24(5): 659–667. DOI: 10.1091/mbc.e12-10-0721 |
| [67] | OTERA H, WANG C X, CLELAND M M, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells[J]. J Cell Biol, 2010, 191(6): 1141–1158. DOI: 10.1083/jcb.201007152 |
| [68] | PALMER C S, OSELLAME L D, LAINE D, et al. MiD49 and MiD51, new components of the mito-chondrial fission machinery[J]. EMBO Rep, 2011, 12(6): 565–573. DOI: 10.1038/embor.2011.54 |
| [69] | ZHAO J, LIU T, JIN S B, et al. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission[J]. EMBO J, 2011, 30(14): 2762–2778. DOI: 10.1038/emboj.2011.198 |
| [70] | FRÖHLICH C, GRABIGER S, SCHWEFEL D, et al. Structural insights into oligomerization and mito-chondrial remodelling of dynamin 1-like protein[J]. EMBO J, 2013, 32(9): 1280–1292. DOI: 10.1038/emboj.2013.74 |
| [71] | GONZÁLEZ-JAMETT A M, MOMBOISSE F, HARO-ACUÑA V, et al. Dynamin-2 function and dys-function along the secretory pathway[J]. Front Endocrinol (Lausanne), 2013, 4: 126. |
| [72] | FERGUSON S M, DE CAMILLI P. Dynamin, a membrane-remodelling GTPase[J]. Nat Rev Mol Cell Biol, 2012, 13(2): 75–88. |
| [73] | LEE J E, WESTRATE L M, WU H X, et al. Multiple dynamin family members collaborate to drive mito-chondrial division[J]. Nature, 2016, 540(7631): 139–143. DOI: 10.1038/nature20555 |
| [74] | HAN X J, LU Y F, LI S A, et al. CaM kinase Iα-induced phosphorylation of Drp1 regulates mito-chondrial morphology[J]. J Cell Biol, 2008, 182(3): 573–585. DOI: 10.1083/jcb.200802164 |
| [75] | BO T, YAMAMORI T, SUZUKI M, et al. Calmodulin-dependent protein kinase Ⅱ (CaMKⅡ) mediates radiation-induced mitochondrial fission by regulating the phosphorylation of dynamin-related protein 1 (Drp1) at serine 616[J]. Biochem Biophys Res, 2018, 495(2): 1601–1607. DOI: 10.1016/j.bbrc.2017.12.012 |
| [76] | MOLINA A J A, WIKSTROM J D, STILES L, et al. Mitochondrial networking protects β-cells from nutrient-induced apoptosis[J]. Diabetes, 2009, 58(10): 2303–2315. DOI: 10.2337/db07-1781 |
| [77] | MAI N, CHRZANOWSKA-LIGHTOWLERS Z M A, LIGHTOWLERS R N. The process of mammalian mitochondrial protein synthesis[J]. Cell Tissue Res, 2017, 367(1): 5–20. DOI: 10.1007/s00441-016-2456-0 |
| [78] | WASILEWSKI M, CHOJNACKA K, CHACINSKA A. Protein trafficking at the crossroads to mito-chondria[J]. Biochim Biophys Acta, 2017, 1864(1): 125–137. DOI: 10.1016/j.bbamcr.2016.10.019 |
| [79] | CHABAN Y, BOEKEMA E J, DUDKINA N V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation[J]. Biochim Biophys Acta, 2014, 1837(4): 418–426. DOI: 10.1016/j.bbabio.2013.10.004 |
| [80] | BARADARAN R, BERRISFORD J M, MINHAS G S, et al. Crystal structure of the entire respiratory complex I[J]. Nature, 2013, 494(7438): 443–448. DOI: 10.1038/nature11871 |
| [81] | EFREMOV R G, BARADARAN R, SAZANOV L A. The architecture of respiratory complex I[J]. Nature, 2010, 465(7297): 441–445. DOI: 10.1038/nature09066 |
| [82] | GUERRERO-CASTILLO S, BAERTLING F, KOWNATZKI D, et al. The assembly pathway of mitochondrial respiratory chain complex I[J]. Cell Metab, 2017, 25(1): 128–139. DOI: 10.1016/j.cmet.2016.09.002 |
| [83] | YANKOVSKAYA V, HORSEFIELD R, TÖRNROTH S, et al. Architecture of succinate dehydrogenase and reactive oxygen species generation[J]. Science, 2003, 299(5607): 700–704. DOI: 10.1126/science.1079605 |
| [84] | SUN F, HUO X, ZHAI Y J, et al. Crystal structure of mitochondrial respiratory membrane protein complex Ⅱ[J]. Cell, 2005, 121(7): 1043–1057. DOI: 10.1016/j.cell.2005.05.025 |
| [85] | SOUSA J S, D'IMPRIMA E, VONCK J. Mitochondrial respiratory chain complexes[M]// HARRIS J, BOEKEMA E. Membrane Protein Complexes: Structure and Function. Singapore: Springer, 2018: 167-227. |
| [86] | IWATA S, LEE J W, OKADA K, et al. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex[J]. Science, 1998, 281(5373): 64–71. DOI: 10.1126/science.281.5373.64 |
| [87] | CROFTS A R, HOLLAND J T, VICTORIA D, et al. The Q-cycle reviewed: how well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex?[J]. Biochim Biophys Acta, 2008, 1777(7-8): 1001–1019. DOI: 10.1016/j.bbabio.2008.04.037 |
| [88] | BOTTANI E, CERUTTI R, HARBOUR M E, et al. TTC19 plays a husbandry role on UQCRFS1 turnover in the biogenesis of mitochondrial respiratory complex Ⅲ[J]. Mol Cell, 2017, 67(1): 96–105. DOI: 10.1016/j.molcel.2017.06.001 |
| [89] | BAREL O, SHORER Z, FLUSSER H, et al. Mitochondrial complex Ⅲ deficiency associated with a homozygous mutation in UQCRQ[J]. Am J Hum Genet, 2008, 82(5): 1211–1216. DOI: 10.1016/j.ajhg.2008.03.020 |
| [90] | ROGER A J, MUÑOZ-GÓMEZ S A, KAMIKAWA R. The origin and diversification of mitochondria[J]. Curr Biol, 2017, 27(21): R1177–R1192. DOI: 10.1016/j.cub.2017.09.015 |
| [91] | BALSA E, MARCO R, PERALES-CLEMENTE E, et al. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain[J]. Cell Metab, 2012, 16(3): 378–386. DOI: 10.1016/j.cmet.2012.07.015 |
| [92] | KADENBACH B. Regulation of mammalian 13-subunit cytochrome c oxidase and binding of other proteins: role of NDUFA4[J]. Trends Endocrinol Metab, 2017, 28(11): 761–770. DOI: 10.1016/j.tem.2017.09.003 |
| [93] | FORNUSKOVA D, STIBUREK L, WENCHICH L, et al. Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b[J]. Biochem J, 2010, 428(3): 363–374. DOI: 10.1042/BJ20091714 |
| [94] | HVTTEMANN M, KADENBACH B, GROSSMAN L I. Mammalian subunit IV isoforms of cytochrome c oxidase[J]. Gene, 2001, 267(1): 111–123. DOI: 10.1016/S0378-1119(01)00385-7 |
| [95] | SINKLER C A, KALPAGE H, SHAY J, et al. Tissue- and condition-specific isoforms of mammalian cytochrome c oxidase subunits: from function to human disease[J]. Oxid Med Cell Longev, 2017, 2017: 1534056. |
| [96] | ZHOU A N, ROHOU A, SCHEP D G, et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM[J]. eLife, 2015, 4: e10180. DOI: 10.7554/eLife.10180 |
| [97] | WALKER J E. The ATP synthase: the understood, the uncertain and the unknown[J]. Biochem Soc Trans, 2013, 41(1): 1–16. DOI: 10.1042/BST20110773 |
| [98] | JONCKHEERE A I, SMEITINK J A M, RODENBURG R J T. Mitochondrial ATP synthase: architecture, function and pathology[J]. J Inherit Metab Dis, 2012, 35(2): 211–225. DOI: 10.1007/s10545-011-9382-9 |
| [99] | GUO R Y, GU J K, ZONG S, et al. Structure and mechanism of mitochondrial electron transport chain[J]. Biomed J, 2018, 41(1): 9–20. DOI: 10.1016/j.bj.2017.12.001 |
| [100] | MOURIER A, MATIC S, RUZZENENTE B, et al. The respiratory chain supercomplex organization is independent of COX7a2l isoforms[J]. Cell Metab, 2014, 20(6): 1069–1075. DOI: 10.1016/j.cmet.2014.11.005 |
| [101] | SCHÄGGER H. Respiratory chain supercomplexes of mitochondria and bacteria[J]. Biochim Biophys Acta, 2002, 1555(1-3): 154–159. DOI: 10.1016/S0005-2728(02)00271-2 |
| [102] | LIU F, LÖSSL P, RABBITTS B M, et al. The interactome of intact mitochondria by cross-linking mass spectrometry provides evidence for coexisting respiratory supercomplexes[J]. Mol Cell Proteomics, 2018, 17(2): 216–232. DOI: 10.1074/mcp.RA117.000470 |
| [103] | SIGNES A, FERNANDEZ-VIZARRA E. Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes[J]. Essays Biochem, 2018, 62(3): 255–270. DOI: 10.1042/EBC20170098 |
| [104] | GUO R Y, ZONG S, WU M, et al. Architecture of human mitochondrial respiratory megacomplex I2Ⅲ2IV2[J]. Cell, 2017, 170(6): 1247–1257. DOI: 10.1016/j.cell.2017.07.050 |
| [105] | GU J K, WU M, GUO R Y, et al. The architecture of the mammalian respirasome[J]. Nature, 2016, 537(7622): 639–643. DOI: 10.1038/nature19359 |
| [106] | LETTS J A, FIEDORCZUK K, SAZANOV L A. The architecture of respiratory supercomplexes[J]. Nature, 2016, 537(7622): 644–648. DOI: 10.1038/nature19774 |
| [107] | BARRIENTOS A, UGALDE C. I function, therefore I am: overcoming skepticism about mitochondrial supercomplexes[J]. Cell Metab, 2013, 18(2): 147–149. DOI: 10.1016/j.cmet.2013.07.010 |
| [108] | LAPUENTE-BRUN E, MORENO-LOSHUERTOS R, ACÍN-PÉREZ R, et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain[J]. Science, 2013, 340(6140): 1567–1570. DOI: 10.1126/science.1230381 |
| [109] | MORENO-LASTRES D, FONTANESI F, GARCÍA-CONSUEGRA I, et al. Mitochondrial complex I plays an essential role in human respirasome assembly[J]. Cell Metab, 2012, 15(3): 324–335. DOI: 10.1016/j.cmet.2012.01.015 |
| [110] | FERNÁNDEZ-VIZARRA E, TIRANTI V, ZEVIANI M. Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects[J]. Biochim Biophys Acta, 2009, 1793(1): 200–211. DOI: 10.1016/j.bbamcr.2008.05.028 |
| [111] | WILLIAMS E G, WU Y B, JHA P, et al. Systems proteomics of liver mitochondria function[J]. Science, 2016, 352(6291): aad0189. DOI: 10.1126/science.aad0189 |
| [112] | PÉREZ-PÉREZ R, LOBO-JARNE T, MILENKOVIC D, et al. COX7A2L is a mitochondrial complex Ⅲ binding protein that stabilizes the Ⅲ2+IV super-complex without affecting respirasome formation[J]. Cell Rep, 2016, 16(9): 2387–2398. DOI: 10.1016/j.celrep.2016.07.081 |