颗粒细胞(granulosa cells,GCs)作为哺乳动物卵巢的主要功能细胞,在卵泡发育过程中发挥至关重要的作用。卵泡发育起始于颗粒细胞的发育,窦前卵泡发育后期,颗粒细胞开始表达FSHR,可使雄激素转变为雌激素,促进卵泡发育,形成优势卵泡[1]。研究发现,卵泡在不同发育阶段都存在卵泡闭锁[2],而卵泡闭锁受多种因素调节,其中卵泡颗粒细胞的凋亡就能够直接诱发卵泡闭锁[3-4]。
microRNA(miRNA)是一种细胞内源性长度约22~24 nt的非编码RNA,通过调控靶基因参与多种细胞过程,如细胞凋亡、分化和增殖[5-6]。利用高通量测序技术从不同物种的卵巢组织中鉴定出大量miRNAs[7-12],研究发现其广泛参与原始卵泡募集[13-14]、优势卵泡选择[15]、颗粒细胞增殖分化[16]、甾体类激素的合成与分泌[17-19]、卵母细胞成熟[20]、排卵[21]以及黄体形成[22]等卵泡发育的各个环节[23]。miR-483-5p和miR-486-5p与人卵丘细胞的增殖和卵泡的发育相关[24],miR-145影响转化生长因子β受体2 (TGFBR2)基因的表达进而调控始基卵泡的募集[13],miR-150通过抑制类固醇合成急性调节蛋白(STAR)基因的表达影响类固醇激素的合成和分泌[17]。miR-214-3p可能通过靶向线粒体融合蛋白-2(MFN2) 和核受体5A1(NR5A1)调控猪颗粒细胞增殖和雌激素的合成[18]。因此,miRNA在动物卵巢周期性变化、激素合成和繁殖过程发挥重要调控作用。
miR-200家族包括miR-200a、miR-200b、miR-200c、miR-141和miR-429,且在脊椎动物高度保守[25]。miR-200a和miR-141可能通过靶向CD36和肿瘤坏死因子α刺激因子-6(TNFAIP6)调控山羊的卵泡发育[26],miR-200b可能通过下调GNAQ反馈调控GnRH的表达来调控绵羊的发情过程[27]。此外,敲除miR-200b和miR-429的雌性小鼠不排卵[28],敲除miR-429a、miR-200a和miR-200b显著降低斑马鱼的精子运动[29]。miR-200s参与动物卵泡发育、发情周期、排卵及精子运动等生物过程,由此可见其与动物生殖密切相关。本课题组前期研究发现,miR-200s在小尾寒羊发情期和间情期卵巢中差异表达[30]。研究证实,miR-200b可以通过直接靶向结合磷酸酶张力蛋白同源物(PTEN)3′UTR抑制卵巢颗粒细胞(KGN)增殖[31]。本试验以miR-200b为研究对象,初步探索其对绵羊卵泡颗粒细胞增殖凋亡过程的影响,为进一步研究miR-200b对绵羊卵泡发育的调控机制奠定基础。
1 材料与方法 1.1 材料DMEM/F12培养基和胎牛血清FBS(Gibco,美国)购自赛默飞世尔科技(Thermo Fisher Scientific)有限公司;CCK8检测试剂盒购自日本同仁化学研究所(Dojindo,日本);HiPerFect Transfection Reagent购自QIAGEN。miR-200b mimic、miR-200b inhhibitor、mimic NC、inhibitor NC由广州锐博生物科技有限公司合成。
1.2 绵羊miR-200b的生物信息学分析通过NCBI(https://www.ncbi.nlm.nih.gov/)及miRBase(http://www.mirbase.org/)数据库查找各物种miR-200b的成熟序列。利用在线工具TargetScan(http://www.targetscan.org/vert_72/)、miRDB(hwz ttp: //www.mirdb.org/index.html)和miRTarBase(http://mirtarbase.cuhk.edu.cn/php/index.php)分别对miR-200b进行靶基因预测,将数据进行分析整理,利用在线工具KOBAS 3.0(http://kobas.cbi.pku.edu.cn/kobas3/?t=1)对靶基因进行Gene Ontology(GO)和Kyoto Encyclopedia of Genes and Genomes(KEGG)分析。
1.3 绵羊卵泡颗粒细胞的分离培养绵羊(小尾寒羊)卵巢采自保定瑞丽肉食品有限公司。将采集的卵巢置于37 ℃生理盐水(含1%双抗)中运回实验室。75%酒精润洗消毒后,预热生理盐水洗涤3次,剪去多余脂肪和系膜,转至超净台。PBS洗3次,利用无菌刀片在无血清培养基中轻轻划破卵泡(3~7 mm),使卵泡液混于培养基中。然后将混合液转移至15 mL离心管,1 500 r·min-1离心10 min,弃上清,PBS洗涤两次,加入2 mL DMEM/F12(1∶1)完全培养基(含10%胎牛血清,1%双抗和50 μg·mL-1丙酮酸钠)吹打混匀后接种于25 cm细胞培养瓶中,再加入3 mL完全培养基。最后置于37 ℃、5% CO2培养箱内预培养过夜。第2天清洗换液,继续培养至细胞汇合度达70%~90%时传代。
1.4 绵羊卵泡颗粒细胞的转染与增殖检测转染前以每孔2×104的密度将颗粒细胞接种于96孔板,每孔100 μL细胞悬液,每组6个重复,37 ℃预培养。用opti-M分别稀释50 nmol·L-1 miR-200b mimic(过表达组)、mimic NC(mimic阴性对照组)和100 nmol·L-1 miR-200b inhibitor(抑表达组)、inhibitor NC(inhibitor阴性对照组),低速漩涡混匀,加入0.75 μL HiPerFect Transfection Reagent,低速漩涡混匀,室温静置10 min,形成转染复合体。然后将转染复合体逐滴加至96孔板中。分别在颗粒细胞转染24、48和72 h后,每孔加10 μL CCK8溶液,37 ℃、5% CO2培养箱内孵育2 h,利用酶标仪检测吸光度(450 nm),观察细胞增殖情况。试验重复3次。
1.5 引物设计采用茎环法设计绵羊miR-200b引物,其反转录引物序列:CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCATCATTA,上游引物序列:GCCGAGTAATACTGCCTGG,下游通用序列:CTCAACTGGTGTCGTGGA,并以U6为内参基因。利用Primer 3.0软件设计其他基因的引物, 并通过NCBI中Primer-BLAST初步查验引物特异性。CDK4、CDK6、CCND1、CCND2、Bax及Bcl-2表达量的检测均以GAPDH为内参基因。引物均由通用生物系统有限公司合成,引物序列详见表 1。
|
|
表 1 qRT-PCR引物序列 Table 1 qRT-PCR primer sequences |
转染前,以每孔2.5×105的密度将颗粒细胞接种于6孔板,每孔2 300 μL细胞悬液,每组3个重复,37 ℃预培养。用opti-M分别稀释50 nmol·L-1 miR-200b mimic、mimic NC和100 nmol·L-1 miR-200b inhibitor、inhibitor NC,低速漩涡混匀,加入12 μL HiPerFect Transfection Reagent,低速漩涡混匀,室温静置10 min,形成转染复合体。然后将转染复合体逐滴加至6孔板中,37 ℃、5% CO2培养箱内继续培养。转染48 h后,收集细胞培养液,PBS清洗细胞2次,利用TRIzolTM Reagent提取RNA。使用PrimeScriptTMRT reagent Kit(宝生物)反转录成cDNA。首先除去DNA,反应体系:RNA 800 μg,5×gDNA Reaction Buffer 2 μL,gDNA Eraser 1 μL,RNase-free dH2O补至10 μL;42 ℃孵育2 min,4 ℃保存。然后进行反转录,20 μL反应体系:上述反应液10 μL,PrimeScript RT Enzyme Mix I 1 μL,RT Primer Mix 1 μL,5×PrimeScript Buffer 2(for Real Time)4 μL,RNase Free dH2 O 4 μL。轻轻混匀,37 ℃孵育15 min,85 ℃加热失活5 s,产物-20 ℃保存。最后使用Forget-Me-NotTM EvagreenⓇ qPCR Master Mix(Biotium)进行qRT-PCR检测,反应体系:2×Forget-Me-NotTM qPCR Master Mix 10 μL,Forget-Me-Not EvaGreen ROX Reference Dye 3 μL,RNase-free dH2 O 4.2 μL,cDNA 2 μL,Forward Primer 0.4 μL,Reverse Primer 0.4 μL。反应条件:95 ℃ 2 min,95 ℃ 30 s,60 ℃ 5 s共40个循环。
1.7 数据统计分析数据均采用SPSS 22.0进行统计分析,使用GraphPad Prism进行作图。qRT-PCR数据采用2-ΔΔCT方法进行计算。结果以“平均值±标准差(Mean±SD)”表示。
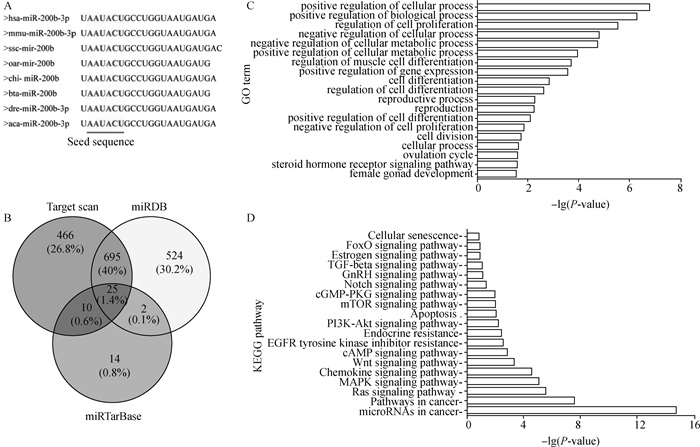
2 结果 2.1 绵羊miR-200b生物信息学分析利用NCBI和miRBase查出各物种miR-200b成熟序列,其种子区域均为AAUACU,说明miR-200b核心序列在各物种间高度保守(图 1A)。利用3种在线工具TargetScan、miRTarBase以及miRDB分别预测出1 196、51和1 246个基因与miR-200b有靶向关系,并用Venny2.1在线网站(https://bioinfogp.cnb.csic.es/tools/venny/)对预测的结果进行交互绘制,如图 1B所示。其中,3个软件预测出25个共同靶基因。GO分析发现,这些靶基因主要富集在细胞的增殖分化、细胞周期及生殖发育过程(图 1C);KEGG分析发现,miR-200b除了参与癌症相关通路,还参与Ras信号通路、MAPK信号通路、PI3K-Akt信号通路、mTOR信号通路及雌激素信号通路等(图 1D),且这些信号通路与细胞周期、细胞生物学过程及生殖过程相关。
|
A.不同物种miR-200b成熟序列;B. miR-200b预测靶基因的韦恩图;C. GO富集分析;D. KEGG通路富集分析 A. miR-200b mature sequence of various species; B. Venn diagram of miR-200b predicted target genes; C. GO enrichment analysis; D. KEGG pathway enrichment analysis 图 1 miR-200b的生物信息学分析 Fig. 1 Bioinformatics analysis of miR-200b |
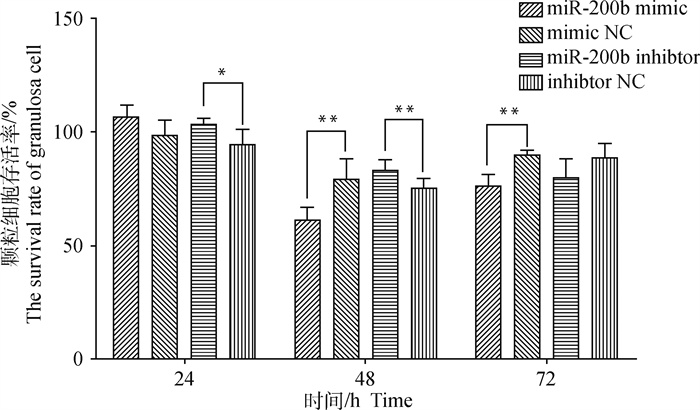
从图 2中可以看出,与mimic NC相比,miR-200b mimic转染24 h颗粒细胞存活率并无显著变化(P>0.05),但48和72 h细胞存活率均极显著降低(P < 0.01);与inhibitor NC相比,miR-200b inhibitor转染24 h细胞存活率显著升高(P < 0.05),48 h极显著升高(P < 0.01),但72 h无显著变化(P>0.05)。随转染时间延长,各组细胞存活率呈“V”型变化趋势,且在48 h达到最低。因此,转染miR-200b可降低颗粒细胞存活率,抑制细胞增殖,并有一定时间效应,本试验选取转染48 h进行后续试验。
|
*. P < 0.05;**. P < 0.01;***. P < 0.001。下同 *. P < 0.05; **. P < 0.01; ***. P < 0.001. The same as below 图 2 转染miR-200b mimic、miR-200b inhibitor及NC的颗粒细胞存活率 Fig. 2 The survival rate of granulosa cell transfected with miR-200b mimic, miR-200b inhibitor and NC |
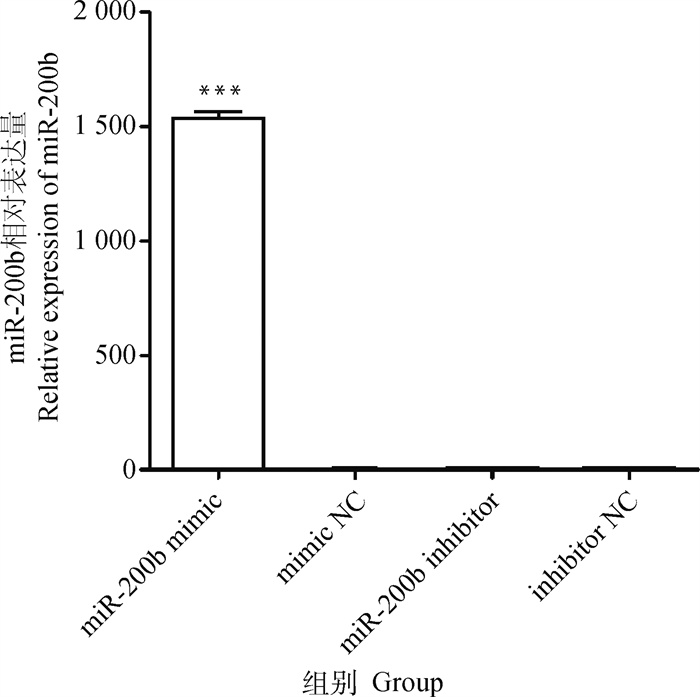
转染miR-200b mimic组的miR-200b表达量是mimic NC组的1 391.50倍(图 3),差异极显著(P < 0.001);而mimic NC、inhibitor NC及miR-200b inhibitor组的miR-200b表达量基本接近,无显著差异(P>0.05)。
|
图 3 miR-200b在卵泡颗粒细胞中的表达水平 Fig. 3 The expression level of miR-200b in follicular granulosa cells |
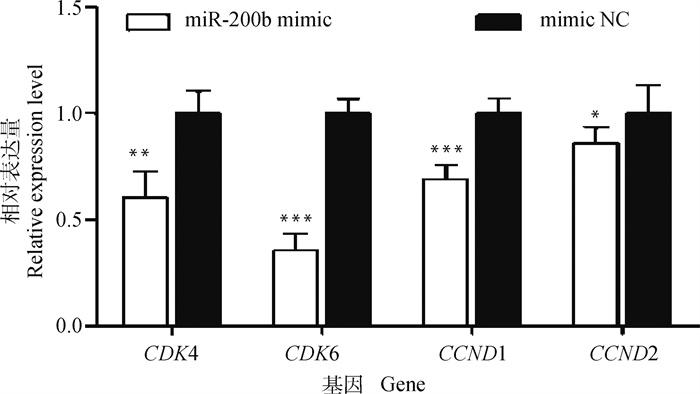
从图 4中可以看出,与mimic NC相比,转染miR-200b mimic极显著下调CDK4(P < 0.01)、CDK6(P < 0.001)及CCND1(P < 0.001)基因表达水平,显著下调CCND2基因的表达水平(P < 0.05)。表明,miR-200b可抑制细胞周期进程。
|
图 4 转染miR-200b mimic和mimic NC的颗粒细胞周期相关基因表达水平 Fig. 4 The expression level of cell cycle related genes in granulosa cells transfected with miR-200b mimic and mimic NC |
从图 5中可以看出,与mimic NC相比,转染miR-200b mimic极显著抑制Bcl-2基因表达水平(P < 0.01),而对Bax基因mRNA表达水平无显著影响(P>0.05)。此外,转染miR-200b mimic极显著下调Bcl-2与Bax的比值(P < 0.001)。
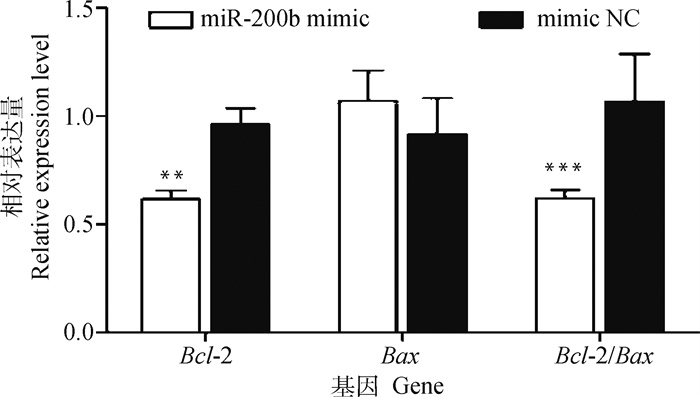
|
图 5 转染miR-200b mimic和mimic NC的颗粒细胞凋亡相关基因表达水平 Fig. 5 The expression level of apoptosis related genes in granulosa cells transfected with miR-200b mimic and mimic NC |
卵泡发育是一个复杂精密的周期性过程,颗粒细胞作为哺乳动物卵巢的主要功能细胞,其生长分化在卵泡发育过程中发挥关键性的作用。通过转录组测序技术,对1和8月龄湖羊的卵巢组织分别进行测序,鉴定出miR-200a、miR-200b和miR-200c为其中3个差异表达的miRNAs,且与1月龄湖羊卵巢相比,8月龄的表达量显著下调,说明miR-200s与绵羊卵巢发育息息相关[32]。通过构建和分析小尾寒羊间情期和发情期卵巢组织miRNA表达谱,筛选出这两个时期差异表达的miRNAs,经进一步分析获得miR-200a、miR-200b和miR-200c这3个显著差异表达的miRNAs,且在发情期较间情期均表达下调[30, 33]。本研究对绵羊miR-200b靶基因进行GO和KEGG富集分析发现,这些基因参与细胞的增殖分化、细胞周期及生殖发育过程,表明miR-200b调控细胞生物学过程。因此,本试验针对miR-200b对绵羊卵泡颗粒细胞周期和凋亡的影响进行了研究。
通过检测细胞存活率,发现各处理组均存在先降后升的现象,这可能与绵羊卵泡颗粒细胞和miR-200b转染时间相关。对于绵羊卵泡颗粒细胞,24 h转染时间较短,转染效率较低,72 h转染时间较长转染可能逐步失效,而48 h转染效果很显著,说明转染效率高,因此后续试验均选择转染48 h。通过检测miR-200b表达量,发现miR-200b mimic组极显著升高,而miR-200b inhibitor组和inhibitor NC组并无显著差异,可能是由于绵羊卵泡颗粒细胞inhibitor选用剂量较低,效果不理想,因此后续试验只选用了mimic及其NC处理组进行过表达研究。研究表明,miRNA影响颗粒细胞增殖和凋亡过程。miR-214-3p能够在转录和翻译水平上调细胞周期相关基因(Cyclin B、Cyclin D、Cyclin E及CDK4)表达从而促进猪颗粒细胞增殖[18],miR-16过表达促进颗粒细胞增殖,诱导细胞周期进程并抑制细胞凋亡[34],而miR-379-5p过表达抑制颗粒细胞增殖[35]。本研究中,miR-200b过表达降低颗粒细胞存活率,并下调CDK4、CDK6、CCND1和CCND2基因表达,而上述4个基因参与细胞周期G1期的进程及G1/S期转化。因此,miR-200b通过抑制绵羊颗粒细胞G1期相关基因表达影响细胞周期,从而抑制细胞增殖。与本试验结果一致,He等[31]发现,过表达miR-200b可抑制人卵巢颗粒细胞(KGN)增殖。
Zhang等[36]对miRNA与颗粒细胞凋亡和卵泡闭锁的调控关系进行了综述分析得出,miRNA调控的卵泡闭锁主要是由颗粒细胞凋亡所致。miR-1275促进猪颗粒细胞凋亡和卵泡闭锁并通过损害LRH-1/CYP19A1轴调控E2分泌[37],miR-204-5p通过靶向Bcl-2调控大鼠卵巢颗粒细胞凋亡[38]。本研究中,miR-200b过表达能从转录水平抑制Bcl-2表达并极显著下调Bcl-2与Bax比值,促进绵羊卵泡颗粒细胞凋亡。Bcl-2家族调控细胞凋亡属于介导细胞凋亡的最重要途径——线粒体途径。Bax的过表达可以加速诱导颗粒细胞的凋亡,而Bcl-2基因过表达可以拮抗Bax基因,抑制卵泡膜细胞及颗粒细胞的凋亡,控制卵母细胞凋亡,进而延缓卵泡闭锁[39]。Bax/Bcl-2的比值可以揭示细胞的生长或凋亡情况[40]。颗粒细胞凋亡是导致发育期卵泡闭锁的关键因素[41-43],本研究中,绵羊卵泡颗粒细胞Bcl-2/Bax比值降低,表明miR-200b促进了细胞凋亡,推测其可能对绵羊卵巢卵泡闭锁具有重要调控作用。
4 结论本研究结果表明,miR-200b通过降低颗粒细胞存活率抑制细胞增殖、促使细胞周期相关基因表达量降低、下调Bcl-2/Bax比值来抑制细胞周期并促进细胞凋亡进而调控绵羊卵泡颗粒细胞。
| [1] |
仝晓丽. 睾酮在体外通过促进颗粒细胞凋亡引起卵泡闭锁的机制研究[D]. 长春: 吉林大学, 2020. TONG X L. Study on the mechanism of follicular atresia induced by testosterone in vitro by promoting granulosa cell apoptosis[D]. Changchun: Jilin University, 2020. (in Chinese) |
| [2] |
郭亚军, 柳苗苗, 付德海, 等. 藏绵羊卵巢组织学及卵泡超微形态的观察[J]. 畜牧兽医学报, 2021, 52(2): 389-398. GUO Y J, LIU M M, FU D H, et al. Observation of ovary histology and ultrastructure of follicles in Tibetan sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(2): 389-398. (in Chinese) |
| [3] |
WANG C L, FAN Y C, TSENG C H, et al. Salmonella Enteritidis infection slows steroidogenesis and impedes cell growth in hen granulosa cells[J]. Avian Dis, 2014, 58(4): 511-517. DOI:10.1637/10846-041414-Reg.1 |
| [4] |
WU Y Q, ZHANG Z H, LIAO X H, et al. High fat diet triggers cell cycle arrest and excessive apoptosis of granulosa cells during the follicular development[J]. Biochem Biophys Res Commun, 2015, 466(3): 599-605. DOI:10.1016/j.bbrc.2015.09.096 |
| [5] |
HSU C Y, HSIEH T H, TSAI C F, et al. Synthetic steroid hormones regulated cell proliferation through microRNA-34a-5p in human ovarian endometrioma[J]. Biol Reprod, 2016, 94(3): 60. |
| [6] |
LIU J Y, TU F, YAO W, et al. Conserved miR-26b enhances ovarian granulosa cell apoptosis through HAS2-HA-CD44-Caspase-3 pathway by targeting HAS2[J]. Sci Rep, 2016, 6: 21197. DOI:10.1038/srep21197 |
| [7] |
YOSHIDA K, YOKOI A, KAGAWA T, et al. Unique miRNA profiling of squamous cell carcinoma arising from ovarian mature teratoma: comprehensive miRNA sequence analysis of its molecular background[J]. Carcinogenesis, 2019, 40(12): 1435-1444. |
| [8] |
ZHANG X D, ZHANG L, SHANG J N, et al. Combined microRNAome and transcriptome analysis of follicular phase and luteal phase in porcine ovaries[J]. Reprod Domest Anim, 2019, 54(7): 1018-1025. DOI:10.1111/rda.13457 |
| [9] |
GU B, LIU H, HAN Y, et al. Integrated analysis of miRNA and mRNA expression profiles in 2-, 6-, and 12-month-old Small Tail Han Sheep ovaries reveals that oar-miR-432 downregulates RPS6KA1 expression[J]. Gene, 2019, 710: 76-90. DOI:10.1016/j.gene.2019.02.095 |
| [10] |
SONG P Y, YUE Q X, FU Q, et al. Integrated analysis of miRNA-mRNA interaction in ovaries of Turpan Black Sheep during follicular and luteal phases[J]. Reprod Domest Anim, 2021, 56(1): 46-57. DOI:10.1111/rda.13848 |
| [11] |
LIU Y, WU X Q, XIE J, et al. Identification of transcriptome differences in goat ovaries at the follicular phase and the luteal phase using an RNA-Seq method[J]. Theriogenology, 2020, 158: 239-249. DOI:10.1016/j.theriogenology.2020.06.045 |
| [12] |
PASQUARIELLO R, MANZONI E F M, FIANDANESE N, et al. Implications of miRNA expression pattern in bovine oocytes and follicular fluids for developmental competence[J]. Theriogenology, 2020, 145: 77-85. DOI:10.1016/j.theriogenology.2020.01.027 |
| [13] |
YANG S H, WANG S, LUO A Y, et al. Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary[J]. Biol Reprod, 2013, 89(5): 126. |
| [14] |
LI T T, LIU X Q, GONG X F, et al. microRNA-92b-3p regulates primordial follicle assembly by targeting TSCI in neonatal mouse ovaris[J]. Cell Cycle, 2019, 18(8): 824-833. DOI:10.1080/15384101.2019.1593648 |
| [15] |
SONTAKKE S D, MOHAMMED B T, MCNEILLY A S, et al. Characterization of microRNAs differentially expressed during bovine follicle development[J]. Reproduction, 2014, 148(3): 271-283. DOI:10.1530/REP-14-0140 |
| [16] |
PANDE H O, TESFAYE D, HOELKER M, et al. MicroRNA-424/503 cluster members regulate bovine granulosa cell proliferation and cell cycle progression by targeting SMAD7 gene through activin signalling pathway[J]. J Ovarian Res, 2018, 11(1): 34. DOI:10.1186/s13048-018-0410-3 |
| [17] |
ZHOU R Y, MIAO Y P, LI Y M, et al. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene[J]. Theriogenology, 2019, 127: 66-71. DOI:10.1016/j.theriogenology.2019.01.003 |
| [18] |
SHI S J, ZHOU X G, LI J J, et al. MiR-214-3p promotes proliferation and inhibits estradiol synthesis in porcine granulosa cells[J]. J Anim Sci Biotechnol, 2020, 11: 94. DOI:10.1186/s40104-020-00500-y |
| [19] |
ZHU L, JING J, QIN S Q, et al. miR-130a-3p regulates steroid hormone synthesis in goat ovarian granulosa cells by targeting the PMEPA1 gene[J]. Theriogenology, 2021, 165: 92-98. DOI:10.1016/j.theriogenology.2021.02.012 |
| [20] |
SINHA P B, TESFAYE D, RINGS F, et al. MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation[J]. J Ovarian Res, 2017, 10(1): 37. DOI:10.1186/s13048-017-0336-1 |
| [21] |
EISENBERG I, NAHMIAS N, PERSKY M N, et al. Elevated circulating micro-ribonucleic acid (miRNA)-200b and miRNA-429 levels in anovulatory women[J]. Fertil Steril, 2017, 107(1): 269-275. DOI:10.1016/j.fertnstert.2016.10.003 |
| [22] |
DONADEU F X, SANCHEZ J M, MOHAMMED B T, et al. Relationships between size, steroidogenesis and miRNA expression of the bovine corpus luteum[J]. Theriogenology, 2020, 145: 226-230. DOI:10.1016/j.theriogenology.2019.10.033 |
| [23] |
贺小云, 刘秋月, 储明星. miRNA调控哺乳动物卵泡发育和卵母细胞成熟的研究进展[J]. 畜牧兽医学报, 2019, 50(11): 2175-2185. HE X Y, LIU Q Y, CHU M X. Advances in miRNA regulating mammalian follicular development and oocyte maturation[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(11): 2175-2185. DOI:10.11843/j.issn.0366-6964.2019.11.001 (in Chinese) |
| [24] |
SHI L, LIU S, ZHAO W Q, et al. miR-483-5p and miR-486-5p are down-regulated in cumulus cells of metaphase Ⅱ oocytes from women with polycystic ovary syndrome[J]. Reprod Biomed Online, 2015, 31(4): 565-572. DOI:10.1016/j.rbmo.2015.06.023 |
| [25] |
HUMPHRIES B, YANG C F. The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy[J]. Oncotarget, 2015, 6(9): 6472-6498. DOI:10.18632/oncotarget.3052 |
| [26] |
ZOU X, LU T T, ZHAO Z F, et al. Comprehensive analysis of mRNAs and miRNAs in the ovarian follicles of uniparous and multiple goats at estrus phase[J]. BMC Genomics, 2020, 21(1): 267. DOI:10.1186/s12864-020-6671-4 |
| [27] |
于要升, 林杉, 王开胜, 等. 绵羊oar-miR-200b对GNAQ基因的表达调控[J]. 石河子大学学报: 自然科学版, 2016, 34(4): 424-430. YU Y S, LIN S, WANG K S, et al. Regulation of gene expression of GNAQ by oar-miR-200b in sheep[J]. Journal of Shihezi University: Natural Science, 2016, 34(4): 424-430. (in Chinese) |
| [28] |
HASUWA H, UEDA J, IKAWA M, et al. MiR-200b and MiR-429 function in mouse ovulation and are essential for female fertility[J]. Science, 2013, 341(6141): 71-73. DOI:10.1126/science.1237999 |
| [29] |
XIONG S T, MA W G, JING J, et al. An miR-200 cluster on chromosome 23 regulates sperm motility in zebrafish[J]. Endocrinology, 2018, 159(5): 1982-1991. DOI:10.1210/en.2018-00015 |
| [30] |
段新崇, 魏彦辉, 李阳, 等. 小尾寒羊间情期和发情期microRNAs差异表达分析[J]. 畜牧兽医学报, 2016, 47(7): 1324-1332. DUAN X C, WEI Y H, LI Y, et al. Analyzing the differential expression of microRNAs in estrus and diestrus of Small Tailed Han Sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(7): 1324-1332. (in Chinese) |
| [31] |
HE T T, SUN Y F, ZHANG Y C, et al. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN[J]. Reprod Biol Endocrinol, 2019, 17(1): 68. DOI:10.1186/s12958-019-0505-8 |
| [32] |
解领丽, 李嫒, 黄万龙, 等. 湖羊卵巢不同发育阶段的miRNA鉴定与分析[J]. 畜牧兽医学报, 2019, 50(7): 1396-1404. XIE L L, LI Y, HUANG W L, et al. Identification and analysis of miRNAs at different developmental stages in Hu Sheep ovaries[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(7): 1396-1404. (in Chinese) |
| [33] |
WANG H, LI X Y, ZHOU R Y, et al. Genome-wide transcriptome profiling in ovaries of small-tail Han sheep during the follicular and luteal phases of the oestrous cycle[J]. Anim Reprod Sci, 2018, 197: 212-221. DOI:10.1016/j.anireprosci.2018.08.031 |
| [34] |
FU X, HE Y L, WANG X F, et al. MicroRNA-16 promotes ovarian granulosa cell proliferation and suppresses apoptosis through targeting PDCD4 in polycystic ovarian syndrome[J]. Cell Physiol Biochem, 2018, 48(2): 670-682. DOI:10.1159/000491894 |
| [35] |
DANG Y J, WANG X Y, HAO Y J, et al. MicroRNA-379-5p is associated with biochemical premature ovarian insufficiency through PARP1 and XRCC6[J]. Cell Death Dis, 2018, 9(2): 106. DOI:10.1038/s41419-017-0163-8 |
| [36] |
ZHANG J B, XU Y X, LIU H L, et al. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis[J]. Reprod Biol Endocrinol, 2019, 17(1): 9. DOI:10.1186/s12958-018-0450-y |
| [37] |
LIU J Y, LI X Y, YAO Y, et al. miR-1275 controls granulosa cell apoptosis and estradiol synthesis by impairing LRH-1/CYP19A1 axis[J]. Biochim Biophys Acta Gene Regul Mech, 2018, 1861(3): 246-257. DOI:10.1016/j.bbagrm.2018.01.009 |
| [38] |
ZHONG P, LIU J, LI H, et al. MicroRNA-204-5p regulates apoptosis by targeting Bcl2 in rat ovarian granulosa cells exposed to cadmium[J]. Biol Reprod, 2020, 103(3): 608-619. DOI:10.1093/biolre/ioaa091 |
| [39] |
STEFANZL G, BERGER D, CERNY-REITERER S, et al. The pan-BCL-2-blocker obatoclax (GX15-070) and the PI3-kinase/mTOR-inhibitor BEZ235 produce cooperative growth-inhibitory effects in ALL cells[J]. Oncotarget, 2017, 8(40): 67709-67722. DOI:10.18632/oncotarget.18810 |
| [40] |
张纯, 徐晓娟, 姚莉娟, 等. 改良多囊卵巢综合征大鼠模型卵泡颗粒细胞中凋亡调控蛋白Bcl-2 Bax表达的研究[J]. 四川中医, 2016, 34(6): 34-37. ZHANG C, XU X J, YAO L J, et al. Research on the expression of apoptosis regulatory proteins Bcl-2, Bax in ovarian follicle granulosa cells of improved PCOS rat model[J]. Journal of Sichuan Traditional Chinese Medicine, 2016, 34(6): 34-37. (in Chinese) |
| [41] |
TILLY J L, KOWALSKI K I, JOHNSON A L, et al. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression[J]. Endocrinology, 1991, 129(5): 2799-2801. DOI:10.1210/endo-129-5-2799 |
| [42] |
BILLIG H, FURUTA I, HSUEH A J. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis[J]. Endocrinology, 1993, 133(5): 2204-2212. DOI:10.1210/endo.133.5.8404672 |
| [43] |
YU Y S, SUI H S, HAN Z B, et al. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors[J]. Cell Res, 2004, 14(4): 341-346. DOI:10.1038/sj.cr.7290234 |
(编辑 郭云雁)



