禽蛋以其丰富的蛋白质和高性价比受到广大消费者的欢迎。未来40年,预计世界人口将增长25%,意味着蛋和蛋产品的需求量将日益增加,统计结果显示,印度和中国的家禽养殖数量将快速增长[1]。蛋清和蛋黄中含有丰富的蛋白质和矿物质,既是人们餐桌上必不可少的一道美食,也是微生物的天然培养基。因此,蛋产品安全问题不容忽视。据估计,全世界每年大约有13亿例非伤寒沙门菌病的发生[2]。禽蛋细菌污染问题不仅不利于消费者的身体健康,而且会对禽蛋产业造成巨大的经济损失[3]。2018年4月,美国政府召回了2亿个疑似被沙门菌污染的鸡蛋[4]。微生物污染鸡蛋主要通过两种途径,垂直污染(产蛋前污染)和水平污染(蛋产出后污染)。垂直污染主要是指母鸡在产蛋前就患病,病原微生物经血液循环进入卵巢或输卵管,从而在鸡蛋形成过程中对鸡蛋造成污染[5]。水平污染是指蛋被产出后,由于粪污、人为触摸或其它非规范化操作造成的污染,例如鸡蛋收集、运输或贮藏等生产环节中造成的污染[6]。随着福利养殖的推广,家禽在散养条件下病原微生物污染鸡蛋的机率会增加。
洁蛋是目前消除或减少蛋壳表面病原体数量的一种常见做法。美国、日本、澳大利亚等国家对鸡蛋进行分级清洗涂膜配以冷链销售。洁蛋可以清洗蛋壳表面的粪污、血斑和羽毛等污渍,也能减少蛋壳表面的细菌数量[7]。巴氏杀菌、紫外线杀菌、沸水杀菌、臭氧及负离子杀菌等方法是常用的物理消毒方式[8]。氯、季铵盐、碳酸钠、过氧化物催化化合物、硫酸锌溶液、甲醛、电解水等是鸡蛋洗涤工艺中常用的消毒剂[9-10]。洗涤剂和刷洗会对蛋壳造成一定的机械损伤,导致蛋壳胶护膜受损,暴露的气孔数增加,需涂膜降低蛋被细菌污染的机率[11-12]。然而,在欧洲,市售鸡蛋一般不经过清洗和涂膜[13],鸡蛋安全建立在生产过程对相关疾病的规范免疫和蛋壳本身具有优质胶护膜的基础上。目前,我国鲜蛋市场的销售主流仍以普通鸡蛋为主。清洗和涂膜相应的增加了蛋产品的成本,售价明显高于普通鲜蛋,一般作为品牌蛋进行销售,其市场份额约占我国鲜蛋市场的5%[14]。洁蛋势必会消耗大量的水,加剧水资源的匮乏[15];而且,洁蛋后的废水残留有粪污和细菌,属于高BOD,养殖场处理这些带菌的污水也会增加生产成本[16]。
胶护膜是蛋壳最外面的一层膜,是蛋抵御细菌跨壳污染的天然屏障[17-18]。选育蛋壳胶护膜品质优良的鸡群,提高蛋壳本身对细菌的免疫力,不仅能够节约人力物力,还可以从根本上减少蛋产品食源性中毒的发生。胶护膜形成于鸡蛋产出前1.5~2.0 h,呈无色透明的薄胶状,主要由碳水化合物、脂肪和一些抗菌糖蛋白组成[19]。胶护膜在鸡蛋产出后几分钟被风干,附着或嵌入蛋壳表面,堵塞气孔,防止细菌入侵和保持鸡蛋内水分[17, 20-21]。胶护膜中还含有多种抗菌蛋白,能有效抑制蛋壳表面细菌的增长[17, 22-23]。胶护膜一般呈片状分布,研究表明,胶护膜越完整,品质越好,其抗菌和保鲜的效果越好[24]。本文在介绍胶护膜结构组成、理化性质及其质量影响因素的基础上,主要综述了国内外以及本团队近年来在胶护膜品质评价、抗菌机理和性状遗传机理方面所取得的研究进展,期望为鸡蛋胶护膜品质的优化选育提供借鉴。
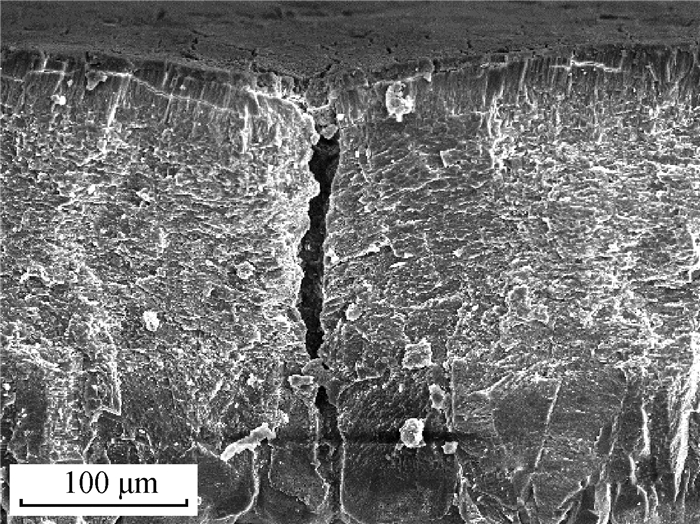
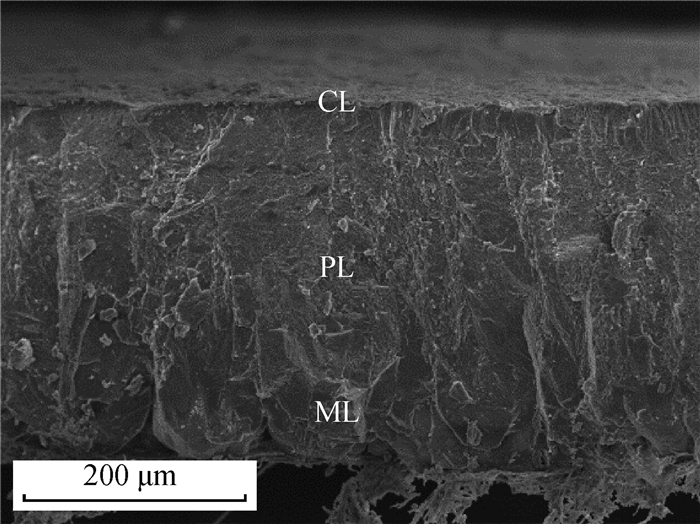
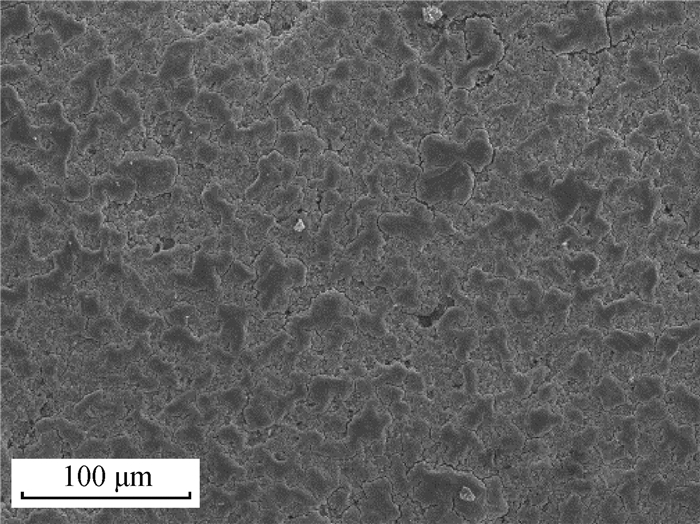
1 蛋壳胶护膜的基本特征 1.1 蛋壳胶护膜的成分及结构蛋壳由内到外可分为乳突层、栅栏层和胶护膜层[25-26](图 1[27])。胶护膜形成于鸡蛋产出前1.5~2.0 h左右[19],由子宫内膜的非纤毛分泌细胞产生,并以颗粒状物质的形式分泌[24]。胶护膜呈无色透明的薄胶状,一般附着或嵌入在蛋壳表面的气孔[28-30]。蛋产出后,胶护膜与空气接触后变干皱缩附着在蛋壳表面。鸡蛋蛋壳胶护膜鲜有完整,多呈斑驳的不均匀分布[24](图 2[27])。胶护膜由钙化(内层)和非钙化的水不溶层(外层)组成[31],钙化内层主要由羟基磷灰石组成,外层水不溶层主要由糖蛋白(90%)、多糖(4%)、脂质(3%)和少部分磷脂组成,蛋白质中半胱氨酸、甘氨酸、谷氨酸、赖氨酸和酪氨酸含量较高[31-32]。藻糖、半乳糖、葡萄糖、己糖胺、甘露糖和唾液酸是多糖的组成部分[25]。
|
图 1 蛋壳横截面乳突层(ML)、栅栏层(PL)、胶护膜层(CL)扫描电镜图(200×)[27] Fig. 1 Scanning electron microscope(SEM) image of the mammillary layer (ML), palisade layer (PL) and cuticle layer (CL) of eggshell cross section (200×)[27] |
|
图 2 扫描电子显微镜下鸡蛋蛋壳表面斑驳的胶护膜(300×)[27] Fig. 2 SEM image of the patchy cuticle on the surface of eggshell (300×)[27] |
胶护膜中含有大量的蛋白质,但绝大部分为水不溶性蛋白。EDTA、盐酸、SDS、尿素均可用于提取蛋壳胶护膜蛋白,不同提取方法的效率不同。通过蛋白质质谱分析,Rose-Martel质谱分析检测到47种蛋白质,其中含量最多的是ovocalyxin-32 (OCX-32)、蛋白抑制剂类似蛋白、ovocleidin-116 (OC-116)、ovocalyxin-36 (OCX-36)、凝集素及少量的溶菌酶C和卵清蛋白[33]。研究发现,胶护膜中的蛋白质与蛋壳基质层的蛋白质重叠,胶护膜中除含有上述蛋白质外,还发现含量丰富的ovocleidin-17 (OC-17)蛋白质[33]。其中溶菌酶C、卵清蛋白、蛋白抑制剂类似蛋白、OCX-32、OC-17、OC-116为抗菌蛋白,可有效地降低革兰阴性菌和革兰阳性菌的跨壳污染[34]。
1.2 蛋壳胶护膜的生物学功能在蛋壳形成过程中,胶护膜对方解石晶体终止生长起着重要的作用[31]。蛋产出前,胶护膜可作为润滑剂帮助鸡蛋在子宫部翻转[35-36],蛋产出后,胶护膜能有效地调节蛋壳内外水气交换,减缓蛋壳内水分蒸发,延长鸡蛋保存时间[29, 37-38],Liu等[12]的研究发现,清洗后的蛋,其保存时间显著低于未经清洗的蛋。胶护膜是天然的抑菌屏障,胶护膜可以从物理和化学两种途径抑制细菌跨壳污染[17, 22, 39]。蛋壳气孔一方面是水气交换的重要场所,另一方面也是微生物进入鸡蛋内部的通道[29-30, 40]。胶护膜形成后覆盖或堵塞蛋壳表面的气孔[41](图 3[27]),阻断细菌跨壳入侵的通道,降低细菌跨壳污染的机率。胶护膜中含有多种抗菌蛋白能有效地抑制蛋壳表面细菌的活性,抵御细菌跨壳污染[42-46]。胶护膜中的纳米球颗粒结构同样决定了胶护膜抗菌功效,胶护膜纳米球颗粒具有疏水的作用,能有效减少水停留在蛋壳表面的数量和面积,有效降低细菌在蛋壳表面繁殖的机率[47]。
Gole等[22]对比沙门菌感染清洗组(去胶护膜组)和对照组(保留胶护膜组)的蛋发现,清洗后蛋的细菌污染率显著高于未经清洗的蛋,说明胶护膜可有效降低细菌跨壳污染。胶护膜成熟度同样对其抗菌有影响,将产蛋后3、6或72 h(分别代表胶护膜未成熟、成熟或干燥)鸡蛋分别浸泡于沙门菌悬液中,20 ℃连续培养21 d,发现蛋产出后6~72 h内蛋壳胶护膜的抗菌效率最高[23]。Bain等[18, 48]用带pGLO荧光质粒的大肠杆菌和沙门菌感染胶护膜品质好和胶护膜品质差的两组鸡蛋,37 ℃培养24 h后紫外灯下观察,发现胶护膜品质好的组其细菌入侵率显著低于胶护膜品质差的组,且该结果与蛋的品种无关。Chen等[20]用带荧光质粒的大肠杆菌浸染白来航、矮小鸡和海兰褐3种鸡蛋,结果同样显示胶护膜质量差的蛋更容易被细菌污染,且统计分析结果表明,当蛋壳厚度不低于324 μm,且胶护膜不透明度大于27.5%时,能起到98%以上的保护效率。有研究发现个别细菌和真菌的糖酵解活性可以消化蛋壳胶护膜层,从而促进微生物进入蛋内[38]。Bain等[48]通过去糖基化试验证明,胶护膜的抑菌功能与其糖基化无关。
2 蛋壳胶护膜品质的评价方法1973年,研究发现,MST胶护膜蓝或豌豆绿能与蛋壳胶护膜特异性结合,可作为检测蛋壳胶护膜品质的指示剂[48]。MST胶护膜蓝是一种安全的指示剂,相关研究表明其既不会对蛋壳造成损伤[23],也不会对鸡胚的发育造成不利影响[48]。将蛋放入配好的染液中浸泡1 min,用缓慢流动的清水冲洗1 min后晾干过夜,根据蛋壳着色的深浅对胶护膜品质进行评估,蛋壳着色越深,代表胶护膜层越厚。在早期的研究中,人为的用肉眼根据蛋壳着色的深浅对胶护膜质量进行判断,用1~12代表染色后蛋壳绿色的深浅,其中1代表染色最浅,12代表染色最深[17]。由于不同的人对于颜色的敏感度不同,且不同蛋壳颜色的蛋之间比较会造成一定的误差,所以该方法只适用于评估胶护膜质量差异较大的蛋,对于着色相近的蛋很难评判。
随着科技的发展,研究人员不断在传统染色法的基础上进行改进,使胶护膜的测定结果更加精确。Leleu等[49]用分光测色仪分别测量MST染色前后蛋壳的L、a、b值,根据公式计算ΔEab*。ΔEab*越大,代表MST的着色效率越高,胶护膜品质越好,反之代表胶护膜的品质越差。Bain等[18]利用反射积分球(ISP-REF)测量蛋壳染色前后的反射率,最佳波长(染色前后最大反射率差值处的波长)处的反射率差值即可代表胶护膜品质。染色前最佳波长处的反射率代表蛋壳颜色的密度,染色前后反射率差值越大,代表蛋壳胶护膜品质越好。上述两种方法可准确比较同一蛋色的胶护膜品质,但该方法还未见运用于不同蛋色间胶护膜质量的比较。
扫描电子显微镜(scanning electron microscope,SEM)不仅可以测量胶护膜的厚度,还可以观察胶护膜的微观结构[22, 24, 35, 50-51]。取一块约1 cm2大小的蛋壳,用导电胶粘在金属台上,真空干燥后喷金,置于SEM下观察,发现鸡蛋的蛋壳胶护膜一般呈不均匀的片状或放射状,胶护膜的厚度范围为0.5~12.5 μm[52]。Rodríguez-Navarro等[38]在2013年用衰减全反射-傅里叶变换红外光谱(attenuated total reflection-Fourier transform infrared spectroscopy,ATR-FTIR)对蛋壳胶护膜化学成分进行分析,根据水、蛋白质、硫酸盐、磷酸盐、碳酸盐、多糖和脂类的吸收峰峰面积决定不同元素的差异,由此对胶护膜质量进行评估。上述两种方法能有效地衡量蛋壳胶护膜品质。但由于SEM和ATR-FTIR设备昂贵,且样品处理费时,所以在实际检测中并不常用。
2018年,Chen等[20]为了比较不同品种鸡蛋(蛋色不同)胶护膜的品质,引入不透明度的原理对胶护膜质量进行评估。用分光测色仪测量MST胶护膜蓝染色前后蛋壳的X、Y、Z值,用公式将X、Y、Z值转化为不透明度α值,不透明度越大代表胶护膜的着色越深,表示胶护膜品质越好,反之代表胶护膜质量越差。其中X、Y、Z为颜色空间模型的三基色,每种颜色都可以表示成X、Y、Z的混合。不透明度与蛋壳底色无关,只与胶护膜着色效率有关。因此,该方法适用于不同颜色以及不同物种禽蛋间胶护膜品质的比较。
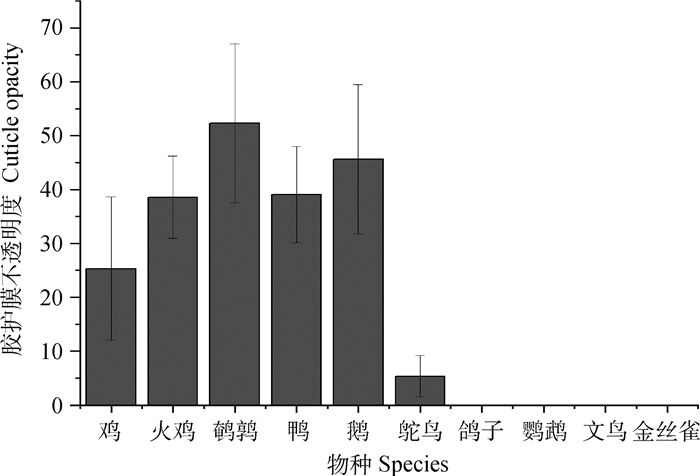
3 蛋壳胶护膜的遗传调控 3.1 蛋壳胶护膜在物种间和物种内的特异性分泌蛋壳胶护膜是蛋抵御细菌跨壳污染的重要屏障,然而不是所有的禽蛋都有胶护膜,例如鸽子和鹦鹉蛋就缺乏胶护膜的保护[41, 51, 53]。不同物种的蛋,其胶护膜品质差异较大,例如鸭、鹅和企鹅蛋的胶护膜品质要显著优于鸡蛋的胶护膜品质[27, 34-35]。Chen等[27]测量了10个不同物种的鸟蛋,各鸟类的蛋壳胶护膜品质见图 4[27],结果显示,鹌鹑的蛋壳胶护膜品质显著优于其他物种,其次为鹅、鸭和火鸡,结果还表明早成鸟(鸡、火鸡、鹌鹑、鸭、鹅、鸵鸟)的蛋壳表面都有胶护膜,而晚成鸟(鸽子、鹦鹉、文鸟、金丝雀)的蛋壳表面都缺乏胶护膜,推测其原因可能与不同鸟类的生存环境有关。同一物种不同品种禽类所产蛋的胶护膜亦存在一定的差异,Chen等[20]测量了白来航、矮小鸡和海兰褐3个品种鸡蛋的胶护膜,发现矮小鸡蛋的胶护膜质量显著优于海兰褐和白莱航蛋。即使是同一品种鸡群中的不同个体,其蛋壳胶护膜品质也存在较大的差异。蛋壳锐端的胶护膜品质显著低于钝端和中部,具体原因尚待探究。
3.2 鸡蛋壳胶护膜候选基因2004年,Dunn等[54]对某肉鸡品种进行基因组测序分析后发现,ovocleidin-116 (OC-116)、ovocalyxin-36 (OCX-36)、osteopontin (SPP1), ovocalyxin-32 (OCX-32), ovotransferrin(卵转铁蛋白)及ovalbumin(卵清蛋白)基因与蛋壳质量显著相关,雌激素受体(estrogen receptor 1, ESR1)和碳酸酐酶(carbonic anhydrase, CAII)基因与蛋壳腺的功能有关。2009年,Dunn等[55]通过重测序在某洛岛红群体中检测上述基因的单核苷酸多态性,将基因多态与胶护膜品质进行关联分析,结果显示,OC-116、OCX-32、卵清蛋白、雌激素受体基因与胶护膜沉积显著相关。OC-116是主要的蛋壳基质蛋白,在母鸡的子宫液中被发现,形成于蛋壳快速矿化阶段,据统计每克蛋壳中含有80 μg的OC-116蛋白质[56],前期研究表明,OC-116与蛋壳强度、蛋壳厚度和蛋形指数有关[57]。OCX-32是分子量为32 ku的抗菌蛋白质,参与蛋壳矿化的终止,存在于蛋壳的栅栏层、垂直晶体层和胶护膜层,在输卵管的峡部和子宫部均可高表达[58-59]。卵清蛋白是一种抗菌蛋白,在蛋清中含量最丰富,在蛋壳形成初期的子宫液中也有存在,主要富集在蛋壳的乳突层中[56, 60]。ESR1基因参与调控复杂的生理机能,与疾病、代谢、炎症和骨质疏松有关[61]。2013年,Bain等[18]用REML拟合线性模型对上述候选基因进一步验证,结果表明,OC-116和ESR1基因与蛋壳胶护膜的沉积显著相关。迄今为止,上述胶护膜候选基因均未开展相关的功能验证,决定胶护膜分泌的基因及相关基因的调控机制还未可知。
3.3 胶护膜与其它蛋壳性状相关的研究李文博等[62]测量了3 283只洛岛红纯系母鸡的胶护膜、蛋壳厚度、蛋壳强度和蛋壳颜色,并估计了胶护膜的遗传力及其与蛋壳相关性状的遗传和表型相关。胶护膜的遗传力为0.40,呈中等遗传力。因此,可通过遗传选育提高鸡群的蛋壳胶护膜品质,从而改善蛋产品安全问题。前人研究认为,胶护膜会直接影响蛋壳强度和蛋壳厚度[63]。李文博等[62]以及Belyavin和Boorman[63]的结果却表明,胶护膜与蛋壳强度和蛋壳厚度之间呈低遗传相关,表明胶护膜性状的选育对蛋壳强度和厚度无不良影响。胶护膜与蛋壳颜色(L*、a*、b*)的3个指标呈现不同程度的遗传相关,分别为-0.49、0.43和-0.27[62]。胶护膜中含有色素成分原卟啉[64],但Wilson等[19]采用激素干扰的方法证明蛋壳色素主要沉积在蛋壳钙化层,胶护膜中极少分布,且胶护膜质量与蛋壳色素无显著相关。
4 影响蛋壳胶护膜质量的因素 4.1 周龄对蛋壳胶护膜的影响胶护膜品质是否会随着母鸡周龄而产生变化一直存在争议。一些学者认为胶护膜厚度随着母鸡周龄的增加而减小,胶护膜品质随着母鸡周龄的增加逐渐变差[38, 49]。Lunam和Ruiz[65]则认为,胶护膜质量是动态变化的,产蛋初期和产蛋末期母鸡所产蛋胶护膜质量显著低于产蛋中期(38周龄)母鸡所产的蛋。本课题组前期研究发现,随着产蛋周龄的增加,胶护膜品质呈下降趋势,但胶护膜品质优良的鸡群所产蛋的胶护膜品质始终保持在较高水平[66]。Bain等[48]追踪了洛岛红蛋鸡和艾维因肉鸡25~50周龄所产蛋的胶护膜品质变化,发现同一个体不同周龄间的胶护膜品质稳定性较好。其他报道也发现胶护膜品质与母鸡周龄无显著相关,胶护膜质量不会随着母鸡周龄的增加而显著变差[24, 67]。
4.2 营养因素对蛋壳胶护膜的影响胶护膜中含有大量的羟基磷灰石,而磷元素参与蛋壳矿化的终止[68-69]。Kusuda等[35]用X射线微区分析了5个物种禽蛋蛋壳胶护膜中氧、碳、钙、磷的含量,发现磷元素主要存在胶护膜层中,且胶护膜品质好的蛋磷元素的含量显著低于胶护膜品质差的蛋。饲料中磷元素是否与胶护膜品质相关还有待进一步探究。
4.3 环境对蛋壳胶护膜的影响蛋壳胶护膜品质与鸟类的生存环境有很大的关系。一般在沼泽、河岸栖息筑巢的鸟类,其胶护膜比栖息在相对干燥环境中鸟类的胶护膜品质更好[35, 53]。这可能是鸟类为了确保后代成功孵育,而进化出了更优质的胶护膜来阻挡潮湿环境中病原微生物的入侵[70-71]。其次,集约化养殖的品系由于其饲养环境的卫生条件优于野生的鸟类,面临的环境压力相对较弱,造成其蛋壳胶护膜质量较差[18]。Samiullah和Roberts[72]分别测量了44、64、73周龄笼养、平养和放养的海兰褐蛋鸡所产蛋的胶护膜,研究发现,平养鸡所产蛋的胶护膜品质最好,笼养鸡所产蛋的胶护膜品质最差。此外,外界环境的刺激,比如对鸡笼的破坏、喂药、惊吓和抓取等都会对笼养鸡群的蛋壳胶护膜品质产生影响[73]。
5 小结和展望蛋壳胶护膜是抵御细菌跨壳污染的重要屏障,可通过物理和化学两种途径阻碍病原物生物的跨壳污染。胶护膜质量评估由之前的粗略分级过渡到现在的精确测量,为胶护膜的研究奠定了基础。蛋壳胶护膜分泌的时间和部位已确认,胶护膜组成成分的研究也相对透彻。然而,决定胶护膜分泌的基因及其调控机理尚不清楚,影响胶护膜分泌的因素尚待探究,如何提高鸡群胶护膜品质的稳定性等问题尚待解决。相信随着国内外学者对胶护膜的深入研究,这些疑问将逐渐被解答,强化胶护膜品质保护鲜蛋不被微生物污染。
| [1] | BAIN M M, NYS Y, DUNN I C. Increasing persistency in lay and stabilising egg quality in longer laying cycles.What are the challenges?[J]. Br Poult Sci, 2016, 57(3): 330–338. DOI: 10.1080/00071668.2016.1161727 |
| [2] | COBURN B, GRASSL G A, FINLAY B B. Salmonella, the host and disease:a brief review[J]. Immunol Cell Biol, 2007, 85(2): 112–118. DOI: 10.1038/sj.icb.7100007 |
| [3] | HALL G, KIRK M D, BECKER N, et al. Estimating foodborne gastroenteritis, Australia[J]. Emerg Infect Dis, 2005, 11(8): 1257–1264. DOI: 10.3201/eid1108.041367 |
| [4] | KRISTINE P.3200 million eggs recalled after nearly two dozen were sickened with Salmonella, officials say[EB/OL]. (2018-04-21).https://www.washingtonpost.com/news/business/wp/2018/04/15/200-million-eggs-recalled-after-nearly-two-dozen-were-sickened-with-salm-onella-officials-say/?utm_term=.9a3f75e68abd. |
| [5] | MESSENS W, GRIJSPEERDT K, HERMAN L. Eggshell penetration by Salmonella:a review[J]. World's Poult Sci J, 2005, 61(1): 71–86. DOI: 10.1079/WPS200443 |
| [6] | MIYAMOTO T, HORIE T, BABA E, et al. Salmonella penetration through eggshell associated with freshness of laid eggs and refrigeration[J]. J Food Prot, 1998, 61(3): 350–353. DOI: 10.4315/0362-028X-61.3.350 |
| [7] | HUTCHISON M L, GITTINS J, WALKER A, et al. An assessment of the microbiological risks involved with egg washing under commercial conditions[J]. J Food Prot, 2004, 67(1): 4–11. |
| [8] |
张京和, 李玉冰, 张永东, 等. 不同洁蛋处理对鸡蛋消毒效果及新鲜度的对比试验[J]. 中国兽医杂志, 2013, 49(8): 70–72.
ZHANG J H, LI Y B, ZHANG Y D, et al. Comparative study of the effect of different eggs cleaning method on the disinfection and the freshness of the eggs[J]. Chinese Journal of Veterinary Medicine, 2013, 49(8): 70–72. DOI: 10.3969/j.issn.0529-6005.2013.08.028 (in Chinese) |
| [9] | PARK C M, HUNG Y C, LIN C S, et al. Efficacy of electrolyzed water in inactivating Salmonella enteritidis and Listeria monocytogenes on shell eggs[J]. J Food Prot, 2005, 68(5): 986–990. DOI: 10.4315/0362-028X-68.5.986 |
| [10] | BIALKA K L, DEMIRCI A, KNABEL S J, et al. Efficacy of electrolyzed oxidizing water for the microbial safety and quality of eggs[J]. Poult Sci, 2004, 83(12): 2071–2078. DOI: 10.1093/ps/83.12.2071 |
| [11] | SAMIULLAH, CHOUSALKAR K K, ROBERTS J R, et al. Effects of egg shell quality and washing on Salmonella Infantis penetration[J]. Int J Food Microbiol, 2013, 165(2): 77–83. DOI: 10.1016/j.ijfoodmicro.2013.05.002 |
| [12] | LIU Y C, CHEN T H, WU Y C, et al. Effects of egg washing and storage temperature on the quality of eggshell cuticle and eggs[J]. Food Chem, 2016, 211: 687–693. DOI: 10.1016/j.foodchem.2016.05.056 |
| [13] | Commission of the European Communities.Commission regulation (EC) No 589/2008[EB/OL]. (2008-06-23).http://data.europa.eu/eli/reg/2008/589/oj. |
| [14] |
张超, 于海鹏, 许振宝, 等. 近期中国鸡蛋市场形势及展望[J]. 农业展望, 2017, 13(10): 14–17, 22.
ZHANG C, YU H P, XU Z B, et al. China's egg market in recent years and its prospect[J]. Agricultural Outlook, 2017, 13(10): 14–17, 22. DOI: 10.3969/j.issn.1673-3908.2017.10.003 (in Chinese) |
| [15] |
孙莹. 生态文明视野下水资源保护及利用分析探讨[J]. 科技创新导报, 2017, 14(33): 105–106.
SUN Y. Analysis and discussion on protection and utilization of water resources from the perspective of ecological civilization[J]. Science and Technology Innovation Herald, 2017, 14(33): 105–106. (in Chinese) |
| [16] |
余周, 胡志伟, 吴佳, 等. 我国水污染现状、危害及处理措施研究[J]. 环境与发展, 2019, 31(6): 61, 63.
YU Z, HU Z W, WU J, et al. Briefly describe the status quo, harm and treatment measures of water pollution in China[J]. Environment and Development, 2019, 31(6): 61, 63. (in Chinese) |
| [17] | BOARD R G, HALLS N A. The cuticle:a barrier to liquid and particle penetration of the shell of the hen's egg[J]. Br Poult Sci, 1973, 14(1): 69–97. DOI: 10.1080/00071667308415999 |
| [18] | BAIN M M, MCDADE K, BURCHMORE R, et al. Enhancing the egg's natural defence against bacterial penetration by increasing cuticle deposition[J]. Anim Genet, 2013, 44(6): 661–668. DOI: 10.1111/age.12071 |
| [19] | WILSON P W, SUTHER C S, BAIN M M, et al. Understanding avian egg cuticle formation in the oviduct:a study of its origin and deposition[J]. Biol Reprod, 2017, 97(1): 39–49. DOI: 10.1093/biolre/iox070 |
| [20] | CHEN X, LI X Z, GUO Y Y, et al. Impact of cuticle quality and eggshell thickness on egg antibacterial efficiency[J]. Poult Sci, 2019, 98(2): 940–948. DOI: 10.3382/ps/pey369 |
| [21] | FRASER A C, BAIN M M, SOLOMON S E. Transmission electron microscopy of the vertical crystal layer and cuticle of the eggshell of the domestic fowl[J]. Br Poult Sci, 1999, 40(5): 626–631. DOI: 10.1080/00071669987016 |
| [22] | GOLE V C, ROBERTS J R, SEXTON M, et al. Effect of egg washing and correlation between cuticle and egg penetration by various Salmonella strains[J]. Int J Food Microbiol, 2014, 182-183: 18–25. DOI: 10.1016/j.ijfoodmicro.2014.04.030 |
| [23] | MUÑOZ A, DOMINGUEZ-GASCA N, JIMENEZ-LOPEZ C, et al. Importance of eggshell cuticle composition and maturity for avoiding trans-shell Salmonella contamination in chicken eggs[J]. Food Control, 2015, 55: 31–38. DOI: 10.1016/j.foodcont.2015.02.028 |
| [24] | ROBERTS J R, CHOUSALKAR K, SAMIULLAH. Egg quality and age of laying hens:implications for product safety[J]. Anim Prod Sci, 2013, 53(12): 1291–1297. DOI: 10.1071/AN12345 |
| [25] | BAKER J R, BALCH D A. A study of the organic material of hen's-egg shell[J]. Biochem J, 1962, 82(2): 352–361. |
| [26] | ARIAS J L, FINK D J, XIAO S Q, et al. Biomineralization and eggshells:cell-mediated acellular compartments of mineralized extracellular matrix[J]. Int Rev Cytol, 1993, 145: 217–250. DOI: 10.1016/S0074-7696(08)60428-3 |
| [27] | CHEN X, LI X Z, HE Z X, et al. Comparative study of eggshell antibacterial effectivity in precocial and altricial birds using Escherichia coli[J]. PLoS One, 2019, 14(7): e0220054. DOI: 10.1371/journal.pone.0220054 |
| [28] | COOKE A S, BALCH D A. Studies of membrane, mammilary cores and cuticle of the hen egg shell[J]. Br Poult Sci, 1970, 11(3): 345–352. DOI: 10.1080/00071667008415825 |
| [29] | SPARKS N H C, BOARD R G. Cuticle, shell porosity and water uptake through hens' eggshells[J]. Br Poult Sci, 1984, 25(2): 267–276. DOI: 10.1080/00071668408454866 |
| [30] | BOARD R G, HALLS N A. Water uptake by eggs of mallards and guinea fowl[J]. Br Poult Sci, 1973, 14(3): 311–314. DOI: 10.1080/00071667308416033 |
| [31] | DENNIS J E, XIAO S Q, AGARWAL M, et al. Microstructure of matrix and mineral components of eggshells from White Leghorn chickens (Gallus gallus)[J]. J Morphol, 1996, 228(3): 287–306. DOI: 10.1002/(SICI)1097-4687(199606)228:3<287::AID-JMOR2>3.0.CO;2-# |
| [32] | MIKSˇÍK I, CHARVÁTOVÁ J, ECKHARDT A, et al. Insoluble eggshell matrix proteins-their peptide mapping and partial characterization by capillary electrophoresis and high-performance liquid chromatography[J]. Electrophoresis, 2003, 24(5): 843–852. DOI: 10.1002/elps.200390106 |
| [33] | MIKSˇÍK I, ERGANG P, PÁCHA J. Proteomic analysis of chicken eggshell cuticle membrane layer[J]. Anal Bioanal Chem, 2014, 406(29): 7633–7640. DOI: 10.1007/s00216-014-8213-x |
| [34] | WELLMAN-LABADIE O, PICMAN J, HINCKE M T. Antimicrobial activity of the Anseriform outer eggshell and cuticle[J]. Comp Biochem Physiol B Biochem Mol Biol, 2008, 149(4): 640–649. DOI: 10.1016/j.cbpb.2008.01.001 |
| [35] | KUSUDA S, IWASAWA A, DOI O, et al. Diversity of the cuticle layer of avian eggshells[J]. J Poult Sci, 2011, 48(2): 119–124. DOI: 10.2141/jpsa.010103 |
| [36] | RAHMAN M A, MORIYAMA A, IWASAWA A, et al. Cuticle formation in quail eggs[J]. Zoolog Sci, 2009, 26(7): 496–499. DOI: 10.2108/zsj.26.496 |
| [37] | PEEBLES E D, PANSKY T, DOYLE S M, et al. Effects of dietary fat and eggshell cuticle removal on egg water loss and embryo growth in broiler hatching eggs[J]. Poult Sci, 1998, 77(10): 1522–1530. DOI: 10.1093/ps/77.10.1522 |
| [38] | RODRÍGUEZ-NAVARRO A B, DOMÍNGUEZ-GASCA N, MUÑOZ A, et al. Change in the chicken eggshell cuticle with hen age and egg freshness[J]. Poult Sci, 2013, 92(11): 3026–3035. DOI: 10.3382/ps.2013-03230 |
| [39] | DE REU K, GRIJSPEERDT K, MESSENS W, et al. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis[J]. Int J Food Microbiol, 2006, 112(3): 253–260. |
| [40] | BOARD R G. Microstructure, water resistance and water repellency of the pigeon egg shell[J]. Br Poult Sci, 1974, 15(4): 415–419. DOI: 10.1080/00071667408416126 |
| [41] | KULSHRESHTHA G, RODRIGUEZ-NAVARRO A, SANCHEZ-RODRIGUEZ E, et al. Cuticle and pore plug properties in the table egg[J]. Poult Sci, 2018, 97(4): 1382–1390. DOI: 10.3382/ps/pex409 |
| [42] | MATSUURA K, TAMURA T, KOBAYASHI N, et al. The antibacterial protein lysozyme identified as the termite egg recognition pheromone[J]. PLoS One, 2007, 2(8): e813. DOI: 10.1371/journal.pone.0000813 |
| [43] | PELLEGRINI A, THOMAS U, BRAMAZ N, et al. Identification and isolation of a bactericidal domain in chicken egg white lysozyme[J]. J Appl Microbiol, 1997, 82(3): 372–378. DOI: 10.1046/j.1365-2672.1997.00372.x |
| [44] | VADEHRA D V, BAKER R C, NAYLOR H B. Distribution of lysozyme activity in the exteriors of eggs from Gallus gallus[J]. Comp Biochem Physiol B Comp Biochem, 1972, 43(3): 503–508. |
| [45] | WELLMAN-LABADIE O, PICMAN J, HINCKE M T. Antimicrobial activity of cuticle and outer eggshell protein extracts from three species of domestic birds[J]. Br Poult Sci, 2008, 49(2): 133–143. DOI: 10.1080/00071660802001722 |
| [46] | XING J, WELLMAN-LABADIE O, GAUTRON J, et al. Recombinant eggshell ovocalyxin-32:expression, purification and biological activity of the glutathione S-transferase fusion protein[J]. Comp Biochem Physiol B Biochem Mol Biol, 2007, 147(2): 172–177. DOI: 10.1016/j.cbpb.2007.01.015 |
| [47] | D'ALBA L, JONES D N, BADAWY H T, et al. Antimicrobial properties of a nanostructured eggshell from a compost-nesting bird[J]. J Exp Biol, 2014, 217(7): 1116–1121. DOI: 10.1242/jeb.098343 |
| [48] | BAIN M M, ZHENG J X, ZIGLER M, et al. Cuticle deposition improves the biosecurity of eggs through the laying cycle and can be measured on hatching eggs without compromising embryonic development[J]. Poult Sci, 2019, 98(4): 1775–1784. DOI: 10.3382/ps/pey528 |
| [49] | LELEU S, MESSENS W, DE REU K, et al. Effect of egg washing on the cuticle quality of brown and white table eggs[J]. J Food Prot, 2011, 74(10): 1649–1654. DOI: 10.4315/0362-028X.JFP-11-013 |
| [50] | RILEY A, STURROCK C J, MOONEY S J, et al. Quantification of eggshell microstructure using X-ray micro computed tomography[J]. Br Poult Sci, 2014, 55(3): 311–320. DOI: 10.1080/00071668.2014.924093 |
| [51] | D'ALBA L, MAIA R, HAUBER M E, et al. The evolution of eggshell cuticle in relation to nesting ecology[J]. Proc Biol Sci, 2016, 283(1836): 20160687. DOI: 10.1098/rspb.2016.0687 |
| [52] | ROSE M L H, HINCKE M T. Protein constituents of the eggshell:eggshell-specific matrix proteins[J]. Cell Mol Life Sci, 2009, 66(16): 2707–2719. DOI: 10.1007/s00018-009-0046-y |
| [53] | FECHEYR-LIPPENS D C, IGIC B, D'ALBA L, et al. The cuticle modulates ultraviolet reflectance of avian eggshells[J]. Biol Open, 2015, 4(7): 753–759. DOI: 10.1242/bio.012211 |
| [54] | DUNN I C, MIAO Y W, MORRIS A, et al. A study of association between genetic markers in candidate genes and reproductive traits in one generation of a commercial broiler breeder hen population[J]. Heredity (Edinb), 2004, 92(2): 128–134. DOI: 10.1038/sj.hdy.6800396 |
| [55] | DUNN I C, JOSEPH N T, BAIN M, et al. Polymorphisms in eggshell organic matrix genes are associated with eggshell quality measurements in pedigree Rhode Island Red hens[J]. Anim Genet, 2009, 40(1): 110–114. DOI: 10.1111/j.1365-2052.2008.01794.x |
| [56] | MANN K, HINCKE M T, NYS Y. Isolation of ovocleidin-116 from chicken eggshells, correction of its amino acid sequence and identification of disulfide bonds and glycosylated Asn[J]. Matrix Biol, 2002, 21(5): 383–387. DOI: 10.1016/S0945-053X(02)00031-8 |
| [57] | HINCKE M T, GAUTRON J, TSANG C P W, et al. Molecular cloning and ultrastructural localization of the core protein of an eggshell matrix proteoglycan, ovocleidin-116[J]. J Biol Chem, 1999, 274(46): 32915–32923. DOI: 10.1074/jbc.274.46.32915 |
| [58] | GAUTRON J, HINCKE M T, MANN K, et al. Ovocalyxin-32, a novel chicken eggshell matrix protein[J]. J Biol Chem, 2001, 276(42): 39243–39252. DOI: 10.1074/jbc.M104543200 |
| [59] | MIKSˇÍK I, ECKHARDT A, SEDLÁKOVÁ P, et al. Proteins of insoluble matrix of avian (Gallus Gallus) eggshell[J]. Connect Tissue Res, 2007, 48(1): 1–8. DOI: 10.1080/03008200601003116 |
| [60] | WANG X Q, KONG R, PAN X X, et al. Role of ovalbumin in the stabilization of metastable vaterite in calcium carbonate biomineralization[J]. J Phys Chem B, 2009, 113(26): 8975–8982. DOI: 10.1021/jp810281f |
| [61] | JIA M, DAHLMAN-WRIGHT K, GUSTAFSSON J. Estrogen receptor alpha and beta in health and disease[J]. Best Pract Res Clin Endocrinol Metab, 2015, 29(4): 557–568. DOI: 10.1016/j.beem.2015.04.008 |
| [62] |
李文博, 陈霞, 苑忠央, 等. 蛋壳胶护膜性状的遗传特性及其与蛋壳品质性状的关联分析[J]. 畜牧兽医学报, 2018, 49(4): 659–666.
LI W B, CHEN X, YUAN Z Y, et al. Genetic characterization of cuticle and its association with eggshell quality traits[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(4): 659–666. (in Chinese) |
| [63] | BELYAVIN C G, BOORMAN K N. The influence of the cuticle on egg-shell strength[J]. Br Poult Sci, 1980, 21(4): 295–298. DOI: 10.1080/00071668008416672 |
| [64] | SAMIULLAH S, ROBERTS J R. The location of protoporphyrin in the eggshell of brown-shelled eggs[J]. Poult Sci, 2013, 92(10): 2783–2788. DOI: 10.3382/ps.2013-03051 |
| [65] | LUNAM C A, RUIZ J. Ultrastructural analysis of the eggshell:contribution of the individual calcified layers and the cuticle to hatchability and egg viability in broiler breeders[J]. Br Poult Sci, 2000, 41(5): 584–592. DOI: 10.1080/713654975 |
| [66] |
时学锋, 李文博, 李兴正, 等. 品种、周龄、保存时间及保存条件对鸡蛋胶护膜品质的影响[J]. 中国家禽, 2018, 40(17): 35–39.
SHI X F, LI W B, LI X Z, et al. Effects of chicken breeds, age, storage time and storage conditions on the quality of egg cuticle[J]. China Poultry, 2018, 40(17): 35–39. (in Chinese) |
| [67] | GOLE V C, CHOUSALKAR K K, ROBERTS J R, et al. Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella Typhimurium strains[J]. PLoS One, 2014, 9(3): e90987. DOI: 10.1371/journal.pone.0090987 |
| [68] | CUSACK M, FRASER A C, STACHEL T. Magnesium and phosphorus distribution in the avian eggshell[J]. Comp Biochem Physiol B Biochem Mol Biol, 2003, 134(1): 63–69. DOI: 10.1016/S1096-4959(02)00185-9 |
| [69] | SIMONS P C M, WIERTZ G. Notes on the structure of membranes and shell in the hen's egg:an electron microscopical study[J]. Z Zellforch Microsk Anat, 1963, 59(4): 555–567. DOI: 10.1007/BF00368728 |
| [70] | WANG J M, FIRESTONE M K, BEISSINGER S R. Microbial and environmental effects on avian egg viability:do tropical mechanisms act in a temperate environment?[J]. Ecology, 2011, 92(5): 1137–1145. DOI: 10.1890/10-0986.1 |
| [71] | PERALTA-SÁNCHEZ J M, MARTÍN-VIVALDI M, MARTÍN-PLATERO A M, et al. Avian life history traits influence eggshell bacterial loads:a comparative analysis[J]. IBIS, 2012, 154(4): 725–737. DOI: 10.1111/j.1474-919X.2012.01256.x |
| [72] | SAMIULLAH S, ROBERTS J R. The eggshell cuticle of the laying hen[J]. World's Poult Sci J, 2014, 70(4): 693–708. DOI: 10.1017/S0043933914000786 |
| [73] | SAMIULLAH S, OMAR A S, ROBERTS J, et al. Effect of production system and flock age on eggshell and egg internal quality measurements[J]. Poult Sci, 2017, 96(1): 246–258. |