2. 中国农业大学动物医院, 北京 100193
2. Veterinary Teaching Hospital, China Agricultural University, Beijing 100193, China
卵巢颗粒细胞瘤(granulosa cell tumor),又称颗粒-卵泡膜细胞瘤(granulosa-theca cell tumor),是一种卵巢性索间质细胞肿瘤(sex cord stromal tumors),主要由不规则排列的卵泡颗粒细胞以及数量不等的卵泡内膜细胞和成纤维细胞组成。颗粒细胞肿瘤是牛和马最常见的卵巢肿瘤类型[1-4],在犬的卵巢肿瘤中,发生率略少于上皮肿瘤[5-7]。猫的卵巢颗粒细胞肿瘤少见,本病例为猫恶性颗粒细胞肿瘤伴发大面积腹腔转移,兽医临床中极其罕见,国内外文献均未见类似报道,本文提供了该病例资料,并分析其组织学特征,为临床诊治和研究提供参考。
1 临床资料与检查方法雌性家猫,15岁,体重2.4 kg。主诉:患猫3月前开始发情,10 d前出现不食和腹围增大。
全血细胞计数检查显示,网织红细胞、嗜酸性粒细胞以及血小板降低,数值分别为9.0×102·μL-1(参考范围3.0×103~50.0×103·μL-1)、1.40×108·L-1(参考范围1.70×108~1.57×109·L-1)、1.20×104·μL-1(参考范围1.51×105~6.00×105·μL-1),白细胞2.530×1010·L-1(参考范围2.87×109~1.702×1010·L-1),其中中性粒细胞2.310×1010·L-1(参考范围1.48×109~1.029×1010·L-1)。
血清肌酐为69 μmol·L-1,肌酸激酶596 U·L-1(参考范围0~314 U·L-1),乳酸3.08 mmol·L-1(参考范围0.60~2.50 mmol·L-1)。血气分析显示呼吸性碱中毒。
超声检查提示:肝、胆囊及肾形态未见明显异常;膀胱结晶;腹腔积液;子宫积液;左肾尾侧可见一大小约4.27 cm×2.63 cm的肿物,内可见血流信号。
放射线检查,可见腹部膨大,腹腔脏器细节不清,胸腔未见明显异常。
腹腔穿刺液呈浅棕色,相对体积质量1.026,有凝固性,黏蛋白阳性,蛋白质含量54 g·L-1,红细胞及退行性中性粒细胞阳性,并可见少量球菌。
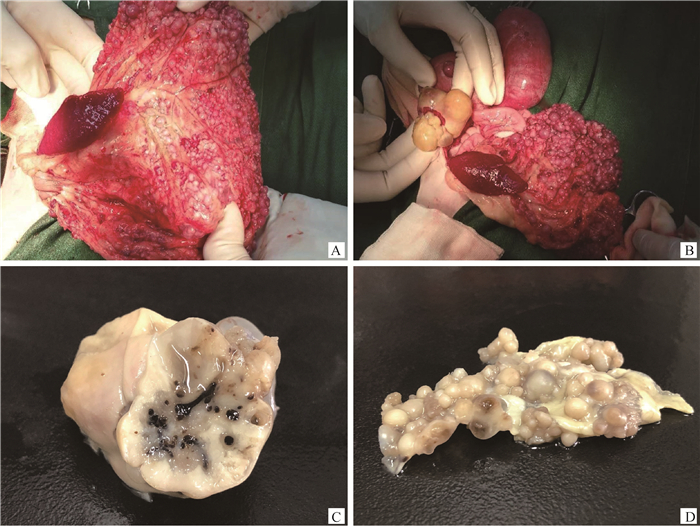
开腹探查,可见大网膜、肠壁及胃壁弥散性分布大量大小不等的球形肿物,柔软光滑,有波动感(图 1A)。左侧卵巢明显增大,色黄,表面形成坚实或柔软的结节(图 1B)。子宫体积增大,内有积液,触之有波动感。
|
A.大网膜,肠壁及胃壁弥散性分布大量大小不等的球形肿物;B.左侧卵巢明显增大,表面形成结节;C.左侧卵巢肿物大小约4.27 cm×2.63 cm,呈卵圆形,切面皮质髓质结构丢失,呈均质实性或囊性,内有少量胶冻样或浆液样内容物;D.大网膜肿物弥散分布于膜两侧,直径0.2~1 cm,切面呈均质实性或囊性,内含浅黄色至褐色浆液性内容物 A. A large number of spherical masses of different sizes are diffusely distributed on large omentum, intestinal wall and stomach wall; B. The left ovary is obviously enlarged and nodules are on the surface; C. The size of the left ovarian mass is about 4.27×2.63 cm, which is oval in shape. The cortical medulla of the cut surface is lost, and it is homogeneous or cystic. There is a small amount of jelly or serous content inside; D. The omental mass is distributed on both sides of the membrane, ranging from 0.2 to 1 cm in diameter. The cut surface is homogeneous or cystic, containing pale yellow to brown serous contents. 图 1 猫卵巢恶性颗粒细胞瘤及大网膜肿瘤转移灶的大体病理学特征 Fig. 1 Gross pathology features of feline ovarian malignant granulosa cell tumor and metastatic focuses |
手术切除卵巢及大网膜,固定于10%福尔马林溶液中,石蜡包埋,2 μm切片,HE染色,光镜观察,采集数码图像。
2 结果 2.1 大体观察左侧卵巢肿物大小约4.27 cm×2.63 cm,呈卵圆形,质地中等,包膜完整,表面可见数个大小不等的球形突起,触感坚实或波动,肿物切面皮质髓质结构丢失,呈均质实性或囊性,内有少量胶冻样或浆液样内容物(图 1C)。大网膜肿物弥散分布于膜两侧,球形,排列紧密,界限清晰,直径0.2~1 cm,表面光滑,触感柔软,切面呈均质实性或囊性,内含浅黄色至褐色浆液性内容物(图 1D)。
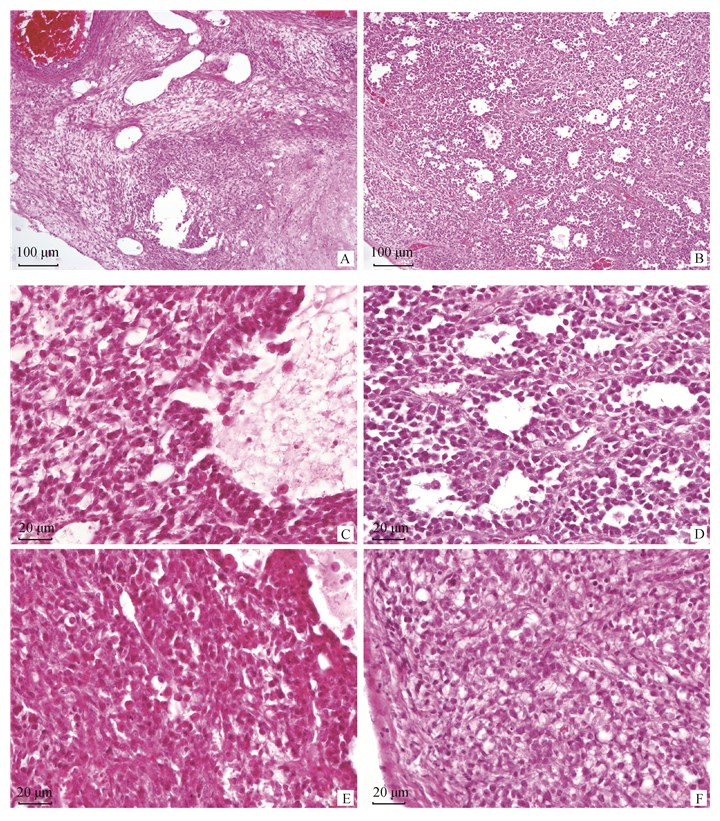
2.2 病理观察低倍镜下,可见肿物由结缔组织被膜包裹,肿物内部正常卵巢的组织结构丢失严重。肿瘤细胞被纤维结缔组织分割为片层状或条索样,肿物浅层可见大量大小不等、形状不规则的管样结构,管腔内可见单个或成团的脱落肿瘤细胞以及少量嗜酸性均质分泌物,个别直径较大管样结构内可见肿瘤细胞及其基底层结缔组织向管腔内突起,形成乳头样、丘样结构。局部区域可见梭形细胞呈旋涡状或交叉束状排列(图 2A、B)。
|
A、B.肿物浅层可见大量大小不等、形状不规则的管样结构,局部区域可见梭形细胞呈旋涡状或交叉束状排列(100×),A.卵巢,B.大网膜;C、D.肿瘤细胞呈卵圆形、多角形,多呈片层状、条索样排列或形成管样结构(400×),C.卵巢,D.大网膜;E、F.肿瘤细胞呈梭形,多呈片状、旋涡状或交叉束状排列(400×),E.卵巢,F.大网膜 A, B. In the shallow layer of the mass, a large number of tube-like structures of different sizes and irregular shapes can be seen. In the local area, the spindle cells can be seen in a spiral or cross-bundle arrangement (100×), A. ovary, B. large omentum; C, D. Tumor cells are oval, polygonal, mostly in the form of lamellar, strip-like or tube-like structures (400×), C. ovary, D. large omentum; E, F. Tumor cells are fusiform, mostly in the form of flakes, spirals or cross-bundles, E. ovary, F. large omentum 图 2 猫卵巢恶性颗粒细胞瘤及大网膜肿瘤转移灶的组织病理学特征 Fig. 2 Histopathology features of feline ovarian malignant granulosa cell tumor and metastatic focuses |
高倍镜下,可见两类肿瘤细胞。一类肿瘤细胞呈卵圆形、多角形,细胞核占比大,呈圆形、卵圆形或多角形,嗜碱性浓染,有一个明显的核仁,有丝分裂象常见,细胞质稀少,弱嗜酸性,该类肿瘤细胞多呈片层状、条索样排列或形成管样结构(图 2C、D)。另一类肿瘤细胞呈梭形,胞核呈卵圆形或梭形,细胞质淡染,部分细胞细胞质内出现颗粒和空泡,该类细胞多呈片状、旋涡状或交叉束状排列,细胞间隙内偶见淋巴样细胞浸润(图 2E、F)。
3 讨论猫的卵巢肿瘤发生率极低[8],据文献报道,Cotchin等调查了571例猫肿瘤病例,仅有1例起源于卵巢,Nielson等在254例发生于猫的肿瘤中只发现了1例卵巢肿瘤[9]。卵巢肿瘤包括性索间质细胞瘤、生殖细胞瘤(germ cell tumors)、上皮细胞瘤(epithelial tumors)和间叶细胞瘤(mesenchymal tumors)等。根据WHO分类方法,猫卵巢性索间质细胞肿瘤可分为颗粒细胞瘤、黄体瘤(luteoma)、卵泡膜细胞瘤(thecoma)三类,其中颗粒细胞瘤发生率最高[10]。
猫卵巢颗粒细胞肿瘤未见于国内报道,发生大范围腹腔内转移的恶性颗粒细胞瘤在国内外文献中均未见报道。随着宠物饲养数量的增加,动物福利意识的增强,可以预见,未来几年国内猫卵巢肿瘤病例会明显增加,因此回顾猫卵巢颗粒细胞瘤相关文献,有望为今后该类肿瘤的诊断治疗和研究提供帮助。
卵巢颗粒细胞瘤是起源于卵泡颗粒细胞的一种卵巢性索间质细胞肿瘤,常发于老年动物,但也可见于其他年龄段,甚至幼龄动物。目前人类和动物的卵巢颗粒细胞瘤病因仍然不明确,但基于人类患者的研究发现,卵巢颗粒细胞瘤与罕见的肿瘤易感性综合征(tumor predisposition syndromes)或长期使用卵巢刺激药物有关[11-13]。
卵巢颗粒细胞可以分泌类固醇激素,是正常卵巢激素的主要来源。卵巢颗粒细胞瘤也有分泌多种类固醇激素的活性[5, 14-16],进而引起患病动物出现间情期延长、间歇性或持续性发情、雄性化等生殖行为改变以及内分泌性脱毛、子宫内膜囊性增生等症状[9, 17]。本病例曾在就诊3月前开始表现出兴奋、食欲减退、臀部上撅扭动等发情表现,在15岁的老龄猫中较为异常,提示可能发生卵巢、子宫等生殖器官的肿瘤或囊肿,手术过程中的大体病理观察和对送检卵巢肿物的组织病理学观察证实了上述推测。
卵巢颗粒细胞瘤通常体积较大,大体外观较多样,表现为多个结节或均质,单侧或双侧,实性或囊性,有或无出血性坏死区域等。囊性区域内通常含有黄色至红色稀薄囊液,囊液的细胞学和激素分析已经运用于人类卵巢颗粒细胞瘤诊断,但尚未有兽医用此方法建立诊断或判断预后。卵巢颗粒细胞瘤由内衬于卵泡样结构的颗粒细胞和起分割支持作用的梭形细胞组成,颗粒细胞排列紊乱,甚至在某些区域无法形成卵泡样结构,而呈片层状、索状、小梁状或巢状排列。值得注意的是,在同一卵巢颗粒细胞瘤内的不同区域可出现不同类型的细胞类型和组织学表现,造成颗粒-卵泡膜细胞瘤、卵巢塞尔托利细胞瘤等易于混淆的别名出现,所以当肿瘤细胞的胚胎学起源和组织学表现不确定时,可以统称为性索间质细胞肿瘤。
卡-埃二氏小体(Call-Exner body)是卵巢颗粒细胞瘤的特征性表现,但仅可见于部分患猫[15]。抑制素-α和Melan-A是卵巢性索间质细胞瘤的有效免疫组化标志物,此外,用于黑色素瘤免疫组化诊断的Melan-A、PNL2、TRP-1和TRP-2等抗体,也可用于颗粒细胞瘤的诊断[18]。
不同动物的卵巢颗粒细胞瘤生物学行为差异很大,马和驴的颗粒细胞瘤通常为良性,犬颗粒细胞瘤较少转移,而猫颗粒细胞瘤相对更易发生转移[9, 19-22]。卵巢颗粒细胞瘤一般可经淋巴道或血道转移到其他脏器,但极少发生腹腔内种植性转移。一般来说,卵巢颗粒细胞瘤如发生转移,则可判断为恶性,另外,肿瘤的实性区域大、卵巢原有结构破坏严重、有丝分裂象常见或颗粒细胞比例高,则恶性程度较高,反之则多为良性。本病例发生大范围腹腔转移,加之肿瘤实性区域较大,卵巢原有结构严重丢失,有丝分裂象常见,圆形的颗粒细胞比例远高于梭形的支持细胞,所以判定为卵巢恶性颗粒细胞瘤。
与其他卵巢肿瘤一样,手术切除是卵巢颗粒细胞瘤的主要治疗手段。手术过程中应仔细检查包括大网膜和隔膜在内的所有浆膜,移除所有疑似肿瘤转移灶并进行组织学检查。有研究报道,化疗对犬卵巢颗粒细胞瘤有效[23-24],铂基化合物可用于治疗人卵巢颗粒细胞瘤[25-27],激素疗法可用于卵巢颗粒细胞瘤病人的控制[28],但以上疗法对猫卵巢颗粒细胞瘤的治疗效果尚未可知。
有关猫卵巢颗粒细胞瘤预后的报道极少。Norris等[9]报道了5例猫恶性卵巢颗粒细胞瘤病例,其中3例死于肿瘤转移,Carpenter等[29]报道了1例疑似复发的猫卵巢颗粒细胞瘤病例,于手术后5个月实施安乐死。猫卵巢颗粒细胞瘤尚未建立良好的预后标准[30],目前,对该肿瘤预后的判断只能参考其他动物:如果肿瘤未转移且完全切除,则预后相对良好,肿瘤发生转移则预后谨慎[31]。
4 结论猫卵巢肿瘤发生率极低,卵巢恶性颗粒细胞瘤更为罕见,对于该病大范围种植性转移的报道在国内外尚为空白。本文报道了1例猫大范围种植性腹腔转移的卵巢颗粒细胞癌案例,观察其组织病理学表现,并回顾了相关文献,希望为今后该类肿瘤的诊断治疗和研究提供帮助。
| [1] | ZACHARY J F, HALIBURTON J C. Malignant granulosa cell tumor in an Angus cow[J]. Vet Pathol, 1983, 20(4): 506–509. DOI: 10.1177/030098588302000417 |
| [2] | MEGANCK V, GOVAERE J, VANHOLDER T, et al. Two atypical cases of granulosa cell tumours in Belgian Blue heifers[J]. Reprod Domest Anim, 2011, 46(4): 746–749. DOI: 10.1111/j.1439-0531.2010.01717.x |
| [3] | MAURICE K T. Diagnosis and surgical remroval of a granulosa-theca cell tumor in a mare[J]. Can Vet J, 2005, 46(7): 644–646. |
| [4] | RAMBAGS B P B, STOUT T A E, RIJKENHUIZEN A B M. Ovarian granulosa cell tumours adherent to other abdominal organs; surgical removal from 2 Warmblood mares[J]. Equine Vet J, 2003, 35(6): 627–632. |
| [5] | PATNAIK A K, GREENLEE P G. Canine ovarian neoplasms:a clinicopathologic study of 71 cases, including histology of 12 granulosa cell tumors[J]. Vet Pathol, 1987, 24(6): 509–514. DOI: 10.1177/030098588702400607 |
| [6] | SIVACOLUNDHU R K, HARA A J, READ R A. Granulosa cell tumour in two speyed bitches[J]. Aust Vet J, 2001, 79(3): 173–176. DOI: 10.1111/j.1751-0813.2001.tb14571.x |
| [7] | AKIHARA Y, SHIMOYAMA Y, KAWASAKO K, et al. Immunohistochemical evaluation of canine ovarian tumors[J]. J Vet Med Sci, 2007, 69(7): 703–708. DOI: 10.1292/jvms.69.703 |
| [8] | HAYES H M JR. The comparative epidemiology of selected neoplasms between dogs, cats and humans. A review[J]. Eur J Cancer, 1978, 14(12): 1299–1308. DOI: 10.1016/0014-2964(78)90111-1 |
| [9] | NORRIS H J, GARNER F M, TAYLOR H B. Pathology of feline ovarian neoplasms[J]. J Pathol, 1969, 97(1): 138–143. DOI: 10.1002/path.1710970118 |
| [10] | MEUTEN D J. Tumors in domestic animals[M]. 5th ed. New Jersey: Wiley-Blackwell, 2017. |
| [11] | JAMIESON S, FULLER P J. Molecular pathogenesis of granulosa cell tumors of the ovary[J]. Endocr Rev, 2012, 33(1): 109–144. |
| [12] | WILLEMSEN W N P, KRUITWAGEN R F P M, BASTIAANS L, et al. Ovarian stimulation and granulosa-cell tumour[J]. Lancet, 1993, 341(8851): 986–988. DOI: 10.1016/0140-6736(93)91071-S |
| [13] | ROSSING M A, DALING J R, WEISS N S, et al. Ovarian tumors in a cohort of infertile women[J]. N Engl J Med, 1994, 331(12): 771–776. DOI: 10.1056/NEJM199409223311204 |
| [14] | ELLENBERGER C, BARTMANN C P, HOPPEN H O, et al. Histomorphological and immunohistochemical characterization of equine granulosa cell tumours[J]. J Comp Pathol, 2007, 136(2-3): 167–176. DOI: 10.1016/j.jcpa.2007.01.011 |
| [15] | GEE E K, DICKEN M, ARCHER R M, et al. Granulosa theca cell tumour in a pregnant mare:concentrations of inhibin and testosterone in serum before and after surgery[J]. N Z Vet J, 2012, 60(2): 160–163. DOI: 10.1080/00480169.2011.645776 |
| [16] | MCCUE P M, ROSER J F, MUNRO C J, et al. Granulosa cell tumors of the equine ovary[J]. Vet Clin North Am Equine Pract, 2006, 22(3): 799–817. DOI: 10.1016/j.cveq.2006.08.008 |
| [17] | SCHLAFER D H, MILLER R B. Pathology of domestic animals[M]. 5th ed. Amsterdam: Elsevier Saunders, 2007. |
| [18] | SMEDLEY R C, LAMOUREUX J, SLEDGE D G, et al. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms[J]. Vet Pathol, 2011, 48(1): 32–40. DOI: 10.1177/0300985810387447 |
| [19] | GELBERG H B, MCENTEE K. Feline ovarian neoplasms[J]. Vet Pathol, 1985, 22(6): 572–576. DOI: 10.1177/030098588502200610 |
| [20] | THEILEN G H, MADEWELL B R. Veterinary cancer medicine[M]. Philadelphia: Lea & Febiger, 1987. |
| [21] | STEIN B S. Tumors of the feline genital tract[J]. J Am Anim Hosp Assoc, 1981, 17: 1022–1025. |
| [22] | ARNBERG J. Extra-ovarian granulosa cell tumor in a cat[J]. Fel Pract, 1980, 10: 26–32. |
| [23] | HAYES A, HARVEY H J. Treatment of metastatic granulosa cell tumor in a dog[J]. J Am Vet Med Assoc, 1979, 174(12): 1304–1306. |
| [24] | GREENE J A, RICHARDSON R C, THORNHILL J A, et al. Ovarian papillary cystadenocarcinoma in a bitch: case report and literature review[J]. J Am Anim Hosp Assoc, 1979, 15: 351–356. |
| [25] | MOORE A S, KIRK C, CARDONA A. Intracavitary cisplatin chemotherapy experience with six dogs[J]. J Vet Intern Med, 1991, 5(4): 227–231. DOI: 10.1111/j.1939-1676.1991.tb00953.x |
| [26] | OLSEN J, KOMTEBEDDE J, LACKNER A, et al. Cytoreductive treatment of ovarian carcinoma in a dog[J]. J Vet Intern Med, 1994, 8(2): 133–135. DOI: 10.1111/j.1939-1676.1994.tb03211.x |
| [27] | PAUTIER P, GUTIERREZ-BONNAIRE M, REY A, et al. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors[J]. Int J Gynecol Cancer, 2008, 18(3): 446–452. DOI: 10.1111/j.1525-1438.2007.01049.x |
| [28] | KORACH J, PERRI T, BEINER M, et al. Promising effect of aromatase inhibitors on recurrent granulosa cell tumors[J]. Int J Gynecol Cancer, 2009, 19(5): 830–833. DOI: 10.1111/IGC.0b013e3181a261d7 |
| [29] | CARPENTER J L, ANDREWS L K, HOLZWORTH J, et al. Diseases of the cat:medicine and surgery[M]. Philadelphia: WB Saunders, 1987. |
| [30] |
ZACHARY J F, MCGAVIN M D.兽医病理学[M].赵德明, 杨利峰, 周向梅, 译. 5版.北京: 中国农业出版社, 2015.
ZACHARY J F, MCGAVIN M D. Pathologic basis of veterinary disease[M].ZHAO D M, YANG L F, ZHOU X M, trans. 5th ed. Beijing: China Agriculture Press, 2015. (in Chinese) |
| [31] | WITHROW S J, VAIL D M. Small animal clinical oncology[M]. 4th ed. Amsterdam: Saunders Elsevier,, 2007. |



