2. 福建省兽医中药与动物保健重点实验室, 福州 350002;
3. 保定职业技术学院, 保定 071051;
4. 河北省中兽药工程技术中心, 保定 071100
2. Fujian Key Laboratory of Traditional Chinese Veterinary Medicine and Animal Health, Fuzhou 350002, China;
3. Baoding Vocational and Technical College, Baoding 071051, China;
4. Engineering Technology Center of Traditional Chinese Veterinary Medicine of Hebei, Baoding 071100, China
随着全球气候逐渐变暖,热应激在现代高密度养殖条件下频繁发生,已经成为影响夏季畜禽生产性能的重要因素,引起畜禽成活率下降、抗病力低下、生产性能下降、畜产品品质降低等问题,带来严重的经济损失。肠道为应激反应的中心器官[1],空肠又是小肠中最长的部分,当热应激发生时,为保护心脑等重要器官而减少胃肠道的血流量[2-3],进而导致肠黏膜缺血缺氧,引发肠绒毛病理性损伤、凋亡和脱落的现象,因此为致病微生物、多种生物大分子和抗原等进入血液循环提供了通道,引起细菌和内毒素位移,造成肠道免疫功能降低和肠道微生态环境失调,是影响肠道正常生理功能的危害因素[4-6]。中兽药在治疗肠黏膜屏障损伤方面有明显的效果,在杨梅梅[7]的研究中发现,中药复方与益生素可以缓解热应激导致的肉鸡肠道绒毛损伤,保护肠黏膜,促进损伤面的修复,对恢复正常的肠道功能有着重要意义。
马齿苋又称五行草、长寿菜,为药食同源草本植物,具有清热解毒、散血消肿、凉血止痢等功效,农村有在夏季给猪饲喂马齿苋的现象[8],能够降低暑热对猪采食和生长的影响,还可治疗仔猪白痢[9]。也有文献报道马齿苋能促进肉鸡的抗氧化能力,促生长[10],马齿苋对冷应激致小鼠胃肠功能紊乱及NO含量具有良好的调节作用[11]。现代研究表明,马齿苋多糖能显著增强雏鸡免疫功能及抗氧化功能[12]。在实验室前期研究[13]中发现马齿苋提取物能够增加肉鸡盲肠中乳酸杆菌和双歧杆菌的丰度,降低大肠杆菌的数量。由于热应激也可导致机体肠道菌群失调和氧化损伤,结合马齿苋具有抗氧化、抗炎等功效,因此本试验重点探讨马齿苋水提物保护热应激致动物肠道损伤的效果,为马齿苋水提物在畜牧业上的广泛应用提供理论依据。
1 材料与方法 1.1 材料与试剂清洁级5~6周龄昆明雄性小鼠,体重29~32 g,购于北京斯贝福生物技术有限公司[生产许可证号:SCKX(京)2016-0002];中药马齿苋购于安国药材市场;维生素C可溶性粉(河南豫正科技有限公司);D-木糖(北京索莱宝科技有限公司);Trizol试剂盒(购自TaKaRa公司);反转录试剂盒(购自TaKaRa公司);TB GreenTM Premix Ex TaqTM(购自TaKaRa公司)。
1.2 马齿苋水提物制备称取100 g马齿苋干燥地上部分,加入10倍量(即1 000 mL)的蒸馏水浸泡2 h,煎煮30 min,过滤,滤渣加适量蒸馏水再煎煮30 min,过滤,合并两次滤液,加热浓缩至50 mL,得马齿苋水提取液(含生药2 g·mL-1),放入4 ℃冰箱中冷藏,备用。
1.3 试验设计5~6周龄健康昆明雄性小鼠40只,随机分为4组(每组10只):空白对照组(control, C)、热应激组(heat stress, HS)、马齿苋水提物组(aqueous extract of purslane, AEP)、维生素C组(vitamin C, Vc)。自购买之日起将小鼠室温饲养,自由采食和饮水适应7 d后,进入正式试验。将空白对照组置于(25±1) ℃室温下饲养;本试验在夏季炎热季节开始,将HS、AEP、Vc组的小鼠置于(40±1) ℃生化培养箱中,每天14:00—14:30进行热应激处理0.5 h,自由采食和饮水,连续热应激6 d。随后将小鼠置于室温下开始给药治疗,其中C组和HS组每天灌服等体积的生理盐水(按体重以0.2 mL·20 g-1给药),AEP组灌服2 g·mL-1的水提马齿苋(按体重以0.2 mL·20 g-1给药),Vc组按体重以0.2 mL·20 g-1的量每天灌服500 mg·kg-1维生素C,连续给药7 d。
1.4 样品采集各组小鼠在治疗7 d后采集样品,处理前每只小鼠灌服0.3 mL 10%的D-木糖溶液,1 h后,眶窦采血,分离血清,处死小鼠,采集空肠,将部分空肠保存在-80 ℃超低温冰箱中备用,另一部分空肠浸入4%甲醛溶液中固定。
1.5 血清D-木糖、葡萄糖含量的测定采用间苯三酚法检测小鼠血清D-木糖含量,具体操作步骤按照说明书进行(南京建成生物工程研究所);采用葡萄糖氧化酶法检测血清葡萄糖水平,具体操作步骤参照说明书进行。
1.6 空肠的组织学观察取每只小鼠相同位置的空肠组织,立即固定在4%甲醛溶液中,常规方法制作石蜡切片,苏木精-伊红(HE)染色,光学显微镜下观察空肠组织的病理变化。并在显微镜下每组选出5个最长绒毛的长度、最深隐窝的深度并计算其比值。采用影像分析软件Image-Pro Plus5.1进行数据收集。
1.7 空肠黏膜中ZO-1、SGLT1和GLUT2 mRNA相对转录水平的测定采用Trizol法提取空肠组织的总RNA。将总RNA充分混匀后,吸取3 μL至0.2 mL的PCR管中经微孔板分光光度计测定A260 nm/A280 nm的比值在1.8~2.0以评价RNA质量。参照TaKaRa反转录试剂盒(with gDNA Eraser)说明书分别将各组提取的总RNA反转录合成cDNA,并以此为模板,应用表 1所列的引物,参照TaKaRa (Tli RNaseH Plus)的说明书进行目的基因和内参基因的荧光定量PCR检测,每个样本检测3个复孔。反应结束后得到各组的Ct值,数据采用2-△△Ct法进行分析。
|
|
表 1 目的基因和内参基因的引物序列 Table 1 Primer sequence of target and house-keeping genes |
本试验所有数据均表示为x±s形式。使用SPSS19.0分析软件进行统计分析,ANOVA法进行组间差异性比较,差异的显著性水平为P < 0.05或P < 0.01。
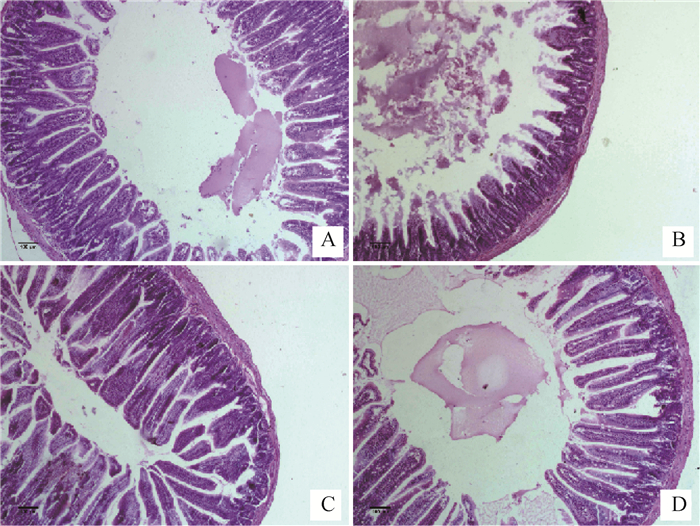
2 结果 2.1 马齿苋水提物对热应激小鼠空肠组织病理学变化的影响由图 1可以看出,C组肠绒毛结构完整、绒毛之间排列整齐(图 1A);HS组空肠黏膜细胞脱落、固有层组织裸露,肠绒毛损伤严重,绒毛表面积减少(图 1B);AEP组(图 1C)与Vc组(图 1D)空肠绒毛出现轻度损伤、断裂,肠绒毛界限清楚,排列较为整齐,与HS组相比,AEP组小鼠肠绒毛损伤在一定程度上得到了恢复。
|
A为C组,B为HS组,C为AEP组,D为Vc组 A is group C, B is HS group, C is AEP group, and D is Vc group 图 1 小鼠空肠组织病理学变化(HE染色,10×) Fig. 1 Histopathological changes of mouse jejunum (HE staining, 10×) |
由表 2数据可知,与C组比较,HS组绒毛高度极显著降低(P<0.01),说明热应激能够导致绒毛长度变短;与HS组相比,AEP组和Vc组的绒毛高度极显著性升高(P<0.01),说明马齿苋水提物能够促使热应激小鼠受损绒毛的恢复。各组之间隐窝深度无显著性差异(P>0.05)。HS组肠绒毛高度与隐窝深度的比值与C组相比极显著降低(P<0.01);AEP组和Vc组与HS组比较,肠绒毛高度与隐窝深度的比值极显著升高(P<0.01)。
|
|
表 2 各组小鼠空肠形态结构的变化(x±s, n=5) Table 2 Changes of morphological structure of jejunum in miceof each group(x±s, n=5) |
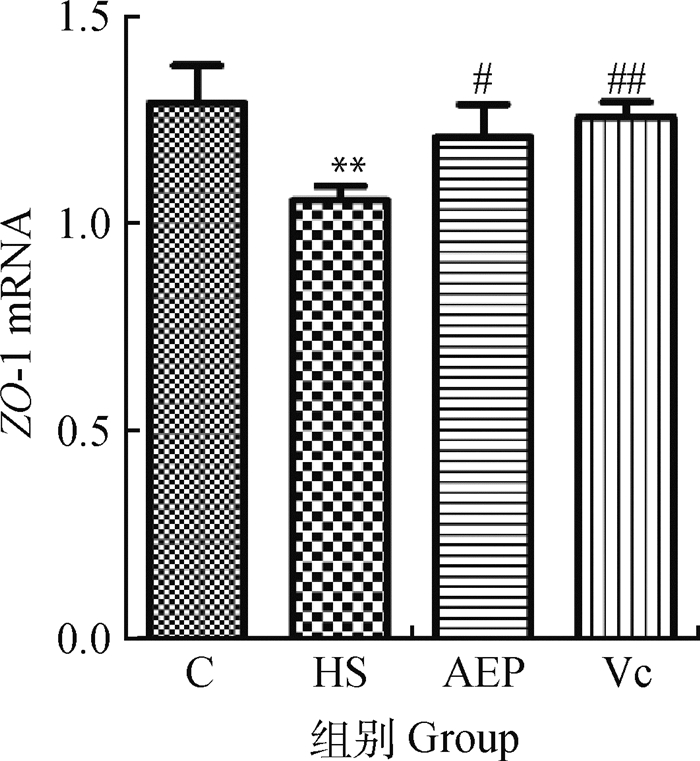
从图 2可以看出,与C组比较,HS组的空肠黏膜ZO-1 mRNA转录量极显著降低(P<0.01);与HS组比较,AEP组的转录水平显著升高(P<0.05),Vc组的转录水平极显著升高(P<0.01)。
|
与C组比较,**表示差异极显著(P<0.01);与HS组比较,#表示差异显著(P<0.05),##表示差异极显著(P<0.01) Compared with C group, ** indicates that the difference is extremely significant (P < 0.01); Compared with HS group, # indicates significant difference (P < 0.05); ## indicates that the difference is extremely significant (P < 0.01) 图 2 小鼠空肠黏膜ZO-1 mRNA的相对转录水平(n=10) Fig. 2 Relative transcription level of ZO-1 mRNA in mouse jejunum (n=10) |
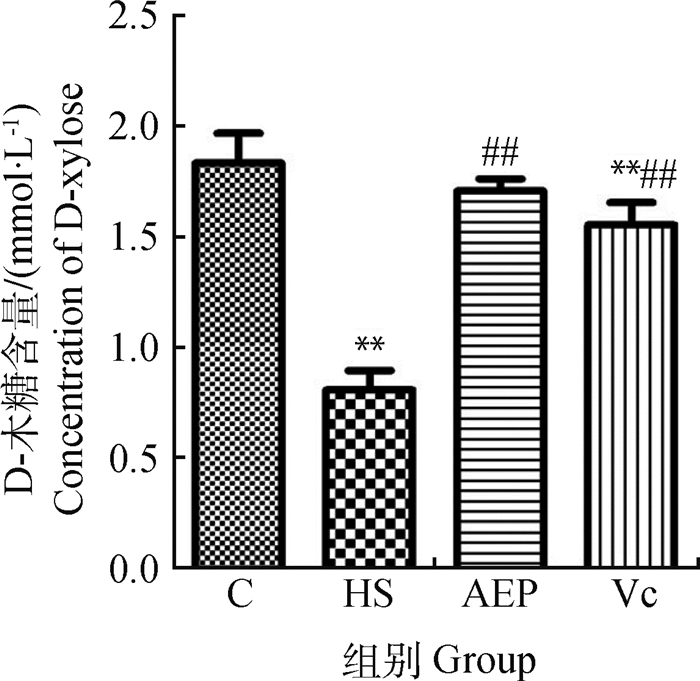
由图 3可知,在治疗7 d后,HS组血清D-木糖含量低于C组,差异极显著(P<0.01),与HS组比较,AEP组和Vc组的含量升高,差异极显著(P<0.01)。
|
与C组比较,**表示差异极显著(P<0.01);与HS组比较,##表示差异极显著(P<0.01) Compared with control group, ** indicates that the difference is extremely significant (P < 0.01); Compared with HS group, ## indicates that the difference is extremely significant (P < 0.01) 图 3 小鼠血清D-木糖的含量(n=10) Fig. 3 D-xylose content in mouse serum (n=10) |
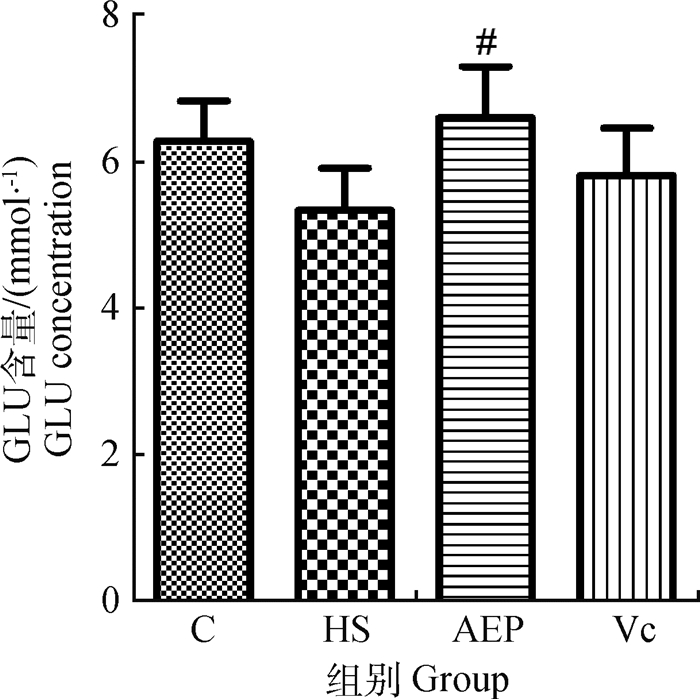
从图 4可以看出,在治疗7 d时,HS组的血清葡萄糖含量与C组比较虽然降低,但二者之间无显著差异(P>0.05);与HS组相比,AEP组的含量显著升高(P<0.05)。
|
与HS组比较,#表示差异显著(P<0.05) Compared with HS group, # indicates significant difference (P < 0.05) 图 4 小鼠血清葡萄糖的含量(n=10) Fig. 4 Glucose content in mouse serum (n=10) |
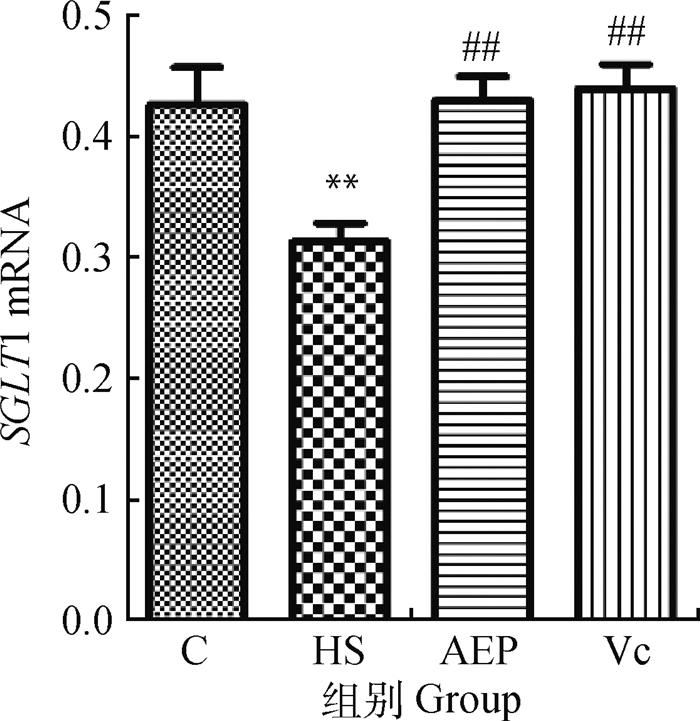
由图 5可知,与C组相比,HS组的SGLT1 mRNA相对转录水平降低,差异极显著(P<0.01);与HS组比较,AEP组和Vc组的SGLT1 mRNA转录量极显著升高(P<0.01)。
|
与C组比较,**表示差异极显著(P<0.01);与HS组比较,##表示差异极显著(P<0.01) Compared with C group, ** indicates that the difference is extremely significant (P < 0.01); Compared with HS group, ## indicates that the difference is extremely significant (P < 0.01) 图 5 小鼠空肠黏膜SGLT1 mRNA相对转录水平(n=10) Fig. 5 Relative transcription level of SGLT1 mRNA in mouse jejunum (n=10) |
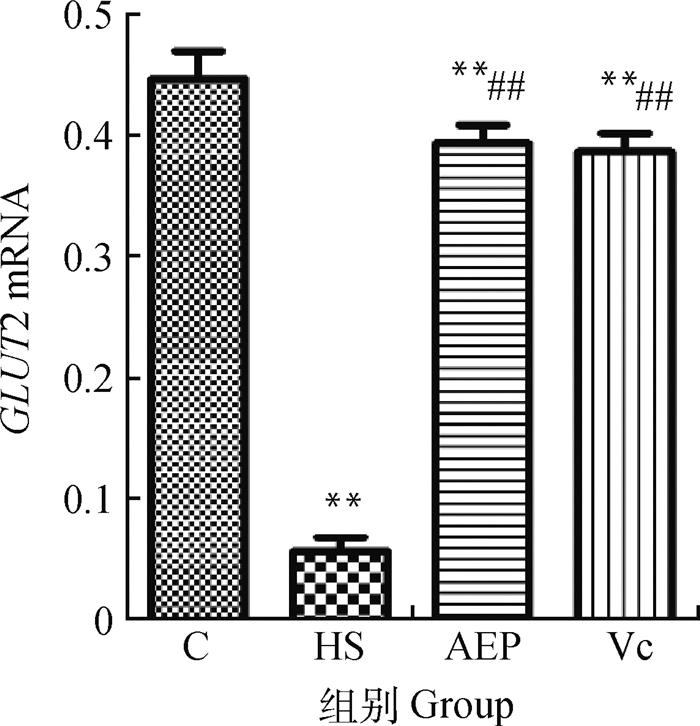
由图 6可知,HS组的空肠黏膜GLUT2 mRNA相对转录水平明显低于C组,差异极显著(P<0.01);AEP组和Vc组的相对转录水平极显著高于HS组(P<0.01)。
|
与C组比较,**表示差异极显著(P<0.01);与HS组比较,##表示差异极显著(P<0.01) Compared with C group, ** indicates that the difference is extremely significant (P < 0.01); Compared with HS group, ## indicates that the difference is extremely significant (P < 0.01) 图 6 小鼠空肠黏膜GLUT2 mRNA相对转录水平(n=10) Fig. 6 Relative transcription level of GLUT2 mRNA in mouse jejunum (n=10) |
紧密连接蛋白是肠道机械屏障中的基础结构,主要包括claudin-1、occludin及ZO-1蛋白等[14]。紧密连接蛋白ZO-1是1986年发现的与紧密连接(TJ)相关的蛋白[15],位于上皮细胞间顶端,是维持黏膜通透性的重要组成成分[16],ZO-1结构的破坏很容易引起肠道疾病[17]。本试验研究表明,热应激的发生可能会导致肠道紧密连接结构被破坏,进而影响肠道机械屏障的正常生理功能。肠黏膜的紧密连接蛋白位于上皮细胞的侧面,很好地连接上皮细胞减少细胞空隙[18],提示当热应激发生时,便可导致肠黏膜通透性加大。然而给予马齿苋水提物治疗后小鼠肠道ZO-1 mRNA转录水平升高,结合空肠的病理切片则可推测马齿苋水提物通过提升紧密连接蛋白ZO-1的含量来缓解热应激对紧密连接结构的破坏,对受损的肠绒毛具有较好的修复作用,这与卢璐等[19]的研究结果基本一致。本试验以抗氧化剂维生素C为阳性对照药,对紧密连接蛋白ZO-1的影响基本与马齿苋水提物的一致,则进一步验证马齿苋水提物对热应激小鼠空肠的作用。
肠绒毛长短的变化影响着绒毛对营养物质的吸收面积[20],小肠隐窝深度反映小肠上皮细胞的生成率,隐窝变浅则表示肠道细胞新陈代谢速度快[21],所以小肠绒毛高度与隐窝深度的比值是衡量肠道结构与功能的重要指标[22]。本试验结果显示,热应激导致肠绒毛表面积减少,绒毛损伤脱落,肠绒毛高度/隐窝深度比值低于空白对照组,则可说明热应激能够促使肠黏膜完整结构被破坏,同时也推测热应激会影响动物机体对营养物质的吸收能力也受到影响,这与前人研究的结果基本一致[23-25]。用药后改善肠黏膜结构,结合对紧密连接蛋白ZO-1的影响,说明马齿苋水提物可通过使肠绒毛长度变长,增加吸收面积,提高VH/CD的比值,来促进热应激小鼠空肠的恢复,提高对营养物质的吸收能力,修复受损的肠黏膜结构。
木糖吸收水平变化常作为判定肠道消化吸收功能的指标。在盛惟等[26]研究中发现,木糖作为五碳糖不经过消化酶的参加直接通过小肠吸收到体内,木糖在吸收入体内后又不经过肝代谢,直接经肾排出,所以在一定时间内可通过检测尿和血中木糖含量即可了解小肠的吸收功能。在本研究中发现,热应激会引起空肠吸收功能下降,给予马齿苋水提物可明显提高血清D-木糖含量并趋于正常,说明马齿苋水提物可能提高热应激小鼠空肠的吸收能力。在哺乳动物体内,小肠黏膜吸收的单糖中葡萄糖占到了80%,葡萄糖又是真核生物的主要能量来源,维持着机体代谢和细胞动态平衡。葡萄糖的吸收主要是经过肠道进入血液,而肠道负责转运葡萄糖的载体主要是Na+-葡萄糖共转运载体(SGLT1)和易化葡萄糖转运载体(GLUT2)。本试验研究中热应激导致肠道葡萄糖转运载体含量降低,则可推测热应激发生时通过影响葡萄糖转运载体在肠道中的含量,从而导致血液中葡萄糖含量降低。SGLT1对葡萄糖在肠道的吸收起到了主导作用[27]。GLUT2参与葡萄糖的吸收释放和重吸收[28],本试验中发现,马齿苋水提物可增加肠道中葡萄糖转运载体SGLT1 mRNA和GLUT2 mRNA的转录量,进而促进血液中葡萄糖的吸收,这与阳性对照药维生素C的抗热应激效果趋于一致,葡萄糖吸收量随SGLT1载体数量而增加,并且主要是SGLT1和GLUT2协同完成[29-30],本试验研究药物马齿苋水提物可能通过增加葡萄糖转运载体的数量而加强空肠的吸收功能,由此说明马齿苋水提物可改善热应激小鼠空肠的吸收能力。
4 结论灌服马齿苋水提物可显著提高热应激小鼠空肠黏膜上皮细胞内紧密连接ZO-1 mRNA的含量,促进空肠绒毛的生长发育,使受损的空肠黏膜结构得到了修复;同时,AEP提高了机体对D-木糖的吸收,增加肠道中SGLT1 mRNA和GLUT2 mRNA的转录量,进而提高热应激小鼠空肠吸收能力。
| [1] |
康磊, 李文立, 姜建阳, 等. 谷氨酰胺对热应激肉鸡盲肠微生物区系的影响[J]. 中国兽医学报, 2013, 33(1): 80–84.
KANG L, LI W L, JIANG J Y, et al. Effects of glutamine on microflora of cecum in broilers suffered from heat stress[J]. Chinese Journal of Veterinary Science, 2013, 33(1): 80–84. (in Chinese) |
| [2] | NABUURS M J A, VAN ESSEN G J, NABUURS P, et al. Thirty minutes transport causes small intestinal acidosis in pigs[J]. Res Vet Sci, 2001, 70(2): 123–127. DOI: 10.1053/rvsc.2001.0448 |
| [3] | SÖDERHOLM J D, PERDUE M H. Ⅱ. Stress and intestinal barrier function[J]. Am J Physiol Gastrointest Liver Physiol, 2011, 280(1): G7–G13. |
| [4] | RADWAN M A, EL-GENDY K S, GAD A F. Oxidative stress biomarkers in the digestive gland of Thebapisana exposed to heavy metals[J]. Arch Environ ContamToxicol, 2010, 58(3): 828–835. DOI: 10.1007/s00244-009-9380-1 |
| [5] | VARASTEH S, BRABER S, KRANEVELD A D, et al. L-arginine supplementation prevents intestinal epithelial barrier breakdown under heat stress conditions by promoting nitric oxide synthesis[J]. Nutr Res, 2018, 57: 45–55. DOI: 10.1016/j.nutres.2018.05.007 |
| [6] | QUINTEIRO-FILHO W M, RIBEIRO A, FERRAZ-DE-PAULA V, et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens[J]. Poult Sci, 2010, 89(9): 1905–1914. DOI: 10.3382/ps.2010-00812 |
| [7] |
杨梅梅.中药复方与益生素对热应激下肉鸡肠道菌群及生长的影响[D].广州: 华南农业大学, 2016.
YANG M M. Effect of traditional Chinese medicine and probiotics on gut microbiomes and growth in broilers under heat stress[D]. Guangzhou: South China Agricultural University, 2016. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10564-1016923231.htm |
| [8] |
朱长生, 王勇飞. 马齿苋的生理作用及在猪饲料中的应用[J]. 饲料广角, 2016(12): 39–41.
ZHU C S, WANG Y F. Physiological effects of purslane and its application in pig feed[J]. Feed Wide Angle, 2016(12): 39–41. DOI: 10.3969/j.issn.1002-8358.2016.12.018 (in Chinese) |
| [9] |
闫港. 加味马齿苋白头翁散对仔猪白痢的治疗[J]. 中国兽医杂志, 2015, 51(5): 104.
YAN G. Treatment of piglet white pupa with Jiawei purslane Baitowengsan[J]. Chinese Journal of Veterinary Medicine, 2015, 51(5): 104. DOI: 10.3969/j.issn.0529-6005.2015.05.044 (in Chinese) |
| [10] |
黄晶, 文生萍, 杨炎林. 马齿苋粉对肉鸡生长性能和抗氧化能力的影响[J]. 中国饲料, 2019(18): 60–63.
HUANG J, WEN S P, YANG Y L. Effects of dietary purslane powder on growth performance and antioxidant status of broiler chickens[J]. China Feed, 2019(18): 60–63. (in Chinese) |
| [11] |
雷梓曦, 简丽梅, 梁聪, 等. 马齿苋多糖对冷应激致小鼠胃肠功能紊乱及NO含量的影响[J]. 湘南学院学报:医学版, 2013, 15(1): 4–7.
LEI Z X, JIAN L M, LIANG C, et al. The effects of purslane polysaccharides on the gastrointestinal mobility and NO concentrations when induced by cold restraint stress[J]. Journal of Xiangnan University: Medical Sciences, 2013, 15(1): 4–7. (in Chinese) |
| [12] |
葛剑, 杨翠军, 孙茂红, 等. 马齿苋多糖对雏鸡免疫器官指数及抗氧化功能的影响[J]. 中国饲料, 2012(9): 15–18.
GE J, YANG C J, SUN M H, et al. Effects of small peptide on the activities of immunoenzymes and disease resistance in Apostichopus japonicus[J]. China Feed, 2012(9): 15–18. DOI: 10.3969/j.issn.1004-3314.2012.09.005 (in Chinese) |
| [13] | ZHAO X H, HE X, YANG X H, et al. Effect of Portulaca oleracea extracts on growth performance and microbial populations in ceca of broilers[J]. Poult Sci, 2016, 92(5): 1343–1347. |
| [14] | VAN ITALLIE C M, ANDERSON J M. Architecture of tight junctions and principles of molecular composition[J]. Semin Cell Dev Biol, 2014, 36: 157–165. DOI: 10.1016/j.semcdb.2014.08.011 |
| [15] | STEVENSON B R, SILICIANO J D, MOOSEKER M S, et al. Identification of ZO-1:a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia[J]. J Cell Biol, 1986, 103(3): 755–766. DOI: 10.1083/jcb.103.3.755 |
| [16] | PIZZUTI D, BORTOLAMI M, MAZZON E, et al. Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet[J]. Dig Liver Dis, 2004, 36(5): 337–341. DOI: 10.1016/j.dld.2004.01.013 |
| [17] | GASSLER N, ROHR C, SCHNEIDER A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions[J]. Am JPhysiol Gastrointest Liver Physiol, 2001, 281(1): G216–G228. DOI: 10.1152/ajpgi.2001.281.1.G216 |
| [18] |
王希, 廖吕钊, 江荣林. 肠上皮细胞紧密连接蛋白的结构功能及其调节[J]. 浙江医学, 2018, 40(8): 895–898.
WANG X, LIAO L Z, JIANG R L. Structure and function of tight junction protein in intestinal epithelial cells[J]. Zhejiang Medical Journal, 2018, 40(8): 895–898. (in Chinese) |
| [19] |
卢璐, 谢建群, 郭春荣. 中药清肠栓对溃疡性结肠炎大鼠结肠黏膜紧密连接蛋白ZO-1、occludin的影响[J]. 世界华人消化杂志, 2011, 19(22): 2322–2327.
LU L, XIE J Q, GUO C R. Qingchang suppository upregulates the expression of tight junction proteins ZO-1 and occludin in experimental ulcerative colitis in rats[J]. World Chinese Journal of Digestology, 2011, 19(22): 2322–2327. (in Chinese) |
| [20] | MITCHELL M A, CARLISLE A J. The effects of chronic exposure to elevated environmental temperature on intestinal morphology and nutrient absorption in the domestic fowl (Gallus domesticus)[J]. Comp Biochem Physiol Part A: Physiol, 1992, 101(1): 137–142. DOI: 10.1016/0300-9629(92)90641-3 |
| [21] |
余进.猪和大鼠小肠黏膜热应激损伤修复机制的研究[D].北京: 北京农学院, 2010.
YU J. Investigation of the mechanism of heat stress-induced damage and repair in pig and rat small intestinal mucosa[D]. Beijing: Beijing Agricultural College, 2010. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10020-2010107907.htm |
| [22] |
毛红霞, 武书庚, 张海军, 等. 植物提取精油混合物对肉仔鸡生长性能、肠道菌群和肠黏膜形态的影响[J]. 动物营养学报, 2011, 23(3): 433–439.
MAO H X, WU S G, ZHANG H J, et al. Effects of plant essential oil mixtures on growth performance, intestinal microflora and intestinal mucosa morphology in broilers[J]. Chinese Journal of Animal Nutrition, 2011, 23(3): 433–439. DOI: 10.3969/j.issn.1006-267x.2011.03.012 (in Chinese) |
| [23] | QUINTEIRO-FILHO W M, GOMES A V S, PINHEIRO M L, et al. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis[J]. Avian Pathol, 2012, 41(5): 421–427. DOI: 10.1080/03079457.2012.709315 |
| [24] | SONG J, XIAO K, KE Y L, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress[J]. Poult Sci, 2014, 93(3): 581–588. DOI: 10.3382/ps.2013-03455 |
| [25] | LAMBERT G P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects[J]. J Anim Sci, 2009, 87(S14): E101–E108. |
| [26] |
盛惟, 黎明, 杨志燕. 木糖吸收实验方法的探讨[J]. 时珍国医国药, 2001, 12(2): 112–113.
SHENG W, LI M, YANG Z Y. The evaluation of xylose absorption experiment[J]. Lishizhen Medicine and Materia Medica Research, 2001, 12(2): 112–113. DOI: 10.3969/j.issn.1008-0805.2001.02.008 (in Chinese) |
| [27] | KELLETT G L, BROT-LAROCHE E. Apical GLUT2: a major pathway of intestinal sugar absorption[J]. Diabetes, 2005, 54(10): 3056–3062. DOI: 10.2337/diabetes.54.10.3056 |
| [28] |
魏娜, 刘瑞. GLUT2在小肠糖吸收中的作用[J]. 现代预防医学, 2010, 37(23): 4425–4427, 4430.
WEI N, LIU R. The role of GLUT2 in intestinal glucose absorption[J]. Modern Preventive Medicine, 2010, 37(23): 4425–4427, 4430. (in Chinese) |
| [29] | GARRIGA C, MORETÓ M, PLANAS J M. Effects of resalination on intestinal glucose transport in chickens adapted to low Na+ intakes[J]. Exp Physiol, 2000, 85(4): 371–378. DOI: 10.1111/j.1469-445X.2000.02039.x |
| [30] |
王修启, 谭会泽, 束刚, 等. 鸡肠道SGLT1和GLUT2 mRNA表达的组织特异性研究[J]. 畜牧兽医学报, 2006, 37(1): 12–17.
WANG X Q, TAN H Z, SHU G, et al. Study on Tissue-specific expression of SGLT1 and GLUT2 mRNA in chicken intestine[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(1): 12–17. DOI: 10.3321/j.issn:0366-6964.2006.01.003 (in Chinese) |



