多糖是由多个单糖分子缩合、失水而成,是一类分子结构复杂且庞大的糖类物质,凡符合高分子化合物概念的碳水化合物及其衍生物均称为多糖。多糖主要来源于高等植物、动物细胞膜以及微生物细胞壁,根据其来源可分为植物多糖、动物多糖以及微生物多糖三大类。常见植物多糖有淀粉、纤维素、果胶等,动物多糖有糖原、肝素、甲壳素等,微生物多糖包括细菌多糖(凝结多糖、半乳葡聚糖、细菌纤维素等)和真菌多糖(酵母多糖、灵芝多糖、香菇多糖等)。多糖类化合物是构成生命的四大基本物质之一,其不仅是细胞的结构物质和能源物质,更是具有调节免疫功能、抗肿瘤、抗病毒、抗感染、抗糖尿病、抗寄生虫、抗菌等多种生理功能的生物活性物质[1-5]。目前,人们已成功地从自然界中提取了大量多糖并广泛应用于食品、饲料、医药等领域[6-8]。国内外对多糖的分离鉴定、提取纯化、结构表征、生物活性研究颇丰[9-10],对其抗菌活性及作用机制也进行了广泛深入的探讨。研究发现,对天然多糖进行化学修饰(硫酸化、羧甲基化、乙酰化等)得到的多糖衍生物比未改性的多糖表现出更好的抗菌性能[11]。多糖抗菌作用机制复杂,除抑制细菌细胞黏附、破坏生物膜、使细胞膜细胞壁损坏或通透性增加外,多糖还作用于细菌核糖体,影响蛋白质的合成以及细菌的物质代谢和营养物质的吸收等[12-15]。本文对国内外关于多糖类化合物的抗菌活性及其抗菌作用机制进行归纳和总结,为进一步深化多糖类抗菌作用机制研究、缓解细菌耐药性的产生、开发新的高效、绿色抗菌药物提供理论基础。
1 多糖类化合物的抗菌作用 1.1 植物多糖多年来,人们从果蔬、花卉种子以及一些药用植物中提取、分离多糖,并对其生物活性进行广泛研究,其中多糖的抗菌作用在国内外备受关注。目前已发现多种植物多糖具有良好抗菌活性,如南瓜水溶性多糖[16]、胡萝卜皮水溶性多糖[17]、橄榄叶多糖[18]、月桂多糖[19]、新鲜玉米须多糖[20]等。据报道,来自人参的酸性多糖PG-F2和来自绿茶的酸性多糖CS-F2对幽门螺杆菌、痤疮丙酸杆菌、金黄色葡萄球菌的最小抑菌浓度(minimum inhibitory concentration,MIC)范围分别为0.1~0.5、0.01~0.1 mg·mL-1[21]。Shao等[22]对浒苔多糖进行化学修饰以及改性后得到的多糖具有比原来更好的抗菌活性,MIC在1~2 mg·mL-1范围内。Jun等[23]研究结果表明,来自墨角藻的岩藻依聚糖F85对几种牙菌斑细菌显示出生长抑制作用,其MIC范围为0.125~1 mg·mL-1。Li等[24]从牡丹籽渣中获得的多糖及其衍生物表现出明显的抑菌活性,对4种被测微生物的抗菌活性顺序为:鼠伤寒沙门氏菌>大肠杆菌>枯草芽孢杆菌>金黄色葡萄球菌,MIC为0.8~2.4 mg·mL-1。部分多糖对耐药菌也有一定的抑制作用,张颖颖等[25]研究发现,罗勒多糖对MRSA(耐甲氧西林金黄色葡萄球菌)的抗菌能力效果最好,其MIC在0.625~1.25 mg·mL-1之间(表 1)。
|
|
表 1 主要植物多糖的抗菌活性 Table 1 Antibacterial activity of major plant polysaccharides |
动物多糖存在和分布极为广泛,几乎存在于所有动物组织器官中。一些具有较好抗菌作用的动物多糖陆续被发现,如星鲨硫酸化多糖[26]、方格星虫多糖[27]以及来源于墨鱼皮肤和肌肉的多糖[28]等。Wang等[29]发现,蚯蚓多糖对大肠杆菌、铜绿假单胞菌、金黄色葡萄球菌的MIC分别为6.25、12.5、6.25 mg·mL-1,而经大肠杆菌诱导后的蚯蚓多糖的MIC为6.25、6.25、3.125 mg·mL-1。Tajdini等[30]用真菌壳聚糖与虾壳聚糖进行抗菌活性对比,其中虾壳聚糖对部分细菌及真菌的MIC值范围为0.05~0.4 mg·mL-1。张晓[31]对两种不同粘度的壳聚糖(CTS1和CTS2)进行了抑菌活性测定,CTS1对大肠杆菌、沙门氏菌、金黄色葡萄球菌、枯草芽孢杆菌的MIC范围是1.28~5.12 mg·mL-1,CTS2为0.64~1.28 mg·mL-1。Krichen等[32]从灰纹三角鱼和星鲨皮中提取的硫酸化多糖对肠沙门氏菌、李斯特菌、金黄色葡萄球菌等有不同的MIC值,其范围分别在6.25~12.5和25~50 mg·mL-1之间。刘玉红[33]测定结果显示:水溶性壳聚糖(WCS)和磺化壳聚糖(SCS)对绿脓杆菌、大肠杆菌、金黄色葡萄球菌的MIC均分别为1、1和2 mg·mL-1,见表 2。
|
|
表 2 主要动物多糖的抗菌活性 Table 2 Antibacterial activity of major animal polysaccharides |
微生物多糖主要源于细菌和真菌,根据多糖在微生物细胞中的位置,可分为胞外多糖、胞壁多糖和胞内多糖。研究表明,微生物多糖具有广谱抗菌活性,其中包括双歧杆菌WBIN03和植物乳杆菌R315的胞外多糖[34]、金针菇多糖和铁配合物[35]、酵母细胞壁多糖[36-37]以及寡聚糖类抗生素(如evernimicin[38])等。Zhu等[39]从蘑菇废料中分离并纯化了一种名为PL的水溶性多糖,并对其纯化得到两种纯化品PL1和PL2,通过纸片扩散法测得PL2对大肠杆菌、金黄色葡萄球菌、藤黄八叠球菌的MIC范围为12.5~100 μg·mL-1。Li和Shah[40]的测定结果显示,杏鲍菇多糖与嗜热链球菌ASCC 1275胞外多糖对大肠杆菌、金黄色葡萄球菌、李斯特菌的MIC分别为1.25~10 mg·mL-1与2.5~5 mg·mL-1,硫酸化后的多糖显示出更强的抗菌活性。嗜热链球菌GST-6产生的胞外多糖与其硫酸化多糖[11]对鼠伤寒沙门氏菌、金黄色葡萄球菌、大肠杆菌的MIC值为 < 2~10 mg·mL-1。此外,Zhang等[41]将灰树花菌SH-05菌株用作锌生物转化的载体以得到细胞内锌多糖,其对金黄色葡萄球菌、大肠杆菌、李斯特菌的MIC为 < 0.625~2.5 mg·mL-1。刘丹丹等[42]发现,地木耳多糖除能抑制细菌外,对啤酒酵母也有抑制作用。Vishwakarma和Vavilala[43]评估了从莱茵衣藻中提取的硫酸化多糖的抗菌性能,其对大肠杆菌、枯草芽孢杆菌、链球菌的MIC为0.42~0.48 mg·mL-1,见表 3。
|
|
表 3 主要微生物多糖的抗菌活性 Table 3 Antibacterial activity of major microbial polysaccharides |
多糖类化合物的抗菌作用机制研究较少,目前已有研究认为多糖类化合物的抗菌作用机制主要为:1)抑制细菌细胞黏附;2)使细菌细胞膜、细胞壁损坏或通透性增加;3)抑制细菌核酸、蛋白质的合成;4)影响细菌代谢;5)抑制生物膜的形成或破坏生物膜;6)阻碍营养物质的吸收,见图 1。
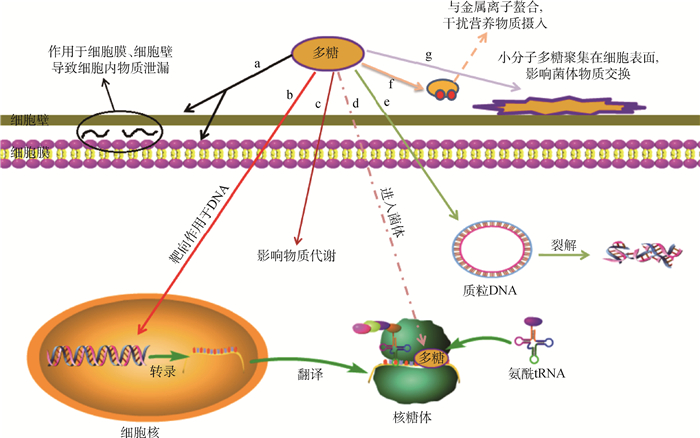
|
a.多糖与菌体细胞膜、细胞壁发生静电作用使细胞膜、细胞壁损坏或使细胞膜发生变形增加其通透性,从而导致菌体内物质泄漏进而发生死亡。b、e.多糖靶向作用于细菌DNA,破坏其遗传系统。C.多糖通过影响细菌体内物质代谢(如糖代谢)发挥杀菌作用。d.多糖与核糖体结合阻碍氨酰tRNA定位,抑制菌体蛋白质的合成。f、g.多糖通过螯合作用与金属阳离子结合或聚集在细胞表面,干扰菌体物质交换 a. The polysaccharide has an electrostatic effect on the cell membrane and cell wall of the bacterial cell, which damages the cell membrane and cell wall or deforms the cell membrane to increase its permeability, resulting in leakage of the material in the bacteria and death. b, e. Polysaccharides target bacterial DNA and disrupt its genetic system.c. Polysaccharides play a bactericidal effect by affecting the substance metabolism (such as sugar metabolism) in bacteria. d. The combination of polysaccharide and ribosome hinders the localization of aminoacyl tRNA and inhibits the synthesis of bacterial protein. f, g. Polysaccharides are combined with metal cations or aggregated on the cell surface through chelation, which interferes with the bacterial substance exchange 图 1 多糖类化合物主要抗菌机制 Fig. 1 The main antibacterial mechanism of polysaccharides |
细菌感染宿主是在其对表面组织黏附作用的基础下进行的,细菌黏附通常是由微生物表面的黏附素与宿主细胞表面发生的碳水化合物-蛋白质相互作用所介导[44],黏附素是细菌的毒力因子之一,与细菌的定居有关,许多富含碳水化合物的多糖与细胞上受体结构相似,与细菌结合后能阻断细菌黏附素与宿主细胞上受体结合,从而发挥很好的抗菌作用。黄芪多糖可通过对呼吸道黏膜的修复作用和抗细菌黏附作用,并通过纤毛运动将吸入的绿脓杆菌从气管清除,从而起到抵抗感染的作用[45]。来自人参的酸性多糖PG-F2、PG-HMW和来自绿茶的酸性多糖CS-F2抑制细菌对细胞黏附的同时,对有益细菌和共生细菌没有影响[21]。张红英等[46]研究发现,板蓝根多糖浓度越大,抑制大肠埃希菌对PK-15细胞的黏附作用越强。Xu等[47]从芦荟中提取的粗多糖AFS分为两部分:AFS-1和AFS-2,AFS-2能有效的抑制幽门螺杆菌附着在MKN45细胞上。据报道,来自未成熟秋葵果实的新鲜水提物可抑制黏附素BabA、SabA、HpaA与特异性受体结合从而抑制幽门螺杆菌对人胃腺癌上皮细胞的黏附[48]。来自秋葵和弯子木根中的果胶多糖也能抑制幽门螺杆菌对人胃腺癌上皮细胞的黏附[49]。Cai等[50]研究发现,来自解淀粉芽孢杆菌JN4的特异性左聚糖型胞外多糖可以通过紧密黏附于肠产毒性大肠杆菌细胞来阻断细菌黏附素和宿主受体之间的相互作用而发挥作用。
2.2 使细菌细胞膜、细胞壁损坏或通透性增加据报道,带正电荷的壳聚糖能与革兰阴性菌外膜中具有阴离子的脂多糖、真菌细胞膜表面的负电荷、革兰阳性菌细胞壁中带负电荷的脂磷壁酸发生静电作用,导致细胞膜或细胞壁损坏、细胞内成分泄露、细菌的功能性紊乱,从而使细菌死亡[51]。Xing等[52]检测到加入正苯基萘胺(1-N-phenylnaphthylamine,NPN)的细菌荧光增强、β-半乳糖苷酶活性增加以及从电镜中可见经油酰壳聚糖纳米粒子处理的细菌细胞表面变得粗糙、发生塌陷现象,表明外膜和内膜通透性均有所增加,进而引起内容物泄露而发挥抗菌作用。与上述结果类似,经壳聚糖处理的大肠杆菌悬浮液在260 nm处的吸光度有所增加、相对荧光度增加,β-半乳糖苷酶活性增加,并且在电子显微镜中可见大肠杆菌外膜发生变化,被一个牙齿状层覆盖,金黄色葡萄球菌的一侧细胞膜或细胞壁被破坏[53]。经白芨多糖处理的大肠杆菌培养液的电导率和蛋白质含量均呈上升趋势,碱性磷酸酶(alkaline phosphatase,AKP)含量呈现先升后降趋势,但均高于对照[54]。Kong等[55]研究发现,随着壳聚糖微球浓度升高,电导率逐渐增大,表明膜的通透性发生改变而导致细胞渗漏,此外,电子显微镜可观察到部分细菌还出现不同程度上的破裂。Zhang等[56]的研究结果也显示出电导率、AKP、β-半乳糖苷酶活性均增加,生长曲线、菌体蛋白、膜蛋白均发生变化。
2.3 抑制细菌核酸、蛋白质的合成多糖类化合物与细菌DNA靶点或核糖体结合可破坏细菌的遗传系统,影响其复制、转录过程或影响翻译过程从而抑制蛋白质的合成。He等[57]研究显示,菌体质粒DNA与从维吉尼亚链霉菌H03发酵液中分离出一种新的多糖结合后分解成段,因此,DNA可能是多糖发挥作用的靶点之一。杜德红等[58]用琼脂糖凝胶电泳分析茶叶多糖及其铈配合物对质粒DNA的裂解作用,结果显示有一定量的线状DNA出现。陈海秀[14]研究发现,分子量较小的岩藻聚糖可在细胞表面形成一层高分子膜,从而造成菌体内部新陈代谢紊乱,红外光谱结果表明,岩藻聚糖也可能对DNA、RNA产生破坏。Qian等[59]研究结果发现,壳聚糖可能插入细菌DNA双螺旋结构的槽中,诱导DNA降解,并与DNA结合,阻碍其生物学功能。早期研究表明,寡糖抗生素阿维拉霉素(avilamycin,AVI),在poly(U)存在情况下能抑制50% Phe-tRNA与70S核糖体结合从而抑制蛋白质的合成[60]。据报道,evernimicin(EVN)与AVI结构类似,且这两种寡糖抗生素均可通过干扰起始因子IF2与50s核糖体亚基结合来抑制起始复合物的形成,从而抑制蛋白质的合成[61]。McNicholas等[62]研究表明,EVN可与50s核糖体亚基结合阻断蛋白质合成过程中肽链的延伸阶段,从而抑制翻译。也有学者猜测,EVN可能通过改变大肠杆菌核糖体A位点的构象或与氨酰tRNA竞争A位点来抑制蛋白质的合成[63]。
2.4 影响细菌代谢研究发现,多糖可能干扰细菌的物质代谢从而减少细菌毒素或抑制细菌生长而发挥抗菌作用。灵芝多糖可以恢复Ⅱ型糖尿病大鼠肠道细菌群落的氨基酸、碳水化合物、炎性物质和核酸代谢紊乱[64]。芍药总多糖能抑制变异链球菌及血链球菌产酸以及产生水不溶性胞外多糖,但抑制产酸作用是否通过细菌无氧糖代谢中的乳酸脱氢酶或其他途径而发挥作用,还需要进一步研究,其抑制产糖的作用机制也尚不清楚[65]。最近,Ji等[66]研究表明,大肠杆菌的生长与1, 6-二磷酸果糖(fructose-1, 6-biphosphate,FBP)的浓度有关。三叶青多糖可干扰6-磷酸果糖(fructose-6-phosphate,F6P)向FBP的转化,导致FBP的浓度低于正常浓度。因此,该多糖可通过阻碍葡萄糖磷酸化的过程来抑制大肠杆菌的增殖[67]。
2.5 抑制生物膜的形成或破坏生物膜生物膜可以增强细菌对恶劣环境条件的耐受性[68],生物膜中的细菌具有极强耐药性以及抵抗机体免疫系统作用的能力。研究表明,半乳聚糖不会干扰细菌细胞与底物的黏附,但会阻止细菌生物量的积累。此外,该半乳聚糖不仅抑制了生物膜的形成,还破坏了部分预先形成的生物膜[69]。植物乳杆菌YW 32胞外多糖抑制大肠杆菌O157等几种致病菌的生物膜形成具有浓度依赖性[70]。分子量为2 000 Da的壳寡糖能有效抑制克罗诺杆菌生物膜[71]。Rubini等[72]研究发现,从海洋生物垃圾中提取的壳聚糖对初始形成的生物膜和预先形成的生物膜的抑制率为80%~85%。
2.6 阻碍营养物质的吸收铁是细菌生长的基本元素,改性多糖的铁结合能力越高,其抑菌作用越强[73]。多糖通过与金属离子螯合可阻碍细菌对营养物质的吸收,进而间接发挥抗菌作用。Dong、Xu、Zhou等[35, 74-75]已证明,具有高铁亲和力的螯合剂具有抗菌活性。Wu等[76]研究发现,用NaClO / NaBr氧化的葡聚糖显示出良好的铁(Ⅲ)螯合能力。Chung等[15]从农业废弃物中提取的壳聚糖能与金属离子螯合,影响细菌营养物质的摄入。Shao等[22]研究表明,经羧甲基化修饰的降解多糖可以通过清除环境中的铁来抑制细菌对铁的吸收而发挥抗菌作用。Wang等[77]研究发现,来自鲍鱼的硫酸化多糖也能抑制铁的吸收。
3 结语随着细菌耐药性的不断发展,寻找更加绿色高效的抗生素替代品成为了新的研究热点。目前,抗菌肽、益生元、植物提取物等抗生素替代品层出不穷。本文介绍的多糖类化合物对革兰阳性、革兰阴性菌以及一些真菌均有抑制作用,可以从多角度抑制或杀灭病原菌。不同种类的多糖对不同的病原菌的抗菌效果不一,多糖的抗菌活性还受到温度、多糖浓度、分子量等多种因素的影响。目前对多糖的生物活性及机制研究成果显著,但其中仍存在一些问题:1)多糖的结构十分复杂,目前对多糖的构效关系、活性成分及其分子机制等研究还有待深入;2)多糖对细菌物质代谢的研究相对匮乏;3)多糖类药物在临床上的应用管理还需规范化。成功解决上述问题还需要付出诸多努力,这将为多糖类化合物替代抗生素在临床上应用提供坚实基础,为人类和动物健康带来新的福祉。
| [1] | YUE Y Y, LI Z H, LI P, et al. Antiviral activity of a polysaccharide from Laminaria japonica against enterovirus 71[J]. Biomed Pharmacother, 2017, 96: 256–262. DOI: 10.1016/j.biopha.2017.09.117 |
| [2] | ZHENG Y F, ZHANG Q, LIU X M, et al. Extraction of polysaccharides and its antitumor activity on Magnolia kwangsiensis Figlar & Noot[J]. Carbohydr Polym, 2016, 142: 98–104. DOI: 10.1016/j.carbpol.2016.01.039 |
| [3] | CHEN L, ZHANG Y P, SHA O, et al. Hypolipidaemic and hypoglycaemic activities of polysaccharide from Pleurotus eryngii in Kunming mice[J]. Int J Biol Macromol, 2016, 93: 1206–1209. DOI: 10.1016/j.ijbiomac.2016.09.094 |
| [4] | JIANG C X, XIONG Q P, LI S L, et al. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis[J]. Carbohydr Polym, 2015, 115: 200–206. DOI: 10.1016/j.carbpol.2014.08.095 |
| [5] | CHEN Y, ZHANG H, WANG Y X, et al. Sulfated modification of the polysaccharides from Ganoderma atrum and their antioxidant and immunomodulating activities[J]. Food Chem, 2015, 186: 231–238. DOI: 10.1016/j.foodchem.2014.10.032 |
| [6] | CAZÓN P, VELAZQUEZ G, RAMíREZ J A, et al. Polysaccharide-based films and coatings for food packaging:a review[J]. Food Hydrocoll, 2017, 68: 136–148. DOI: 10.1016/j.foodhyd.2016.09.009 |
| [7] |
吴秋玉, 郑远鹏, 林志谦, 等. 黄芪多糖作为饲料添加剂及免疫增强剂在畜牧生产中的应用[J]. 中国饲料, 2018(2): 30–33.
WU Q Y, ZHENG Y P, LIN Z Q, et al. The application of Astragalus polysaccharides as feed additive and immunopotentiator in animal production[J]. China Feed, 2018(2): 30–33. (in Chinese) |
| [8] |
董群, 方积年. 多糖在医药领域中的应用[J]. 中国药学杂志, 2001, 36(10): 649–652.
DONG Q, FANG J N. Applications of polysaccharides in medicine[J]. Chinese Pharmaceutical Journal, 2001, 36(10): 649–652. DOI: 10.3321/j.issn:1001-2494.2001.10.001 (in Chinese) |
| [9] | YAN J K, WANG W Q, WU J Y. Recent advances in Cordyceps sinensis polysaccharides:mycelial fermentation, isolation, structure, and bioactivities:a review[J]. J Funct Foods, 2014, 6: 33–47. DOI: 10.1016/j.jff.2013.11.024 |
| [10] | YUAN Z, SHI Y, CAI F, et al. Isolation and identification of polysaccharides from Pythium arrhenomanes and application to strawberry fruit (Fragaria ananassa Duch.) preservation[J]. Food Chem, 2020, 309: 125604. DOI: 10.1016/j.foodchem.2019.125604 |
| [11] | ZHANG J, CAO Y Q, WANG J, et al. Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6[J]. Carbohydr Polym, 2016, 146: 368–375. DOI: 10.1016/j.carbpol.2016.03.063 |
| [12] | LIU Z Q, ZHANG Z H, QIU L, et al. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04[J]. J Dairy Sci, 2017, 100(9): 6895–6905. DOI: 10.3168/jds.2016-11944 |
| [13] | WANG Z C, XUE R H, CUI J W, et al. Antibacterial activity of a polysaccharide produced from Chaetomium globosum CGMCC 6882[J]. Int J Biol Macromol, 2019, 125: 376–382. DOI: 10.1016/j.ijbiomac.2018.11.248 |
| [14] |
陈海秀.海洋硫酸多糖抗菌活性研究及在肉类保鲜中的应用[D].厦门: 集美大学, 2016.
CHEN H X.Study on antibacterial activity of marine sulfate polysaccharide and its application in meat preserving[D].Xiamen: Jimei University, 2016.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10390-1018177358.htm |
| [15] | CHUNG Y C, WANG H L, CHEN Y M, et al. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens[J]. Bioresour Technol, 2003, 88(3): 179–184. DOI: 10.1016/S0960-8524(03)00002-6 |
| [16] | QIAN Z G. Cellulase-assisted extraction of polysaccharides from Cucurbita moschata and their antibacterial activity[J]. Carbohydr Polym, 2014, 101: 432–434. DOI: 10.1016/j.carbpol.2013.09.071 |
| [17] | GHAZALA I, SILA A, FRIKHA F, et al. Antioxidant and antimicrobial properties of water soluble polysaccharide extracted from carrot peels by-products[J]. J Food Sci Technol, 2015, 52(11): 6953–6965. DOI: 10.1007/s13197-015-1831-2 |
| [18] | KHEMAKHEM I, ABDELHEDI O, TRIGUI I, et al. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves[J]. Int J Biol Macromol, 2018, 106: 425–432. DOI: 10.1016/j.ijbiomac.2017.08.037 |
| [19] | CHMIT M, KANAAN H, HABIB J, et al. Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon[J]. Asian Pac J Trop Med, 2014, 7(S1): S546–S552. |
| [20] |
徐彬, 连帅, 袁建彬, 等. 新鲜玉米须多糖提取工艺及体外抗菌、抗氧化活性研究[J]. 饲料研究, 2018(5): 48–53.
XU B, LIAN S, YUAN J B, et al. Study on the extraction technology and antibacterial and antioxidant activity of fresh corn silk polysaccharide[J]. Feed Research, 2018(5): 48–53. (in Chinese) |
| [21] | LEE J H, SHIM J S, LEE J S, et al. Inhibition of pathogenic bacterial adhesion by acidic polysaccharide from green tea (Camellia sinensis)[J]. J Agric Food Chem, 2006, 54(23): 8717–8723. DOI: 10.1021/jf061603i |
| [22] | SHAO L L, XU J, SHI M J, et al. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera[J]. Food Chem, 2017, 237: 481–487. DOI: 10.1016/j.foodchem.2017.05.119 |
| [23] | JUN J Y, JUNG M J, JEONG I H, et al. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria[J]. Mar Drugs, 2018, 16(9): 301. DOI: 10.3390/md16090301 |
| [24] | LI X L, THAKUR K, ZHANG Y Y, et al. Effects of different chemical modifications on the antibacterial activities of polysaccharides sequentially extracted from peony seed dreg[J]. Int J Biol Macromol, 2018, 116: 664–675. DOI: 10.1016/j.ijbiomac.2018.05.082 |
| [25] |
张颖颖, 付业佩, 王萍. 罗勒多糖抗菌作用研究[J]. 山东化工, 2017, 46(20): 6–7.
ZHANG Y Y, FU Y P, WANG P. Research on the antibacterial action of Ocimum basilicum L. polysaccharide[J]. Shandong Chemical Industry, 2017, 46(20): 6–7. DOI: 10.3969/j.issn.1008-021X.2017.20.003 (in Chinese) |
| [26] | ABDELHEDI O, NASRI R, SOUISSI N, et al. Sulfated polysaccharides from common smooth hound:extraction and assessment of anti-ACE, antioxidant and antibacterial activities[J]. Carbohydr Polym, 2016, 152: 605–614. DOI: 10.1016/j.carbpol.2016.07.048 |
| [27] |
夏乾峰, 谭河林, 覃西, 等. 方格星虫多糖抗菌活性的初步研究[J]. 中国热带医学, 2007, 7(12): 2192–2193.
XIA Q F, TAN H L, QIN X, et al. Preliminary observation on the antibacterial activity on polysaccharides in Sipunculus nudus[J]. China Tropical Medicine, 2007, 7(12): 2192–2193. DOI: 10.3969/j.issn.1009-9727.2007.12.007 (in Chinese) |
| [28] | JRIDI M, NASRI R, MARZOUGUI Z, et al. Characterization and assessment of antioxidant and antibacterial activities of sulfated polysaccharides extracted from cuttlefish skin and muscle[J]. Int J Biol Macromol, 2019, 123: 1221–1228. DOI: 10.1016/j.ijbiomac.2018.11.170 |
| [29] | WANG C, SUN Z J, LIU Y Q, et al. Earthworm polysaccharide and its antibacterial function on plant-pathogen microbes in vitro[J]. Eur J Soil Biol, 2007, 43(S1): S135–S142. |
| [30] | TAJDINI F, AMINI M A, NAFISSI-VARCHEH N, et al. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus[J]. Int J Biol Macromol, 2010, 47(2): 180–183. DOI: 10.1016/j.ijbiomac.2010.05.002 |
| [31] |
张晓.甲壳素/壳聚糖的分子修饰及其抗氧化和抑菌性能研究[D].杭州: 浙江工商大学, 2013.
ZHANG X.Modification of chitin/chitosan and evaluation of their antioxidant activity and antibacterial activity in vitro[D].Hangzhou: Zhejiang Gongshang University, 2013.(in Chinese) http://d.wanfangdata.com.cn/Thesis/Y2292003 |
| [32] | KRICHEN F, KAROUD W, SILA A, et al. Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins[J]. Int J Biol Macromol, 2015, 75: 283–289. DOI: 10.1016/j.ijbiomac.2015.01.044 |
| [33] |
刘玉红.磺化壳聚糖对细菌及其生物被膜抑制作用的研究[D].杭州: 浙江工商大学, 2019.
LIU Y H.The inhibiting effects of sulfonated chitosan on bacteria and its biofilm[D].Hangzhou: Zhejiang Gongshang University, 2019.(in Chinese) http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&filename=1019091465.nh |
| [34] | LI S J, HUANG R H, SHAH N P, et al. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315[J]. J Dairy Sci, 2014, 97(12): 7334–7343. DOI: 10.3168/jds.2014-7912 |
| [35] | DONG Y R, CHENG S J, QI G H, et al. Antimicrobial and antioxidant activities of Flammulina velutipes polysacchrides and polysacchride-iron(Ⅲ) complex[J]. Carbohydr Polym, 2017, 161: 26–32. DOI: 10.1016/j.carbpol.2016.12.069 |
| [36] | SANTOVITO E, GRECO D, LOGRIECO A F, et al. Eubiotics for food security at farm level:yeast cell wall products and their antimicrobial potential against pathogenic bacteria[J]. Foodborne Pathog Dis, 2018, 15(9): 531–537. DOI: 10.1089/fpd.2018.2430 |
| [37] |
周胜男.酵母细胞壁抗菌性能及干预DON细胞毒性的研究[D].武汉: 武汉轻工大学, 2017.
ZHOU S N.Study on antibacterial activity of yeast cell wall and its effect on DON cell cytotoxicity[D].Wuhan: Wuhan Polytechnic University, 2017.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10496-1018067577.htm |
| [38] | TERAKUBO S, TAKEMURA H, YAMAMOTO H, et al. Antimicrobial activity of everninomicin against clinical isolates of Enterococcus spp., Staphylococcus spp., and Streptococcus spp. tested by Etest[J]. J Infect Chemother, 2001, 7(4): 263–266. DOI: 10.1007/s101560170025 |
| [39] | ZHU H J, SHENG K, YAN E F, et al. Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate[J]. Int J Biol Macromol, 2012, 50(3): 840–843. DOI: 10.1016/j.ijbiomac.2011.11.016 |
| [40] | LI S Q, SHAH N P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275[J]. Food Chem, 2014, 165: 262–270. DOI: 10.1016/j.foodchem.2014.05.110 |
| [41] | ZHANG C, GAO Z, HU C L, et al. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05[J]. Int J Biol Macromol, 2017, 95: 778–787. DOI: 10.1016/j.ijbiomac.2016.12.003 |
| [42] |
刘丹丹, 朱志学, 马健, 等. 地木耳多糖的抗氧化活性与抗菌活性研究[J]. 食品安全质量检测学报, 2019, 10(4): 921–926.
LIU D D, ZHU Z X, MA J, et al. Research on the antioxidant and antibacterial activity of polysaccharides from Nostoc commune[J]. Journal of Food Safety & Quality, 2019, 10(4): 921–926. DOI: 10.3969/j.issn.2095-0381.2019.04.017 (in Chinese) |
| [43] | VISHWAKARMA J, VAVILALA S L. Evaluating the antibacterial and antibiofilm potential of sulphated polysaccharides extracted from green algae Chlamydomonas reinhardtii[J]. J Appl Microbiol, 2019, 127(4): 1004–1017. DOI: 10.1111/jam.14364 |
| [44] | WITTSCHIER N, LENGSFELD C, VORTHEMS S, et al. Large molecules as anti-adhesive compounds against pathogens[J]. J Pharm Pharmacol, 2007, 59(6): 777–786. DOI: 10.1211/jpp.59.6.0004 |
| [45] |
余丹凤, 孔繁智, 朱婉萍, 等. 黄芪多糖抗呼吸道绿脓杆菌感染的实验研究[J]. 中国中西医结合急救杂志, 2007, 14(2): 76–79.
YU D F, KONG F Z, ZHU W P, et al. Experimental study on anti-pseudomonas aeruginosa infection in trachea by astragalus polysaccharides (黄芪多糖)[J]. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care, 2007, 14(2): 76–79. DOI: 10.3321/j.issn:1008-9691.2007.02.004 (in Chinese) |
| [46] |
张红英, 王学兵, 赵现敏, 等. 板蓝根多糖抑制致病性大肠埃希菌细胞黏附的试验研究[J]. 微生物学杂志, 2010, 30(1): 61–63.
ZHANG H Y, WANG X B, ZHAO X M, et al. Radix isatis polysaccharides to inhibit adhesion of pathogenic E. coli to cell[J]. Journal of Microbiology, 2010, 30(1): 61–63. DOI: 10.3969/j.issn.1005-7021.2010.01.013 (in Chinese) |
| [47] | XU C, RUAN X M, LI H S, et al. Anti-adhesive effect of an acidic polysaccharide from Aloe vera L. var. chinensis (Haw.) Berger on the binding of Helicobacter pylori to the MKN-45 cell line[J]. J Pharm Pharmacol, 2010, 62(12): 1753–1759. DOI: 10.1111/j.2042-7158.2010.01181.x |
| [48] | MESSING J, THÖLE C, NIEHUES M, et al. Antiadhesive Properties of Abelmoschus esculentus (Okra) Immature Fruit Extract against Helicobacter pylori Adhesion[J]. PLoS One, 2014, 9(1): e84836. DOI: 10.1371/journal.pone.0084836 |
| [49] | INNGJERDINGEN K T, THÖLE C, DIALLO D, et al. Inhibition of Helicobacter pylori adhesion to human gastric adenocarcinoma epithelial cells by aqueous extracts and pectic polysaccharides from the roots of Cochlospermum tinctorium A. Rich. and Vernonia kotschyana Sch. Bip. ex Walp[J]. Fitoterapia, 2014, 95: 127–132. DOI: 10.1016/j.fitote.2014.03.009 |
| [50] | CAI G L, LIU Y F, LI X M, et al. New levan-type exopolysaccharide from Bacillus amyloliquefaciens as an antiadhesive agent against enterotoxigenic Escherichia coli[J]. J Agric Food Chem, 2019, 67(28): 8029–8034. DOI: 10.1021/acs.jafc.9b03234 |
| [51] |
付俊锋, 毛国梁. 壳聚糖的抗菌能力研究进展[J]. 化工科技, 2018, 26(4): 66–72.
FU J F, MAO G L. Research progress on antimicrobial of chitosan and its derivatives[J]. Science & Technology in Chemical Industy, 2018, 26(4): 66–72. DOI: 10.3969/j.issn.1008-0511.2018.04.014 (in Chinese) |
| [52] | XING K, CHEN X G, KONG M, et al. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus[J]. Carbohydr Polym, 2009, 76(1): 17–22. DOI: 10.1016/j.carbpol.2008.09.016 |
| [53] | LIU H, DU Y M, WANG X H, et al. Chitosan kills bacteria through cell membrane damage[J]. Int J Food Microbiol, 2004, 95(2): 147–155. DOI: 10.1016/j.ijfoodmicro.2004.01.022 |
| [54] |
代建丽, 周斯荻, 刘铭轩, 等. 白芨多糖的抑菌作用研究[J]. 安徽农学通报, 2015, 21(19): 19–21.
DAI J L, ZHOU S D, LIU M X, et al. Study on the antibacterial activity of Bletilla striata polysaccharide[J]. Anhui Agricultural Science Bulletin, 2015, 21(19): 19–21. DOI: 10.3969/j.issn.1007-7731.2015.19.008 (in Chinese) |
| [55] | KONG M, CHEN X G, LIU C S, et al. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli[J]. Colloids Surf B:Biointerfaces, 2008, 65(2): 197–202. DOI: 10.1016/j.colsurfb.2008.04.003 |
| [56] | ZHANG Y, WU Y T, ZHENG W, et al. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae[J]. J Funct Foods, 2017, 38: 273–279. DOI: 10.1016/j.jff.2017.09.047 |
| [57] | HE F, YANG Y, YANG G, et al. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03[J]. Food Control, 2010, 21(9): 1257–1262. DOI: 10.1016/j.foodcont.2010.02.013 |
| [58] |
杜德红, 汪东风, 孙继鹏, 等. 茶叶多糖及其铈配合物对质粒DNA及有机磷农药的降解作用[J]. 中国稀土学报, 2005, 23(1): 118–121.
DU D H, WANG D F, SUN J P, et al. Effect of complex coordinating tea polysaccharide with cerium on degradation of plasmid DNA and organophosphorous pesticides[J]. Journal of the Chinese Rare Earth Society, 2005, 23(1): 118–121. DOI: 10.3321/j.issn:1000-4343.2005.01.025 (in Chinese) |
| [59] | QIAN B J, JUNG J, ZHAO Y Y. Impact of acidity and metal ion on the antibacterial activity and mechanisms of β-and α-chitosan[J]. Appl Biochem Biotechnol, 2015, 175(6): 2972–2985. DOI: 10.1007/s12010-014-1413-1 |
| [60] | WOLF H. Avilamycin, an inhibitor of the 30 S ribosomal subunits function[J]. FEBS Lett, 1973, 36(2): 181–186. DOI: 10.1016/0014-5793(73)80364-3 |
| [61] | KRUPKIN M, WEKSELMAN I, MATZOV D, et al. Avilamycin and evernimicin induce structural changes in rProteins uL16 and CTC that enhance the inhibition of A-site tRNA binding[J]. Proc Natl Acad Sci U S A, 2016, 113(44): E6796–E6805. DOI: 10.1073/pnas.1614297113 |
| [62] | McNICHOLAS P M, NAJARIAN D J, MANN P A, et al. Evernimicin binds exclusively to the 50S ribosomal subunit and inhibits translation in cell-free systems derived from both gram-positive and gram-negative bacteria[J]. Antimicrob Agents Chemother, 2000, 44(5): 1121–1126. DOI: 10.1128/AAC.44.5.1121-1126.2000 |
| [63] | ADRIAN P V, ZHAO W J, BLACK T A, et al. Mutations in ribosomal protein L16 conferring reduced susceptibility to evernimicin (SCH27899):implications for mechanism of action[J]. Antimicrob Agents Chemother, 2000, 44(3): 732–738. DOI: 10.1128/AAC.44.3.732-738.2000 |
| [64] | CHEN M Y, XIAO D, LIU W, et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats[J]. Int J Biol Macromol, 2019. DOI: 10.1016/j.ijbiomac.2019.11.047 |
| [65] |
李贺.芍药总多糖对口腔致龋细菌毒力因子作用的体外实验研究[D].乌鲁木齐: 新疆医科大学, 2013.
LI H.Study on effect of polysaccharides from Paeonia sinjiangensis K.Y. Pan on oral cariogenic bacteria virulences in vitro[D].Urumqi: Xinjiang Medical University, 2013.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10760-1014431704.htm |
| [66] | JI F F, SHEN Y, TANG L H, et al. Determination of intracellular metabolites concentrations in Escherichia coli under nutrition stress using liquid chromatography-tandem mass spectrometry[J]. Talanta, 2018, 189: 1–7. DOI: 10.1016/j.talanta.2018.06.050 |
| [67] | CHEN X, TAO L, RU Y, et al. Antibacterial mechanism of Tetrastigma hemsleyanum Diels et Gilg's polysaccharides by metabolomics based on HPLC/MS[J]. Int J Biol Macromol, 2019, 140: 206–215. DOI: 10.1016/j.ijbiomac.2019.08.097 |
| [68] | RABIN N, ZHENG Y, OPOKU-TEMENG C, et al. Biofilm formation mechanisms and targets for developing antibiofilm agents[J]. Future Med Chem, 2015, 7(4): 493–512. DOI: 10.4155/fmc.15.6 |
| [69] | GRISHIN A V, KARYAGINA A S. Polysaccharide galactan inhibits Pseudomonas aeruginosa biofilm formation but protects pre-formed biofilms from antibiotics[J]. Biochemistry (Moscow), 2019, 84(5): 509–519. DOI: 10.1134/S0006297919050055 |
| [70] | WANG J, ZHAO X, YANG Y W, et al. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32[J]. Int J Biol Macromol, 2015, 74: 119–126. DOI: 10.1016/j.ijbiomac.2014.12.006 |
| [71] | LU J, CHEN Q M, PAN B L, et al. Efficient inhibition of Cronobacter biofilms by chitooligosaccharides of specific molecular weight[J]. World J Microbiol Biotechnol, 2019, 35(6): 87. DOI: 10.1007/s11274-019-2662-5 |
| [72] | RUBINI D, VARTHAN P V, JAYASANKARI S, et al. Suppressing the phenotypic virulence factors of Uropathogenic Escherichia coli using marine polysaccharide[J]. Microb Pathog, 2020, 141: 103973. DOI: 10.1016/j.micpath.2020.103973 |
| [73] | LI Y T, CHEN B J, WU W D, et al. Antioxidant and antimicrobial evaluation of carboxymethylated and hydroxamated degraded polysaccharides from Sargassum fusiforme[J]. Int J Biol Macromo, 2018, 118: 1550–1557. DOI: 10.1016/j.ijbiomac.2018.06.196 |
| [74] | XU B, KONG X L, ZHOU T, et al. Synthesis, iron(Ⅲ)-binding affinity and in vitro evaluation of 3-hydroxypyridin-4-one hexadentate ligands as potential antimicrobial agents[J]. Bioorg Med Chem Lett, 2011, 21(21): 6376–6380. DOI: 10.1016/j.bmcl.2011.08.097 |
| [75] | ZHOU Y J, LIU M S, OSAMAH A R, et al. Hexadentate 3-hydroxypyridin-4-ones with high iron(Ⅲ) affinity:design, synthesis and inhibition on methicillin resistant Staphylococcus aureus and Pseudomonas strains[J]. Eur J Med Chem, 2015, 94: 8–21. DOI: 10.1016/j.ejmech.2015.02.050 |
| [76] | WU H, SHANG-GUAN D C, LU Q M, et al. Oxidation of dextran using H2O2 and NaClO/NaBr and their applicability in iron chelation[J]. Int J Biol Macromol, 2020, 144: 615–623. DOI: 10.1016/j.ijbiomac.2019.12.104 |
| [77] | WANG L L, SONG S, ZHANG B, et al. A sulfated polysaccharide from abalone influences iron uptake by the contrary impacts of its chelating and reducing activities[J]. Int J Biol Macromol, 2019, 138: 49–56. DOI: 10.1016/j.ijbiomac.2019.07.072 |



