我国家禽品种资源丰富,现有地方鸡品种114个。这些品种资源在长期的选择进化过程中形成了多样化的生产力类型及丰富的遗传多样性,是我国家禽库极其宝贵的遗传资源。
微卫星DNA标记、线粒体DNA序列变异等已被广泛应用于地方鸡种的遗传进化及起源分析[1-2],然而这些DNA标记在整个鸡基因组中的覆盖范围是极其微小的,其所代表的基因组遗传变异信息量非常有限。新一代测序(next-generation sequencing,NGS)技术的发展实现了SNP分子标记的高通量检测[3],为从基因组水平上重新审视鸡的遗传进化提供了更为高效准确的研究方法。简化基因组测序(reduced-representation genome sequencing,RRGS)能鉴定出合适数量、广泛覆盖全基因组范围的变异位点,具有成本相对较低、适于大规模群体研究的特点,被广泛应用于遗传图谱构建、全基因组关联分析和群体遗传分析等领域[4-6]。
本研究利用简化基因组RAD-seq测序鉴定地方鸡种SNP标记,揭示地方鸡种的遗传多样性和遗传结构,鉴定基因组中受选择区域,发掘重要种质特性基因,为开展地方鸡种质特性评价、品种资源保护及开发利用提供科学依据。
1 材料与方法 1.1 试验材料19个地方鸡品种(狼山鸡(LS)、安义瓦灰鸡(WH)、固始鸡(GS)、仙居鸡(XJ)、白耳黄鸡(BE)、东乡绿壳蛋鸡(DX)、金湖乌凤鸡(JH)、文昌鸡(WC)、大围山微型鸡(WX)、藏鸡(ZZ)、茶花鸡(CH)、瓢鸡(PJ)、惠阳胡须鸡(HX)、大骨鸡(DG)、萧山鸡(XS)、鹿苑鸡(LY)、边鸡(BJ)、北京油鸡(BY)、河南斗鸡(DJ))、2个引入鸡品种(隐性白羽肉鸡RW、安卡红鸡AK,作为进化树构建的外群)均来自于国家级地方鸡种基因库保种群。每个品种按照家系选取30个个体(10公、20母),个体间无亲缘关系。翅静脉采血1.0~1.5 mL,柠檬酸钠(ACD)抗凝,-20 ℃保存备用。
1.2 试验方法 1.2.1 RAD-seq简化基因组测序常规酚-氯仿法提取个体基因组DNA,构建pair-end文库(300~500 bp)进行EcoRⅠ(G ^ AATTC)和NlaⅢ (Hin1IICATG ^)双酶切RAD-seq简化基因组测序,每个品种个体独立测序。测序由南京集思慧远生物科技有限公司完成。
1.2.2 SNP质控SNP的鉴定采用GATK和samtools程序。质控条件包括:Q20>95%,ddRAD depth>60%,在所有样品中的SNP Call rate>70%,在单个鸡种中的SNP Call rate>90%,MAF>0.05。
1.2.3 统计分析连锁不平衡(LD)分析采用Haploview软件,观察杂合度(Ho)、近交系数(Fis)和群体分化指数(Fst)计算采用PopGen软件,群体聚类分析应用MEGA软件UPGMA聚类法。选择信号检测采用PLINK软件,以100 kb为窗口、10 kb为step进行滑动的ZHp选择信号分析,ZHp转换参照Rubin等[7]的方法,在所有个体上为纯合的位点剔除,Z/W性染色体位点不作分析。
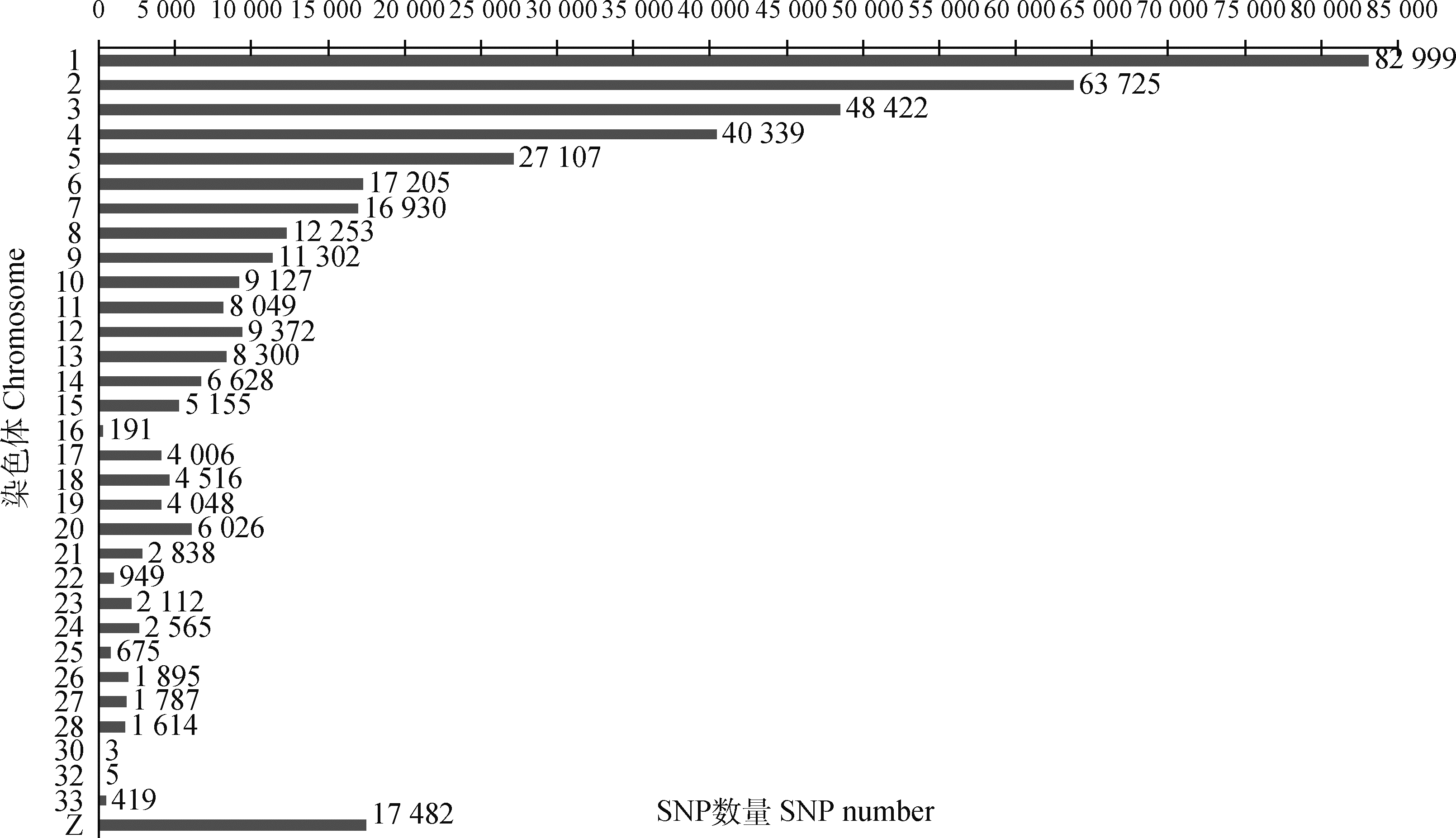
2 结果 2.1 19个地方鸡种的基因组SNP鉴定经过两步数据质控,在19个地方鸡种33个常染色体中鉴定出400 562个SNPs,在性染色体Z上鉴定出17 482个SNPs,合计418 044个(图 1)。总体上,SNP数量与染色体长度呈正相关,大染色体上SNP数量较多(其中1~9号染色体上SNP数量均超过10 000个),小染色体上SNP数量较少。两个引入品种隐性白羽肉鸡和安卡红鸡中分别鉴定出319 051、317 155个基因组SNPs。
|
图 1 不同染色体上SNPs数量 Fig. 1 SNPs number on different chromosomes |
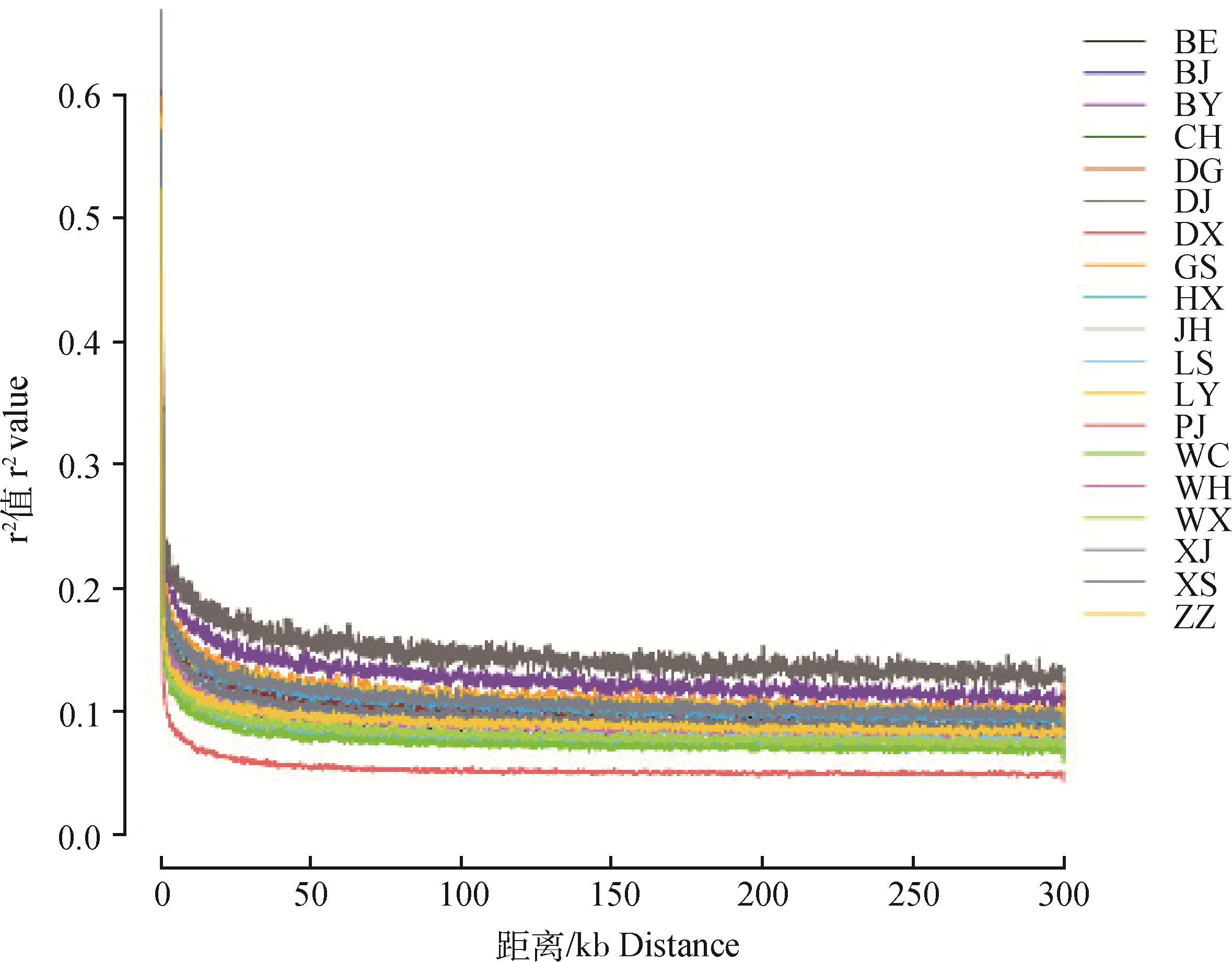
19个地方鸡种的遗传多样性和近交系数统计见表 1。结果表明,瓢鸡和文昌鸡的遗传多样性最为丰富,观察杂合度(Ho)分别为0.246 8、0.243 0,核苷酸多样度(Pi)分别为0.278 1、0.265 5。河南斗鸡的遗传多样性相对匮乏,Ho为0.156 0,Pi为0.175 2,与LD连锁不平衡分析结果(图 2)相一致。东乡绿壳蛋鸡和边鸡的近交系数Fis最高,均超过0.160 0。
|
|
表 1 19个地方鸡种观察杂合度、核苷酸多样度和近交系数 Table 1 Ho, Pi and Fis statistics of 19 indigenous chicken breeds |
|
图 2 LD随距离增长的衰减图 Fig. 2 LD decay with distance |
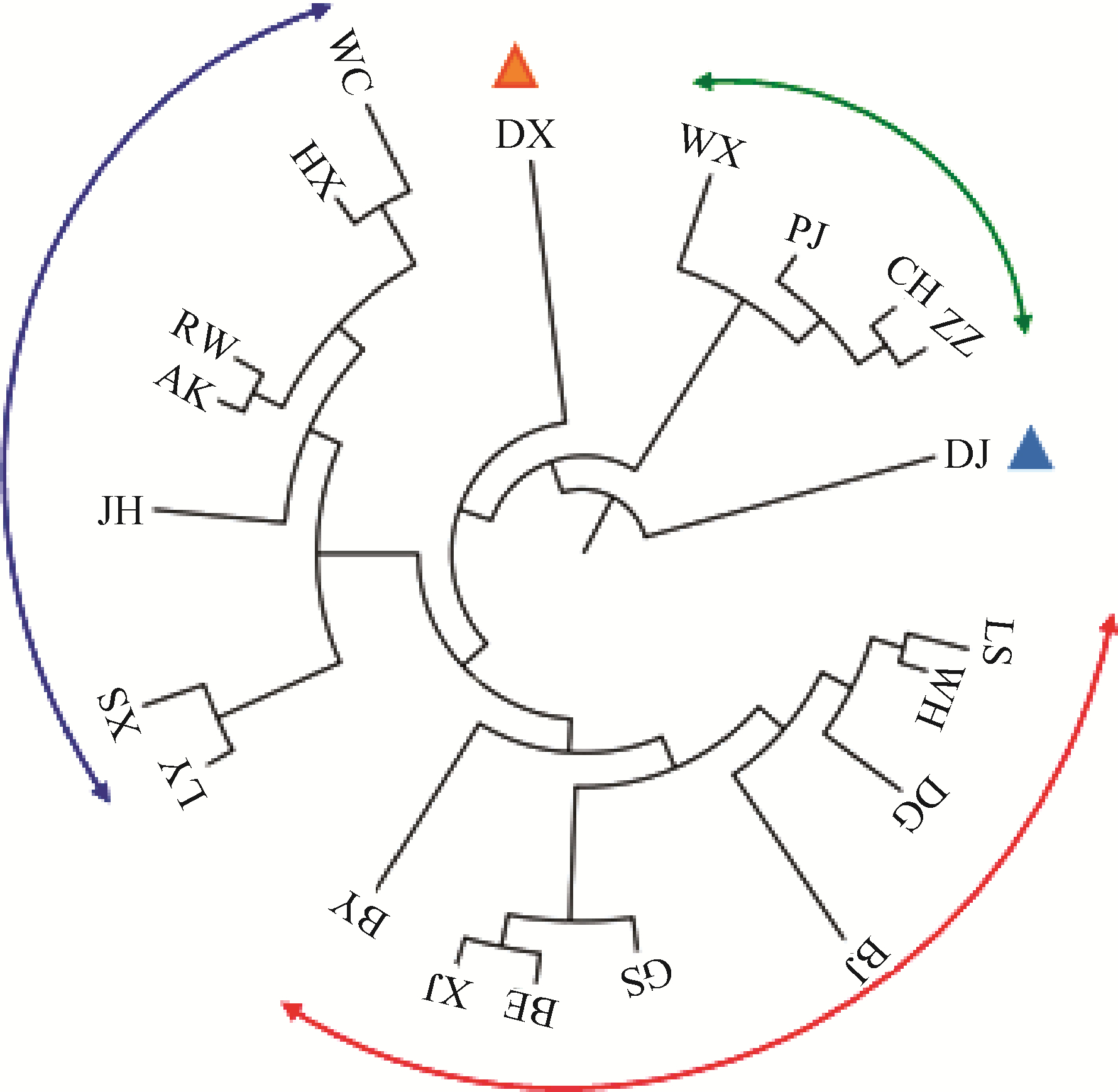
19个地方鸡种间遗传分化系数(Fst)和基因流(Nm)见表 2。结果表明,河南斗鸡与其它品种间的遗传分化均处于较高水平,与其中15个品种的Fst>0.200 0、Nm < 1.000 0。瓢鸡与文昌鸡(0.077 1)、惠阳胡须鸡(0.083 8)、藏鸡(0.088 9)、大围山微型鸡(0.092 9),惠阳胡须鸡与文昌鸡(0.089 4),藏鸡与茶花鸡(0.092 6)间的遗传分化最低,Fst < 0.100 0;萧山鸡与鹿苑鸡(0.101 6),瓢鸡与茶花鸡(0.103 4)间的遗传分化也处于较低水平。相应的,这些品种间的基因流较高,Nm>0.240 0。遗传聚类分析见图 3,总体上聚为5类:第一类包括云南地区的4个品种,瓢鸡、大围山微型鸡、藏鸡和茶花鸡;第二类包括狼山鸡、安义瓦灰鸡、大骨鸡、边鸡、仙居鸡、固始鸡、白耳黄鸡和北京油鸡;第三类包括萧山鸡、鹿苑鸡、金湖乌凤鸡、惠阳胡须鸡和文昌鸡,两个引入品种隐性白羽肉鸡和安卡红鸡也聚入此类,但表现为相对独立的分支;东乡绿壳蛋鸡与河南斗鸡则分别形成了独立的类别。
|
|
表 2 19个地方鸡种间遗传分化系数(下三角)和基因流(上三角) Table 2 Fst (lower triangle) and Nm (upper triangle) for 19 indigenous chicken breeds |
|
图 3 19个地方鸡种遗传聚类图(两个引入品种作外群) Fig. 3 Phylogenetic tree of 19 indigenous chicken breeds (two introduced chicken breeds as outgroups) |
以100 kb为窗口、10 kb为step进行滑动的ZHp选择信号分析,将19个地方鸡种合并为一个群体,结果发现,合并群体9个常染色体上的26个区域受到选择作用(ZHp阈值< -4.0),包含31个受选择基因(表 3)。
|
|
表 3 19个地方鸡种基因组受选择区域和基因 Table 3 Selected genome regions and genes in 19 indigenous chicken breeds |
本研究利用RAD-seq技术在19个地方鸡种中鉴定出418 044个SNPs标记,进一步证实了我国地方鸡种丰富的遗传多样性。这些标记信息将为构建地方鸡种SNP芯片提供基础,也更适用于开展地方鸡种的分子标签、遗传进化及关联分析等研究。
3.2 地方鸡种群体的遗传多样性和遗传结构分析基于观察杂合度和核苷酸多样度两个统计量,在19个地方鸡种中,瓢鸡和文昌鸡的遗传多样性最为丰富,与这两个鸡种群体多样化的表型相吻合[8-9]。东乡绿壳蛋鸡和边鸡的近交系数处于较高水平,提示在保种过程中应严格控制近交水平的过快增加。总体上,遗传聚类分析结果与19个地方鸡种间遗传分化、基因交流,品种形成历史和地理分布相一致[10]。由于所处的特殊地理位置和生态条件,云南省的4个鸡种形成了明显的区域性独立分支。斗鸡主要用于玩赏,加之驯养方法非常保守,在品种形成过程中与其他鸡种间鲜有基因交流;东乡绿壳蛋鸡是一类特殊遗传资源,绿壳蛋性状在地方鸡种中较为少见,这些因素造成河南斗鸡和东乡绿壳蛋鸡分别聚为两个独立的分支。鹿苑鸡与萧山鸡的主产地浙江省萧山市与江苏省的张家港市之间的地理距离较近,仙居鸡和白耳黄鸡在浙江、江西两省均有分布,云南省(茶花鸡)与西藏地区(藏鸡)相邻,广东省(惠阳胡须鸡)与香港地区(文昌鸡)贸易往来频繁,而且产地相似的文化、生活习惯为这些鸡种之间的交流提供了便利。北京油鸡、边鸡、大骨鸡、狼山鸡和安义瓦灰鸡尽管聚为一类,但在分支结构上仍保持相对独立,金湖乌凤鸡在其所在类别中亦是如此,表明这些品种间较远的遗传距离和较大的遗传差异。
3.3 地方鸡种的选择信号分析自然选择和人工选择作用会在动物基因组上留下选择信号,受选择区域会表现出高度的遗传分化,核苷酸多样度降低和长范围内的连锁不平衡[11],通过选择信号分析可以发掘重要功能基因。目前,研究者通过基因组测序或芯片检测,对不同物种开展了选择信号研究[12-15]。针对鸡而言,Rubin等[7]重测了家鸡、红原鸡及商品化鸡的基因组序列,发现了大量选择性清除片段,并在家鸡中鉴定了与代谢调节和生殖光周期调控相关的促甲状腺激素受体基因TSHR。Elferink等[16]利用芯片检测了商业化品系鸡、荷兰和中国部分地方鸡种的基因组变异,发现26个区域显示出强烈的选择信号。Wang等[17-18]基于重测序发现,藏鸡群体中受到选择作用的基因在钙离子信号通路中显著富集,大量视觉相关基因在家鸡中受到正选择作用。然而,针对我国地方鸡种的基因组选择信号检测研究较少。
选择信号检测中一个关键要素是确定适宜的基因组信号窗口和阈值,本研究借鉴已有的鸡基因组选择信号分析经验,采用ZHp分析方法,在安卡红鸡和隐性白羽肉鸡两个国外标准品种群体中以肉鸡品系(品种)已鉴定的受选择基因作为验证标准。发现以100 kb为窗口、10 kb为step计算滑动的ZHp值能检测出已发现的受选择基因BCO2(Chr24: 6050000-6150000)、IGF-1(Chr1: 55350000-55490000)、ROBO2(Chr1: 96820000-96940000)、TBC1D1(Chr4: 70070000-70220000)等[7, 16],表明了分析窗口和阈值选择的可靠性。另一个关键要素是降低随机漂变效应对选择信号的影响,本研究将19个地方鸡种合并为一个群体,由于各鸡种用于分析的样本数量相同,故不作权重分配。
结果表明,合并群体9个常染色体上的26个区域受到选择作用,包含31个受选择基因。已有的研究表明,这些受选择基因广泛参与免疫系统调节、生殖机能调控、应激响应及代谢等生物学过程。TRIM62基因能有效抑制禽白血病ALV-J和禽网状内皮组织增生症病毒的复制[19],RORC基因是调控Th17细胞产生的关键分子开关[20],S100A10基因调节炎性刺激响应过程中巨噬细胞的募集和迁移[21],NIT1基因是T细胞的负调节因子[22]。KDM3B基因是维持雄性小鼠正常精子发生、性行为及雌性小鼠生殖机能所必需的[23-24]。TDRKH基因参与精子发生过程和piRNA生成[25],EIF4G3基因突变会导致雄性小鼠不育和精母细胞减数分裂抑制[26],FKBP5是雄激素的直接靶基因[27]。TIA1基因参与细胞和组织应激颗粒的生成,是进化上保守的应对环境压力的响应机制[28]。USP48基因在保持基因组稳定性和DNA修复方面发挥重要作用[29-30],ECE-1基因与耐低氧有关[31-32]。TEAD3基因调节肌细胞生成分化[33-34],MICAL基因是肌动蛋白细胞骨架动态变化的关键调节因子[35-36]。PCYOX1和AGMO基因[37-38]调节脂质代谢,SRPK1基因抑制脂肪生成[39]。另外,LHFPL5基因与听力有关[40],推测自然条件下躲避天敌和散养条件下的人工饲喂呼唤对此起到了重要的选择作用,形成了地方鸡种机灵敏感的特质。诸多受选择基因的功能尚未有清晰的注释,需要进一步的深入研究。
4 结论利用基因组SNP标记能更全面准确地揭示地方鸡种的遗传多样性和遗传结构,选择作用主要体现在对地方鸡种抗逆抗病特性、配子活力及行为等方面的塑造。
| [1] | QU L J, LI X Y, XU G F, et al. Evaluation of genetic diversity in Chinese indigenous chicken breeds using microsatellite markers[J]. Science in China Series C Life Sciences, 2006, 49(4): 332–341. DOI: 10.1007/s11427-006-2001-6 |
| [2] |
高玉时, 唐修君, 屠云洁, 等. 基于线粒体COⅠ基因15个鸡种的DNA编码研究[J]. 中国农业科学, 2011, 44(3): 587–594.
GAO Y S, TANG X J, TU Y J, et al. Studies on the DNA barcoding of fifteen chicken breeds by mtDNA COⅠ gene[J]. Scientia Agricultura Sinica, 2011, 44(3): 587–594. DOI: 10.3864/j.ssn.0578-1752.2011.03.020 (in Chinese) |
| [3] | DAVEY J W, HOHENLOHE P A, ETTER P D, et al. Genome-wide genetic marker discovery and genotyping using next-generation sequencing[J]. Nat Rev Genet, 2011, 12(7): 499–510. DOI: 10.1038/nrg3012 |
| [4] | HOHENLOHE P A, BASSHAM S, ETTER P D, et al. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags[J]. PLoS Genet, 2010, 6(2): e1000862. DOI: 10.1371/journal.pgen.1000862 |
| [5] | EMERSON K J, MERZ C R, CATCHEN J M, et al. Resolving postglacial phylogeography using high-throughput sequencing[J]. Proc Natl Acad Sci U S A, 2010, 107(37): 16196–16200. DOI: 10.1073/pnas.1006538107 |
| [6] | HOHENLOHE P A, AMISH S J, CATCHEN J M, et al. Next-generation RAD sequencing identifies thousands of SNPs for assessing hybridization between rainbow and westslope cutthroat trout[J]. Mol Ecol Resour, 2011, 11(S1): 117–122. |
| [7] | RUBIN C J, ZODY M C, ERIKSSON J, et al. Whole-genome resequencing reveals loci under selection during chicken domestication[J]. Nature, 2010, 464(7288): 587–592. DOI: 10.1038/nature08832 |
| [8] |
贡潘偏抽, 刘丽仙, 李大林, 等. 基于线粒体DNA控制区(mtDNA D-loop)序列分析瓢鸡的遗传多样[J]. 云南农业大学学报, 2011, 26(2): 211–214, 223.
GONGPAN P C, LIU L X, LI D L, et al. The investigation of genetic diversity of Piao Chicken based on mitochondrial DNA D-loop region sequence[J]. Journal of Yunnan Agricultural University, 2011, 26(2): 211–214, 223. DOI: 10.3969/j.issn.1004-390X(n).2011.02.012 (in Chinese) |
| [9] |
顾玉兰, 刘小林, 张建勤. 文昌鸡群体内遗传变异分析[J]. 西北农业学报, 2008, 17(4): 28–31.
GU Y L, LIU X L, ZHANG J Q. Genetic variation in Wenchang chicken[J]. Acta Agriculturae Boreali- Occidentalis Sinica, 2008, 17(4): 28–31. DOI: 10.3969/j.issn.1004-1389.2008.04.007 (in Chinese) |
| [10] |
陈宽维, 李慧芳, 王金玉, 等. 华东27个地方鸡品种(品系)的遗传变异[J]. 畜牧兽医学报, 2006, 37(1): 7–11.
CHEN K W, LI H F, WANG J Y, et al. Study on genetic diversity of 27 indigenous chicken breeds or strains in East China[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(1): 7–11. DOI: 10.3321/j.issn:0366-6964.2006.01.002 (in Chinese) |
| [11] | SABETI P C, VARILLY P, FRY B, et al. Genome-wide detection and characterization of positive selection in human populations[J]. Nature, 2007, 449(7164): 913–918. DOI: 10.1038/nature06250 |
| [12] | HUERTA-SÁNCHEZ E, JIN X, AS AN, et al. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA[J]. Nature, 2014, 512: 194–197. DOI: 10.1038/nature13408 |
| [13] | LI M Z, TIAN S L, JIN L, et al. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars[J]. Nat Genet, 2013, 45(12): 1431–1438. DOI: 10.1038/ng.2811 |
| [14] | YANG S B, LI X L, LI K, et al. A genome-wide scan for signatures of selection in Chinese indigenous and commercial pig breeds[J]. BMC Genet, 2014, 15(1): 7. DOI: 10.1186/1471-2156-15-7 |
| [15] | WEI C H, WANG H H, LIU G, et al. Genome-wide analysis reveals population structure and selection in Chinese indigenous sheep breeds[J]. BMC Genomics, 2015, 16(1): 194. DOI: 10.1186/s12864-015-1384-9 |
| [16] | ELFERINK M G, MEGENS H J, VEREIJKEN A, et al. Signatures of selection in the genomes of commercial and non-commercial chicken breeds[J]. PLoS One, 2012, 7(2): e32720. DOI: 10.1371/journal.pone.0032720 |
| [17] | WANG M S, LI Y, PENG M S, et al. Genomic analyses reveal potential independent adaptation to high altitude in Tibetan Chickens[J]. Mol Biol Evol, 2015, 32(7): 1880–1889. DOI: 10.1093/molbev/msv071 |
| [18] | WANG M S, ZHANG R W, SU L Y, et al. Positive selection rather than relaxation of functional constraint drives the evolution of vision during chicken domestication[J]. Cell Res, 2016, 26(5): 556–573. |
| [19] | LI L, FENG W G, CHENG Z Q, et al. TRIM62-mediated restriction of avian leukosis virus subgroup J replication is dependent on the SPRY domain[J]. Poult Sci, 2019, 98(11): 6019–6025. DOI: 10.3382/ps/pez408 |
| [20] | ZHANG S, TAKAKU M, ZOU L Y, et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation[J]. Nature, 2017, 551(7678): 105–109. DOI: 10.1038/nature24283 |
| [21] | O'CONNELL P A, SURETTE A P, LIWSKI R S, et al. S100A10 regulates plasminogen-dependent macrophage invasion[J]. Blood, 2010, 116(7): 1136–1146. DOI: 10.1182/blood-2010-01-264754 |
| [22] | ZHANG H B, HOU Y J, HAN S Y, et al. Mammalian nitrilase 1 homologue Nit1 is a negative regulator in T cells[J]. Int Immunol, 2009, 21(6): 691–703. DOI: 10.1093/intimm/dxp038 |
| [23] | LIU Z L, OYOLA M G, ZHOU S L, et al. Knockout of the histone demethylase Kdm3b decreases spermatogenesis and impairs male sexual behaviors[J]. Int J Biol Sci, 2015, 11(12): 1447–1457. DOI: 10.7150/ijbs.13795 |
| [24] | LIU Z L, CHEN X, ZHOU S L, et al. The histone H3K9 demethylase Kdm3b is required for somatic growth and female reproductive function[J]. Int J Biol Sci, 2015, 11(5): 494–507. DOI: 10.7150/ijbs.11849 |
| [25] | SAXE J P, CHEN M J, ZHAO H Y, et al. Tdrkh is essential for spermatogenesis and participates in primary piRNA biogenesis in the germline[J]. EMBO J, 2013, 32(13): 1869–1885. DOI: 10.1038/emboj.2013.121 |
| [26] | SUN F Y, PALMER K, HANDEL M A. Mutation of Eif4g3, encoding a eukaryotic translation initiation factor, causes male infertility and meiotic arrest of mouse spermatocytes[J]. Development, 2010, 137(10): 1699–1707. DOI: 10.1242/dev.043125 |
| [27] | MAGEE J A, CHANG L W, STORMO G D, et al. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element[J]. Endocrinology, 2006, 147(1): 590–598. |
| [28] | SUN Y J, ZHANG P, ZHENG H, et al. Chicken RNA-binding protein T-cell internal antigen-1 contributes to stress granule formation in chicken cells and tissues[J]. J Vet Sci, 2018, 19(1): 3–12. DOI: 10.4142/jvs.2018.19.1.3 |
| [29] | UCKELMANN M, DENSHAM R M, BAAS R, et al. USP48 restrains resection by site-specific cleavage of the BRCA1 ubiquitin mark from H2A[J]. Nat Commun, 2018, 9(1): 229. DOI: 10.1038/s41467-017-02653-3 |
| [30] | VELIMEZI G, ROBINSON-GARCIA L, MUÍOZ-MARTÍNEZ F, et al. Map of synthetic rescue interactions for the Fanconi anemia DNA repair pathway identifies USP48[J]. Nat Commun, 2018, 9(1): 2280. DOI: 10.1038/s41467-018-04649-z |
| [31] | WANG Y D, ZHANG J, LI C H, et al. Molecular cloning, sequence characteristics, and tissue expression analysis of ECE1 gene in Tibetan pig[J]. Gene, 2015, 571(2): 237–244. DOI: 10.1016/j.gene.2015.06.054 |
| [32] | KHAMAISI M, TOUKAN H, AXELROD J H, et al. Endothelin-converting enzyme is a plausible target gene for hypoxia- inducible factor[J]. Kidney Int, 2015, 87(4): 761–770. DOI: 10.1038/ki.2014.362 |
| [33] | FIGEAC N, MOHAMED A D, SUN C S, et al. VGLL3 operates via TEAD1, TEAD3 and TEAD4 to influence myogenesis in skeletal muscle[J]. J Cell Sci, 2019, 132(13): jcs225946. DOI: 10.1242/jcs.225946 |
| [34] | JOSHI S, DAVIDSON G, LE GRAS S, et al. TEAD transcription factors are required for normal primary myoblast differentiation in vitro and muscle regeneration in vivo[J]. PLoS Genet, 2017, 13(2): e1006600. DOI: 10.1371/journal.pgen.1006600 |
| [35] | VANONI M A. Structure-function studies of MICAL, the unusual multidomain flavoenzyme involved in actin cytoskeleton dynamics[J]. Arch Biochem Biophys, 2017, 632: 118–141. DOI: 10.1016/j.abb.2017.06.004 |
| [36] | FRÉMONT S, ROMET-LEMONNE G, HOUDUSSE A, et al. Emerging roles of MICAL family proteins-from actin oxidation to membrane trafficking during cytokinesis[J]. J Cell Sci, 2017, 130(9): 1509–1517. DOI: 10.1242/jcs.202028 |
| [37] | HERRERA-MARCOS L V, LOU-BONAFONTE J M, MARTINEZ-GRACIA M V, et al. Prenylcysteine oxidase 1, a pro-oxidant enzyme of low density lipoproteins[J]. Front Biosci (Landmark Ed), 2018, 23: 1020–1037. DOI: 10.2741/4631 |
| [38] | WATSCHINGER K, WERNER E R. Alkylglycerol monooxygenase[J]. IUBMB Life, 2013, 65(4): 366–372. DOI: 10.1002/iub.1143 |
| [39] | LIN J C. Multi-posttranscriptional regulations lessen the repressive effect of SRPK1 on brown adipogenesis[J]. Biochim Biophys Acta:Mol Cell Biol Lipids, 2018, 1863(5): 503–514. DOI: 10.1016/j.bbalip.2018.02.004 |
| [40] | LIAQAT K, CHIU I, LEE K, et al. Novel missense and 3'-UTR splice site variants in LHFPL5 cause autosomal recessive nonsyndromic hearing impairment[J]. J Hum Genet, 2018, 63(11): 1099–1107. DOI: 10.1038/s10038-018-0502-3 |



