在我国,随着人们生活质量和消费水平的提高,膳食结构已由温饱型向品质型转变,消费者对肉品质的要求越来越高。肌内脂肪(intramuscular fat,IMF)含量是影响肉品质的关键因素之一,决定着肉的嫩度、多汁性及风味等感官品质[1]。肌内脂肪沉积主要受到动物的日龄、性别、品种、营养水平以及遗传等因素影响,但越来越多的研究报道证实,肠道菌群也可调控动物肌内脂肪的沉积[2-3]。
众所周知,肠道微生物对宿主营养物质的消化、吸收及代谢起着重要的调控作用。近年来,宏基因组测序等技术使人们对肠道菌群特征有了一定了解,在脂质代谢方面,已有研究证实,肠道微生物群会影响动物机体的脂肪代谢及沉积水平[4],但深层次的代谢物、功能酶及信号分子等方面的数据尚不多[5]。随着代谢组学技术及多组学联合技术的应用,在肠道中发现了数千种微生物代谢物,同时, 在宿主组织内也检测到其中的一些代谢产物[6],这表明,肠道微生物同宿主的互作或许是通过代谢产物等实现的。在脂肪代谢方面,已有研究表明,可由微生物产生的短链脂肪酸、胆汁酸、三甲胺类、色氨酸衍生物以及促炎细菌产生的因子如脂多糖等代谢产物等介导[4, 7]。为更好地明确肠道菌群及其代谢物与脂肪沉积之间的关系及互作效应,本文对近年来相关研究进行了系统综述。
1 肌内脂肪肌内脂肪是沉积在肌纤维和肌束之间的白色脂肪,主要成分为磷脂、甘油三酯和胆固醇,与肉品质密切相关,决定着肉的嫩度、多汁性和风味等感官品质[1]。研究显示,肌内脂肪可通过两种途径改善肉的嫩度和风味,一是肌内脂肪在氧化时能够溶解肌纤维束,进而提高肉的嫩度和多汁性;二是肌内脂肪中含有较高水平的磷脂,能够通过美拉德(Mailard)反应产生风味物质[8]。
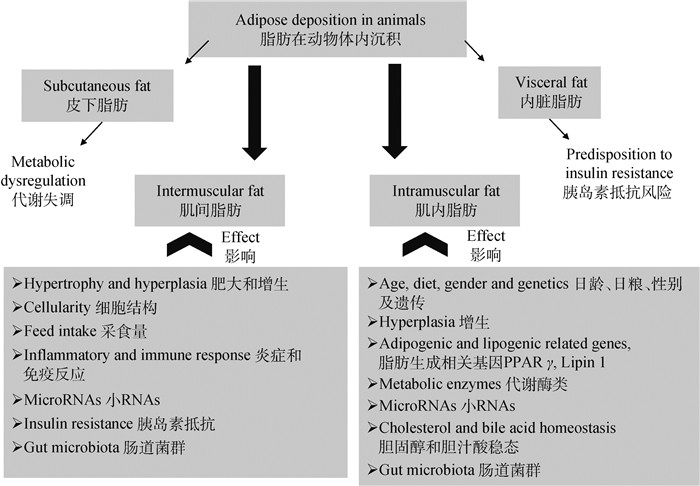
肌内脂肪含量过低会引起肉类口感干涩、风味下降。肌内脂肪沉积主要来自于三方面:肌内脂肪细胞数量的增加、肌内脂肪细胞甘油三酯的积累以及肌纤维内的脂滴沉积[9],而脂肪细胞数量多在动物出生前就已经确定[10],因此,肌内脂肪的沉积主要源于脂肪细胞中甘油三酯的累积[9]。如图 1所示,肌内脂肪沉积主要与动物的日龄、性别、品种、营养水平以及遗传等因素有关,但近年来的研究报道证实,肠道菌群也可引起动物肌内脂肪沉积的改变[2-3]。
|
图 1 影响肌间脂肪及肌内脂肪沉积的因素[11] Fig. 1 Major factors affecting the deposition of intermuscular fat and intramuscular fat [11] |
大量研究表明,肠道微生物能够调控脂肪代谢并影响血液及组织中脂肪沉积水平[4, 12-14]。在模式动物中的研究发现,高脂饮食诱导的无菌小鼠空肠菌群即使在低脂饮食下也表现出更高的脂肪吸收效率,且上调了小鼠近端小肠脂肪吸收相关基因,推测小肠菌群可能是小鼠脂肪吸收的重要调节因子[13]。脂质组学分析表明,同无菌小鼠比较,肠道微生物群影响了小鼠组织和血清中的脂质组成,并加速了循环中甘油三酯的去除率[15]。斑马鱼上的研究也表明,肠道菌群增加了肠道上皮细胞的脂肪累积[16]。还有研究表明,肠道微生物可能是减少脂肪沉积的潜在干预靶点。Gao等[17]研究发现,高脂饮食老鼠饲喂西藏开菲尔(Tibet kefir)牛奶后,正向调控了肠道中Firmicutes/Bacteroidetes比例以及Akkermansia、Escherichia和Oscillospira丰度,并且在转录水平下调了脂蛋白脂肪酶(lipoprotein lipase,LPL)和血管生成素样蛋白4(angiopoietin-like protein-4,Angptl4)表达丰度,降低了腹部脂肪沉积和血清甘油三酯水平。
在养殖动物中的研究发现,肉鸡十二指肠和盲肠中的的菌群对脂肪沉积的影响较大,而盲肠中Methanobacterium和Mucispirillum schaedleri的丰度分别与腹部脂肪沉积有显著正和负的相关性[18],通过调节肠道菌群或能影响肉鸡的脂肪沉积。进一步研究发现,鸡肠道脂肪代谢水平还受到不同肠型(enterotypes,ET)的影响,Ochrobactrum(19.4%)+Rhodococcus(14.7%)肠型的血清甘油三酯和皮下、腹部脂肪含量最高,这与其中的微生物功能主要为多糖降解和利用有关[19]。在德州驴的研究中也有类似的发现,肠道中的微生物参与了脂肪代谢[20]。与此同时,益生菌的研究为肠道菌群在调节宿主脂肪沉积中的作用提供了进一步的证据。研究发现,饲喂由6株乳酸杆菌+3株双歧杆菌组成的复合益生菌制剂显著降低了非酒精性脂肪肝大鼠肝、血清中甘油三酯及机体脂肪沉积水平[21];在高脂日粮的小鼠中饲喂假双歧杆菌(Bifidobacterium pseudolongum)也可改善肠道厚壁菌门与拟杆菌门的比例,增加丁酸菌和双歧杆菌属细菌的丰度,进而降低机体甘油三酯的沉积[22];添加双歧杆菌还可缓解内毒素脂多糖引起的内毒素血症,降低机体脂肪沉积[23]。动物养殖试验发现,应用发酵豆粕饲喂日本鹌鹑后,显著降低了血清中胆固醇和低密度脂蛋白水平,但对甘油三酯水平却没有影响[24],然而,经口灌胃复合益生菌制剂(包含9株益生菌:Aspergillus oryzae PXN 68、Lactobacillus acidophilus PXN 35、L. rhamnosus PXN 54、L. plantarum PXN 47、L. bulgaricus PXN 39、Bifidobacterium bifidum PXN 23、Enterococcus faecium PXN 33、Streptococcus thermophilus PXN 66和Candida pintolopesii PXN 70,活菌数2 × 109 cfu·g-1)则显著降低了鹌鹑血清中甘油三酯浓度[25]。肉鸡日粮中添加益生菌Saccharomyces cerevisiae或复合菌制剂(Lactobacillus acidophilus+Bacillus subtilis+Aspergillus oryzae)都显著降低了血清中总脂质和胆固醇含量[26],补充益生菌也可显著降低生长肥育猪血清中甘油三酯、低密度脂蛋白和尿素氮的水平[27]。由此可见,肠道微生物影响了动物脂肪沉积,而且不同的菌群特征对动物脂肪沉积的影响各异。
2.2 肠道菌群对肌内脂肪沉积的影响多个研究表明,动物肌内脂肪的沉积也受到肠道菌群的调控[2-3, 28-29]。在养殖生产中,报道较多的是添加益生菌或直接饲喂富含益生菌的发酵饲料对动物脂肪沉积水平及胴体品质等方面的影响。肉鸡上的研究发现,日粮中补充约氏乳杆菌(Lactobacillus johnsonu BS15)能够平衡肠道菌群、改善肠道发育,提高肉鸡的生长性能并降低脂肪沉积[30],而日粮中补充丁酸梭菌(Clostridium butyricum)则显著增加了21日龄肉鸡腿肌肌内脂肪含量[3],丁酸梭菌的饲喂也显著提高了北京肉鸭胸肌肌肉的红度、肌苷酸及肌内脂肪含量[29]。在猪上的研究发现,添加8%的发酵饲料可显著增加生长肥育猪眼肌面积、a*值、大理石纹评分、肌苷酸和肌内脂肪含量[31];发酵中草药的应用则提高了生长肥育猪背最长肌中的n-3多不饱和脂肪酸含量,而降低了n-6/n-3脂肪酸的比例[32]。然而,微生物对动物肌内脂肪沉积影响的报道并不一致。Xue等[33]研究发现,枯草芽胞杆菌的补充并没有对慢性热应激肉鸡胸部肌肉脂肪沉积产生影响,在日粮中添加经过益生菌发酵的窄叶泽泻(Alisma canaliculatum)显著提高了肉鸡胸肌粗蛋白含量但降低了粗脂肪含量,同时改变了肌肉脂肪酸组成[34]。Inatomi[35]应用三株益生菌Bacillus mesentericus TO-A、Clostridium butyricum TO-A和Streptococcus faecalis T-110组成的复合菌剂饲喂肉鸡显著降低了腹部脂肪及腿肌肌内脂肪含量。这或许同动物状态、品种及应用的微生物菌种有关。此外,组学技术对肌内脂肪沉积相关微生物的研究有很大的帮助,通过对500份猪盲肠和粪便样品进行高通量测序及相关性分析发现,119个与肌内脂肪沉积显著相关的可操作分类单元(operational taxonomic units,OTUs),多数属于与多糖降解和氨基酸代谢有关的细菌(如Prevotella、Treponema、Bacteroides和Clostridium)[2]。综上可见,肠道微生物在动物的脂肪消化、吸收及沉积过程起着重要的作用,进一步影响肌内脂肪沉积。然而,目前关于肠道菌群调节动物肌内脂肪沉积机制的研究还较少,微生物代谢产物的高通量测定和分析(代谢组学技术)或许为两者互作解释提供了可能。
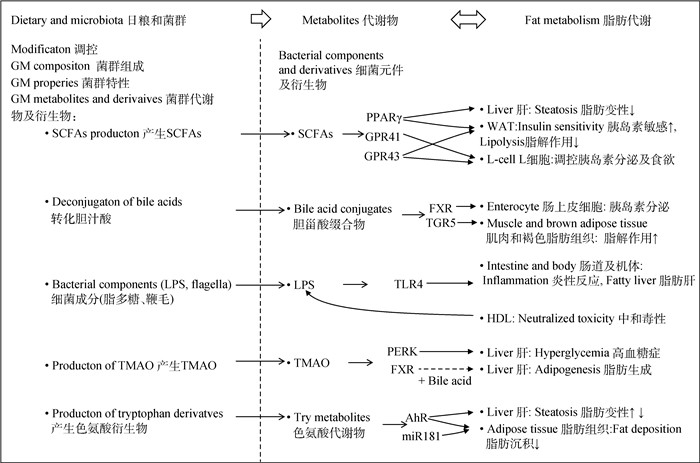
3 微生物功能代谢物与脂肪代谢肠道微生物能够完成许多宿主无法完成的转化过程,这些过程可以生成或调控某些代谢底物或信号分子,对动物的代谢和健康具有重要意义。然而,关于动物肌内脂肪沉积相关的微生物菌群、优势群落基因及功能代谢物的研究报道较少。相关研究主要集中在微生物功能代谢物同脂肪代谢的互作方面,如图 2所示,肠道菌群对宿主脂肪代谢的影响可能通过肠道菌群产生的短链脂肪酸、胆汁酸、脂多糖、三甲胺类、色氨酸及其衍生物等代谢产物介导。
|
GM.肠道菌群;SCFAs:短链脂肪酸;LPS.脂多糖;TMAO.氧化三甲胺;PPARγ.过氧化物酶体增殖物活化受体γ;GPR41/43.G蛋白偶联受体41/43;WAT.白色脂肪组织;FXR.法尼酯衍生物X受体;TGR5. G蛋白偶联胆汁酸受体5;TLR4.toll样受体4;HDL.高密度脂蛋白;PERK.蛋白激酶R样内质网激酶;AhR.芳香族化合物核受体;miR181.小RNA-181 GM.gut microbiota; SCFAs.short-chain fatty acids; LPS.lipopolysaccharide; TMAO.trimethylamine oxide; PPARγ.peroxisome proliferator-activated receptor γ; GPR41/43.G-protein coupled receptors 41/43;WAT.white adipose tissue; FXR.nuclear receptor farnesoid X receptor; TGR5.Takeda-G-protein coupled bile acid receptor 5;TLR4.toll-like receptor 4;HDL.high density lipoprotein; PERK.protein kinase R-like ER kinase; AhR.aryl hydrocarbon receptor; miR181.mircoRNA-181 图 2 肠道微生物及其代谢物同脂肪代谢的关联机制[4, 36-37] Fig. 2 Illustration of the gut microbiota and its metabolites and fat metabolism crosstalk[4, 36-37] |
乙酸、丙酸和丁酸等短链脂肪酸(short-chain fatty acids,SCFAs)是肠道细菌发酵纤维类物质产生的代谢物,一般而言,丁酸和丙酸浓度较低,而乙酸浓度则较高。短链脂肪酸对宿主的新陈代谢具有很重要的作用,可作为能量产生、脂肪生成、糖异生和胆固醇合成的底物[38],还可以充当信号分子影响动物脂肪代谢。研究发现,不同SCFAs对动物脂肪代谢的调控存在差异。副干酪乳杆菌(Lactobacillus paracasei)分泌的L-乳酸通过产生丙二酰辅酶A促进了肠上皮细胞脂肪储存,而大肠杆菌分泌的乙酸激活的AMPK/PGC-1α/PPARα通路可促进脂肪氧化[39];正常初生重和低初生重仔猪呈现出不同的脂肪代谢模式和肠道炎性状态,这或许同肠道菌群差异引起的SCFAs变化有关[40]。SCFAs对动物肌内脂肪沉积影响方面,蒲俊宁等[41]对生长肥育猪研究发现,随着日粮中直链/支链淀粉比的升高,显著提高了盲肠丁酸、结肠丙酸、总挥发性脂肪酸含量及盲肠食糜中双歧杆菌数量,然而,猪背最长肌肌内脂肪含量却显著降低,G蛋白偶联受体43(G protein-coupled receptor 43,GPR43)表达量显著升高,这表明提高饲粮直链/支链淀粉比增加了后肠SCFAs含量并影响动物肌内脂肪沉积。在牦牛中的研究发现,随着日粮能量水平的升高,牦牛瘤胃中乙酸浓度及乙酸/丙酸比显著降低,进而上调了脂肪酸合成基因和脂肪酸转运基因的表达丰度,促进肌内脂肪沉积,并且改善肉品质[42]。
外源直接补充SCFAs也可调控动物脂肪沉积[43]。在小鼠上研究发现,丁酸钠能够增强肌肉细胞线粒体功能并且促进脂肪酸β氧化,进而降低采食高脂日粮小鼠肌肉脂肪异常沉积[44]。肉鸡日粮中添加乙酸盐显著提高了腿肌中饱和脂肪酸含量以及n-6:n-3脂肪酸比例[45],丁酸的饲喂则显著降低了肉鸡腹部脂肪的沉积[46],丁酸甘油酯的应用显著降低了肉鸡血清中胆固醇水平,但对甘油三酯水平无显著影响[47],邱洪仪[48]研究则发现,日粮中添加三丁酸甘油酯和包被丁酸钠降低了脂多糖引起的肉鸡腿肌肌内脂肪过量沉积,改善了在应激状态下的肉品质。而在断奶仔猪日粮中补充乙酸、丙酸和丁酸组成的混合脂肪酸后,显著降低了血清和肝中甘油三酯水平,抑制了脂肪沉积[49]。在影响机体脂肪代谢机制方面,首先,肠道微生物发酵产生的SCFAs可作为动物额外的能量来源,对动物的能量代谢产生影响,进而影响机体脂肪沉积[50]。研究发现,丁酸作为结肠上皮细胞的能量来源,可降低葡萄糖的氧化分解,从而促进机体脂肪分解、减少机体脂肪沉积[51]。Turnbaugh等[52]研究表明,与“瘦菌群”定植的无菌小鼠相比,“肥胖菌群”定植的小鼠机体脂肪沉积增加,且差异菌群功能多涉及淀粉、蔗糖及半乳糖等能量代谢途径,可进一步分解前肠残留的碳水化合物,产生SCFAs,提高宿主从饮食中获取能量的能力。其次,SCFAs还可作为信号分子,在白色脂肪组织(white adipose tissue, WAT)和肠上皮L细胞(L-cell)中通过GPR43和GPR41对机体脂肪代谢进行调控(图 2)。白色脂肪组织中,SCFAs通过GPR43介导降低了脂肪细胞的脂解作用[4],还可防止因高脂饮食引起的小鼠脂肪过度沉积[53];SCFAs可激活肠上皮细胞GRP41/43,促进L细胞分泌胰高血糖素样肽-1(glucagon like peptide-1,GLP-1)和酪酪肽(peptide YY,PYY),影响胰岛素分泌和动物食欲,进而对机体脂肪代谢进行调控[4],研究发现,乙酸及丙酸的摄入会增加血浆中GLP-1和PYY含量,从而降低能量摄入,减少机体脂肪沉积[54-55]。此外,丁酸和丙酸还可激活肝和脂肪组织中过氧化物酶体增殖物活化受体γ(peroxisome proliferator-activated receptor γ,PPARγ)信号通路影响脂肪代谢[56](图 2),使机体能量消耗增加,减少肝中甘油三酯的积累[57]。由上可知,在肠道、肝、脂肪和肌肉等靶器官中,SCFAs可通过GPR43/41和PPARγ等受体途径调控机体脂肪代谢。
3.2 胆汁酸及衍生物与脂肪代谢原发性胆汁酸由胆固醇合成,在肝中可与牛磺酸或甘氨酸结合,正常储存在胆囊中,进食后释放到十二指肠中,有助于脂肪乳化。微生物转化在调节胆汁酸方面也起着重要的作用,可通过脱氢、脱羟基和差向异构化作用增加胆汁酸库的多样性(如胆酸、去氧胆酸、胆石酸和脱氧胆酸等)。机体内通过7α-脱羧作用(7α-dehydroxylation)可生成包括脱氧胆酸和胆石酸等次生胆汁酸,而相关的酶系在几种厚壁菌(Firmicutes)特别是梭状芽胞杆菌(Clostridium scindens)中发现[58]。同时,编码胆汁盐水解酶(bile salt hydrolases,BSHs)的系统发育群包括了厚壁菌门(如乳酸菌、梭状芽胞杆菌)、放线菌门(双歧杆菌)和拟杆菌门的微生物属[59]。此外,肠道中的SCFAs对胆汁酸的代谢也会产生影响,研究发现,菊粉的添加改变了生长肥育猪盲肠中初级胆汁酸和总胆汁酸的含量及组成比例,这同Lactobacillaceae、Lachnospiraceae、Ruminococcaceae等几个科的微生物发酵不同日粮纤维产生不同的SCFAs有关[60]。在抗生素处理菌群缺失的小鼠中,后肠SCFAs特别是丁酸盐含量显著降低,可影响二级胆汁酸池,乃至机体胆汁酸代谢[61]。还有研究表明,色氨酸衍生物吲哚-3-乙酸对宿主胆汁酸代谢也具有调控作用[37]。
胆汁酸除了乳化脂肪外,还可作为信号分子调控动物脂肪代谢。在饲养试验中,胆汁酸作为添加剂可改善动物脂类物质消化吸收、提高动物生长性能并改善胴体品质,但其衍生物对肌内脂肪沉积影响的研究报道较少。肉鸡饲养试验发现,在日粮中添加60和80 mg·kg-1的胆汁酸可有效提高肉鸡肠道脂肪酶和脂蛋白脂酶活性,改善肉鸡生长性能和胴体性状[62]。水产动物上的研究表明,高脂日粮中添加胆汁酸降低了草鱼肝和肌肉脂肪沉积、促进了蛋白质合成[63],同时,欧洲鳗鲡日粮中添加胆汁酸也发现了类似的结果[64]。
胆汁酸及衍生物影响动物脂肪代谢的可能机制方面,如图 2所示,其可作为信号分子结合肠道上皮细胞和肠L细胞的法尼酯衍生物X受体(nuclear receptor farnesoid X receptor,FXR)和G蛋白偶联胆汁酸受体5(Takeda-G-protein coupled bile acid receptor 5,TGR5),通过激活FXR可引起小鼠脂肪变性、脂肪沉积及体重增加[65],而TGR5信号通路的激活可诱导肠内分泌L细胞释放GLP-1,从而改善肥胖小鼠的肝和胰腺功能[66]。微生物的多种转化作用极大地丰富了肠道中的胆汁酸库,并使其同受体的亲和性发生改变[67],进而影响动物脂肪沉积。Li等[68]使用肠道特异性FXR-/-小鼠进行研究发现,FXR信号的抑制与乳酸杆菌(Lactobacillus)和梭菌(Clostridium)的菌活降低以及BSH和7α-脱羟化酶活性的下降有关。而当无菌小鼠肠道内的细菌BSH过表达时,也观察到类似的相关性[69]。然而,在养殖动物方面的报道还较少,尚需进一步研究。
3.3 脂多糖与脂肪代谢脂多糖(lipopolysaccharides,LPS)为革兰阴性菌细胞壁的结构化合物,如图 2所示,LPS可通过激活toll样受体4(toll-like receptor 4,TLR4)诱导炎症反应。TLR4可在肠上皮细胞、肝细胞和脂肪细胞等多种细胞上表达,缺乏TLR4和CD14的小鼠对高胰岛素血症、胰岛素抵抗和高脂饮食及脂多糖引起的脂肪变性具有抗性[70]。在肠道屏障功能受损的情况下可发生LPS移位,从而导致血液中LPS水平升高,引发机体的炎症反应,导致代谢紊乱、血脂异常及胰岛素抵抗等[71]。研究表明,小鼠肝内脂肪变性是由高脂肪饮食引起的,并与菌群失调和肠道通透性增加有关[70]。化学诱导大鼠结肠炎增加了血液中LPS水平,并在高脂肪饮食期间恶化了脂肪性肝炎[72]。研究发现,LPS对动物脂肪代谢影响存在剂量效应,在大鼠中研究表明,低剂量LPS增加了肝中极低密度脂蛋白(very low density lipoprotein,VLDL)合成,而高剂量LPS则降低了脂蛋白分解[73];怀孕大鼠在LPS(0.79 mg·kg-1)作用下,血清和肝总胆固醇、甘油三酯、低密度脂蛋白和胆固醇水平显著增加,同时发现,肝的脂质代谢及相关信号通路基因表达丰度异常[74]。
LPS对养殖动物多为负面影响,能够引起动物发生炎症反应,导致肉鸡平均日增重和日采食量下降[75],还能够引起肉鸡腿肌肌内脂肪过量沉积,导致鸡肉品质发生变化,而添加短链脂肪酸(三丁酸甘油酯和包被丁酸钠)可以缓解其作用[76],在乌鸡中的研究表明,LPS注射2周后,乌鸡生产性能极显著下降,显著降低了胸肌、腿肌脂肪沉积量,且减少了肌间脂肪及皮下脂肪含量[77],这些结果的差异或许同LPS作用持续时间不同有关。反刍动物上的研究发现,长期低剂量LPS灌注引起犊母牛血糖浓度降低和脂肪分解增强[78],利用代谢组学的手段研究发现,LPS处理后奶山羊血清中甘油三酯、非酯化脂肪酸、总胆固醇、高密度脂蛋白和低密度脂蛋白的含量显著降低,并检测到69种代谢物变量,涉及到氨基酸、脂肪和碳水化合物等代谢途径[79]。可见,LPS能够导致机体脂肪代谢发生紊乱,这对动物的健康及畜产品品质都存在不利影响。体外试验也有类似的发现,LPS处理促使奶牛原代肝细胞发生凋亡,而且降低了脂肪酸合成相关基因的mRNA表达丰度,提高了脂肪酸转运相关基因的表达丰度[80]。此外,血浆中的脂蛋白,特别是高密度脂蛋白(high density lipoprotein,HDL)还可中和LPS的毒性作用[81],研究发现,在LPS攻毒之前注入HDL可以减少促炎细胞因子的释放[82],这或许为缓解LPS毒性提供了新的思路。同时,也有研究发现,短链脂肪酸可以通过激活GPR41/43和抑制HDACs通路缓解LPS的毒性作用[83]。
3.4 三甲胺类与脂肪代谢肠道菌群代谢含有甲胺的营养素如胆碱、卵磷脂及左旋肉碱等可产生三甲胺,其在肝中经黄素单加氧酶(flavin monooxygenases,FMO)催化形成氧化三甲胺。黄素单加氧酶3是将三甲胺转化为氧化三甲胺的主要酶,FMO3的下调降低了血浆中氧化三甲胺水平,并影响脂肪和胆固醇代谢[84]。研究发现,氧化三甲胺在脂肪代谢、蛋白质稳定、胰岛素抵抗及糖代谢等过程中发挥重要作用[4]。在人类中的研究表明,氧化三甲胺水平同心血管疾病的发生相关[85-86],可通过抑制胆固醇逆转运和胆汁酸合成引发动脉粥样硬化,还可通过激活内质网蛋白激酶R样内质网激酶(protein kinase R-like ER kinase,PERK)信号通路引发高血糖症[87]。同时,氧化三甲胺还影响着动物脂肪沉积和能量代谢,研究发现,氧化三甲胺能够通过影响胆汁酸介导的FXR信号通路促进肝脂肪生成[88]。Gao等[89]研究也发现,高脂日粮小鼠饲喂氧化三甲胺后胰岛素受体底物2和丝氨酸/苏氨酸蛋白激酶mRNA表达丰度降低。
在养殖动物中,三甲胺类参与脂肪代谢调控方面的研究报道相对较少,但其前体物质如胆碱、卵磷脂及左旋肉碱则已成功应用于养殖中,在调控动物脂肪代谢、改善肉品质[90]、提升动物生产性能及抗氧化等方面取得了较好的效果[12, 91]。Li等[92]研究发现,胆碱可通过抑制脂肪酸从头合成途径降低子宫内发育迟缓猪的肌内脂肪过量沉积,改善肌肉品质。L-肉碱的添加能显著降低肉鸡皮下脂肪含量,但通过降低肉碱棕榈酰转移酶的活性提高胸肌肌内脂肪含量[93]。体外试验研究发现,添加胆碱缓解了非酯化脂肪酸诱导的犊牛肝细胞脂肪沉积症状,并检测到74个差异蛋白(主要参与PPAR通路、RNA转运等代谢通路)、14个差异代谢物(主要参与不饱和脂肪酸合成、花生四烯酸代谢等)[94],这表明,胆碱或许通过上述通路调控牛肝内的脂肪代谢。
3.5 色氨酸及衍生物与脂肪代谢肠道中微生物群能将色氨酸转化成多种代谢物,如吲哚、吲哚乙酸、吲哚醛、吲哚乳酸和吲哚丙烯酸酯[95-96]。已发现的色氨酸转化相关微生物包括产生吲哚的细菌:革兰阴性菌(Bacteroides spp.、Escherichia coli、Desulfovibrio vulgaris、Fusobacterium nucleatum及Vibrio cholerae),革兰阳性菌(Clostridium spp.和Enterococcus faecalis);产生吲哚醛的细菌:Lactobacillus reuteri和L. acidophilus;产生吲哚酯的细菌:Clostridium sporogenes、Peptostreptococcus russellii、P. stomatis及P. anaerobius[96-97]。此外,其他代谢产物也对色氨酸转化产生影响,研究表明,在肠道上皮和免疫细胞中通过吲哚胺2,3-双加氧酶1(indoleamine 2, 3-dioxygenase 1,IDO1)催化产生如犬尿氨酸(kynurenine,Kyn)等色氨酸代谢物[98-99],而丁酸则可诱导IDO1的表达[100]。
色氨酸及衍生的微生物代谢产物能够影响动物脂肪代谢。研究发现,与低脂饮食相比,高脂组小鼠的吲哚乙酸水平降低,肝细胞内细胞因子介导的脂肪生成减弱,这表明,色氨酸微生物代谢物在脂肪代谢中具有潜在的保护作用[101]。外源补充色氨酸可降低大鼠饲粮中氨基酸的分解代谢,促进蛋白质合成和脂肪酸氧化,降低机体脂肪沉积[102]。在养殖动物中,色氨酸作为一种常见的添加剂可以调控动物脂肪代谢、改善动物生产性能和产品品质,蛋鸡中的研究发现,色氨酸能够影响机体脂肪代谢,显著降低蛋鸡肝和腹部脂肪沉积[103],肉鸡中的研究表明,饲粮中添加适量色氨酸提高了肉鸡胸肌比重,降低了腹脂沉积[104]。Jiang等[105]研究发现,适宜的色氨酸水平能够显著提升草鱼肌肉中脂肪、蛋白质和胶原含量,改善草鱼肉质。
调控机制方面,已有研究发现,色氨酸及衍生的微生物代谢产物能够激活肝中芳香族化合物核受体(aryl hydrocarbon receptor,AhR),作为AhR激动剂通过改善肠屏障功能和促进肠激素GLP-1的分泌,进而改善饮食诱导的代谢损伤,特别是葡萄糖代谢异常和肝脂肪变性[95],但是肝中AhR受体过表达反而造成肝脂肪变性,提示代谢物作用可能具有组织特异性和剂量依赖效应[5]。此外,促进采食和脂肪沉积的microRNA-181也是吲哚及其代谢物的靶位点,在小鼠中的研究发现,吲哚及其代谢物硫酸吲哚酚通过AhR和microRNA-181介导了相关信号通路,降低了小鼠脂肪细胞中的脂质积累[106]。在养殖动物中色氨酸及其衍生物是否也是通过AhR及microRNA-181通路调控脂肪代谢还需要进一步验证。
4 小结综上可知,肠道微生物能够影响动物脂肪代谢及肌内脂肪沉积,越来越多的研究表明,微生物的代谢物是其功能的体现者,通过产生功能代谢产物可实现对动物脂肪沉积的调控互作。虽然,目前关于肠道微生物代谢物对宿主脂肪代谢的调控方面已有较多研究,但其深层机制探讨的研究报道却非常有限,尤其是功能代谢物对肌内脂肪影响的相关研究尚处于初级阶段。目前,存在的主要研究难点如下:1)肠道微生物产生的代谢物众多,同脂肪沉积相关的菌群特异代谢物和引起代谢物变化的关键菌群筛选难度较大;2)存在多种功能代谢物的相互影响及协同调控机制;3)已掌握的具有生理作用的功能代谢物尚不全面,多数代谢物或许是未知的或无法获得的。这使得肠道菌群-代谢物与动物脂肪代谢及肌内脂肪沉积间相互调控的机制尚不十分明确,还需要进一步深入研究。
随着代谢组学技术,尤其是多组学联合技术的应用普及,为解析肠道微生物及其功能代谢物提供了强有力手段。同时,借助如代谢物注释和基因整合(metabolite annotation and gene integration,MAGI)工具将基因序列与代谢组学数据进行关联[107],可产生多层次(mRNA转录、蛋白翻译及表观修饰等)的数据集合(datasets),还可应用无菌动物、特异改造的工程细菌(代谢酶敲除或过表达)、人工合成化合物等以进一步探明肠道菌群-功能代谢物对机体脂肪沉积的调控作用机制[37],进而筛选提高肌内脂肪相关的添加剂,通过营养手段调控肠道菌群,为增加肌内脂肪沉积、改善动物肉品质提供新的思路和方法。
| [1] | BLANCHARD P J, WILLIS M B, WARKUP C C, et al. The influence of carcass backfat and intramuscular fat level on pork eating quality[J]. J Sci Food Agric, 2000, 80(1): 145–151. |
| [2] | FANG S M, XIONG X W, SU Y, et al. 16S rRNA gene-based association study identified microbial taxa associated with pork intramuscular fat content in feces and cecum lumen[J]. BMC Microbiol, 2017, 17(1): 162. |
| [3] |
赵旭, 丁晓, 杨在宾, 等. 丁酸梭菌对肉鸡腿肌脂肪代谢的影响[J]. 动物营养学报, 2017, 29(8): 2884–2892.
ZHAO X, DING X, YANG Z B, et al. Effects of Clostridium butyricum on thigh muscle lipid metabolism of broilers[J]. Chinese Journal of Animal Nutrition, 2017, 29(8): 2884–2892. (in Chinese) |
| [4] | SCHOELER M, CAESAR R. Dietary lipids, gut microbiota and lipid metabolism[J]. Rev Endocr Metab Dis, 2019, 20(4): 461–472. |
| [5] | KOH A, BÄCKHED F. From association to causality:the role of the gut microbiota and its functional products on host metabolism[J]. Mol Cell, 2020, 78(4): 584–596. |
| [6] | UCHIMURA Y, FUHRER T, LI H, et al. Antibodies set boundaries limiting microbial metabolite penetration and the resultant mammalian host response[J]. Immunity, 2018, 49(3): 545–559.e5. |
| [7] | BUSNELLI M, MANZINI S, BOUKADIRI A, et al. Microbiota composition affects lipid metabolism and intestinal homeostasis[J]. Nutr Metab Cardiovas Dis, 2017, 27(1): e11. |
| [8] | HAN G, LIAO H R, LIU X X, et al. Study on the fat-related genes of chicken[J]. Int J Biol, 2009, 1(1): 41–44. |
| [9] | ESSÉN-GUSTAVSSON B, KARLSSON A, LUNDSTRÖM K, et al. Intramuscular fat and muscle fibre lipid contents in halothane-gene-free pigs fed high or low protein diets and its relation to meat quality[J]. Meat Sci, 1994, 38(2): 269–277. |
| [10] | TANG Q Q, OTTO T C, LANE M D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage[J]. Proc Natl Acad Sci U S A, 2004, 101(26): 9607–9611. |
| [11] | HAUSMAN G J, BASU U, DU M, et al. Intermuscular and intramuscular adipose tissues:bad vs.good adipose tissue[J]. Adipocyte, 2014, 3(4): 242–255. |
| [12] | LIN Z D, HAN F L, LU J T, et al. Influence of dietary phospholipid on growth performance, body composition, antioxidant capacity and lipid metabolism of Chinese mitten crab, Eriocheir sinensis[J]. Aquaculture, 2020, 516: 734653. |
| [13] | MARTINEZ-GURYN K, HUBERT N, FRAZIER K, et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids[J]. Cell Host Microbe, 2018, 23(4): 458–469. |
| [14] | KINDT A, LIEBISCH G, CLAVEL T, et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice[J]. Nat Commun, 2018, 9(1): 3760. |
| [15] | VELAGAPUDI V R, HEZAVEH R, REIGSTAD C S, et al. The gut microbiota modulates host energy and lipid metabolism in mice[J]. J Lipid Res, 2010, 51(5): 1101–1112. |
| [16] | SHENG Y, REN H, LIMBU S M, et al. The presence or absence of intestinal microbiota affects lipid deposition and related genes expression in zebrafish (Danio rerio)[J]. Front Microbiol, 2018, 9: 1124. |
| [17] | GAO J, DING G Q, LI Q, et al. Tibet kefir milk decreases fat deposition by regulating the gut microbiota and gene expression of Lpl and Angptl4 in high fat diet-fed rats[J]. Food Res Int, 2019, 121: 278–287. |
| [18] | WEN C L, YAN W, SUN C J, et al. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens[J]. ISME J, 2019, 13(6): 1422–1436. |
| [19] | YUAN Z Y, YAN W, WEN C L, et al. Enterotype identification and its influence on regulating the duodenum metabolism in chickens[J]. Poult Sci, 2020, 99(3): 1515–1527. |
| [20] | LIU G Q, BOU G, SU S F, et al. Microbial diversity within the digestive tract contents of Dezhou donkeys[J]. PLoS One, 2019, 14(12): e0226186. |
| [21] | LIANG Y J, LIANG S, ZHANG Y P, et al. Oral administration of compound probiotics ameliorates HFD-induced gut microbe dysbiosis and chronic metabolic inflammation via the G protein-coupled receptor 43 in non-alcoholic fatty liver disease rats[J]. Probiotics Antimicrob Prot, 2019, 11(1): 175–185. |
| [22] | BO T B, WEN J, ZHAO Y C, et al. Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food[J]. J Steroid Biochem Mol Biol, 2020, 198: 105602. |
| [23] | CHEN X H, FU Y, WANG L L, et al. Bifidobacterium longum and VSL#3® amelioration of TNBS-induced colitis associated with reduced HMGB1 and epithelial barrier impairment[J]. Dev Comp Immunol, 2019, 92: 77–86. |
| [24] | JAZI V, ASHAYERIZADEH A, TOGHYANI M, et al. Fermented soybean meal exhibits probiotic properties when included in Japanese quail diet in replacement of soybean meal[J]. Poult Sci, 2018, 97(6): 2113–2122. |
| [25] | SEIFI K, KARIMI TORSHIZI M A, RAHIMI S, et al. Efficiency of early, single-dose probiotic administration methods on performance, small intestinal morphology, blood biochemistry, and immune response of Japanese quail[J]. Poult Sci, 2017, 96(7): 2151–2158. |
| [26] | HUSSEIN E, SELIM S. Efficacy of yeast and multi-strain probiotic alone or in combination on growth performance, carcass traits, blood biochemical constituents, and meat quality of broiler chickens[J]. Livest Sci, 2018, 216: 153–159. |
| [27] | LIU T Y, SU B C, WANG J L, et al. Effects of probiotics on growth, pork quality and serum metabolites in growing-finishing pigs[J]. J Northeast Agric Univ (Engl Ed), 2013, 20(4): 57–63. |
| [28] | SUDIKAS G, KULPYS J, JEREŠIUNAS A, et al. The influence of probiotics on carcass, meat and fat quality in pigs[J]. Vet Med Zoot, 2010, 52(74): 79–86. |
| [29] | LIU Y H, LI Y Y, FENG X C, et al. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks[J]. Poult Sci, 2018, 97(9): 3218–3229. |
| [30] | WANG H S, NI X Q, QING X D, et al. Live probiotic Lactobacillus johnsonu BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers[J]. Front Microbiol, 2017, 8: 1073. |
| [31] | HAO L H, SU W F, ZHANG Y, et al. Effects of supplementing with fermented mixed feed on the performance and meat quality in finishing pigs[J]. Anim Feed Sci Technol, 2020, 266: 114501. |
| [32] | AHMED S T, MUN H S, ISLAM M M, et al. Effects of dietary natural and fermented herb combination on growth performance, carcass traits and meat quality in grower-finisher pigs[J]. Meat Sci, 2016, 122: 7–15. |
| [33] | XUE S W, HU J Y, CHENG H W, et al. Effects of probiotic supplementation and postmortem storage condition on the oxidative stability of M. Pectoralis major of laying hens[J]. Poult Sci, 2019, 98(12): 7158–7169. |
| [34] | HOSSAIN M E, KO S Y, KIM G M, et al. Evaluation of probiotic strains for development of fermented Alisma canaliculatum and their effects on broiler chickens[J]. Poult Sci, 2012, 91(12): 3121–3131. |
| [35] | INATOMI T. Growth performance, gut mucosal immunity and carcass and intramuscular fat of broilers fed diets containing a combination of three probiotics[J]. Sci Postprint, 2015, 1(2): e52. |
| [36] | MOKKALA K, HOUTTU N, CANSEV T, et al. Interactions of dietary fat with the gut microbiota:evaluation of mechanisms and metabolic consequences[J]. Clin Nutr, 2020, 39(4): 994–1018. |
| [37] | CHEN F D, STAPPENBECK T S. Microbiome control of innate reactivity[J]. Curr Opin Immunol, 2019, 56: 107–113. |
| [38] | DEN BESTEN G, LANGE K, HAVINGA R, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids[J]. Am J Physiol Gastrointest Liver Physiol, 2013, 305(12): G900–G910. |
| [39] | ARA U ' JO J R, TAZI A, BURLEN-DEFRANOUX O, et al. Fermentation products of commensal bacteria alter enterocyte lipid metabolism[J]. Cell Host Microbe, 2020, 27(3): 358–375. |
| [40] | HUANG S M, WU Z H, LI T T, et al. Perturbation of the lipid metabolism and intestinal inflammation in growing pigs with low birth weight is associated with the alterations of gut microbiota[J]. Sci Total Environ, 2020, 719: 137382. |
| [41] |
蒲俊宁, 王华杰, 陈代文, 等. 饲粮直链/支链淀粉比对育肥猪生长性能、营养物质表观消化率、肠道食糜菌群数量与挥发性脂肪酸浓度以及肌内脂肪含量的影响[J]. 动物营养学报, 2018, 30(12): 4874–4885.
PU J N, WANG H J, CHEN D W, et al. Effects of dietary amylose/amylopectin ratio on growth performance, nutrient apparent dgestibility, intestinal microflora number and volatile fatty acid concentrations and intramuscular fat content of finishing pigs[J]. Chinese Journal of Animal Nutrition, 2018, 30(12): 4874–4885. (in Chinese) |
| [42] | KANG K, MA J, WANG H Z, et al. High-energy diet improves growth performance, meat quality and gene expression related to intramuscular fat deposition in finishing yaks raised by barn feeding[J]. Vet Med Sci, 2020. DOI: 10.1002/vms3.306 |
| [43] | NICOLUCCI A C, HUME M P, MARTÍNEZ I, et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity[J]. Gastroenterology, 2017, 153(3): 711–722. |
| [44] | HONG J, JIA Y M, PAN S F, et al. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice[J]. Oncotarget, 2016, 7(35): 56071–56082. |
| [45] | OMIDI M, KHOSRAVINIA H, MASOURI B. Independent and combined effects of Satureja khuzistanica essential oils and dietary acetic acid on fatty acid profile in thigh meat in male broiler chicken[J]. Poult Sci, 2020, 99(4): 2266–2274. |
| [46] | AGHAZADEH A M, TAHAYAZDI M. Effect of butyric acid supplementation and whole wheat inclusion on the performance and carcass traits of broilers[J]. South Afr J Anim Sci, 2011, 42(3): 241–248. |
| [47] | POURAZIZ S, AGHDAM SHAHRYAR H, CHEKANI-AZAR S. Effects of dietary Saccharomyces cerevisiae and butyric acid glycerides on performance and serum lipid level of broiler chickens[J]. Kafkas Univ Vet Fak Derg, 2013, 19(5): 903–907. |
| [48] |
邱洪仪.三丁酸甘油酯和包被丁酸钠对慢性应激肉鸡肠道形态结构、二糖酶活性及脂质代谢的影响[D].武汉: 武汉轻工大学, 2013.
QIU H Y.The effect of tributyrin and sodium butyrate on intestinal morphological structure, mucosa disaccharidase activity and lipid metabolism in broilers challenged with lipopolysaccharide[D].Wuhan: Wuhan Polytechnic University, 2013.(in Chinese) |
| [49] | JIAO A R, DIAO H, YU B, et al. Oral administration of short chain fatty acids could attenuate fat deposition of pigs[J]. PLoS One, 2018, 13(5): e0196867. |
| [50] | LEY R E, TURNBAUGH P J, KLEIN S, et al. Human gut microbes associated with obesity[J]. Nature, 2006, 444(7122): 1022–1023. |
| [51] | HE B, MOREAU R. Lipid-regulating properties of butyric acid and 4-phenylbutyric acid:Molecular mechanisms and therapeutic applications[J]. Pharmacol Res, 2019, 144: 116–131. |
| [52] | TURNBAUGH P J, LEY R E, MAHOWALD M A, et al. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature, 2006, 444(7122): 1027–1031. |
| [53] | MCNELIS J C, LEE Y S, MAYORAL R, et al. GPR43 potentiates β-cell function in obesity[J]. Diabetes, 2015, 64(9): 3203–3217. |
| [54] | CHAMBERS E S, VIARDOT A, PSICHAS A, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults[J]. Gut, 2015, 64(11): 1744–1754. |
| [55] | FREELAND K R, WOLEVER T M S. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α[J]. Br J Nutr, 2010, 103(3): 460–466. |
| [56] | ALEX S, LANGE K, AMOLO T, et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ[J]. Mol Cell Biol, 2013, 33(7): 1303–1316. |
| [57] | DEN BESTEN G, BLEEKER A, GERDING A, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation[J]. Diabetes, 2015, 64(7): 2398–2408. |
| [58] | RIDLON J M, HYLEMON P B. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7α-dehydroxylating intestinal bacterium[J]. J Lipid Res, 2012, 53(1): 66–76. |
| [59] | JONES B V, BEGLEY M, HILL C, et al. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome[J]. Proc Natl Acad Sci U S A, 2008, 105(36): 13580–13585. |
| [60] | WU W D, ZHANG L, XIA B, et al. Bioregional alterations in gut microbiome contribute to the plasma metabolomic changes in pigs fed with inulin[J]. Microorganisms, 2020, 8(1): 111. |
| [61] | ZARRINPAR A, CHAIX A, XU Z Z, et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism[J]. Nat Commun, 2018, 9: 2872. |
| [62] | LAI W Q, HUANG W G, DONG B, et al. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens[J]. Poult Sci, 2018, 97(1): 196–202. |
| [63] | ZHOU J S, CHEN H J, JI H, et al. Effect of dietary bile acids on growth, body composition, lipid metabolism and microbiota in grass carp (Ctenopharyngodon idella)[J]. Aquacult Nutr, 2018, 24(2): 802–813. |
| [64] |
赵盼月.饲料中添加胆汁酸对欧洲鳗鲡幼鱼生长、血清生化指标、肠道菌群及肝脏代谢的影响[D].厦门: 集美大学, 2019.
ZHAO P Y.Effects of dietary bile acid supplementation on growth performance, serum biochemical parameters, intestinal flora and liver metabolism of Juvenile European Eel (Anguilla anguilla)[D].Xiamen: Jimei University, 2019.(in Chinese) |
| [65] | PARSÉUS A, SOMMER N, SOMMER F, et al. Microbiota-induced obesity requires farnesoid X receptor[J]. Gut, 2017, 66(3): 429–437. |
| [66] | THOMAS C, GIOIELLO A, NORIEGA L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis[J]. Cell Metab, 2009, 10(3): 167–177. |
| [67] | WAHLSTR M A, SAYIN S I, MARSCHALL H U, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism[J]. Cell Metab, 2016, 24(1): 41–50. |
| [68] | LI F, JIANG C T, KRAUSZ K W, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity[J]. Nat Commun, 2013, 4: 2384. |
| [69] | JOYCE S A, MACSHARRY J, CASEY P G, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut[J]. Proc Natl Acad Sci U S A, 2014, 111(20): 7421–7426. |
| [70] | CANI P D, AMAR J, IGLESIAS M A, et al. Metabolic endotoxemia initiates obesity and insulin resistance[J]. Diabetes, 2007, 56(7): 1761–1772. |
| [71] | MANCO M, PUTIGNANI L, BOTTAZZO G F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk[J]. Endocr Rev, 2010, 31(6): 817–844. |
| [72] | MAO J W, TANG H Y, ZHAO T, et al. Intestinal mucosal barrier dysfunction participates in the progress of nonalcoholic fatty liver disease[J]. Int J Clin Exp Pathol, 2015, 8(4): 3648–3658. |
| [73] | READ T E, HARRIS H W, GRUNFELD C, et al. The protective effect of serum lipoproteins against bacterial lipopolysaccharide[J]. Eur Heart J, 1993, 14(Suppl K): 125–129. |
| [74] | YU S Y, WEN Y, LI J M, et al. Prenatal lipopolysaccharide exposure promotes dyslipidemia in the male offspring rats[J]. Front Physiol, 2018, 9: 542. |
| [75] | YANG L, LIU G, ZHU X Q, et al. The anti-inflammatory and antioxidant effects of leonurine hydrochloride after lipopolysaccharide challenge in broiler chicks[J]. Poult Sci, 2019, 98(4): 1648–1657. |
| [76] | XIONG J, QIU H, BI Y, et al. Effects of dietary supplementation with tributyrin and coated sodium butyrate on intestinal morphology, disaccharidase activity and intramuscular fat of lipopolysaccharide-challenged broilers[J]. Braz J Poultry Sci, 2018, 20(4): 707–716. |
| [77] |
齐仁立, 解芸菲, 孙婵, 等. 大肠埃希菌LPS感染对乌鸡脂肪代谢及相关调控基因表达的影响[J]. 西北农林科技大学学报:自然科学版, 2011, 39(8): 1–8.
QI R L, XIE Y F, SUN C, et al. Effects of the inflammation mediated by Escherichia coli LPS on the lipid metabolism and the expression of genes of black-bone chicken[J]. Journal of Northwest A & F University:Natural Science Edition, 2011, 39(8): 1–8. (in Chinese) |
| [78] | STEIGER M, SENN M, ALTREUTHER G, et al. Effect of a prolonged low-dose lipopolysaccharide infusion on feed intake and metabolism in heifers[J]. J Anim Sci, 1999, 77(9): 2523–2532. |
| [79] | WANG L F, JIA S D, YANG G Q, et al. Study on the effect of lipopolysaccharide on hepatic metabolism in dairy goat liver[J]. Scientia Agricultura Sinica, 2015, 48(18): 3701–3710. |
| [80] | WANG Y Y, LI H P, WANG X J, et al. Attenuated mRNA expression of lipid metabolism genes in primary hepatocytes following lipopolysaccharide treatment in dairy cows[J]. Genet Mol Res, 2015, 14(2): 3718–3728. |
| [81] | EICHBAUM E B, HARRIS H W, KANE J P, et al. Chylomicrons can inhibit endotoxin activity in vitro[J]. J Surg Res, 1991, 51(5): 413–416. |
| [82] | PAJKRT D, DORAN J E, KOSTER F, et al. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia[J]. J Exp Med, 1996, 184(5): 1601–1608. |
| [83] | MENG L, VAN ESCH B C A M, HENRICKS P A J, et al. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs[J]. Front Pharmacol, 2018, 9: 533. |
| [84] | SHIH D M, WANG Z N, LEE R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis[J]. J Lipid Res, 2015, 56(1): 22–37. |
| [85] | TANG W H W, WANG Z N, LEVISON B S, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk[J]. N Engl J Med, 2013, 368(17): 1575–1584. |
| [86] | WANG Z N, TANG W H W, BUFFA J A, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide[J]. Eur Heart J, 2014, 35(14): 904–910. |
| [87] | CHEN S F, HENDERSON A, PETRIELLO M C, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction[J]. Cell Metab, 2019, 30(6): 1141–1151.e5. |
| [88] | TAN X Y, LIU Y, LONG J A, et al. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease[J]. Mol Nutr Food Res, 2019, 63(17): e1900257. |
| [89] | GAO X, LIU X F, XU J, et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet[J]. J Biosci Bioeng, 2014, 118(4): 476–481. |
| [90] | MENG Q W, SUN S S, SUN Y C, et al. Effects of dietary lecithin and L-carnitine on fatty acid composition and lipid-metabolic genes expression in subcutaneous fat and longissimus thoracis of growing-finishing pigs[J]. Meat Sci, 2018, 136: 68–78. |
| [91] | BOLLATTI J M, ZENOBI M G, ARTUSSO N A, et al. Timing of initiation and duration of feeding rumen-protected choline affects performance of lactating Holstein cows[J]. J Dairy Sci, 2020, 103(5): 4174–4191. |
| [92] | LI W, LI B, LV J Q, et al. Choline supplementation improves the lipid metabolism of intrauterine-growth-restricted pigs[J]. Asian Australas J Anim Sci, 2018, 31(5): 686–695. |
| [93] | XU Z R, WANG M Q, MAO H X, et al. Effects of L-carnitine on growth performance, carcass composition, and metabolism of lipids in male broilers[J]. Poult Sci, 2003, 82(3): 408–413. |
| [94] |
王艳辉.胆碱对奶牛肝细胞脂质沉积中蛋白谱和代谢谱的影响[D].大庆: 黑龙江八一农垦大学, 2017.
WANG Y H.Proteomics and metabolomics research on the hepatic lipidosis of dairy cows with choline[D].Daqing: Heilongjiang Bayi Agricultural University, 2017.(in Chinese) |
| [95] | ZELANTE T, IANNITTI R G, CUNHA C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22[J]. Immunity, 2013, 39(2): 372–385. |
| [96] | WLODARSKA M, LUO C W, KOLDE R, et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation[J]. Cell Host Microbe, 2017, 22(1): 25–37.e6. |
| [97] | DODD D, SPITZER M H, VAN TREUREN W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites[J]. Nature, 2017, 551(7682): 648–652. |
| [98] | LAMAS B, RICHARD M L, LEDUCQ V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands[J]. Nat Med, 2016, 22(6): 598–605. |
| [99] | NIKOLAUS S, SCHULTE B, AL-MASSAD N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases[J]. Gastroenterology, 2017, 153(6): 1504–1516.e2. |
| [100] | GURAV A, SIVAPRAKASAM S, BHUTIA Y D, et al. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions[J]. Biochem J, 2015, 469(2): 267–278. |
| [101] | KRISHNAN S, DING Y F, SAEDI N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages[J]. Cell Rep, 2018, 23(4): 1099–1111. |
| [102] | RUAN Z, YANG Y H, WEN Y M, et al. Metabolomic analysis of amino acid and fat metabolism in rats with L-tryptophan supplementation[J]. Amino Acids, 2014, 46(12): 2681–2691. |
| [103] |
周斌.日粮色氨酸水平对蛋鸡生产性能与蛋品质的影响及其机理探讨[D].杭州: 浙江大学, 2010.
ZHOU B.Research on the effects of dietary Tryptophan levels on the production performance and egg quality in laying hens and approach to the mechanism[D].Hangzhou: Zhejiang University, 2010.(in Chinese) |
| [104] | WANG B, MIN Z Z, YUAN J M. Apparent ileal digestible tryptophan requirements of 22- to 42-day-old broiler chicks[J]. J Appl Poult Res, 2016, 25(1): 54–61. |
| [105] | JIANG W D, WEN H L, LIU Y, et al. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella):avoid tryptophan deficiency or excess[J]. Food Chem, 2016, 199: 210–219. |
| [106] | VIRTUE A T, MCCRIGHT S J, WRIGHT J M, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs[J]. Sci Transl Med, 2019, 11(496): eaav1892. |
| [107] | ERBILGIN O, RVBEL O, LOUIE K B, et al. MAGI:a method for metabolite annotation and gene integration[J]. ACS Chem Biol, 2019, 14(4): 704–714. |



