猪繁殖与呼吸综合征(porcine reproductive and respiratory syndrome,PRRS)每年给世界范围内的生猪养殖业造成巨大的经济损失,其病原是猪繁殖与呼吸综合征病毒(porcine reproductive and respiratory syndrome virus,PRRSV)。PRRSV是动脉炎病毒科的单股正链RNA病毒,基因组全长约15 kb,可分为欧洲型PRRSV(Ⅰ型)和美洲型PRRSV(Ⅱ型)两种基因型[1]。PRRSV基因组变异速度快,核苷酸取代率高达4.7×10-2~9.8×10-2碱基·年-1,是已知变异速度最快的RNA病毒之一[2-3]。欧洲型PRRSV和美洲型PRRSV间仅共享约60%的核苷酸相似性、50%~80%的氨基酸相似性[4-6]。此外,PRRSV毒株众多,易于发生重组而形成新的PRRSV毒株,具备更加复杂的基因组特性和致病性,这也为PRRS的防控带来了更大的挑战[7-11]。虽然PRRS疫苗已退出国家强制免疫,但疫苗接种仍是我国大部分猪场防控PRRS的主要手段。目前我国PRRS疫苗种类较多,大致可分为以高致病性PRRSV毒株(HP-PRRSV)为种毒的HP-PRRS疫苗和以经典毒株为种毒的经典PRRS疫苗。
由猪圆环病毒2型(porcine circovirus type 2,PCV2)引起的猪圆环病毒相关疾病(porcine circovirus type 2 associated diseases,PCVAD)是断奶仔猪多系统衰竭综合征(PMWS)、猪呼吸道综合征(PRDC)及猪皮炎肾病综合征(PDNS)等临床综合性疾病的统称[12]。仔猪感染PCV2后易于继发其他疾病,特别是PRRS等[13-14]。疫苗接种是防控PCV2的主要手段[15]。
猪瘟(classical swine fever, CSF),又称经典猪瘟,是由猪瘟病毒(classical swine fever virus, CSFV)引起的一种热性、烈性猪传染病,具有高发病率和高死亡率[16-17]。猪场常用的猪瘟减毒活疫苗为传代细胞源猪瘟活疫苗和脾淋源猪瘟活疫苗[18-19]。
PRRSV、PCV2、CSFV往往给猪场带来巨大的经济损失。更重要的是PRRS作为免疫抑制性疾病,常与PCV2或CSFV感染相伴而发,甚至发生PRRSV、PCV2、CSFV的共感染[20-23]。疫苗免疫是猪场防控PRRS等疾病的主要手段。虽然有部分报道分析了HP-PRRSV、PCV2、CSFV疫苗免疫后的效果[24-27],但考虑到临床上经典PRRSV(VR2332)减毒活疫苗的广泛使用,探究经典PRRSV减毒活疫苗(VR2332)对PCV2疫苗和CSF疫苗免疫的干扰作用、比较不同免疫方式对仔猪生长性能的影响,可以为经典PRRSV减毒活疫苗(VR2332)与PCV2、CSF疫苗的联合免疫提供一定的数据支持,为规模化猪场制定科学合理的免疫程序提供一定的经验参考。
1 材料与方法 1.1 试验动物本试验主要于2019—2020年在西北农林科技大学动物医学院及山东某规模化猪场完成。试验用猪为该场二元母猪所产的仔猪。在试验前对仔猪进行了PRRSV、PCV2、CSFV抗体检测(未进行相关病原检测),挑选PRRSV、PCV2、CSFV抗体为阴性的仔猪进行试验。
1.2 疫苗及抗体检测试剂盒经典PRRSV减毒活疫苗(VR2332)、PCV2灭活疫苗(CP08株)均购自勃林格殷格翰公司、猪瘟减毒活疫苗(传代细胞源)购自中牧实业股份有限公司。PRRSV抗体X3检测试剂盒、CSFV抗体检测试剂盒均购自爱德士(IDEXX)公司;PCV2抗体检测试剂盒购自荷兰百测公司。
1.3 试验方案将100头健康状况良好的仔猪随机分为A、B、C、D 4组,每组25头,用于评估经典PRRSV减毒活疫苗免疫对PCV2疫苗诱导抗体产生的影响。另选100头健康状况良好的仔猪随机分为E、F、G、H四组,每组25头,用于评估经典PRRSV减毒活疫苗免疫对CSF疫苗诱导抗体产生的影响。各组仔猪具体免疫方案如表 1所示。
|
|
表 1 仔猪免疫方案 Table 1 Immunization program for piglets |
A组于21日龄时免疫经典PRRSV减毒活疫苗,7 d后再免疫PCV2疫苗;B组于28日龄时同期分点注射经典PRRSV减毒活疫苗和PCV2疫苗;C组于28日龄时仅免疫PCV2疫苗;D组于28日龄时仅免疫经典PRRSV减毒活疫苗。E组于28日龄时免疫经典PRRSV减毒活疫苗,12 d后再免疫CSF疫苗;F组于28日龄时同期分点注射经典PRRSV减毒活疫苗和CSF疫苗;G组于40日龄时仅免疫CSF疫苗;H组于28日龄时仅免疫经典PRRSV减毒活疫苗。免疫4周后,采集各组仔猪的前腔静脉血液,分离血清,贮存于-20 ℃备用。待所有血清收集完毕后,同批次检测PRRSV、PCV2、CSFV抗体水平,比较不同组的抗体产生水平。
于免疫前、免疫后4周,称量各组仔猪的体重,计算各组仔猪的平均日增重:平均日增重=(终末体重-初始体重)/间隔时间(d),以比较不同免疫方案对仔猪生长性能的影响。
1.4 判定标准根据IDEXX抗体检测试剂盒说明书的判定标准:当S/P≥0.4时,PRRSV抗体为阳性;当S/P < 0.4时,PRRSV抗体为阴性。根据荷兰百测公司PCV2抗体检测试剂盒说明书的判定标准:当S/P≥ 0.4时,PCV2抗体为阳性;当S/P < 0.4时,PCV2抗体为阴性。根据IDEXX抗体检测试剂盒说明书的判定标准:当猪瘟抗体阻断率>40%时,CSFV抗体为阳性;当40%≥猪瘟抗体阻断率≥30% 时,CSFV抗体为可疑;当猪瘟抗体阻断率<30%时,CSFV抗体为阴性。
1.5 数据统计方法运用GraphPad Prism 5软件对试验数据进行统计分析,用T检验法进行不同组数据差异显著性分析。当P < 0.05时,判定为差异显著;当P < 0.01时,判定为差异极显著;P>0.05时判为差异不显著。
2 结果 2.1 经典PRRSV减毒活疫苗免疫对PCV2疫苗诱导抗体产生的影响如前所述,A组在经典PRRSV减毒活疫苗接种7 d后再注射PCV2疫苗,B组同期分点注射经典PRRSV减毒活疫苗及PCV2疫苗,C组仅接种PCV2疫苗,D组仅接种经典PRRSV减毒活疫苗。结果显示A、B、D组均产生了较高水平的PRRSV抗体,且相互间无显著性差异,共同免疫组A、B组的PRRSV抗体水平甚至略高于D组单独免疫组(P>0.05, 图 1a)。A、B、C组经免疫后均产生了较高水平的PCV2抗体;其中A组PCV2抗体水平最高,显著高于B组;C组PCV2抗体水平略高于B组,但无显著性差异(P>0.05, 图 1b)。以上结果表明经典PRRSV减毒活疫苗免疫并未对PCV2诱导的抗体产生造成明显干扰。
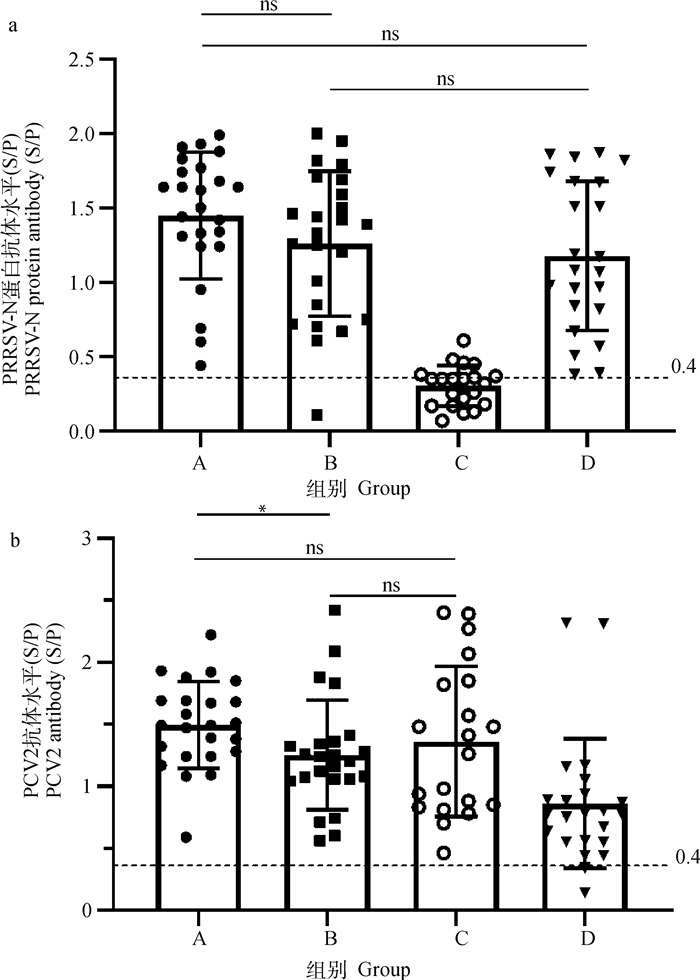
|
A. PRRSV免疫7 d后再免疫PCV2;B. 同期分点免疫PRRSV和PCV2;C. 仅免疫PCV2;D. 仅免疫PRRSV;ns. P>0.05;*. P < 0.05;***. P < 0.01。图 3同 A. The PCV2 vaccine is administered 7 days after PRRSV immunization; B. Simultaneous immunization with PRRSV and PCV2; C. Immunization with PCV2 vaccine; D. Immunization with PRRSV vaccine; ns. P>0.05; *. P < 0.05; ***. P < 0.01. The same as Fig. 3 图 1 A、B、C、D组仔猪PRRSV(a)、PCV2(b)抗体水平 Fig. 1 PRRSV(a), PCV2(b) antibody levels of piglets in groups A, B, C, and D |
如前所述,E组在经典PRRSV减毒活疫苗接种12 d后再注射CSF疫苗,F组同期分点注射经典PRRS减毒活疫苗及CSF疫苗,G组仅接种CSF疫苗,H组仅接种经典PRRSV减毒活疫苗。结果显示,共同免疫组E、F及单独免疫组H组均产生了高水平的PRRSV抗体,其中H组PRRSV抗体水平最高,显著高于F组(P < 0.05),而略高于E组(P>0.05),而共同免疫组E、F间并无显著性差异(图 2a)。共同免疫组E、F和单独免疫组G组均产生了高水平的CSFV抗体,且相互间无显著性差异(图 2b)。以上结果表明,与经典PRRSV减毒活疫苗共同免疫并未干扰CSF疫苗诱导的抗体产生,甚至还略微提高了其抗体水平(P>0.05)。
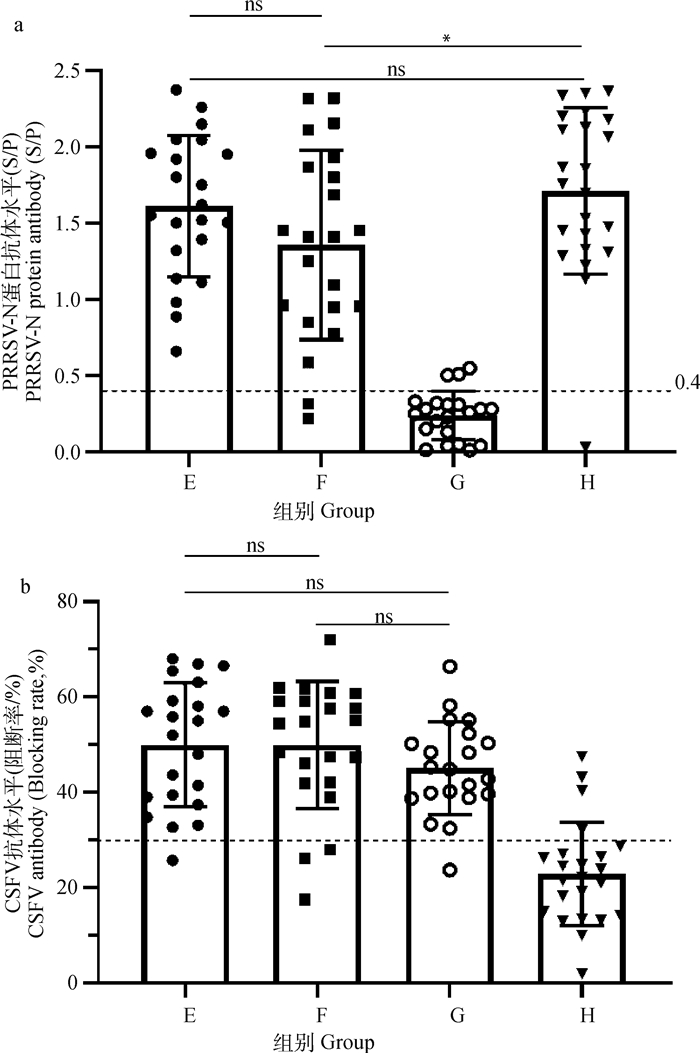
|
E. PRRSV免疫12 d后再免疫CSFV;F. 同期分点免疫PRRSV和CSFV;G. 仅免疫CSFV;H. 仅免疫PRRSV;ns. P>0.05;*. P < 0.05;***. P < 0.01。图 3同 E. The CSFV vaccine is administered 12 days after PRRSV immunization; F. Simultaneous immunization with PRRSV and CSFV; G. Immunization with CSFV vaccine; H. Immunization with PRRSV vaccine; ns. P>0.05; *. P < 0.05; ***. P < 0.01. The same as Fig. 3 图 2 E、F、G、H组仔猪PRRSV(a)、CSFV(b)抗体水平 Fig. 2 PRRSV(a), CSFV(b) antibody levels of piglets in E, F, G, and H groups |
为进一步探究不同免疫方式对仔猪生长性能的影响,分别在免疫前及免疫后28 d时采集A、B、C、D和E、F、G、H各组仔猪的体重数据,计算各组仔猪的平均日增重,结果如图 3所示。
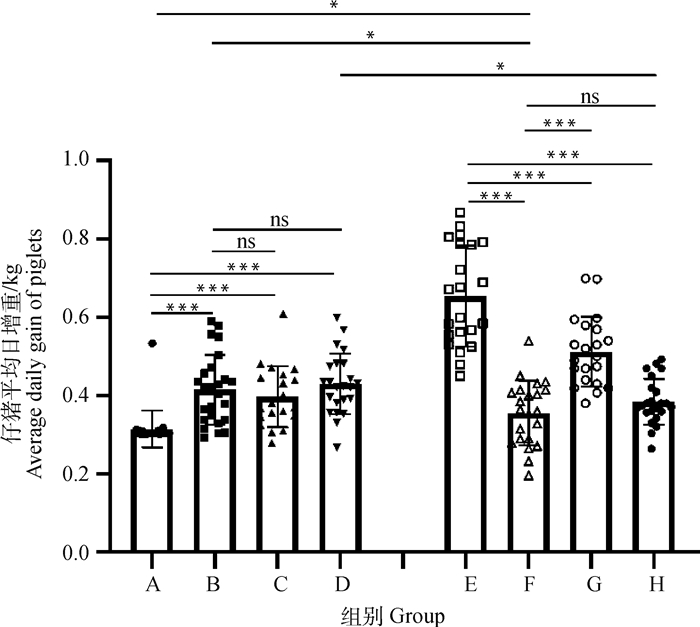
|
图 3 A、B、C、D和E、F、G、H组仔猪平均日增重 Fig. 3 Average daily gain of piglets in groups A, B, C, D, E, F, G and H |
在A~D中,A组的平均日增重最低,显著低于B、C、D组;D组的平均日增重最高,但相较于B组并无显著差异。
在E~H中,E组具有最高的平均日增重,显著高于F、G、H组;F组的平均日增重最低。
3 讨论猪繁殖与呼吸综合征、猪圆环病毒病、猪瘟是危害生猪产业的三种重要传染病,严重影响猪场的经济效益,是猪病防控的重点。尽管有多种针对这三种重要病原体的防控方案,但疫苗免疫仍是国内使用最多,也是效果最好的方式[28]。PRRS作为免疫抑制性疾病,会对猪体的免疫功能产生特异性或非特异性的抑制作用。有报道称PRRSV减毒活疫苗免疫可能会对其他疫苗的免疫产生干扰[29-32]。基于PRRSV毒株多样、经典PRRSV减毒活疫苗的广泛使用、临床上PRRSV、PCV2、CSFV常以混合感染的形式出现等原因,探究经典PRRSV减毒活疫苗免疫对PCV2、CSFV疫苗免疫的干扰情况,在有效防控的基础上筛选对仔猪生长性能更有利的免疫方案是实现生猪绿色健康养殖的重要举措,对养猪生产具有非常重要的指导意义。
陆佳丽等[25]评价了同时与分别接种HP-PRRSV疫苗和PCV2疫苗诱导的抗体产生水平,发现无论是同时免疫还是分别免疫,均产生了高水平的PRRSV抗体及PCV2抗体,且无显著性差异。本研究的结论与其并不完全一致,免疫经典PRRSV减毒活疫苗7 d后再免疫PCV2疫苗与同期分点免疫经典PRRSV减毒活疫苗和PCV2疫苗均产生了针对两种病毒的高水平抗体。进一步比较发现,两种组合方式免疫产生的PRRSV抗体无显著性差异,而间隔免疫组的PCV2抗体高于同期分点免疫组。与单独免疫PCV2疫苗组相比,两种组合方式免疫均未影响PCV2的抗体水平。意外的是单独免疫经典PRRSV减毒活疫苗组也检出了PCV2抗体阳性。基于仔猪在免疫前抗体检测均为阴性,作者推测该组仔猪可能感染了PCV2野毒。鉴于未在试验开始前对供试仔猪进行相关抗原检测,推测可能是仔猪在试验开始前处于低水平的带毒状态,但考虑到所有供试仔猪均来自该场本场,且供试仔猪随机进行分组,试验均在该场进行,降低了不同组间的误差,虽然仍对试验结果造成了影响,但作者认为其并未对研究结论造成本质上的影响。当然,这充分说明了在试验前对供试仔猪进行相关抗原的核酸检测的必要性。
李彩虹等[33]的研究中用HP-PRRSV JXA1-R疫苗与CSF疫苗对种公猪、后备母猪、育肥猪进行了共同免疫,结果显示HP-PRRSV JXA1-R疫苗的免疫可能会降低猪群中的CSFV抗体,而提高PRRSV抗体水平。徐孝会等[34]发现同时免疫两种疫苗时,CSF疫苗或口蹄疫疫苗会促进HP-PRRSV JXA1-R疫苗诱导的抗体水平。朱华贤等[26]在试验组仔猪49日龄时接种HP-PRRSV减毒活疫苗,56日龄时又免疫了CSF疫苗,对照组仅在56日龄时免疫了CSF疫苗。结果显示试验组CSFV抗体水平明显低于对照组,表明HP-PRRSV JXA1-R疫苗免疫会对CSF疫苗的免疫造成干扰,CSF疫苗免疫应在PRRS疫苗免疫后7 d以上进行。本研究中,与单独免疫CSF疫苗组相比,无论免疫经典PRRSV减毒活疫苗(VR2332)12 d后再免疫CSF疫苗,还是使用经典PRRSV减毒活疫苗与CSF疫苗同期分点免疫,均未影响猪群的CSFV抗体水平,甚至还略微提高了CSFV的抗体水平。作者推测PRRS疫苗对CSF疫苗诱导抗体产生的影响可能与PRRSV疫苗原型毒株的类型有关,这还需要更进一步的试验来验证。但与单独免疫CSF疫苗组相比,与CSF疫苗同期分点免疫显著降低了经典PRRSV减毒活疫苗诱导的PRRSV抗体水平,而CSF疫苗的免疫在经典PRRSV减毒活疫苗免疫12 d后再进行时,则可以产生与经典PRRSV减毒活疫苗单独免疫相似水平的PRRSV抗体,且抗体处于较高水平(S/P值>1.35)。
高杏[15]比较了PCV2疫苗免疫与不免疫对猪生长的影响,发现免疫PCV2疫苗有助于提升猪的日增重。本研究未与正常组进行平均日增重的比较,但比较了不同免疫方案组仔猪的平均日增重水平。无论与单独免疫PCV2疫苗组(单独免疫组)相比,还是与同期分点免疫经典PRRSV减毒活疫苗与PCV2疫苗组(同期分点免疫组)相比,免疫经典PRRSV减毒活疫苗7 d后再免疫PCV2疫苗组(间隔免疫组)显著均降低了仔猪的平均日增重。而同期分点免疫组仔猪的平均日增重与单独免疫组相比差异不显著,但显著高于间隔时间免疫组。相反,无论与单独免疫CSF疫苗组相比,还是与同期分点免疫经典PRRSV减毒活疫苗与CSF疫苗组相比,免疫经典PRRSV减毒活疫苗12 d后再免疫CSF疫苗均显著提高了仔猪的平均日增重。
疫苗接种在诱导抗体产生的同时,往往会不同程度地激活细胞免疫,细胞免疫是机体清除病原微生物的重要手段。本研究仅在体液免疫水平探究了PRRSV减毒活疫苗(VR2332)免疫对PCV2、CSF疫苗免疫诱导抗体产生的影响,并未进行细胞免疫相关的评估。且目前关于这3类疫苗免疫,特别是共同免疫下对机体细胞免疫影响的报道较少。Martelli等[35]探究了单独接种PCV2疫苗或与PRRSV-1疫苗共同接种对猪体液免疫和细胞免疫的影响,发现共同免疫PRRSV-1和PCV2疫苗不会干扰特定的体液免疫和细胞介导的免疫反应。孙雪等[36]比较了PRRSV减毒活疫苗(VR2332)与灭活苗同时免疫或单独免疫的效果,发现在免疫42 d后,同时免疫组引起猪淋巴细胞增殖速度高于单独免疫组;而免疫后56 d时, 虽都呈下降趋势,但单独免疫减毒活疫苗组具有更高的淋巴细胞转化率。这些结果似乎表明共同免疫在产生高水平相关抗体的同时,对机体细胞免疫的影响可能是一个较为复杂的情况。鉴于细胞免疫在清除病原体中的关键作用,联合免疫对猪细胞免疫的影响值得更多的研究。
综上,在制定联合免疫方案时,除了考虑抗体水平外,还应参考免疫方案对仔猪生长性能的影响,综合选择科学、高效、性价比高的联合免疫方案。
4 结论1) 经典PRRSV减毒活疫苗(VR2332)不影响PCV2灭活苗诱导的抗体产生,且二者同期分点免疫能使仔猪具备更高的平均日增重。2)经典PRRSV减毒活疫苗(VR2332)不影响CSF疫苗诱导的抗体产生,但间隔12 d免疫时效果更好,且能使仔猪具备更高的平均日增重。3)28日龄时同期分点免疫经典PRRSV减毒活疫苗(VR2332)和PCV2疫苗,12 d后再免疫CSF疫苗可能是比较理想的联合免疫方案,但仍需要进一步验证。
| [1] |
BENFIELD D A, NELSON E, COLLINS J E, et al. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332)[J]. J Vet Diagn Invest, 1992, 4(2): 127-133. DOI:10.1177/104063879200400202 |
| [2] |
JOHNSON C R, GRIGGS T F, GNANANDARAJAH J, et al. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses[J]. J Gen Virol, 2011, 92(Pt 5): 1107-1116. |
| [3] |
HANADA K, SUZUKI Y, NAKANE T, et al. The origin and evolution of porcine reproductive and respiratory syndrome viruses[J]. Mol Biol Evol, 2005, 22(4): 1024-1031. DOI:10.1093/molbev/msi089 |
| [4] |
ALLENDE R, LEWIS T L, LU Z, et al. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions[J]. J Gen Virol, 1999, 80(Pt 2): 307-315. |
| [5] |
NELSEN C J, MURTAUGH M P, FAABERG K S. Porcine reproductive and respiratory syndrome virus Comparison: divergent evolution on two continents[J]. J Virol, 1999, 73(1): 270-280. DOI:10.1128/JVI.73.1.270-280.1999 |
| [6] |
MARDASSI H, MOUNIR S, DEA S. Identification of major differences in the nucleocapsid protein genes of a Québec strain and European strains of porcine reproductive and respiratory syndrome virus[J]. J Gen Virol, 1994, 75(Pt 3): 681-685. |
| [7] |
GUO Z H, CHEN X X, LI R, et al. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: a molecular epidemiological perspective[J]. Virol J, 2018, 15(1): 2. DOI:10.1186/s12985-017-0910-6 |
| [8] |
ZHOU L, KANG R M, YU J F, et al. Genetic Characterization and pathogenicity of a novel recombined porcine reproductive and respiratory syndrome virus 2 among NADC30-Like, JXA1-Like, and MLV-like strains[J]. Viruses, 2018, 10(10): 551. DOI:10.3390/v10100551 |
| [9] |
ZHAO J, ZHU L, HUANG J B, et al. Genetic characterization of a novel recombined porcine reproductive and respiratory syndrome virus 2 among NADC30-like, JXA1-like and TJ-like strains[J]. Vet Med Sci, 2021, 7(3): 697-704. DOI:10.1002/vms3.402 |
| [10] |
XIE C Z, WANG Z, ZHOU H, et al. Genetic characterization of a new NSP2-deletion porcine reproductive and respiratory syndrome virus in China[J]. Microb Pathog, 2021, 150: 104729. DOI:10.1016/j.micpath.2021.104729 |
| [11] |
LI Y, XU G X, DU X Q, et al. Genomic characteristics and pathogenicity of a new recombinant strain of porcine reproductive and respiratory syndrome virus[J]. Arch Virol, 2021, 166(2): 389-402. DOI:10.1007/s00705-020-04917-8 |
| [12] |
ROSELL C, SEGALES J, RAMOS-VARA J A, et al. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome[J]. Vet Rec, 2000, 146(2): 40-43. DOI:10.1136/vr.146.2.40 |
| [13] |
张晓艳. 一例保育猪蓝耳与圆环病毒病并发诊治分析[J]. 中国畜禽种业, 2020, 16(1): 88-89. ZHANG X Y. Diagnosis and treatment analysis of a case of PRRSV and PCV2 in nursery pigs[J]. The Chinese Livestock and Poultry Breeding, 2020, 16(1): 88-89. (in Chinese) |
| [14] |
李晶, 王贵平, 吴建辉, 等. 一例高致病性蓝耳病病毒与猪圆环病毒2型混合感染的诊治[J]. 湖南畜牧兽医, 2019(4): 18-21. LI J, WANG G P, WU J H, et al. Diagnosis and treatment of a mixed infection of HP-PRRS virus and PCV2[J]. Hunan Journal of Animal Science & Veterinary Medicine, 2019(4): 18-21. (in Chinese) |
| [15] |
高杏. 接种猪圆环疫苗对猪生长的影响[J]. 国外畜牧学-猪与禽, 2019, 39(2): 24-25. GAO X. The effect of inoculation of PCV2 vaccine on pig growth[J]. Animal Science Abroad (Pigs and Poultry), 2019, 39(2): 24-25. DOI:10.3969/j.issn.1001-0769.2019.02.009 (in Chinese) |
| [16] |
祖立闯, 谢金文, 王文秀, 等. 我国猪瘟疫苗免疫现状及抗体检测方法综述[J]. 养猪, 2018(3): 109-112. ZU L C, XIE J W, WANG W X, et al. A review of the current situation of swine fever vaccine immunity and antibody detection methods in China[J]. Swine Production, 2018(3): 109-112. (in Chinese) |
| [17] |
仇华吉, 童光志, 沈荣显. 猪瘟兔化弱毒疫苗——半个世纪的回顾[J]. 中国农业科学, 2005, 38(8): 1675-1685. QIU H J, TONG G Z, SHEN R X. The Lapinized Chinese strain of classical swine fever virus: a retrospective review spanning half a century[J]. Scientia Agricultura Sinica, 2005, 38(8): 1675-1685. DOI:10.3321/j.issn:0578-1752.2005.08.026 (in Chinese) |
| [18] |
刘武刚, 张海雷, 周绪斌, 等. 在猪蓝耳病病毒和猪圆环病毒感染猪场利用猪瘟E2基因工程亚单位疫苗控制猪瘟的案例分析[J]. 猪业科学, 2019, 36(8): 70-72. LIU W G, ZHANG H L, ZHOU X B, et al. Case analysis of CSFV E2 genetic engineering subunit vaccine to control swine fever in PRRSV and PCV2 infected pig farms[J]. Swine Industry Science, 2019, 36(8): 70-72. DOI:10.3969/j.issn.1673-5358.2019.08.028 (in Chinese) |
| [19] |
李娇, 祖立闯, 王文秀, 等. 我国猪瘟疫苗研究与应用进展[J]. 养猪, 2020(5): 109-112. LI J, ZU L C, WANG W X, et al. Progress in research and application of swine fever vaccine in China[J]. Swine Production, 2020(5): 109-112. DOI:10.3969/j.issn.1002-1957.2020.05.035 (in Chinese) |
| [20] |
牛绪东, 任禾, 冯学俊, 等. 猪瘟、蓝耳病和圆环病毒病混合感染的诊断与防控[J]. 黑龙江畜牧兽医, 2018(12): 134-136, 247-248. NIU X D, REN H, FENG X J, et al. Diagnosis, prevention and control of mixed infection of CSF, PRRS and PCV2[J]. Heilongjiang Animal Science and Veterinary Medicine, 2018(12): 134-136, 247-248. (in Chinese) |
| [21] |
李鑫, 舒相华, 刘永波, 等. 一起猪繁殖与呼吸综合征病毒、猪瘟病毒和猪圆环病毒2型混合感染的诊断[J]. 黑龙江畜牧兽医, 2018(7): 118-120, 244. LI X, SHU X H, LIU Y B, et al. Diagnosis of a mixed infection of PRRSV, CSFV and PCV2[J]. Heilongjiang Animal Science and Veterinary Medicine, 2018(7): 118-120, 244. (in Chinese) |
| [22] |
曾学文. 猪蓝耳病与猪瘟混感的诊断与防控[J]. 畜牧兽医科技信息, 2020(8): 172. ZENG X W. Diagnosis, prevention and control of PRRS and CSF mixed infection[J]. Chinese Journal of Animal Husbandry and Veterinary Medicine, 2020(8): 172. DOI:10.3969/J.ISSN.1671-6027.2020.08.161 (in Chinese) |
| [23] |
吴瑞通. 猪瘟与猪蓝耳病混合感染防控措施[J]. 畜牧兽医科学, 2020(4): 24-25. WU R T. Prevention and control measures of combined infection of CSF and PRRS[J]. Graziery Veterinary Science, 2020(4): 24-25. (in Chinese) |
| [24] |
郭亮明, 邢红燕. 4种圆环病毒病疫苗对母猪的免疫效果[J]. 养殖与饲料, 2019(8): 78-80. GUO L M, XING H Y. Immune effects of four PCV2 vaccines on sows[J]. Animals Breeding and Feed, 2019(8): 78-80. DOI:10.3969/j.issn.1671-427X.2019.08.035 (in Chinese) |
| [25] |
陆桂丽, 吴国梁, 夏俊, 等. 仔猪接种猪繁殖与呼吸综合征和猪瘟疫苗的免疫效力观察[J]. 动物医学进展, 2013, 34(11): 119-122. LU G L, WU G L, XIA J, et al. Observe on immunological efficacy against PRRS and CSF vaccines in piglets[J]. Progress in Veterinary Medicine, 2013, 34(11): 119-122. DOI:10.3969/j.issn.1007-5038.2013.11.028 (in Chinese) |
| [26] |
朱华贤, 陈建祥, 厉金炳, 等. 高致病性猪蓝耳病、圆环病毒病疫苗对猪瘟疫苗免疫效果的影响试验[J]. 浙江畜牧兽医, 2014, 39(5): 21-23. ZHU H X, CHEN J X, LI J B, et al. The effect of HP-PRRS and PCV2 vaccine on the immune effect of CSFV vaccine[J]. Zhejiang Journal Animal Science and Veterinary Medicine, 2014, 39(5): 21-23. DOI:10.3969/j.issn.1005-7307.2014.05.007 (in Chinese) |
| [27] |
邹昌进, 刘紫微, 胡仕凤. 规模猪场不同圆环病毒疫苗和蓝耳病疫苗联合免疫效果比较[J]. 湖南畜牧兽医, 2015(6): 16-19. ZOU C J, LIU Z W, HU S F. Comparison of combined immunization effects of different circovirus vaccines and PRRS vaccines in large-scale pig farms[J]. Hunan Animal Science and Veterinary Medicine, 2015(6): 16-19. DOI:10.3969/j.issn.1006-4907.2015.06.006 (in Chinese) |
| [28] |
吴丽艳, 赵永旭, 李慧. 仔猪圆环疫苗免疫程序研究[J]. 中国动物保健, 2020, 22(9): 71-73. WU L Y, ZHAO Y X, LI H. Study on the immunization program of circovirus vaccine[J]. China Animal Health, 2020, 22(9): 71-73. DOI:10.3969/j.issn.1008-4754.2020.09.059 (in Chinese) |
| [29] |
李华, 杨汉春, 黄芳芳, 等. 猪繁殖与呼吸综合征病毒感染抑制猪瘟疫苗的免疫应答[J]. 中国兽医学报, 2001, 21(3): 219-222. LI H, YANG H C, HUANG F F, et al. Infection of porcine reproductive and respiratory syndrome virus inhibits immune response against hog cholera virus vaccine[J]. Chinese Journal of Veterinary Science, 2001, 21(3): 219-222. DOI:10.3969/j.issn.1005-4545.2001.03.003 (in Chinese) |
| [30] |
陈秋勇. 哺乳仔猪免疫蓝耳病弱毒疫苗对猪瘟免疫抗体水平的影响[J]. 福建畜牧兽医, 2013, 35(6): 20-21. CHEN Q Y. The effect of attenuated PRRS vaccine in suckling piglets on the level of swine fever immune antibody[J]. Journal of Animal Husbandry and Veterinary Medicine Fujian, 2013, 35(6): 20-21. DOI:10.3969/j.issn.1003-4331.2013.06.010 (in Chinese) |
| [31] |
WANG X L, MU G H, DANG R Y, et al. Up-regulation of IL-10 upon PRRSV vaccination impacts on the immune response against CSFV[J]. Vet Microbiol, 2016, 197: 68-71. DOI:10.1016/j.vetmic.2016.11.007 |
| [32] |
SURADHAT S, KESDANGSAKONWUT S, SADA W, et al. Negative impact of porcine reproductive and respiratory syndrome virus infection on the efficacy of classical swine fever vaccine[J]. Vaccine, 2006, 24(14): 2634-2642. DOI:10.1016/j.vaccine.2005.12.010 |
| [33] |
李彩虹, 何文, 何子双. 高致病性猪蓝耳病疫苗对猪瘟和蓝耳病抗体水平的影响[J]. 安徽农业科学, 2016, 44(8): 110-112. LI C H, HE W, HE Z S. Effects of high pathogenic porcine reproductive and respiratory Syndrome (HP-PRRS) vaccine on serum antibody level of Classical Swine Fever (CSF) and PRRS[J]. Journal of Anhui Agricultural Sciences, 2016, 44(8): 110-112. DOI:10.3969/j.issn.0517-6611.2016.08.039 (in Chinese) |
| [34] |
徐孝会, 徐雨, 腾统, 等. 猪瘟、口蹄疫疫苗对高致病性猪蓝耳病疫苗免疫的影响[J]. 贵州畜牧兽医, 2017, 41(2): 10-13. XU X H, XU Y, TENG T, et al. The vaccine of classical swine fever virus, foot and mouth disease virus affect vaccine immunization of highly pathogenic blue-ear pig disease[J]. Guizhou Journal of Animal Husbandry & Veterinary, 2017, 41(2): 10-13. DOI:10.3969/j.issn.1007-1474.2017.02.004 (in Chinese) |
| [35] |
MARTELLI P, ARDIGÒ P, FERRARI L, et al. Concurrent vaccinations against PCV2 and PRRSV: study on the specific immunity and clinical protection in naturally infected pigs[J]. Vet Microbiol, 2013, 162(2-4): 558-571. DOI:10.1016/j.vetmic.2012.11.016 |
| [36] |
孙雪, 赵一霏, 李帅, 等. 不同免疫程序对猪蓝耳病疫苗免疫效果的影响[J]. 中国兽医学报, 2017, 37(10): 1847-1851. SUN X, ZHAO Y F, LI S, et al. The evaluation of different immunization schedules for PRRS vaccine effect[J]. Chinese Journal of Veterinary Science, 2017, 37(10): 1847-1851. (in Chinese) |
(编辑 白永平)



