2. 贵州大学动物科学学院, 贵州省动物遗传育种与繁殖重点实验室, 贵阳 550025;
3. 贵州省种畜禽种质测定中心, 贵阳 550018;
4. 贵州省农业科学院畜牧兽医研究所, 贵阳 550025;
5. 贵阳市花溪区农业局, 贵阳 550025
2. Guizhou Province Key Laboratory of Animal Genetics, Breeding and Reproduction, College of Animal Science, Guizhou University, Guiyang 550025, China;
3. Guizhou Tesitng Center for Livestock and Poultry Germplasm, Guiyang 550018, China;
4. Institute of Animal Husbandry and Veterinary, Guizhou Academy of Agricultural Sciences, Guiyang 550025, China;
5. Agricultural Bureau of Huaxi District, Guiyang 550025, China
许多干细胞或细胞亚群在不同的发育阶段受到大量特定基因的调控,其关键是转录因子精准调控基因表达的模式具有复杂性和特异性,而转录因子激活剂、阻遏物及其功能修饰剂之间相互作用的组合又进一步增加了调控网络的复杂多样性[1]。CCAAT/增强子结合蛋白A(CCAAT/enhancer binding protein alpha, CEBPA),别名C/EBP、RcC/EBP-1、C/EBPα[2],该基因基序上存在激活剂/阻遏物结合位点,能通过组织特异性和信号依赖性精准调节基因表达的转录因子。肌内脂肪(intramuscular fat,IMF)含量是影响肉质的重要因素,前体脂肪细胞分化则是IMF沉积不可或缺的步骤,其过程包括前体脂肪细胞指数扩增和分化两个阶段。细胞分化属于核心阶段,其过程是一系列的转录级联事件[3]。在这个转录级联系统中,CEBPA作为核心转录调控因子与脂肪细胞分化相关基因相互作用共同调节其分化。CEBPA基因在脂肪和胆固醇代谢相对较旺盛的组织中有较高的表达水平[4-6],参与细胞的信号传导、增殖、分化、能量代谢、应激反应和血液生成等生命活动[7]。由于CEBPA基因在生命进程中既能作为激活剂又有阻遏抑制功效,已有大量研究开始将其与人类肥胖症、糖尿病和白血病等疾病进行联系[8]。
成熟miRNA和相关蛋白组合形成RISC复合体(RNA-induced silencing complex,RISC),RISC复合体与靶基因mRNA 3′UTR或5′UTR结合,以切割靶mRNA或抑制翻译间接调节基因表达[9],在RNA的基础上行使自身的生物学功能,参与生命过程中一系列的重要进程,如早期发育、细胞增殖、细胞凋亡、脂肪代谢和细胞分化等生命活动[10-11]。随着研究的不断深入,越来越多的miRNA被发现在脂肪细胞分化过程中扮演着重要角色。miR-150能提高脂肪源性干细胞(adipose-derived stem cells,ASC)中C/EBPα的表达,促进脂肪沉积[12];而miR-326会直接抑制C/EBPα的表达,削弱该细胞的脂肪沉积能力[13]。绵羊基质血管组分(stromal vascular fractions,SVFs)中的miR-124-3p能作为成脂过程中的调节剂,通过直接作用于C/EBPα来影响脂肪沉积[14];miR-25通过直接结合C/EBPα抑制3T3-L1脂肪形成[15]。此外,CEBPA还能作为miRNA调节剂,通过与miRNA启动子区特异性结合,间接调控脂肪的生成[16]。当前仅在人、绵羊脂肪干细胞以及3T3-L1细胞系发现部分miRNA能直接结合CEBPA基因调控脂肪沉积过程,在牛肌内前体脂肪细胞中是否还存在其他能直接作用于CEBPA基因的miRNA,以及这部分miRNA对牛肌内前体脂肪细胞增值和分化的影响尚未见报道。
本研究综合运用多个生物信息学软件筛选调控牛CEBPA基因的miRNA,结合qRT-PCR、双荧光素酶报告系统、Western blot、CCK-8等生物学试验探究候选miRNA对牛肌内前体脂肪细胞分化与增殖的影响, 明确miRNA与CEBPA基因调控关系对肌内脂肪沉积效果的遗传基础,为加速关岭牛品种改良、提高关岭牛肌内脂肪含量、生产高档牛肉奠定基础。
1 材料与方法 1.1 材料1.1.1 试验材料 试验动物为贵州省关岭牛,选取饲养管理条件相同且健康的关岭牛7头(2岁公、母牛各3头,1日龄犊牛1头),屠宰前停料、停水12 h,屠宰后迅速采集心、肝、脾、肺、肾、脂肪、腰大肌、背最长肌、股二头肌、大肠、小肠、下丘脑、睾丸、卵巢和子宫共15个组织(用于提取RNA),1日龄关岭牛犊牛耳静脉注射速眠新麻醉处死后,取背最长肌(用于提取RNA和分离培养肌内前体脂肪细胞)。用于提取RNA的样品放入液氮罐中冻存,带回实验室后置于-80 ℃冰箱保存;用于分离培养肌内前体脂肪细胞的背最长肌,经75%酒精和PBS清洗后,浸泡在含1%双抗的PBS中并置于冰盒,3 h内带回实验室立刻进行分离培养。
1.1.2 主要试剂 氯仿购自宏达尔生物科技有限公司;pmir GLO载体购自Promgea公司;HEK-293T购自ATCC;pMD-19 T Vector购自宝生物工程(大连)有限公司;PBS、Ⅰ型胶原酶、DMEM/F12培养基、青链霉素、胰岛素、地塞米松、油红O染料、胰蛋白酶、3-异丁基-1-甲基黄嘌呤、BCA蛋白定量试剂盒、Opti-MEMTM培养基均购自上海索莱宝生物科技有限公司;Trizol试剂、无水乙醇、DL2000 DNA Marker、2×Es Taq MasterMix、核酸染料、反转录试剂盒(HiFiScript cDNA Synthesis Kit)均购自康为世纪生物有限公司;SYBR Green qPCR Master Mix (No ROX)购自美国MCE公司;miRNA反转录试剂盒(miRcute Plus miRNA First-Strand cDNA Kit)和荧光定量试剂盒(miRcute Plus miRNA qPCR Kit)均购自天根生化科技(北京)有限公司;蛋白Marker、蛋白上样缓冲液、PVDF膜均购自北京碧云天公司;山羊抗兔IgG购自中杉公司;发光试剂购自德国roche公司;GAPDH(KC-5G5)购自康成公司;CCK-8检测试剂盒购自大连美仑生物技术有限公司;miRNA模拟物由上海吉玛制药技术有限公司合成;LipofectamineTM 3000试剂购自Thermo Fisher。
1.2 方法1.2.1 生物信息学预测与分析 利用Starbase[17]、MiRWalk[18]、miRSystem[19]和TargetScan[20] 4个在线软件预测能直接调节CEBPA基因的miRNA。用Venny 2.1对4个软件的预测结果做韦恩图取交集,以聚焦调节CEBPA基因的miRNA。
1.2.2 引物设计与合成 根据GenBank公布的牛CEBPA mRNA序列(登录号:NM_176784.2),利用Primer 3.0和NCBI设计CEBPA基因荧光定量引物;利用miRprimer软件设计miRNA荧光定量引物(miRNA下游引物为试剂盒自带的通用下游引物);以牛GAPDH基因(登录号:NM _001034034)作为CEBPA基因内参,参照王军等[21]使用的U6基因作为miRNA内参,引物由上海生工生物工程技术服务有限公司合成(表 1)。
|
|
表 1 引物序列 Table 1 Primer sequences |
1.2.3 CEBPA-3′UTR双荧光素酶报告载体构建及双荧光素酶活性检测 为确定用于构建载体的序列,先以关岭牛背最长肌cDNA为模板,通过普通PCR反应,扩增出miRNA结合区域的CEBPA-3′UTR-1片段。将扩增产物送上海生工生物工程股份有限公司测序,根据测序结果截取适当长度用于构建CEBPA-3′UTR双荧光素酶报告载体。序列由北京擎科生物科技有限公司合成,在5′和3′分别添加Sac I和Xho I酶切位点(酶切位点用下划线斜体标注,miRNA结合位点用下划线加粗标注)。
野生型CEBPA 3′UTR(CEBPA-3′UTR-WT):5′-GAGTCTCGACCCCTAGTCAGATCAGAAAGCTAGGTCGAGGGTTAGCTAGGAGGACATATGTTCATGGTGGAAGAGGGGAACCTAGAGATCTGGTCTGGGGGTCAGTAGGGGGTGGTGAAGGGACACTGGGACCATCAGCCTTGTCTGTACTGTATGTCGCCAGCATTGCCATATACACATGAAGCACAATCAGTCCATCCCAGAGGGACCGAAGTTATGAGAAGCTTTCCAAATATTTTGCTTTATCAGCCGACATCAACACTTGTATCTGGCCTCTCTGTGTCCCTGCGGTGCCTTGTGCAATG TGA ATGT GCACGTCTATGCTAAACCACCATTTTATTTGGTTTTTGTTTTGTTTTGGTTTTGCTCAGAAACTTGCCAGAATGAGGCTCTTCGTCAGCAGCTGCAGGGAGGGTCTGCGGCTGTCTTTCCTTTTGGTCTCGAG-3′;
突变型CEBPA 3′UTR(CEBPA-3′UTR-MUT):5′-GAGTCTCGACCCCTAGTCAGATCAGAAAGCTAGGTCGAGGGTTAGCTAGGAGGACATATGTTCATGGTGGAAGAGGGGAACCTAGAGATCTGGTCTGGGGGTCAGTAGGGGGTGGTGAAGGGACACTGGGACCATCAGCCTTGTCTGTACTGTATGTCGCCAGCATTGCCATATACACATGAAGCACAATCAGTCCATCCCAGAGGGACCGAAGTTATGAGAAGCTTTCCAAATATTTTGCTTTATCAGCCGACATCAACACTTGTATCTGGCCTCTCTGTGTCCCTGCGGTGCCTTGT CGTTACACTTACTGCACGTCTATGCTAAACCACCATTTTATTTGGTTTTTGTTTTGTTTTGGTTTTGCTCAGAAACTTGCCAGAATGAGGCTCTTCGTCAGCAGCTGCAGGGAGGGTCTGCGGCTGTCTTTCCTTTTGGTCTCGAG-3′。
将合成片段与双酶切后的Pmir-GLO载体进行连接、转化和测序后得到野生型和突变型CEBPA-3′UTR双荧光素酶报告质粒。将HEK-293T细胞悬液等量接种至24孔细胞培养板中,待细胞汇合度达到70%时参照Thermo Fisher LipofectamineTM 3000试剂说明书进行转染。LipofectamineTM3000 1 μL、重组质粒500 ng、miRNA mimic或miRNA NC终浓度为50 nmol·L-1。试验分为两组,每组设3个重复:CEBPA野生型组(共转染Pmir-CEBPA- WT和miRNA mimic,以共转染Pmir-CEBPA-WT和miRNA NC为对照组)、CEBPA突变型组(共转染Pmir-CEBPA-MUT和miRNA mimic,以共转染Pmir-CEBPA-MUT和miRNA NC为对照组)。共转染48 h后进行双荧光素酶活性检测,操作步骤参照Dual-Luciferase®双荧光素酶报告基因检测试剂盒说明书检测荧光活性变化。
1.2.4 牛肌内脂肪前体细胞的分离培养、诱导分化和转染 参照张萌萌等[22]和苑洪霞[23]的方法进行牛肌内前体脂肪细胞的分离和培养,以差速贴壁法纯化肌内前体脂肪细胞。参照相奥琪[24]的方法配制诱导分化和维持分化培养基,当六孔板中F4代细胞培养完全汇合2 d后用诱导分化培养基诱导2 d(记加诱导分化培养基时为第0天),再换成维持培养基培养至第8天。六孔板中细胞完全汇合2 d后,根据Thermo Fisher LipofectamineTM 3000试剂说明书进行转染,转染48 h后分别提取细胞RNA和蛋白。
1.2.5 CEBPA基因与miRNA荧光定量分析 利用Trizol法提取组织、细胞总RNA,按照HiFiScript cDNA Synthesis Kit试剂盒与miRcute Plus miRNA First-Strand cDNA Kit试剂盒分别进行普通反转录和miRNA反转录。CEBPA基因与miRNA基因荧光定量反应体系和反应条件参照相应试剂盒说明书进行设置,每个样品重复3次。
1.2.6 CEBPA蛋白表达量检测 提取转染miRNA模拟物48 h后的细胞蛋白,以每孔上样量50 μg进行SDS-PAGE。将蛋白转移至PVDF膜自然晾干后,置于95%乙醇中固定,再用0.01 mol·L-1 PBST洗5 min。用5%脱脂奶粉封闭液中封闭非特异性蛋白,室温下约4 h,再用0.01 mol·L-1 PBST洗3次,每次15 min。加I抗体(1:1 000,用0.01 mol·L-1 PBST配制),室温下3~4 h,再用0.01 mol·L-1 PBST洗3次,每次15 min。加HRP标记的二抗(1:1 000,用0.01 mol·L-1 PBST配制),室温下1~2 h,再用0.01 mol·L-1 PBST洗3次,每次15 min。将PVDF置于化学发光试剂中增强反应1~3 min,Blo-RAO扫描拍照。
1.2.7 CCK-8检测 将细胞悬液等量接种到96孔板中(每孔100 μL),并置于37 ℃、5% CO2的细胞培养箱中培养。24 h后分别转染miRNA mimic和miRNA NC,每组设置5个重复。在待测孔中加入10 μL CCK-8试剂后孵育2 h,再用酶标仪检测450 nm处吸光度。
1.2.8 数据分析 采用2-△△CT法计算CEBPA mRNA、miRNA表达水平;运用Image J软件分析Western blot试验结果。试验数据用SPSS 23.0软件进行单因素方差分析,分析结果用“X ±SD”表示,并用GraphPad Prism作图。
2 结果 2.1 调控CEBPA基因的miRNA预测通过聚焦4个在线软件的预测结果(图 1A),发现miR-23、miR-181、miR-101、miR-144、miR-186和miR-326与CEBPA基因3′UTR存在潜在作用关系。C/EBPβ(CEBPB)、C/EBPδ(CEBPD)和PPARγ(PPARG)对前体脂肪细胞分化过程均发挥一定作用[25-28],为提高筛选精度,排除这些基因中可能存在调控关系的miRNA,故选择miR-23和miR-181进行下一步试验。bta-miR-23和bta-miR-181家族中的miRNA种子序列里均有相似碱基能与CEBPA 3′UTR相结合(图 1B),最终以bta-miR-23a、bta-miR-23b-3p、bta-miR-181a、bta-miR- 181b、bta-miR-181c、bta-miR-181d作为候选miRNA。
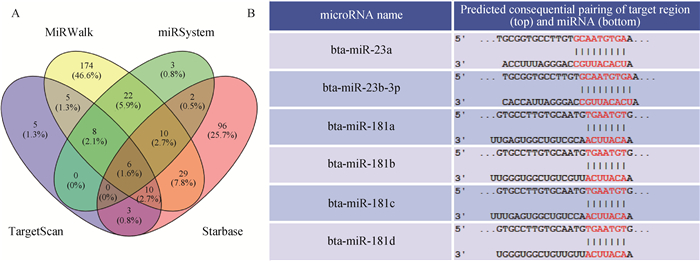
|
A. miRNA聚焦预测结果;B.候选miRNA与CEBPA基因3′UTR结合位点 A.miRNA focus prediction results; B. The binding site of candidate miRNA and CEBPA gene 3′UTR 图 1 与CEBPA存在潜在调控关系的miRNA预测结果 Fig. 1 Prediction results of miRNA with potential regulatory relationship with CEBPA |
通过qRT-PCR发现(图 2),CEBPA mRNA在肌肉组织(背最长肌、股二头肌、腰大肌)、睾丸、卵巢中的相对表达量极显著低于心、肝、脾、肺、脂肪等组织(P < 0.01);CEBPA基因在心中的表达量最高,极显著高于除下丘脑外的其他组织(P < 0.01);CEBPA基因在下丘脑呈高表达,极显著高于除心、肺、脂肪以外的组织(P < 0.01);同时,还发现CEBPA基因在2周岁关岭牛背最长肌的表达量极显著高于1日龄犊牛(P < 0.01);CEBPA基因在睾丸中表达量极显著高于卵巢(P < 0.01),且在母牛不同生殖器官中的表达量存在极显著差异,在子宫中表达量极显著高于卵巢(P < 0.01)。
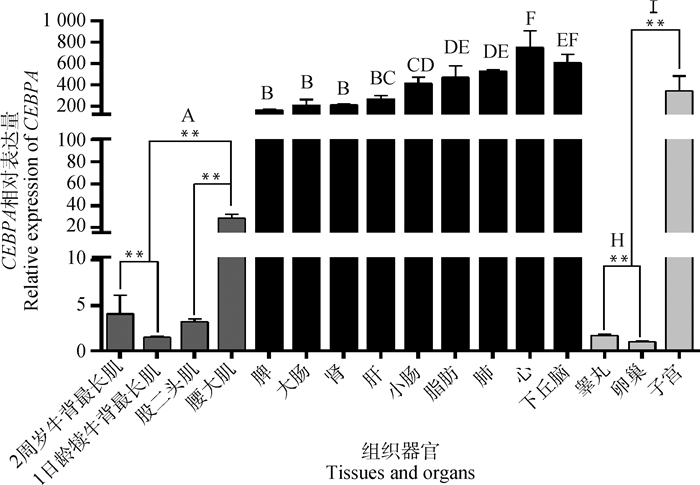
|
上标相同字母表示差异不显著(P>0.05),上标不同大写字母或**表示差异极显著(P < 0.01),上标不同小写字母或*表示差异显著(P < 0.05),下同 The same letter on the top indicates that the difference is not significant (P>0.05), the superscript different capital letters or ** means the difference is extremely significant (P < 0.01), and the superscript different lowercase letters or * means the difference is significant(P < 0.05), the same as below 图 2 CEBPA mRNA在关岭牛不同组织中的表达差异性 Fig. 2 Expression differences of CEBPA Gene in different tissues of Guanling cattles |
当前,大量探究miRNA与目标基因3′UTR作用关系的试验采用的均是双荧光素酶报告系统[29-30]。双荧光素酶报告系统检测结果如图 3所示,miR-23a+CEBPA-3′UTR-WT组荧光活性极显著低于miR-NC+CEBPA-3′UTR-WT组(P < 0.01),且显著低于miR-23a+CEBPA-3′UTR-MUT组(P < 0.05),说明bta-miR-23a与CEBPA基因mRNA的3′UTR具有直接调控关系;miR-23b-3p+CEBPA-3′UTR-WT组、miR-181a+CEBPA-3′UTR-WT组转染后荧光活性均极显著低于miR-NC+CEBPA-3′UTR-WT组和miR-mimic+CEBPA-3′UTR-MUT组(P < 0.01),表明miR-23b-3p、miR-181a均能抑制CEBPA基因mRNA 3′UTR活性。
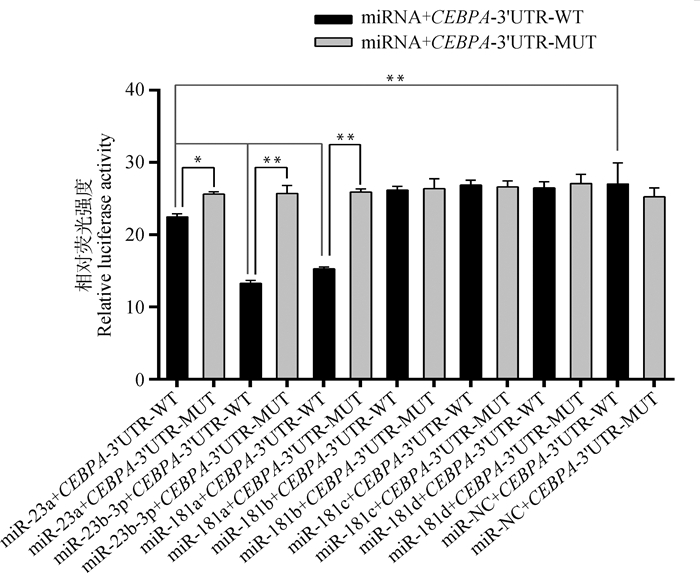
|
图 3 双荧光素酶检测结果 Fig. 3 Dual luciferase test results |
利用“鸡尾酒法”诱导细胞分化,检测诱导0、2、6、8 d时CEBPA mRNA、bta-miR-23a、bta-miR-23b-3p和bta-miR-181a表达量,其结果见图 4。CEBPA基因在分化初期呈低表达,随着分化时间逐渐升高,第8天的表达量极显著高于第0、2、6天(P < 0.01),而bta-miR-23a、bta-miR-23b-3p、bta-miR-181a在细胞分化初期呈现高表达,极显著高于其他时期(P < 0.01)。在表达量变化趋势上,bta-miR-23b-3p、bta-miR-181a的在细胞不同分化时期的表达量与CEBPA基因的表达趋势相反;bta-miR-23a除在分化初期高表达外,第2、6、8天处于较低表达水平,且这3个时间段间的表达水平差异不显著(P>0.05)。
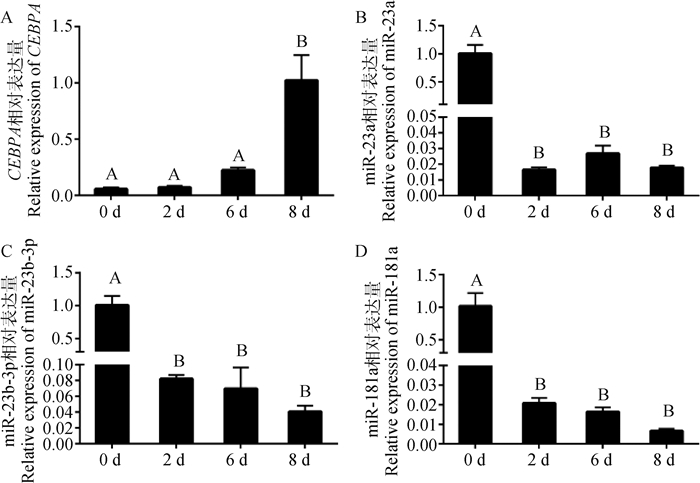
|
图 4 CEBPA、miR-23b-3p、miR-23a和miR-181a在牛肌内前体脂肪细胞不同分化时期的表达量变化 Fig. 4 Changes in the expression levels of CEBPA, miR-23b-3p, miR-23a and miR-181a in cattle intramuscular precursor adipocytes at different stages of differentiation |
过表达miRNA 48 h后,miRNA、CEBPA mRNA与蛋白变化情况分别见图 5、6。由图 5B可知,3个miRNA mimic均能极显著降低CEBPA mRNA表达量(P < 0.01),其中,miR-23b-3p比miR-23a、miR-181a降低CEBPA表达效果更强,但未达显著水平(P>0.05)。由图 6B可知,过表达miR-23a、miR-23b-3p、miR-181a均能极显著降低CEBPA蛋白表达(P < 0.01),在过表达后对CEBPA蛋白抑制效果最强的是miR-23b-3p组,表明miR-23a、miR-23b-3p、miR-181a均能抑制牛肌内前体脂肪细胞中CEBPA基因的表达。由图 7可观察到,分别过表达miR-23a、miR-23b-3p、miR-181a后牛肌内前体脂肪细胞中脂滴数均少于miR-NC组,表明miR-23a、miR-23b-3p、miR-181a均能降低牛肌内前体脂肪细胞中脂滴含量。由此可知,miR-23a、miR-23b-3p、miR-181a均能通过抑制CEBPA基因的表达来调节牛肌内前体脂肪细胞分化。
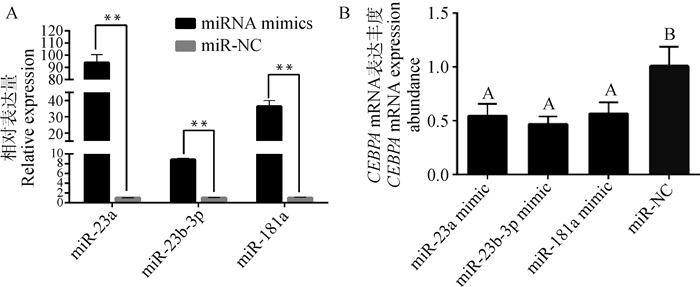
|
A.过表达miRNA后荧光定量PCR检测miRNA变化;B.过表达miRNA后荧光定量PCR检测CEBPA表达量 A. Fluorescence quantitative PCR to detect changes in miRNA after overexpression of miRNA; B.Detection of CEBPA expression by fluorescence quantitative PCR after overexpression of miRNA 图 5 过表达miRNA后牛肌内前体脂肪细胞miRNA和CEBPA mRNA表达量变化 Fig. 5 Changes of miRNA and CEBPA mRNA expression in cattle intramuscular precursor adipocytes after overexpression of miRNA |
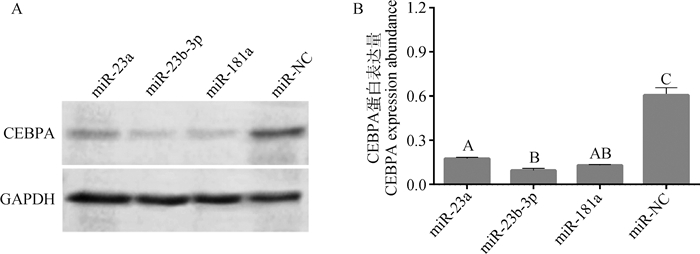
|
A.过表达miR-23a、miR-23b-3p、miR-181a后CEBPA的Western blot条带;B.过表达miR-23a、miR-23b-3p、miR-181a后CEBPA蛋白的相对表达量 A. Western blot band of CEBPA after overexpression of miR-23a, miR-23b-3p, miR-181a; B. Relative expression of CEBPA protein after overexpression of miR-23a, miR-23b-3p and miR-181a 图 6 过表达miR-23a、miR-23b-3p、miR-181a后CEBPA蛋白相对表达量变化 Fig. 6 Changes in relative expression of CEBPA protein after overexpression of miR-23a, miR-23b-3p and miR-181a |
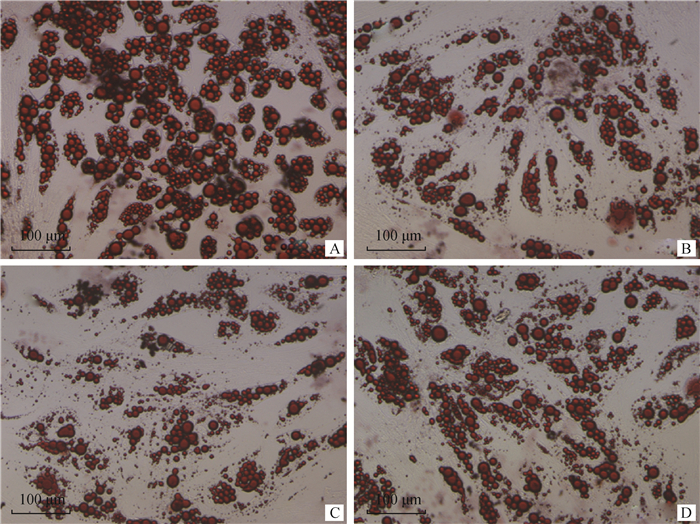
|
A~D.分别过表达miR-NC、miR-23a、miR-23b-3p、miR-181a后油红O染色图 A-D. Oil red O staining diagram after overexpression of miR-NC, miR-23a, miR-23b-3p, miR-181a 图 7 在牛肌内前体脂肪细胞中过表达miR-23a、miR-23b-3p、miR-181a后诱导分化8 d时油红O染色结果(200×) Fig. 7 Oil red O staining results after 8 days of overexpression of miR-23a, miR-23b-3p and miR-181a in cattle intramuscular precursor adipocytes (200×) |
运用CCK-8检测过表达miRNA后对细胞增的影响,试验结果如图 8所示,转染24 h后,3个处理组细胞增殖效果较miRNA NC组无显著变化(P>0.05);转染48 h后,3个处理组细胞增殖效果较转染miRNA NC组呈极显著增强(P < 0.01),而转染miR-23a mimic、miR-23b-3p mimic对细胞增殖效果显著高于miR-181a mimic(P < 0.05);转染72 h后,3个处理组对细胞增殖影响差异不显著(P>0.05)。CCK-8检测结果表明,bta-miR-23a、bta-miR-23b-3p、bta-miR-181a仅在关岭牛肌内前体脂肪细胞增殖过程中阶段性的发挥促进作用。
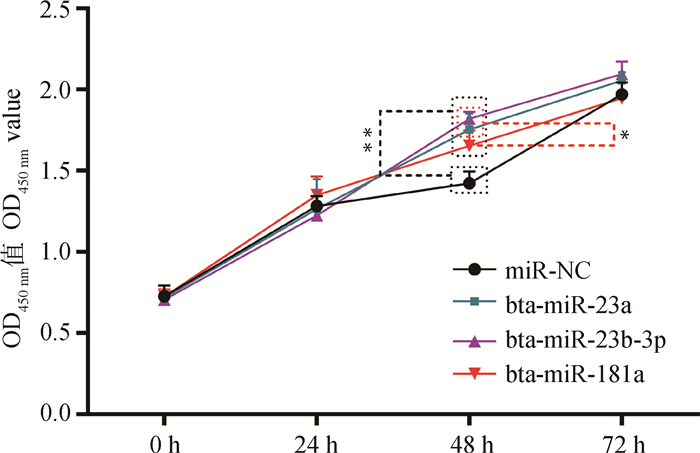
|
图 8 过表达miRNA对牛肌内前体脂肪细胞增殖的影响 Fig. 8 Effect of overexpression of miRNA on the proliferation of cattle intramuscular precursor adipocytes |
CEBPA基因作为重要的转录调节因子,广泛存在于机体各类组织中[31]。Williams等[32]研究表明,CEBPA基因在肝和脂肪组织中有较高表达水平,本研究也发现CEBPA基因在关岭牛心、肝、脾、肺、脂肪中的表达水平极显著高于肌肉(背最长肌、股二头肌、腰大肌)组织(P < 0.01)。此外,下丘脑是调控食欲和维持能量代谢稳态的关键区域[33],本研究在下丘脑中检测到CEBPA基因呈高表达,这与该基因的信号传导功能有关[34]。CEBPA基因参与卵巢促性腺激素生成以及睾丸的损伤修复[35-36],本试验在关岭牛卵巢、子宫和睾丸中均检测到CEBPA基因有不同程度的表达。这表明,CEBPA基因是机体正常生殖活动不可或缺的因子。
动物基因3′UTR包含多个miRNA特定结合区域[37],多个miRNA协同作用于靶基因,通过调节基因表达影响基因功能,进而调控信号传导、增殖分化、IMF沉积等过程。已有大量研究采用“生物信息学+生物试验学”方法验证miRNA与其靶基因的调控关系[38-40]。本研究利用多个在线软件筛选出6个与牛CEBPA基因具有潜在调控关系的miRNA,并通过双荧光素酶活性检测证实bta-miR-23a、bta-miR-23b-3p、bta-miR-181a能直接作用于CEBPA mRNA的3′UTR。Bi等[41]已证实巨噬细胞中的miR-181a能直接调节C/EBPα基因。
CEBPα表达水平与细胞分化时间呈线性增长关系[3],本研究发现CEBPA基因在分化初期呈低表达,随着分化时间逐渐升高,这与牟彦双等[42]和张萌萌等[22]的研究结果一致。此外,CEBPα在分化初期表达水平较低,而bta-miR-23a、bta-miR-23b-3p、bta-miR-181a在分化初期呈现高表达且极显著高于其他时期(P < 0.01),整个分化时期bta-miR-23b-3p、bta-miR-181a与CEBPA基因的表达趋势相反。Shen等[43]指出,miR-23a在3T3-L1细胞分化0 d时呈高表达状态,而在分化期间miR-23a的表达降低了4倍以上。过表达miR-23a、miR-23b-3p、miR-181a后,均能极显著降低CEBPA基因mRNA和蛋白表达量(P < 0.01),3个miRNA仅在细胞增殖过程中发挥阶段性促进作用。miR-23a在脂肪细胞分化过程中起抑制作用[43-44],miR-23b-3p、miR-181a通过直接作用于关键转录因子调控多种细胞周期和分化[45-48]。
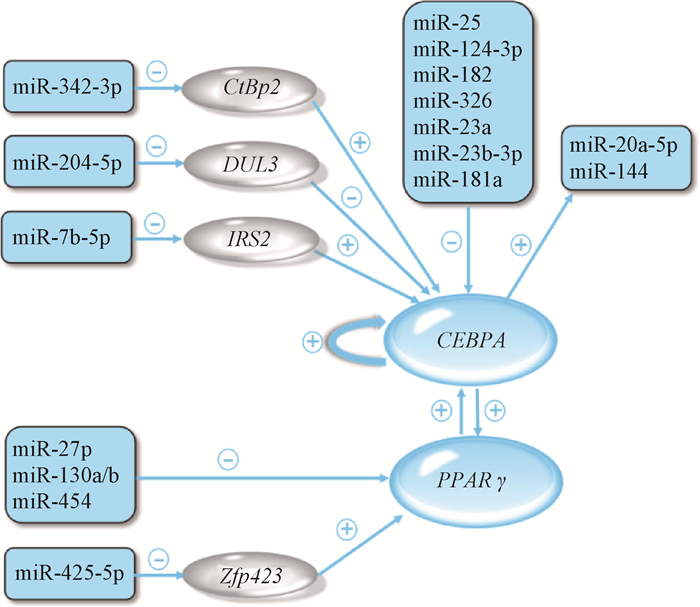
miRNA、转录因子以及调控转录因子的miRNA共同参与对CEBPA表达调控过程。一些miRNA能直接作用于CEBPA影响其表达,如miR-181a、miR-124-3p、miR-326、miR-182、miR-25是CEBPA基因的直接调控miRNA[41, 49-51];另一些miRNA则通过结合对CEBPA表达有影响的转录因子,间接介导对CEBPA的调控,如miR-342-3p、miR-204-5p、miRNA-7b-5负调控转录因子来影响CEBPA表达[52-54]。在脂肪细胞分化过程中,CEBPA与PPARγ相互促进,形成一个正反馈环[55]。因此,miRNA对PPARγ的调节效果会间接影响CEBPA表达。已有研究表明,miR-454、miR-130a/b、miR-425-5p、miR-27b能直接或间接抑制PPARγ[56-59]。此外,CEBPA基因也能通过与miRNA基因启动区结合,进而积极调节miRNA的表达[16, 60]。本研究确认了miR-23a、miR-23b-3p、miR-181a能负调控CEBPA基因,基于已有的报道和本研究结果,构建出miRNA与CEBPA之间通路如图 9所示。
|
⊕表示正调控;⊖表示负调控 ⊕means positive regulation; ⊖means negative regulation 图 9 miRNA直接或间接调控CEBPA的通路图 Fig. 9 Pathways of miRNA regulating CEBPA directly or indirectly |
CEBPA mRNA在关岭牛内脏组织、肌肉组织、脂肪、下丘脑以及部分生殖器官中均有表达。生物信息学筛选和生物学试验结果表明,bta-miR-23a、bta-miR-23b-3p、bta-miR-181a能阶段性的促进牛肌内前体脂肪细胞增殖,并负调控CEBPA基因抑制牛肌内前体脂肪细胞分化。
| [1] | TSUKADA J, YOSHIDA Y, KOMINATO Y, et al. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP)transcription factors is a multifaceted highly-regulated system for gene regu-lation[J]. Cytokine, 2011, 54(1): 6–19. DOI: 10.1016/j.cyto.2010.12.019 |
| [2] |
皇甫一凡.C/EBPα基因诱导牛成肌细胞转分化的研究[D].杨凌: 西北农林科技大学, 2013.
HUANGPU Y F.C/EBPα induce the trans-differentiation of bovine myoblasts[D]. Yangling: Northwest A&F University, 2013.(in Chinese) |
| [3] | SIERSBÆK R, NIELSEN R, MANDRUP S. Transcriptional networks and chromatin remodeling controlling adipogenesis[J]. Trends Endocrinol Metab, 2012, 23(2): 56–64. DOI: 10.1016/j.tem.2011.10.001 |
| [4] | DAVIES G E, SABATAKOS G, CRYER A, et al. The ovine CCAAT-enhancer binding protein δ gene:cloning, characterization, and species-specific autoregulation[J]. Biochemical and Biophysical Research Communications, 2000, 271(2): 346–352. DOI: 10.1006/bbrc.2000.2630 |
| [5] | CAO Z, UMEK R M, MCKNIGHT S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells[J]. Genes Dev, 1991, 5(9): 1538–1552. DOI: 10.1101/gad.5.9.1538 |
| [6] |
孙伟芳.呼伦贝尔羊两个品系不同部位脂肪细胞形态及脂肪代谢相关基因表达差异分析[D].成都: 四川农业大学, 2017.
SUN W F.Differential analysis of adipocyte morphology and fat metabolism-reated genes expression in two strains of Hulun buir sheep[D]. Chengdu: Sichuan Agricultural University, 2017.(in Chinese) |
| [7] | NERLOV C, ZIFF E B. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-alpha on the serum albumin promoter[J]. Genes Dev, 1994, 8(3): 350–362. DOI: 10.1101/gad.8.3.350 |
| [8] |
黄明捷, 陈祥, 张勇, 等. CEBPA基因的生物学效应及其在畜禽生产中的研究进展[J]. 中国畜牧杂志, 2020, 56(1): 44–50.
HUANG M J, CHEN X, ZHANG Y, et al. Biological effects of CEBPA gene and its research progress in animal husbandry[J]. Chinese Journal of Animal Science, 2020, 56(1): 44–50. (in Chinese) |
| [9] | FILIPOWICZ W, BHATTACHARYYA S N, SONENBERG N. Mechanisms of post-transcriptional regulation by microRNAs:are the answers in sight?[J]. Nat Rev Genet, 2008, 9(2): 102–114. DOI: 10.1038/nrg2290 |
| [10] | VISHNOI A, RANI S. MiRNA biogenesis and regulation of diseases:an overview[J]. Methods Mol Biol (Clifton, N.J.), 2017, 1509: 1–10. |
| [11] |
金晓露, 杨建香, 李真, 等. 乳腺发育及泌乳相关miRNA研究进展[J]. 遗传, 2013, 35(6): 695–702.
JIN X L, YANG J X, LI Z, et al. Progress on the miRNA related with mammary gland development and lactation[J]. Hereditas (Beijing), 2013, 35(6): 695–702. (in Chinese) |
| [12] | LI X, ZHAO Y, LI X Q, et al. MicroRNA-150 modulates adipogenic differentiation of adipose-derived stem cells by targeting notch3[J]. Stem Cells Int, 2019, 2019: 2743047. |
| [13] | FENG Y T, ZHOU L T, PENG Y, et al. The role of miR-326 in adipogenic differentiation of human adipose-derived stem cells by targeting C/EBPα in vitro[J]. Anat Rec (Hoboken), 2020, 303(7): 2054–2060. DOI: 10.1002/ar.24281 |
| [14] | PAN Y, JING J, QIAO L, et al. MiRNA-seq reveals that miR-124-3p inhibits adipogenic differentiation of the stromal vascular fraction in sheep via targeting C/EBPα[J]. Domest Anim Endocrin, 2018, 65: 17–23. DOI: 10.1016/j.domaniend.2018.05.002 |
| [15] | LIANG W C, WANG Y, LIANG P P, et al. MiR-25 suppresses 3T3-L1 adipogenesis by directly targeting KLF4 and C/EBPα[J]. J Cell Biochem, 2015, 116(11): 2658–2666. DOI: 10.1002/jcb.25214 |
| [16] | ZHOU J, YANG J Y, WANG X C. A Novel regulatory circuit "C/EBPα/miR-20a-5p/TOB2" regulates adipogenesis and lipogenesis[J]. Front Endocrinol (Lausanne), 2020, 10: 894. DOI: 10.3389/fendo.2019.00894 |
| [17] | LI J H, LIU S, ZHOU H, et al. StarBasev 2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein- RNA interaction networks from large-scale CLIP-Seq data[J]. Nucleic Acids Res, 2014, 42(1): D92–97. |
| [18] | NIKPAYAM E, TASHARROFI B, SARRAFZADEH S, et al. The role of long non-coding RNAs in ovarian cancer[J]. Iran Biomed J, 2017, 21(1): 3–15. DOI: 10.18869/acadpub.ibj.21.1.3 |
| [19] | LU T P, LEE C Y, TSAI M H, et al. MiRSystem:an integrated system for characterizing enriched functions and pathways of microRNA targets[J]. PLoS One, 2012, 7(8): e42390. DOI: 10.1371/journal.pone.0042390 |
| [20] | LEWIS B P, SHIH I H, JONES-RHOADES M W, et al. Prediction of mammalian microRNA targets[J]. Cell, 2003, 115(7): 787–798. DOI: 10.1016/S0092-8674(03)01018-3 |
| [21] |
王军, 燕晓晓, 丁赫, 等. LPS诱导牛子宫内膜细胞miRNA差异表达分析[J]. 中国兽医杂志, 2017, 53(10): 10–12, 16.
WANG J, YAN X X, DING H, et al. Lipopolysaccharide-induced differential expression of miRNAs in bovine endometrial cells[J]. Chinese Journal of Veterinary Medicine, 2017, 53(10): 10–12, 16. (in Chinese) |
| [22] |
张萌萌, 魏胜娟, 王艺如, 等. 牛肌内前体脂肪细胞的分离培养及分化相关基因的表达规律研究[J]. 畜牧与兽医, 2018, 50(5): 1–6.
ZHANG M M, WEI S J, WANG Y R, et al. Primary culture and expression of differentiation-related genes in bovine intramuscular preadipocytes[J]. Animal Husbandry & Veterinary Medicine, 2018, 50(5): 1–6. (in Chinese) |
| [23] |
苑洪霞.LYRM1与LYRM2基因对白洗猪脂肪沉积的影响研究[D].贵阳: 贵州大学, 2018.
YUAN H X.Study on the effect of LYRM1 and LYRM2 gene on the fat deposition of Baixi pig[D]. Guiyang: Guizhou University, 2018.(in Chinese) |
| [24] |
相奥琪.IGFBP5对肌内脂肪细胞和肌细胞脂肪沉积的影响及其机制[D].杨凌: 西北农林科技大学, 2019.
XIANG A Q.The function and mechanism of IGFBP5 on fat deposition in intramuscular adipocytes and myocytes[D]. Yangling: Northwest A&F University, 2019.(in Chinese) |
| [25] | CHUNG K Y, JOHNSON B J. Melengestrol acetate enhances adipogenic gene expression in cultured muscle-derived cells[J]. J Anim Sci, 2009, 87(12): 3897–3904. DOI: 10.2527/jas.2008-1645 |
| [26] | STEPHENS J M. The fat controller:adipocyte development[J]. PLoS Biol, 2012, 10(11): e1001436. DOI: 10.1371/journal.pbio.1001436 |
| [27] | KANG S, AKERBLAD P, KIVIRANTA R, et al. Regulation of early adipose commitment by Zfp521[J]. PLoS Biol, 2012, 10(11): e1001433. DOI: 10.1371/journal.pbio.1001433 |
| [28] | WU J, BOSTRÖM P, SPARKS L M, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human[J]. Cell, 2012, 150(2): 366–376. DOI: 10.1016/j.cell.2012.05.016 |
| [29] | YACHI K, TSUDA M, KOHSAKA S, et al. MiR-23a promotes invasion of glioblastoma via HOXD10-regulated glial-mesenchymal transition[J]. Signal Transduct Target Ther, 2018(3): 33. |
| [30] | ZHANG J, HOU L D, LIANG R, et al. CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling[J]. Mol Cancer, 2019, 18(1): 80. DOI: 10.1186/s12943-019-1015-1 |
| [31] |
盘道兴, 王振, 杨茂林, 等. 不同品种猪PPARγ和C/EBPα基因表达规律与肌内脂肪含量的相关[J]. 中国农业科学, 2017, 50(1): 171–182.
PAN D X, WANG Z, YANG M L, et al. Association of the PPARγ and C/EBPα gene expression with intramuscular fat content in different varieties of pig[J]. Scientia Agricultura Sinica, 2017, 50(1): 171–182. (in Chinese) |
| [32] | WILLIAMS S C, CANTWELL C A, JOHNSON P F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro[J]. Genes Dev, 1991, 5(9): 1553–1567. DOI: 10.1101/gad.5.9.1553 |
| [33] |
任晓莹, 梁向艳, 刘媛, 等. 游离脂肪酸受体4调控能量代谢的途径与分子机制[J]. 医学综述, 2020, 26(4): 636–640.
REN X Y, LIANG X Y, LIU Y, et al. Pathways and molecular mechanism of free fatty acid receptor 4 in metabolism regulation[J]. Medical Recapitulate, 2020, 26(4): 636–640. DOI: 10.3969/j.issn.1006-2084.2020.04.003 (in Chinese) |
| [34] |
汤惠.MT2受体通过C/EBPα/miR-125b信号通路改善阿尔茨海默病模型鼠中树突异常[D].武汉: 华中科技大学, 2019.
TANG H.Activation of MT2 receptor ameliorates dendritic abnormalities in Alzheimer's disease via C/EBPα/miR-125b pathway[D]. Wuhan: Huazhong University of Science and Technology, 2019.(in Chinese) |
| [35] | PIONTKEWITZ Y, ENERBÄCK S, HEDIN L. Expression and hormonal regulation of the CCAAT enhancer binding protein-alpha during differentiation of rat ovarian follicles[J]. Endocrinology, 1993, 133(5): 2327–2333. DOI: 10.1210/endo.133.5.8404685 |
| [36] | YAO P L, LIN Y C, RICHBURG J H. Transcriptional suppression of sertoli cell Timp2 in rodents following mono-(2-ethylhexyl) phthalate exposure is regulated by CEBPA and MYC[J]. Biology of Reproduction, 2011, 85(6): 1203–1215. DOI: 10.1095/biolreprod.111.093484 |
| [37] | STARK A, BRENNECKE J, RUSSELL R B, et al. Identification of drosophila microRNA targets[J]. PLoS Biol, 2003, 1(3): E60. DOI: 10.1371/journal.pbio.0000060 |
| [38] |
金连丰, 于广璞, 孙霞, 等. 牛GHR基因与miR-139靶向关系的验证[J]. 畜牧兽医学报, 2016, 47(12): 2398–2404.
JIN L F, YU G P, SUN X, et al. Targeting verification between Bta-GHR and miR-139[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(12): 2398–2404. DOI: 10.11843/j.issn.0366-6964.2016.12.009 (in Chinese) |
| [39] |
赵艳艳, 乔利英, 景炅婕, 等. miR-142和miR-144靶向FoxO1基因调节绵羊前体脂肪细胞分化的研究[J]. 畜牧兽医学报, 2018, 49(4): 675–684.
ZHAO Y Y, QIAO L Y, JING J J, et al. Study on the differentiation regulation of miR-142 and miR-144 on ovine preadipocytes by targeting FoxO1 gene[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(4): 675–684. (in Chinese) |
| [40] |
王书芳, 潘洋洋, 任端阳, 等. miR-200c和miR-429靶向调节绵羊皮下脂肪细胞中SCD1表达的研究[J]. 畜牧兽医学报, 2019, 50(7): 1347–1357.
WANG S F, PAN Y Y, REN D Y, et al. Regulation of SCD1 gene expression by miR-200c and miR-429 in ovine subcutaneous adipocytes[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(7): 1347–1357. (in Chinese) |
| [41] | BI J, ZENG X X, ZHAO L, et al. MiR-181a induces macrophage polarized to M2 phenotype and promotes M2 macrophage-mediated tumor cell metastasis by targeting KLF6 and C/EBPα[J]. Mol Ther Nucleic Acids, 2016, 5: e368. DOI: 10.1038/mtna.2016.71 |
| [42] |
牟彦双, 王宇祥, 李辉. 油酸对鸡前脂肪细胞分化过程中基因表达的影响[J]. 东北农业大学学报, 2013, 44(12): 46–51.
MU Y S, WANG Y X, LI H. Effect of oleate on gene expression during differentiation of chicken preadipocyte[J]. Journal of Northeast Agricultural University, 2013, 44(12): 46–51. DOI: 10.3969/j.issn.1005-9369.2013.12.009 (in Chinese) |
| [43] | SHEN L Y, ZHANG Y, DU J J, et al. MicroRNA-23a regulates 3T3-L1 adipocyte differentiation[J]. Gene, 2016, 575(2): 761–764. DOI: 10.1016/j.gene.2015.09.060 |
| [44] |
关龙.牛间充质干细胞成肌/成脂分化中差异miRNAs的鉴定及miR-23a调控机制研究[D].北京: 中国农业科学院, 2017.
GUAN L.Identification of differentially expressed miRNAs during MSCs adipogenesis and myogenesis in cattle and regulation mechanism of miR-23a in adipogenesis[D]. Beijing: Chinese Academy of Agricultural Sciences, 2017.(in Chinese) |
| [45] | HILDEBRAND J, RVTZE M, WALZ N, et al. A comprehensive analysis of microRNA expression during human keratinocyte differentiation in vitro and in vivo[J]. J Invest Dermatol, 2011, 131(1): 20–29. DOI: 10.1038/jid.2010.268 |
| [46] | HAM O, SONG B W, LEE S Y, et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase a signaling[J]. Biomaterials, 2012, 33(18): 4500–4507. DOI: 10.1016/j.biomaterials.2012.03.025 |
| [47] | MERCATELLI N, FITTIPALDI S, DE PAOLA E, et al. MiR-23-TrxR1 as a novel molecular axis in skeletal muscle differentiation[J]. Sci Rep, 2017, 7: 7219. DOI: 10.1038/s41598-017-07575-0 |
| [48] | TAYLOR M A, SOSSEY-ALAOUI K, THOMPSON C L, et al. TGF-β upregulates miR-181a expression to promote breast cancer metastasis[J]. J Clin Invest, 2013, 123(1): 150–163. DOI: 10.1172/JCI64946 |
| [49] | WURM A A, ZJABLOVSKAJA P, KARDOSOVA M, et al. Disruption of the C/EBPα-miR-182 balance impairs granulocytic differentiation[J]. Nat Commun, 2017, 8(1): 46. DOI: 10.1038/s41467-017-00032-6 |
| [50] | HU X X, FENG J, HUANG X W, et al. Histone deacetylases up-regulate C/EBPα expression through reduction of miR-124-3p and miR-25 in hepatocellular carcinoma[J]. Biochem Biophys Res Commun, 2019, 514(3): 1009–1016. DOI: 10.1016/j.bbrc.2019.05.024 |
| [51] | XU C F, YU C H, LI Y M. Regulation of hepatic microRNA expression in response to ischemic preconditioning following ischemia/reperfusion injury in mice[J]. OMICS, 2009, 13(6): 513–520. DOI: 10.1089/omi.2009.0035 |
| [52] | WANG L, XU L, XU M, et al. Obesity-associated miR-342-3p promotes adipogenesis of mesenchymal stem cells by suppressing CtBP2 and releasing C/EBPα from CtBP2 binding[J]. Cell Physiol Biochem, 2015, 35(6): 2285–2298. DOI: 10.1159/000374032 |
| [53] | HE H, CHEN K, WANG F, et al. miR-204-5p promotes the Adipogenic differentiation of human adipose-derived mesenchymal stem cells by modulating DVL3 expression and suppressing Wnt/β-catenin signaling[J]. Int J Mol Med, 2015, 35(6): 1587–1595. DOI: 10.3892/ijmm.2015.2160 |
| [54] | LI Q Q, WEI Q, ZHAI X C. MiRNA-7b-5p attenuates the progression of osteoporosis by inhibiting adipose differentiation of hMSCs via regulating IRS2[J]. Eur Rev Med Pharmacol Sci, 2019, 23(21): 9207–9214. |
| [55] | ROSEN E D, MACDOUGALD O A. Adipocyte differentiation from the inside out[J]. Nat Rev Mol Cell Biol, 2006, 7(12): 885–896. |
| [56] | KARBIENER M, FISCHER C, NOWITSCH S, et al. MicroRNA miR-27b impairs human adipocyte differentiation and targets PPARγ[J]. Biochem Biophys Res Commun, 2009, 390(2): 247–251. DOI: 10.1016/j.bbrc.2009.09.098 |
| [57] |
张铭琪.miR-454在牛乳腺上皮细胞中靶向PPAR-γ对甘油三酯合成的影响及其机制研究[D].长春: 吉林大学, 2019.
ZHANG M Q.MiR-454 regulates triglyceride synthesis in bovine mammary epithelial cells by targeting PPAR-γ[D]. Changchun: Jilin University, 2019.(in Chinese) |
| [58] | MA X Y, WEI D W, CHENG G, et al. Bta-miR-130a/b regulates preadipocyte differentiation by targeting PPARG and CYP2U1 in beef cattle[J]. Mol Cell Probes, 2018, 42: 10–17. |
| [59] |
占猛斯.miR-425对3T3-L1细胞分化的抑制作用研究[D].武汉: 华中农业大学, 2016.
ZHAN M S.Study on the inhibition of miR-425 to differentiation of 3T3-L1 cell[D]. Wuhan: Huazhong Agricultural University, 2016.(in Chinese) |
| [60] | CHEN G H, WU K, ZHAO T, et al. MiR-144 mediates high fat-induced changes of cholesterol metabolism via direct regulation of C/EBPα in the liver and isolated hepatocytes of yellow catfish[J]. J Nutr, 2020, 150(3): 464–474. |



