金黄色葡萄球菌(Staphylococcus aureus, S. aureus)是奶牛乳腺炎的主要病原菌,此外,还是世界第三大食源性致病菌,对畜牧养殖业和人类公共卫生健康构成巨大威胁[1-2]。奶牛乳腺炎导致奶牛的产奶量和乳品质量大幅降低,是制约奶牛养殖业绿色发展的一个最主要问题,对奶牛养殖业造成巨大的经济损失。临床症状通常表现为乳房红肿、硬结、乳汁水样变性甚至血乳等,严重影响奶产量与牛奶质量,持续性感染甚至可使乳房“报废”。其传播途径多种多样,不仅可以在牛群间水平传播,也可以通过挤奶设备和器具、挤奶工人等传播[3]。
目前,对奶牛乳腺炎的治疗主要以抗生素为主。但临床上长期对抗生素的大量使用导致的细菌耐药性现象日益严重,多重耐药性S. aureus较为常见,这给牛乳腺炎的防治带来了极大困难[4]。生物被膜是细菌产生的可以把细菌细胞包裹起来的网状聚合基质,细菌首先吸附在物体表面,然后经聚集、成熟和剥离形成三维结构的聚合物,生物被膜的产生大大增强了S. aureus对外界不良环境的抵抗力,对其在牛群间的传播和持续性感染发挥重要作用[5]。S. aureus形成生物被膜后,可极大增强其对环境消毒剂、宿主免疫系统和抗菌药物的耐受性[6-7],使得奶牛乳腺炎的防控难度增加。
此外,S. aureus是食源性疾病的常见致病菌,其分泌的葡萄球菌肠毒素进入食物后能引起食物中毒。葡萄球菌肠毒素对热有较高耐受性,烹饪通常不能使其失活,即使巴氏消毒过程能杀灭该病原菌,但其肠毒素生理活性仍然存在,是引起食源性中毒的主要原因[8]。agr基因座在调控S. aureus毒力因子的表达过程中具有重要作用,根据自诱导肽氨基酸序列多态性及其受体的不同,目前,已知的有4种agr类型(Ⅰ~Ⅳ)[9]。agr分型是临床S. aureus流行病学调查的重要分析工具。作者在本文中对336株奶牛乳腺炎性S. aureus的生物被膜、耐药性、毒素基因和agr基因型进行了研究,并对检测的毒素基因和agr基因型之间的关系进行分析,旨在为更好地治疗和预防奶牛乳腺炎提供科学依据。
1 材料与方法 1.1 试验菌株本试验336株S. aureus于2016年5月—2019年4月从甘肃、江苏、黑龙江和山西4个省的20个奶牛养殖场采集的乳腺炎奶样中分离获得,保存于中国农业科学院兰州畜牧与兽药研究所奶牛疾病研究创新团队。
1.2 主要试剂与器材TSB肉汤培养基、试验所用药敏纸片和MHA琼脂基础均购自Oxoid公司,Multiskan GO全波长酶标仪购自ThermoFisher SCIENCE公司,无水葡萄糖购自国药集团化学试剂有限公司,结晶紫染色液购自Solarbio公司,96孔平底细胞培养板购自Costar公司,隔水式恒温培养箱购自上海一恒科学仪器有限公司,Bacterial DNA Kit购自Omega公司,SEDI PCR仪为威泰克公司产品。
1.3 S. aureus生物被膜形成能力的检测用96孔微量滴定板法定量检测336株S. aureus生物被膜的产生情况[10]。取-80 ℃低温保存的S. aureus冻存菌液,吸取20 μL接入2 mL TSB肉汤培养管中进行复苏,置37 ℃恒温培养箱中静置培养18 h+30 min。孵育:将培养后的菌液与含1%葡萄糖的无菌TSB按1:100的比例加入到96孔微量滴定板内,每孔终体积为200 μL,每株菌设置3个平行组,另设置6个孔作为阴性对照,阴性对照孔仅含200 μL的1%葡萄糖的无菌TSB肉汤,将96孔细胞培养板在35~37 ℃培养箱内培养24 h+30 min。洗脱:弃去孔内容物,用300 μL PBS(pH 7.2~7.4)洗3次,轻拍96孔细胞培养板,室温下干燥。固定:加入150 μL分析纯甲醇,室温放置20 min,弃去甲醇,轻拍以干燥微量滴定板。染色:加入150 μL结晶紫染液,室温放置15 min。弃去染液,用自来水轻轻洗去孔内残留的染液,室温下干燥。然后每孔轻轻加入150 μL 95%的乙醇,室温下,静置30 min。测量:在570 nm波长处,检测样品的OD值。
取每株受试菌的平均吸光度OD570 nm值作为每株菌生物被膜的吸光度值OD,计算6个阴性对照孔的平均吸光度值ODc。当OD≤ODc时,无生物被膜产生(-);ODc<OD≤2 ODc,形成弱的生物被膜(+);2 ODc<OD≤4 ODc,生物被膜形成能力中等(++);4 ODc<OD,形成强生物被膜(+++)。
1.4 药物敏感性检测采用药敏纸片法对336株S. aureus进行药物敏感性检测,本研究所用14种抗生素药敏纸片:青霉素、头孢西丁、庆大霉素、卡那霉素、红霉素、四环素、环丙沙星、呋喃妥因、克林霉素、甲氧苄氨嘧啶、氯霉素、利福平、喹奴普丁/达福普汀和利奈唑胺。各药敏纸片含量及相应的耐药折点见表 2,根据2013版CLSI判读标准[11],将所有受试S. aureus分为敏感S(敏感)、I(中度敏感)或R(耐药)。
1.5 生物被膜与黏附素基因检测取1.5 mL TSB过夜培养的S. aureus菌悬液,根据Bacterial DNA Kit操作说明提取细菌基因组DNA作为PCR反应的模板。25 μL的PCR反应体系包括12.5 μL Premix TaqTM,正、反向引物各1 μL,9.5 μL 16S rRNA-free水,1 μL模板DNA。PCR扩增条件为94 ℃预变性5 min;94 ℃变性30 s,55 ℃(icaA和icaD)、60 ℃(clfA和bap)、52 ℃(fnbA)和54 ℃(cna)退火30 s,72 ℃延伸45 s (icaA和icaD),1 min(clfA和bap)和15 s(fnbA和cna);72 ℃终延伸10 min。取10 μL的PCR反应产物在10 g·L-1琼脂糖凝胶上电泳,鉴定目的基因。生物被膜与黏附素基因引物如表 1所示。
|
|
表 1 试验中用到的引物序列与退火温度 Table 1 Primer sequences and annealing temperature used in the present study |
5个经典葡萄球菌肠毒素基因(sea、seb、sec、sed和see)和中毒性休克综合征毒素基因(tst)引物见表 1。分别用两套多重PCR法对肠毒素基因进行检测。多重PCR反应条件为94 ℃预变性4 min;94 ℃变性30 s;55 ℃(sea、seb、sec)、60 ℃(sed和see)退火30 s,72 ℃延伸30 s,25个循环;最后,72 ℃延伸7 min。tst基因的检测与黏附素基因的扩增条件相似。
1.7 agr分型用多重PCR法对336株S. aureus进行agr分型,根据特异性agr等位基因引物(表 2),将所有S. aureus分为agr Ⅰ、agr Ⅱ、agr Ⅲ和agr Ⅳ型[12]。
|
|
表 2 奶牛乳腺炎性S. aureus药敏试验结果 Table 2 Antimicrobial susceptibility testing of S. aureus from bovine mastitis |
结晶紫定量检测结果显示,分离自牛乳腺炎的336株S.aureus均能产生生物被膜。其中, 生物被膜形成能力中等的S. aureus有175株(++),占52.1%,黏附能力较强,生物被膜形成能力强的S. aureus有161株(+++),占47.9%,黏附能力极强。
2.2 S. aureus的药敏试验336株S. aureus对青霉素表现出高度耐药,耐药率达91.7%,其次是红霉素、卡那霉素、克林霉素和庆大霉素,耐药率分别为89.6%、72.9%、66.7%和60.4%,所有S. aureus菌株对呋喃妥因和利奈唑胺表现为敏感,详见表 2。
2.3 生物被膜表型与相关基因及agr基因型分布试验结果显示,所有S. aureus均至少携带1种生物被膜或者黏附素基因。其中,检出率最高的是fnbA基因,检出率达99.7%,其次, 是icaD、icaA、clfA和cna,检出率分别为98.2%、89.6%、86.0%和56.0%,bap基因检出率最低,仅为14.6%。agr分型与生物被膜基因检测结果显示,奶牛乳腺炎性 S. aureus主要为agr Ⅰ型,占66.5%~91.8%,其次是agr Ⅱ型和agr Ⅲ型,agr Ⅳ型流行率较低,详见表 3。
|
|
表 3 金黄色葡萄球菌生物被膜表型与相关基因及agr基因型分布 Table 3 Distribution of biofilm phenotype, related genes and agr genotype of S. aureus strains |
在本研究检测的5个经典肠毒素基因中,sea的检出率最高(26.5%,89/336),seb与sec的检出率分别为8.3%(28/336)和6.8%(23/336),未检测到sed和see基因,毒素基因tst的检出率占8.3%(28/336)。S. aureus分型结果显示,agr Ⅰ、agr Ⅱ、agr Ⅲ和agr Ⅳ型S. aureus的流行率分别为77.1%、14.0%、4.8%和2.1%。agr基因型与其携带毒素基因种类的关系如图 1所示,agr Ⅰ型S. aureus携带的毒素基因最多,agr Ⅱ型S. aureus携带2种毒素基因,agr Ⅲ只携带sea基因,而agr Ⅳ型S. aureus无毒素基因携带潜力。
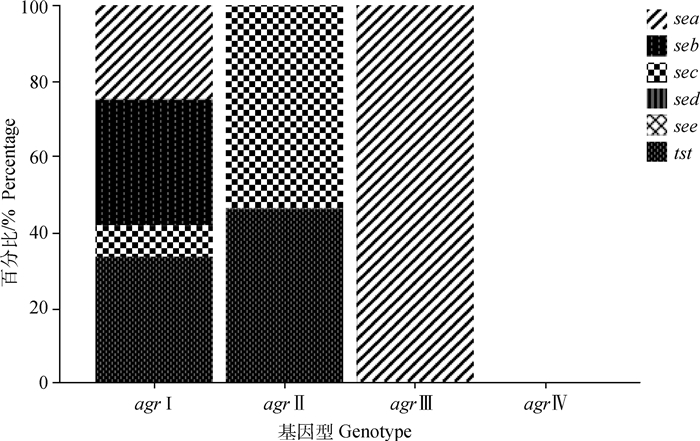
|
图 1 牛源S.aureus毒素基因与agr基因型的关系 Fig. 1 Relationship between virulence gene and agr genotype in S.aureus isolates from cows |
在世界奶牛养殖业中,S. aureus是最常见的传染性牛乳腺炎致病菌之一,不同地区的乳源性S.aureus流行率不同。目前,S. aureus对奶牛乳腺的致病机制尚不完全清楚,对由S. aureus引起的慢性或复发性牛乳腺炎缺乏有效的治疗方法,这与S. aureus自身复杂的毒力机制有关。生物被膜的形成是细菌的一种重要的自我保护毒力机制[13]。细菌形成生物膜后,通过减缓细菌的生长,降低细菌的新陈代谢及抗生素对生物膜的渗透,能有效逃避免疫系统的识别和抗生素的清除[14]。
生物被膜形成的初始阶段主要与ica操纵子调控的胞间黏附多糖有关,其中, icaA与icaD基因在生物被膜的形成的过程中发挥重要作用[15]。本研究中,336株牛源S. aureus均能形成生物被膜,其中形成+++和++生物被膜的菌株分别占47.9%和52.1%,与Yang等[16]和Felipe等[17]的报道基本一致。试验结果显示,icaD与icaA基因的检出率分别为98.2%和89.6%,而Khoramrooz等[18]对80株奶牛乳腺炎性S. aureus的研究发现,icaD与icaA的检出率分别为87.5%和56.3%,与本研究结果存在一定差异,这可能与S. aureus来源及试验样本量的大小不同有关。除胞间黏附多糖外,纤连蛋白结合蛋白(FnbA)、纤维蛋白原结合蛋白(ClfA)、胶原结合蛋白(Can)和生物膜相关蛋白(Bap)等多种黏附分子也介导了细菌对宿主细胞的黏附。PCR检测结果显示,fnbA的检出率达99.7%,Pereyra等[19]研究指出,fnbA基因的流行率分别为100.0%,与本研究结果一致,说明fnbA基因在介导S. aureus对乳腺上皮细胞的黏附过程中发挥重要作用。clfA在牛乳腺炎性S. aureus中的检出率区域差异较大(30.4%~100.0%)。本研究发现,牛源clfA的检出率为86.0%,与伍晔晖等[20]的报道一致,同时,can和bap基因的检出率分别为56.0%,14.6%,试验未检测到bhp基因。
药敏试验结果显示,所有试验菌株对青霉素的耐药性非常严重,耐药率达91.7%,对红霉素(89.6%)、克林霉素(66.7%)和庆大霉素(60.4%)也表现出较高水平的耐药,所有S. aureus菌株对利奈唑胺表现为敏感,这与孟丹等[21]研究结果一致,而与Shi等[22]研究有较大差异,表明同种菌株在不同地区对同一种药物的耐药性不同,这可能与不同地区临床给药方式和给药频率有关。提示在临床治疗时,应先进行药物敏感性检测,以选用合适的药物进行治疗,提高治疗效果。
目前,关于奶牛源S. aureus的agr分子分型的研究国内鲜有报道。试验结果显示,我国奶牛源S. aureus的agr分子类型呈多样性分布,但以agr Ⅰ型(77.1%)为主,这与Khoramrooz等[18]研究结果一致,而王琼等[23]报道指出,agr Ⅱ型S. aureus的流行性最高,分析原因可能与S. aureus菌株来源不同有关。据报道,agr Ⅳ型S. aureus的流行率较低,一般为0.4%~3.7%,通常情况下检测不出agr Ⅳ型S. aureus,本研究中,agr Ⅳ型的检出率仅为2.1%,与前人的研究结果相符[24]。此外,agr Ⅱ与agr Ⅲ型S. aureus的检出率分别占14.0%和4.8%,比Fabres-Klein等[24]的结果低,可能是因为地域不同所致。本研究检测的葡萄球菌肠毒素基因中,sea的检出率为26.5%,其次是seb与sec,检出率分别为8.3%和6.8%,进一步分析发现,与其他agr基因型的S. aureus相比,agr Ⅰ型S. aureus更具有携带多种毒素基因的潜力,agr Ⅳ型S. aureus无毒素基因携带潜力,而Zhang等[25]指出,agr Ⅱ型S. aureus携带的毒素基因数量较多,其次是agr Ⅰ型S. aureus,分析原因可能与样本来源不同有关。本研究中,毒素基因tst的检出率为8.3%,低于孟丹等[21]的研究结果,表明我国不同地区牛源S. aureus的致病潜力有一定差异。因此,开展奶牛乳腺炎S. aureus毒素基因、分子分型和耐药性研究对提高临床治疗效果、减少耐药菌株的产生和加强食源性疾病的监测及保障公共卫生安全具有重要的意义。
4 结论奶牛乳腺炎性S. aureus具有较强的生物被膜形成能力,对常见的抗菌药物耐药严重且毒素基因分布多样,agr Ⅰ型是奶牛乳腺炎性S. aureus主要的基因型且具有携带多种毒素基因的能力,其潜在威胁应引起重视。
| [1] | COELHO S M O, PEREIRA I A, SOARES L C, et al. Short communication: profile of virulence factors of Staphylococcus aureus isolated from subclinical bovine mastitis in the state of Rio de Janeiro, Brazil[J]. J Dairy Sci, 2011, 94(7): 3305–3310. DOI: 10.3168/jds.2010-3229 |
| [2] | HENNEKINNE J A, DE BUYSER M L, DRAGACCI S. Staphylococcus aureus and its food poisoning toxins:characterization and outbreak investigation[J]. FEMS Microbiol Rev, 2012, 36(4): 815–836. DOI: 10.1111/j.1574-6976.2011.00311.x |
| [3] | DE VLIEGHER S, FOX L K, PIEPERS S, et al. Invited review: mastitis in dairy heifers: nature of the disease, potential impact, prevention, and control[J]. J Dairy Sci, 2012, 95(3): 1025–1040. DOI: 10.3168/jds.2010-4074 |
| [4] |
张行, 杨峰, 李新圃, 等. 牛源耐甲氧西林金黄色葡萄球菌耐药性与外排泵相关基因的检测及相关性研究[J]. 畜牧兽医学报, 2019, 50(11): 2326–2332.
ZHANG H, YANG F, LI X P, et al. Study on relationship between antimicrobial resistance and efflux pump related genes of methicillin-resistant Staphylococcus aureus in dairy cow[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(11): 2326–2332. (in Chinese) |
| [5] | COSTERTON J W, STEWART P S, GREENBERG E P. Bacterial biofilms:a common cause of persistent infections[J]. Science, 1999, 284(5418): 1318–1322. DOI: 10.1126/science.284.5418.1318 |
| [6] | SONG M H, LI Q Q, ZHANG Y, et al. Biofilm formation and antibiotic resistance pattern of dominant Staphylococcus aureus clonal lineages in China[J]. J Food Safety, 2017, 37(2): e12304. DOI: 10.1111/jfs.12304 |
| [7] | THIRAN E, DI CICCIO P A, GRABER H U, et al. Biofilm formation of Staphylococcus aureus dairy isolates representing different genotypes[J]. J Dairy Sci, 2018, 101(2): 1000–1012. DOI: 10.3168/jds.2017-13696 |
| [8] | ARGUDÍN M Á, MENDOZA M C, RODICIO M R. Food poisoning and Staphylococcus aureus enterotoxins[J]. Toxins, 2010, 2(7): 1751–1773. DOI: 10.3390/toxins2071751 |
| [9] | JARRAUD S, MOUGEL C, THIOULOUSE J, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease[J]. Infect Immun, 2002, 70(2): 631–641. |
| [10] | STEPANOVIĆ S, VUKOVIĆ D, HOLA V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci[J]. APMIS, 2007, 115(8): 891–899. DOI: 10.1111/j.1600-0463.2007.apm_630.x |
| [11] | Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing. Twenty-third informational supplement[S]. Wayne, PA: CLSI, 2013. |
| [12] | SHOPSIN B, MATHEMA B, ALCABES P, et al. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians[J]. J Clin Microbiol, 2003, 41(1): 456–459. |
| [13] | MELCHIOR M B, FINK-GREMMELS J, GAASTRA W. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture[J]. J Vet Med B Infect Dis Vet Public Health, 2006, 53(7): 326–332. DOI: 10.1111/j.1439-0450.2006.00962.x |
| [14] | STEWART P S, COSTERTON J W. Antibiotic resistance of bacteria in biofilms[J]. Lancet, 2001, 358(9276): 135–138. DOI: 10.1016/S0140-6736(01)05321-1 |
| [15] | VASUDEVAN P, NAIR M K M, ANNAMALAI T, et al. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation[J]. Vet Microbiol, 2003, 92(1-2): 179–185. DOI: 10.1016/S0378-1135(02)00360-7 |
| [16] | YANG F, LIU L H, WANG L, et al. Penicillin-resistant characterization of Staphylococcus aureus isolated from bovine mastitis in Gansu, China[J]. J Integr Agr, 2017, 16(8): 1874–1878. DOI: 10.1016/S2095-3119(16)61531-9 |
| [17] | FELIPE V, MORGANTE C A, SOMALE P S, et al. Evaluation of the biofilm forming ability and its associated genes in Staphylococcus species isolates from bovine mastitis in Argentinean dairy farms[J]. Microb Pathog, 2017, 104: 278–286. DOI: 10.1016/j.micpath.2017.01.047 |
| [18] | KHORAMROOZ S S, MANSOURI F, MARASHIFARD M, et al. Detection of biofilm related genes, classical enterotoxin genes and agr typing among Staphylococcus aureus isolated from bovine with subclinical mastitis in southwest of Iran[J]. Microb Pathog, 2016, 97: 45–51. DOI: 10.1016/j.micpath.2016.05.022 |
| [19] | PEREYRA E A L, PICECH F, RENNA M S, et al. Detection of Staphylococcus aureus adhesion and biofilm-producing genes and their expression during internalization in bovine mammary epithelial cells[J]. Vet Microbiol, 2016, 183: 69–77. DOI: 10.1016/j.vetmic.2015.12.002 |
| [20] |
伍晔晖, 孟庆玲, 乔军, 等. 奶牛源金黄色葡萄球菌新疆流行株生物被膜形成及相关基因的分布与转录水平[J]. 南方农业学报, 2019, 50(8): 1829–1835.
WU Y H, MENG Q L, QIAO J, et al. Analysis of biofilm formation, related genes distribution and transcription level in Xinjiang isolates of Staphylococcus aureus from cows[J]. Journal of Southern Agriculture, 2019, 50(8): 1829–1835. (in Chinese) |
| [21] |
孟丹, 孟庆玲, 乔军, 等. 奶牛源金黄色葡萄球菌新疆流行株的耐药特性、毒力基因及分子分型[J]. 畜牧兽医学报, 2018, 49(1): 181–194.
MENG D, MENG Q L, QIAO J, et al. Study on resistance characteristics, virulence genes and molecular classification of Staphylococcus aureus isolated from cows in Xinjiang[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(1): 181–194. (in Chinese) |
| [22] | SHI D, HAO Y, ZHANG A, et al. Antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis in China[J]. Transbound Emerg Dis, 2010, 57(4): 221–224. |
| [23] |
王琼, 唐俊妮, 汤承, 等. 针对不同来源金黄色葡萄球菌分离菌株的agr I-IV分型初探[J]. 西南民族大学学报:自然科学版, 2014, 40(3): 354–357.
WANG Q, TANG J N, TANG C, et al. Investigation of the agr Ⅰ-Ⅳ groups in Staphylococcus aureus isolates from five different sources[J]. Journal of Southwest University for Nationalities:Natural Science Edition, 2014, 40(3): 354–357. (in Chinese) |
| [24] | FABRES-KLEIN M H, CAIZER SANTOS M J, CONTELLI KLEIN R, et al. An association between milk and slime increases biofilm production by bovine Staphylococcus aureus[J]. BMC Vet Res, 2015, 11(1): 3. DOI: 10.1186/s12917-015-0319-7 |
| [25] | ZHANG Y, XU D Y, SHI L, et al. Association between agr type, virulence factors, biofilm formation and antibiotic resistance of Staphylococcus aureus isolates from pork production[J]. Front Microbiol, 2018, 9: 1876. DOI: 10.3389/fmicb.2018.01876 |



