绵羊痘病(sheep pox)是由痘病毒科(Poxviridae)、山羊痘病毒属(Capripoxvirus)中的绵羊痘病毒(sheep pox virus, SPV)引起羊的一种急性、热性、高度接触性传染病,以皮肤、黏膜和内脏形成痘疹为特征[1-2]。本病起源于中亚地区,后蔓延至世界各地,现已广泛分布于非洲、亚洲、欧洲、大洋洲等地区,特别是北非、中东和部分亚洲国家流行较严重[2-17]。该病在我国内蒙古、青海、新疆、甘肃、贵州等地均有报道[2, 15, 18-19]。绵羊痘在自然情况下仅发生于绵羊,其中,细毛羊较粗毛羊易感,且病情严重,羔羊(4~8月龄)较成年羊易感,症状更严重,病死率也高[2, 5]。本病一年四季皆可发生,但以春、冬常见[5, 20]。病毒主要通过呼吸道感染,也可通过损伤的皮肤或黏膜侵入机体[5, 15]。健康羊可通过接触病羊或病羊排出的痂皮感染,甚至可通过吸血昆虫传播[15]。SPV在干燥条件下具有很强的抵抗力,能够在干燥痂皮中存活数月至数年,在干燥羊舍内可存活6~8月[21]。SPV对热、酸、碱等较敏感。近年来,随着我国各地养羊业的快速发展,各地绵羊痘的发病也呈现多发和流行态势,给养羊业带来了巨大的威胁和严重的经济损失。
2019年1月,甘肃省景泰县某羊场(约200只)养殖的30~40只3~6月龄小尾寒羊发病,病羊体温升高至41~42 ℃,精神不振,食欲减退,鼻腔流较多黏稠鼻液。随后,病羊鼻唇、眼部、腹部及四肢内侧出现红斑、丘疹及灰白色结节。最初只有个别羊发病,但很快蔓延至全群,并导致羔羊大量死亡。根据临诊检查疑似绵羊痘。为进一步确诊此病和深入探讨该病的病理变化特点与发生机制,本研究对该羊场病羊进行了一系列实验室诊断和病理学研究。
1 材料与方法 1.1 病料来源6只发病小尾寒羊(4♀、2♂)来自甘肃景泰县某羊场,临诊病理检查后,颈部局部剪毛,酒精消毒,颈静脉采血,迅速送实验室分离血清。随后,在严格的消毒条件下,对6例病羊放血处死,无菌切取皮肤、瘤胃黏膜、肺等组织样品,无菌袋保存,于液氮中冷却后再移至实验室-70 ℃冰箱保存备用。在放血处死病羊后30 min内,自肺分别切取数块组织(1 mm3),于2%多聚甲醛-2.5%戊二醛溶液中预固定,4 ℃冰箱保存。同时,分别切取心、肝、脾、肺、肾、肠系膜淋巴结及瘤胃等组织数块(1 cm3),于4%多聚甲醛磷酸缓冲液(0.1 mol·L-1,pH 7.4)固定。
1.2 主要试剂羊痘间接ELISA抗体检测试剂盒(中国农业科学院兰州兽医研究所);DNA分子质量标准、PCR试剂盒、上、下游引物(RPO30F1:5′-GCCTCGAGATGGATGATGAT-3′,RPO30F2: 5′- GCGGATCCACTTTTTTCTACAGCTCT-3′)、DNA提取试剂盒及凝胶回收试剂盒均为天根生化科技(北京)有限公司产品;Macchiavello法染色试剂盒(包涵体染色试剂盒)(长沙,达尔锋生物科技有限公司)。DMEM高糖培养基、胎牛血清为Hyclone公司的产品。其余试剂均为国产分析纯。
1.3 羊痘间接ELISA抗体检测取已包被的ELISA板,加入待测血清与对照血清,共设立8孔,2孔待测血清用血清样品稀释液1:100稀释,2孔标准阴性血清,2孔标准阳性血清,每孔直接加入100 μL;2孔空白对照,每孔加100 μL样品稀释液。置于37 ℃温箱内孵育1 h。然后,取出酶标板甩干,用洗涤液(1×PBST)洗涤3次,甩干。用血清稀释液将兔抗羊-HRP结合物按1:300稀释,每孔加入100 μL;置于37 ℃温箱内孵育1 h。取出酶标板甩干,再用洗涤液(1×PBST)洗涤3次,甩干。每孔加入底物(TMB)溶液100 μL,置37 ℃避光处静置反应10 min后,每个孔加入50 μL终止液。在450 nm波长处,测定酶标板各孔的光吸收值。试剂盒检测判断标准:OD450 nm≥0.25,抗体为阳性;OD450 nm≤0.2,抗体为阴性;当检测值0.25>OD450 nm >0.20,判定阳性可疑,则重复检测1次,若OD450 nm≥0.23,判定为阳性,OD450 nm<0.23则判为阴性。
1.4 PCR检测1.4.1 样品处理 在生物安全环境下,将采集的动物病料(皮肤、胃黏膜及肺痘疹结节)从-70 ℃的冰箱取出,用缓冲液清洗干净,置乳钵中,剪碎,添加灭菌石英砂研磨,加入0.01 mol·L-1灭菌PBS(pH为7.6~7.8)制成1:5悬液,置4 ℃冰箱浸毒过夜;然后,-20 ℃冻融2次,8 000 r·min-1离心5 min,吸取上清液提取总DNA。
1.4.2 样品DNA提取与检测 按试剂盒说明书要求从痘疹样病灶组织提取样品DNA,以此为模板,建立50 μL扩增体系(扩增预混液25 μL,RPO30F1上游引物1 μL,RPO30F2下游引物1 μL,DNA模板5 μL,水18 μL),扩增目的片段。反应条件:94 ℃预变性5 min;94 ℃ 50 s,57 ℃ 50 s,72 ℃ 50 s,共35个循环;72 ℃延伸8 min。PCR扩增产物经1.0%琼脂糖凝胶电泳10~20 min,紫外灯下观察电泳条带判定结果(绵羊痘病毒完整ORF 585 bp,山羊痘病毒为606 bp。如扩增到585 bp条带,说明是绵羊痘病毒)。
1.5 病理组织学观察6只病羊组织病料固定后,常规石蜡包埋切片,制备3~4 μm连续切片。分别采用HE染色、高碘酸-Schiff氏(PAS)染色、Masson三色染色、甲基绿派洛宁(Methyl green-Pyronin,MGP)染色;同时,采用Macchiavello法染色试剂盒,按试剂盒说明书要求进行包涵体染色。采用OLYMPUS BX-UCB/ BX61图像分析系统观察、拍照。
1.6 透射电镜观察电镜材料预固定结束后,采用1 mL·L-1锇酸后固定,丙酮乙醇梯度脱水,Epon812包埋,LKB8800型超薄切片机切片,醋酸铀、硝酸铅双层染色,日本JEM-100CX透射电子显微镜观察、拍照。
1.7 数据处理采用SPSS 22.0对相关试验数据进行方差分析处理,数据用“x±s”表示。
2 结果 2.1 羊痘间接ELISA抗体检测根据测定结果显示,1号病羊血清样初次测定OD450 nm为0.25>OD450 nm=0.246 8>0.20,判定阳性可疑,重复测定1次,OD450 nm =0.263 2>0.23,因此,判定为阳性;2号血清样初次测定OD450 nm =0.341 1>0.25,因此,判定为阳性;3号血清样初次测定OD450 nm为0.25>OD450 nm =0.238 0>0.20判定阳性可疑,重复测定1次,OD450 nm =0.238 4>0.23,因此,也为阳性;4号血清样初次测定OD450为0.25>OD450 nm =0.242 3>0.20,判定阳性可疑,重复测定为OD450 nm =0.248 0>0.23,判定为阳性;5号血清初次检测OD450 nm =0.315 7>0.25,判定为阳性;6号血清样初次测定OD450 nm =0.446 6>0.25,判定为阳性。所以,6例病羊均初步确诊为羊痘。
2.2 PCR检测2份皮肤痘疹样品PCR检测结果显示,检测样品均扩增出585 bp左右的片段,与预期的基因片段长度相符(图 1),测序结果经过NCBI blast比对为SPV,说明该检测样品中有SPV核酸存在,结合羊痘间接ELISA抗体检测结果,证明本次小尾寒羊疫病为绵羊痘。
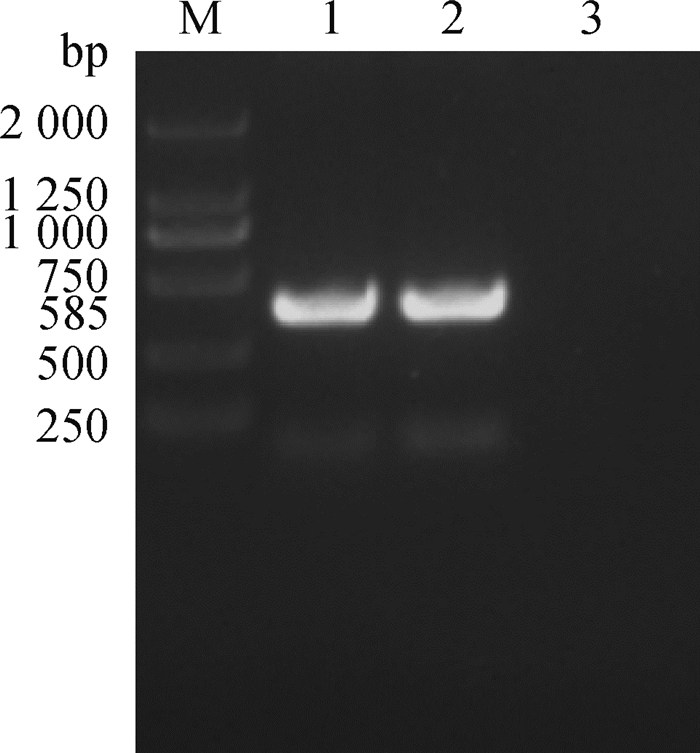
|
M. DNA相对分子质量标准;1、2.病料;3.阴性对照 M. DNA marker; 1, 2. Sample; 3. Negative control 图 1 PCR扩增 Fig. 1 PCR amplification |
6只发病小尾寒羊为3月龄左右羔羊。病羊精神沉郁、体温升高(41~42 ℃)。在局部皮肤,尤其无毛或少毛处,如眼周围、鼻、唇、乳腺、四肢内侧、尾、会阴等处,可见充血、红斑及数量不等的圆形痘疹。严重时,尾内侧痘疹局部感染化脓或溃疡(图 2A)。部分病羊舌及口腔黏膜有坏死及痘疹,鼻腔常有多量黏稠、灰白色分泌物,鼻腔和气管黏膜也可见坏死与痘疹。6只病羊瘤胃等前胃黏膜常有大小不等痘疹与坏死病灶(图 2B),黏膜不同程度充血、水肿,表面有多量黏稠分泌物;皱胃呈急性卡他性胃炎。
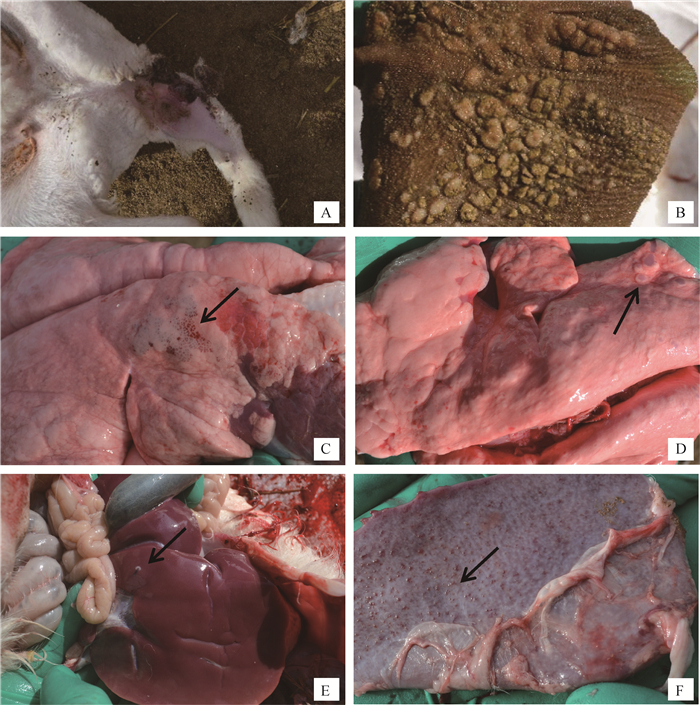
|
A.病羊尾内侧痘疹及溃疡;B.病羊瘤胃黏膜面有大量痘疹与坏死病灶;C.肺膈叶呈渗出-增生性结节的痘疹(↑);D.肺膈叶呈增生-坏死性结节的痘疹(↑);E.肝表面灰白色结节状痘疹(↑);F.脾表面大量结节状痘疹(↑) A. Pox lesions and ulcers on the tail root; B. A large number of pox lesions and necrosis lesions on the rumen mucosa; C. Exudation-proliferation nodules in the lung diaphragm (↑); D. Necrosis-proliferation nodules in the lung diaphragm (↑); E. Pox lesions on the liver surface (↑); F. Nodular pox lesions on the spleen surface (↑) 图 2 病羊临诊及剖检病理变化 Fig. 2 The clinical pathological changes of sick sheep |
6只病羊程度不等肺肿大,气管与支气管有大量灰白色黏稠分泌物,肺叶(尤其膈叶)表面与切面有明显呈散在或灶状分布的痘疹病灶,包括渗出-增生性结节和增生-坏死性结节。渗出-增生性结节为肺痘疹的初期表现,呈米粒大、暗红色、微突出于肺表面、质地较硬实(图 2C);增生-坏死性结节,呈大小不等灰白色或灰红色圆形或不正圆形病灶,与周围肺组织界限明显,微突出于肺表面,质地坚实,切面干燥(图 2D)。同时,病羊肺尖叶、心叶常见较大范围灰红色肺炎实变区。肝、脾、肾轻度肿大,也常散在数量不等圆形、灰白色结节状痘疹病灶(图 2E、F)。病羊心呈现程度不等扩张、心肌纤维变性和坏死。
2.4 病理组织学变化6只病羊眼观皮肤、口腔黏膜及胃黏膜等的痘疹病灶,在病理组织学上表现为局部黏膜上皮细胞和皮肤表皮细胞大量增生,且发生程度不等水泡变性和气球样变(图 3A),使局部显著增厚,位于皮肤的痘疹常伴发角化不全或角化过度。变性黏膜上皮和表皮细胞胞质内有时可见大小不一的嗜酸性包涵体。黏膜固有层和皮肤真皮层充血、水肿、炎性细胞浸润,血管周围和胶原纤维束间有大量绵羊痘细胞浸润,绵羊痘细胞呈星形、梭形和不规则圆形,细胞体积较大,细胞核呈卵圆形或不规则形,染色体常边集,其细胞质内常有一个或几个嗜酸性或嗜酸性颗粒状包涵体(图 3B)。时间较长时,痘疹局部黏膜固有层和皮肤真皮层发生大范围凝固性坏死。
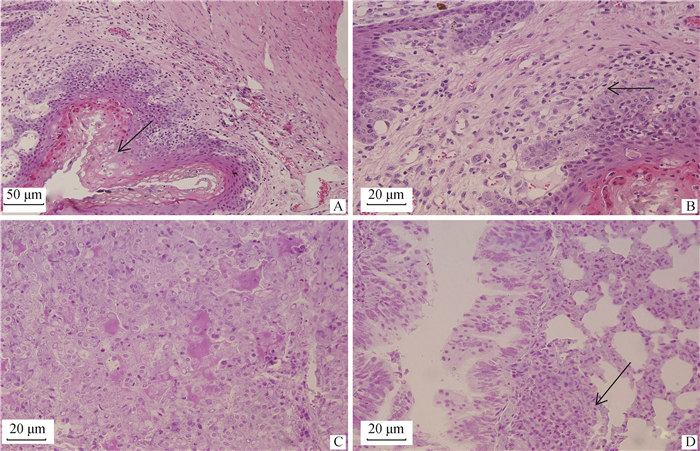
|
A.瘤胃黏膜局部上皮细胞大量增生、水泡变性,固有层散在数量不等的绵羊痘细胞(↑),100×;B.瘤胃黏膜局部固有层散在数量不等的绵羊痘细胞(↑),400×;C.肺渗出-增生性结节局部,肺泡Ⅱ型上皮细胞大量增生,肺泡腔充满浆液、脱落的上皮和炎性细胞,肺泡隔散在多量巨噬细胞,400×;D.渗出-增生性结节局部,细支气管黏膜上皮增生,上皮细胞胞质有嗜酸性包涵体,细支气管周围有大量绵羊痘细胞,羊痘细胞胞质有嗜酸性包涵体(↑),400× A. The epithelial cells of the rumen mucosa proliferation and vacuolar degeneration, lamina propria scattered varying amounts of sheep pox cells (↑), 100×; B. Varying number of sheep pox cells (↑) scattered in the lamina propria of the rumen mucosa, 400×; C. Exudation-proliferation nodules, alveolar type Ⅱ epithelial cells proliferate massively, the alveolar cavities are full of serous fluid, exfoliated epithelial cells and inflammatory cells, a large number of macrophages in alveolar septum, 400×; D. Exudation-proliferation nodules, bronchiolar epithelial proliferation, eosinophilic inclusions in the epithelial cells, a large number of sheep pox cells around the bronchiole, and the eosinophilic inclusions (↑) in the cytoplasm of sheep pox cells, 400× 图 3 病羊病理组织学变化(HE) Fig. 3 The histopathologic changes of sick sheep (HE) |
6只病羊肺呈现痘疹和肺炎病变。肺痘疹也表现为两类痘疹结节变化:渗出-增生性结节和增生-坏死性结节。渗出-增生性结节,局部肺泡隔高度充血,有大量嗜中性粒细胞和巨噬细胞浸润,肺泡腔充满浆液、脱落的上皮和炎性细胞,肺泡Ⅱ型上皮细胞增生(图 3C)。局部细支气管黏膜上皮细胞增生,增生的上皮细胞内可见嗜酸性包涵体,管腔中也有大量浆液、脱落的上皮细胞和炎性细胞(图 3D)。血管周围和细支气管周围有多少不等绵羊痘细胞,绵羊痘细胞胞质内也常有嗜酸性包涵体(图 3D)。增生-坏死性结节,病变中央区肺组织坏死,坏死区周围肺组织呈现与渗出-增生性结节基本类似病变,但无明显肺泡腔内浆液渗出。除痘疹病变外,见继发感染病变,呈程度不等的化脓性肺炎和大叶性肺炎病变。
6只病羊肝、脾、肾呈程度不等的充血、变性病变。痘疹病灶病理组织学观察主要表现为组织细胞变性、坏死。同时,局部有大量单核巨噬细胞增生和绵羊痘细胞聚集,其细胞胞质内也常有嗜酸性包涵体。病羊心充血、出血,呈程度不等变质性心肌炎。病羊全身淋巴结呈程度不等急性浆液性淋巴结炎或出血性淋巴结炎。
2.5 超微结构变化2只病羊肺超微结构观察显示,病变局部肺泡Ⅱ型上皮细胞增生,上皮细胞核浓缩、染色质聚集,线粒体程度不等肿胀、嵴断裂、空泡化,细胞内常见大小不等、形态不一、界限明显的胞质内包涵体,包涵体周围有明显膜包裹(图 4)。
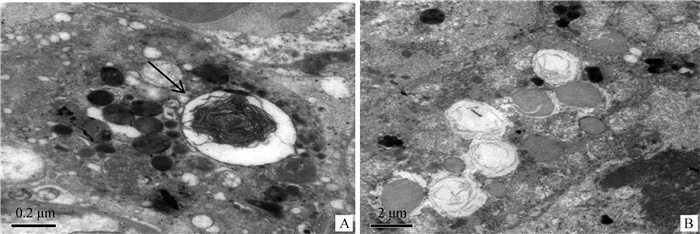
|
A.肺泡隔增生的Ⅱ型上皮细胞,细胞内可见多个界限明显的胞质包涵体(↑),15 000×;B.肺泡上皮细胞内,线粒体肿胀、嵴断裂、空泡化,8 000× A. Alveolar type Ⅱ epithelial cells proliferation, inclusions in the cytoplasm(↑), 15 000×; B. Mitochondrial swelling, fractured cristae and cavitation, 8 000× 图 4 肺超微结构变化 Fig. 4 The ultrastructural changes of lung |
本研究对样品血清和病料分别进行间接ELISA抗体检测和PCR检测。羊痘间接ELISA抗体检测显示,6只病羊样品均为阳性,表明6只病羊均被羊痘病毒感染,血清存在羊痘病毒抗体;进一步对病羊组织样品提取病毒基因组DNA进行PCR检测,均扩增出585 bp左右的目的基因片段,与预期的片段相符,与对照比较证明被检样品中有绵羊痘病毒(SPV)存在。结合ELISA和PCR检测结果,证明本次甘肃省景泰县羊场小尾寒羊疫情是由SPV引起的绵羊痘。
有研究表明[22-40],皮肤以及呼吸道和消化道黏膜的病理损伤是绵羊痘重要的病变,本研究也同样观察到上述部位的痘疹、坏死、溃疡等病变。但除上述部位病变外,研究发现,小尾寒羊发生绵羊痘时,实质器官肺、肝、脾、肾等痘疹病变表现更加突出。本研究中6只病羊肺、肝、脾、肾均出现明显的痘疹病变。病理剖检观察,肺痘疹病变主要出现在肺膈叶,呈散在或灶状分布,包括2种形式的痘疹,渗出-增生性结节和增生-坏死性结节。前者,呈米粒大、暗红色、微突出于肺表面;后者呈大小不等圆形或不正圆形病灶,灰白色或灰红色,质地坚实的结节状病灶。肝、脾、肾等的痘疹病灶呈散在的大小不等的灰白色结节状病灶。病理组织学观察,肺2种痘疹病变的差异是渗出-增生性结节局部有明显多量的急性炎性渗出物,如浆液等;而增生-坏死性结节局部则组织呈广泛凝固性坏死,无明显炎性渗出物,但两者共同的变化是病变局部出现大量绵羊痘细胞聚集,甚至在病灶局部血管周围、细支气管周围密集。而绵羊痘细胞胞质有明显的嗜酸性包涵体。肝、脾、肾等的痘疹病灶则表现增生-坏死性结节,同样局部出现大量绵羊痘细胞。因此,这种具有胞质包涵体的特殊形态的绵羊痘细胞在痘疹病变局部的大量出现是组织病理诊断的重要依据。
绵羊痘细胞也被称为“cellulus claveleulus”,最初由Borrel[41]于1903年发现,认为它们可能是巨噬细胞;1959年, Plowright等[42]描述绵羊痘细胞为真皮中的大细胞,拥有空泡化细胞核,偶有细胞质包涵体[32],并认为绵羊痘细胞是纤维细胞,或是未分化的血管周细胞。Al-Attar等[34]认为绵羊痘细胞类似于单核细胞、巨噬细胞或成纤维细胞。本研究认为,绵羊痘细胞是SPV病毒感染单核-巨噬细胞进一步变化形成的特有形态。SPV在复制过程中引起病羊机体全身性的单核-巨噬细胞系统活化,单核-巨噬细胞进行非特异性细胞免疫和辅助激活特异性免疫系统,而绵羊痘细胞胞质中嗜酸性包涵体也是SPV大量复制的证据。由于绵羊痘病毒是以细胞质为DNA复制起点的病毒,因此,其包涵体的形成则应该位于细胞质中。本研究从组织病理到超微病理都均有发现,绵羊痘细胞内的包涵体也都位于细胞质中。目前,关于细胞内的包涵体,有学者认为是病毒成分的蓄积;也有学者认为是病毒粒子合成装配的场所或发育部位。SPV的遗传物质DNA是在细胞质中复制,因此,在SPV感染中细胞中,细胞质内包涵体应为病毒合成装配的场所或发育部位。至于绵羊痘细胞的产生、分化、增殖等机制有待进一步深入研究。
4 结论结合ELISA和PCR检测结果,证明本次甘肃省景泰县羊场小尾寒羊疫情是由SPV引起的绵羊痘。除局部皮肤病变外,消化道黏膜和肺等实质器官的痘疹病变是小尾寒羊绵羊痘具有代表性的病理变化,尤其痘疹病灶中大量绵羊痘细胞的出现具有重要证病性。
| [1] |
陈溥言.
兽医传染病学[M]. 6版. 北京: 中国农业出版社, 2015: 56-57.
CHEN P Y. Veterinary lemology[M]. 6th ed. Beijing: China Agriculture Press, 2015: 56-57. (in Chinese) |
| [2] |
李杨, 颜新敏, 吴国华, 等. 羊痘的诊断与疫苗研究进展[J]. 中国兽医杂志, 2017, 53(6): 86–88.
LI Y, YAN X M, WU G H, et al. Progress in diagnosis and vaccine research of capripox[J]. Chinese Journal of Veterinary Medicine, 2017, 53(6): 86–88. (in Chinese) |
| [3] | ROHIT J, SUBHA G. Sheep pox and goat pox:the animal diseases of importance for transboundary control[J]. Int J Contemp Pathol, 2017, 3(1): 29–30. DOI: 10.5958/2395-1184.2017.00007.9 |
| [4] | GITAO C G, MBINDYO C, OMANI R, et al. Review of sheep pox disease in sheep[J]. J Vet Med Res, 2017, 4(1): 1068. |
| [5] | MIRZAIE K, BARANI S M, BOKAIE S. A review of sheep pox and goat pox:perspective of their control and eradication in Iran[J]. J Adv Vet Anim Res, 2015, 2(4): 373–381. DOI: 10.5455/javar.2015.b117 |
| [6] | MUBASHIR A R, SHOWKAT A A, RAHEEQA R, et al. Outbreak of sheep pox among cross bred sheep in Kashmir valley[J]. The Pharma Innov J, 2020, 9(8): 50–53. |
| [7] | HURISA T T, JING Z Z, JIA H J, et al. A review on sheeppox and goatpox:insight of epidemiology, diagnosis, treatment and control measures in Ethiopia[J]. J Infect Dis Epidemiol, 2018, 4(3): 57. |
| [8] | YILMAZ E F, ARAYICI P P, MAHARRAMOV A M, et al. The approaches to designing of new generation vaccines against the sheep pox disease[J]. Biotechnol Acta, 2016, 9(6): 7–15. DOI: 10.15407/biotech9.06.007 |
| [9] | GALE P, KELLY L, SNARY E L. Qualitative assessment of the entry of capripoxviruses into Great Britain from the European Union through importation of ruminant hides, skins and wool[J]. Microb Risk Anal, 2016, 1: 13–18. DOI: 10.1016/j.mran.2015.07.001 |
| [10] | MAKSYUTOV R A, GAVRILOVA E V, AGAFONOV A P, et al. An outbreak of sheep pox in Zabajkalskij kray of Russia[J]. Transbound Emerg Dis, 2015, 62(4): 453–456. DOI: 10.1111/tbed.12176 |
| [11] | HAEGEMAN A, ZRO K, SAMMIN D, et al. Investigation of a possible link between vaccination and the 2010 sheep pox epizootic in Morocco[J]. Transbound Emerg Dis, 2016, 63(6): e278–e287. DOI: 10.1111/tbed.12342 |
| [12] | KARDJADJ M. Prevalence, distribution, and risk factor for sheep pox and goat pox (SPGP) in Algeria[J]. Trop Anim Health Prod, 2017, 49(3): 649–652. DOI: 10.1007/s11250-017-1220-0 |
| [13] | MAHMOUD A Z E, KHALAFALLA A I, ABDELLATIF M M. An epidemiological study of sheep and goat pox outbreaks in the Sudan[J]. Food Biol, 2016, 5: 1–5. |
| [14] | HAMOUDA M, AL-HIZAB F, EL-SABAGH I. Clinical, pathological and molecular diagnosis of sheeppox virus in Saudi Arabia[J]. J Anim Plant Sci, 2017, 27(1): 91–97. |
| [15] | YAN X M, CHU Y F, WU G H, et al. An outbreak of sheep pox associated with goat poxvirus in Gansu province of China[J]. Vet Microbiol, 2012, 156(3-4): 425–428. DOI: 10.1016/j.vetmic.2011.11.015 |
| [16] | BOLAJOKO M B, ADEDEJI A J, DASHE G D, et al. Molecular epidemiology and economic impact of goat pox on small holder sheep and goats farmers in north central Nigeria[J]. Small Rumin Res, 2019, 179: 75–78. DOI: 10.1016/j.smallrumres.2019.09.013 |
| [17] | KALI K, KARDJADJ M, TOUAGHIT N, et al. Understanding the epidemiology of sheep-pox outbreaks among vaccinated Algerian sheep and post vaccination evaluation of the antibodies kinetics of the commercially used vaccine[J]. Comp Immunol Microbiol Infect Dis, 2019, 65: 128–131. DOI: 10.1016/j.cimid.2019.05.014 |
| [18] | BHANUPRAKASH V, INDRANI B K, HOSAMANI M, et al. The current status of sheep pox disease[J]. Comp Immunol Microbiol Infect Dis, 2006, 29(1): 27–60. DOI: 10.1016/j.cimid.2005.12.001 |
| [19] |
陈轶霞. 羊痘病毒基因组及其功能研究概况[J]. 中国兽医学报, 2015, 35(2): 340–344.
CHEN Y X. Research Progress on genome and function of capripox virus[J]. Chinese Journal of Veterinary Science, 2015, 35(2): 340–344. (in Chinese) |
| [20] | MALESIOS C, KOSTOULAS P, DADOUSIS K, et al. An early warning indicator for monitoring infectious animal diseases and its application in the case of a sheep pox epidemic[J]. Stoch Environ Res Risk Assess, 2017, 31(2): 329–337. DOI: 10.1007/s00477-016-1316-5 |
| [21] | BABIUK S, PARKYN G, COPPS J, et al. Evaluation of an ovine testis cell line (OA3. Ts) for propagation of capripoxvirus isolates and development of an immunostaining technique for viral plaque visualization[J]. J Vet Diagn Invest, 2007, 19(5): 486–491. DOI: 10.1177/104063870701900505 |
| [22] | BEYTUT E. Sheep pox virus induces proliferation of type Ⅱ pneumocytes in the lungs[J]. J Comp Pathol, 2010, 143(2-3): 132–141. DOI: 10.1016/j.jcpa.2010.01.014 |
| [23] |
赵琳, 李萌, 罗静, 等. 绵羊痘病毒的检测与遗传进化分析[J]. 动物医学进展, 2017, 38(12): 9–14.
ZHAO L, LI M, LUO J, et al. Detection and phylogenetic analysis of sheep poxvirus[J]. Progress in Veterinary Medicine, 2017, 38(12): 9–14. DOI: 10.3969/j.issn.1007-5038.2017.12.003 (in Chinese) |
| [24] | MANIMARAN K, MAHAPRABHU R, JAISREE S, et al. An outbreak of sheep pox in an organized farm of Tamil Nadu, India[J]. Indian J Anim Res, 2017, 51(1): 162–164. |
| [25] | YUNE N, ABDELA N. Epidemiology and economic importance of sheep and goat pox:a review on past and current aspects[J]. J Vet Sci Technol, 2017, 8(2): 430. |
| [26] | KARAPINAR Z, ILHAN F, DINCER E, et al. Pathology and phylogenetic analysis of capripoxvirus in naturally infected sheep sheeppox virus[J]. Pak Vet J, 2017, 37(1): 78–84. |
| [27] | SUMANA K, REVANAIAH Y, APSANA R, et al. Molecular characterization of sheeppox virus from outbreaks in Karnataka, India[J]. Vet World, 2020, 13(2): 386–391. DOI: 10.14202/vetworld.2020.386-391 |
| [28] | BABIUK S, BOWDEN T R, BOYLE D B, et al. Capripoxviruses:an emerging worldwide threat to sheep, goats and cattle[J]. Transbound Emerg Dis, 2008, 55(7): 263–272. DOI: 10.1111/j.1865-1682.2008.01043.x |
| [29] | GULBAHAR M Y, CABALAR M, GUL Y, et al. Immunohistochemical detection of antigen in lamb tissues naturally infected with sheeppox virus[J]. J Vet Med B Infect Dis Vet Public Health, 2000, 47(3): 173–181. DOI: 10.1046/j.1439-0450.2000.00321.x |
| [30] | HAN M G, KWEON C H, MO I P, et al. Pathogenicity and vaccine efficacy of a thymidine kinase gene deleted infectious laryngotracheitis virus expressing the green fluorescent protein gene[J]. Arch Virol, 2002, 147(5): 1017–1031. DOI: 10.1007/s00705-001-0794-y |
| [31] | CHEHIDA F B, AYARI-FAKHFAKH E, CAUFOUR P, et al. Sheep pox in Tunisia:current status and perspectives[J]. Transbound Emerg Dis, 2018, 65(1): 50–63. DOI: 10.1111/tbed.12656 |
| [32] | MURRAY M, MARTIN W B, KÖYLV A. Experimental sheep pox. A histological and ultrastructural study[J]. Res Vet Sci, 1973, 15(2): 201–208. DOI: 10.1016/S0034-5288(18)33829-3 |
| [33] | DAGLEISH M P, BENAVIDES J, CHIANINI F. Immunohistochemical diagnosis of infectious diseases of sheep[J]. Small Rumin Res, 2010, 92(1-3): 19–35. DOI: 10.1016/j.smallrumres.2010.04.003 |
| [34] | AL-ATTER, Al-ASAVED. Pathologic observations on sheep and goat pox[J]. Libyan Vet Med, 2010, 1(1): 182–190. |
| [35] | SOUNDARARAJAN C, NAGARAJAN K, PRAKASH M A. Pathological features of sheep pox seen in Madras Red sheep in Tamil Nadu[J]. Indian Vet J, 2017, 94(7): 41–43. |
| [36] | ROY P, PURUSHOTHAMAN V, SREEKUMAR C, et al. Sheep pox disease outbreaks in Madras Red and Mechery breeds of indigenous sheep in Tamilnadu, India[J]. Res Vet Sci, 2008, 85(3): 617–621. DOI: 10.1016/j.rvsc.2008.03.011 |
| [37] | CHANIE M. Clinical and histopathological study of sheep pox in Ethiopia[J]. Int J Nat Sci, 2011, 1(4): 89–92. |
| [38] | MAHMOUD M A, KHAFAGI M H. Detection, identification, and differentiation of sheep pox virus and goat pox virus from clinical cases in Giza Governorate, Egypt[J]. Vet World, 2016, 9(12): 1445–1449. DOI: 10.14202/vetworld.2016.1445-1449 |
| [39] | PLOWRIGHT W, MACLEOD W G, FERRIS R D. The pathogenesis of sheep pox in the skin of sheep[J]. J Comp Pathol, 2012, 146(2-3): 97–105. DOI: 10.1016/j.jcpa.2011.12.004 |
| [40] | MANJUNATHAREDDY G B, SUMANA K, APSANA R, et al. Investigation of malignant form of sheep pox outbreak in fattening lambs in Mandya, Karnataka[J]. Indian J Vet Pathol, 2017, 41(3): 184–188. DOI: 10.5958/0973-970X.2017.00045.1 |
| [41] | BORREL A. Epitheloses infectieuses et epitheliomas[J]. Ann Inst Pasteur, 1903, 17: 81–122. |
| [42] | PLOWRIGHT W, MACLEOD W G, FERRIS R D. The pathogenesis of sheep pox in the skin of sheep[J]. J Comp Pathol, 1959, 69: 400–413. DOI: 10.1016/S0368-1742(59)80039-4 |



