2. 中国科学院微生物研究所, 北京 100101
2. Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
山竹,又称凤果,是藤黄科藤黄属常绿乔木山竹的果实,享有“上帝之果”的美誉,在泰国山竹一直作为传统医药用于治疗腹痛、腹泻、痢疾等疾病[1-2]。α-倒捻子素作为山竹果壳的主要代谢产物,具有显著的抗炎、抗菌和抗肿瘤的作用[3-5]。LPS是革兰阴性菌的主要致病成分,常被作为炎症模型的刺激因子通过TLR4-NF-κB信号通路,调控PGE2、IL-6、TNF-α、IL-1β等相关炎性因子[6-7]。关于LPS进入细胞的机制,有研究表明LPS可以与细胞表面的TLR4受体结合从而激活下游的信号通路[8-9]。核转录因子NF-κB是调节炎症反应重要的转录因子,静息时NF-κB与其抑制蛋白IκB结合,并且无生物学活性[10-12]。当细胞受炎症介质或LPS刺激后IκB对NF-κB的抑制作用被解除,NF-κB复合物解离并转移至细胞内,与特异性DNA相应位点结合后诱导相关基因转录,促进细胞因子的释放,从而引起全身炎症反应促进[13-15]。有研究报道,LPS可以引起RAW264.7巨噬细胞分泌PGE2、IL-6、TNF-α、IL-1β增加[16-17]。肠黏膜结构的完整性是肠黏膜屏障的基础,而单层的肠上皮细胞(intestinal epithelial cells,IECs)作为第一道防线,可以阻止物理、化学及生物因子对其造成损伤并引发炎症性肠病IBD[18-19]。据报道,α-倒捻子素可以抑制LPS刺激不同细胞引起的IL-6、IL-8和IL-1β分泌增多,进而抑制NF-κB信号通路的激活[6, 20]。但是,α-倒捻子素对LPS引起的IEC-6炎症的抑制作用及其作用机制尚未见报道。
在本研究中建立了LPS刺激后IEC-6炎症模型,通过α-倒捻子素对炎症相关的指标检测,拟探讨TLR4-NF-κB信号通路的变化研究其抗炎机制,为开发保健品提供参考。
1 材料与方法 1.1 材料IEC-6细胞株(CRL21592),购自北京协和细胞资源中心(Cell Resource Center,IBMS,CAMS/PUMC)。DMEM完全培养基由89%含酚红DMEM培养液(Gibco,美国)+10%胎牛血清(Gibco)+1%双抗组成(Gibco)。055:B5 LPS(Sigma,美国);α-倒捻子素>98%(同田,上海);CCK-8(同仁,日本);ELISA试剂盒(云克隆,武汉);总RNA提取试剂盒(OMEGA,美国);反转录试剂盒(Promega,美国);SuperReal PreMix Plus (SYBR Green)荧光定量试剂盒(天根,北京);pIκBα、pp65、β-actin(CST,美国);羊抗兔二抗(Lincoln,美国);核蛋白和胞质蛋白提取试剂盒(凯基,南京);BCA蛋白含量检测试剂盒(凯基,南京);Annexin V-FITC和Propidium Iodide(Becton Dickinson,美国);引物由上海生工生物工程合成。
1.2 方法 1.2.1 CCK-8检测细胞活力调整IEC-6细胞密度为1×105 cell·mL-1,并以96孔板孵育过夜。以5 μg·mL-1 LPS刺激细胞不同时间0、3、6、12、24 h,之后每孔加入10 μL CCK-8避光孵育2 h,使用酶标仪测定在450 nm处的吸光度(TECAN,奥地利)以确定模型组LPS浓度;5 μmol·L-1的α-倒捻子素与细胞共培养不同的时间(0、0.5、1、1.5、2 h),之后用CCK-8的方法检测细胞活力以确定加药组药物浓度,方法同上。
1.2.2 流式细胞术检测细胞凋亡调整IEC-6细胞密度为1×105cell·mL-1,并接种到培养瓶中培养过夜。模型组直接以LPS(5 μg·mL-1)刺激IEC-6细胞24 h,加药组以5 μmol·L-1的α-倒捻子素预处理1 h后再用LPS(5 μg·mL-1)刺激IEC-6细胞24 h。收集细胞并调节浓度为1×106 cell·mL-1依次加入5 μL Annexin V-FITC和Propidium Iodide,流式细胞仪(Becton Dickinson,美国)检测细胞凋亡。
1.2.3 ELISA检测细胞因子调整IEC-6细胞密度为1×105cell·mL-1,并以96孔板孵育过夜。模型组直接以LPS(5 μg·mL-1)刺激IEC-6细胞24 h,加药组以5 μmol·L-1的α-倒捻子素预处理1 h后再用LPS(5 μg·mL-1)刺激IEC-6细胞24 h。取细胞上清,根据ELISA试剂盒说明书分别测定PGE2、IL-6、IL-1β、TNF-α在450 nm处OD值,并根据各自的标准曲线计算细胞因子浓度。
1.2.4 Q-PCR检测细胞因子mRNA同“1.2.3”处理IEC-6细胞。使用总RNA提取试剂盒提取IEC-6细胞总RNA,微量紫外光分光光度计(JENWAY,英国)A260 nm/A280 nm为标准检测总RNA浓度和完整性。反应体系共20 μL:1 μL cDNA,上下游引物各1 μL,6.7 μL DEPC水,10 μL SuperReal PreMix Plus,0.3 μL ROX Reference Dye。β-肌动蛋白与靶基因平行扩增并用作标准化对照。反应条件:95 ℃预变性10 min,95 ℃变性30 s,60 ℃退火30 s,延伸1 min,40个循环,用2-ΔΔCt分析。表 1列出了用Q-PCR的5种基因特异性寡核苷酸引物。
|
|
表 1 基因引物列表 Table 1 Gene primer list |
同“1.2.3”处理IEC-6细胞。使用核蛋白和胞质蛋白提取试剂盒提取细胞质和细胞核蛋白,并用BCA蛋白含量检测试剂盒定量检测蛋白浓度。12% SDS-PAGE检测pIκB和pp65,使用β-actin校正印迹,以校正蛋白质加载的差异。使用Image J(National Institutes of Health,美国)从三个单独的试验获得了免疫印迹信号的密度测量值。
1.2.6 数据分析应用SPSS软件进行方差分析(ANOVA),凡标有不同字母即为差异显著(P<0.05)。试验数据表示为“x±sx”。
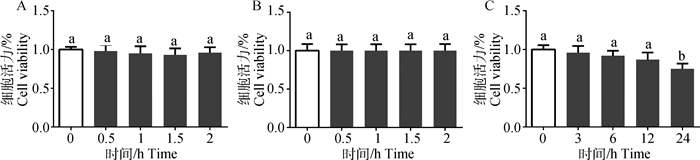
2 结果 2.1 α-倒捻子素对IEC-6细胞毒性试验为了检验α-倒捻子素对IEC-6细胞的毒性作用,5 μmol·L-1的α-倒捻子素与细胞共培养0~2 h,并以细胞活力为检测指标进行筛选。结果发现,5 μmol·L-1的α-倒捻子素对IEC-6细胞没有明显的毒性作用(图 1A),未经药物处理的IEC-6细胞的细胞活力也没有发生改变(图 1B),因此选用5 μmol·L-1的α-倒捻子素对细胞进行预处理。
|
A. α-倒捻子素浓度5 μmol·L-1;B.正常细胞;C. LPS浓度5 μg·mL-1。图中相同字母表示差异不显著(P>0.05),不同字母表示差异显著(P<0.05)。下图同 A. α-mangostin concentration 5 μmol·L-1; B. Normal cell; C. LPS concentration 5 μg·mL-1. The difference in the figure with the same letter is not significant (P > 0.05), and the difference between the different letters is significant (P < 0.05). The same as below 图 1 α-倒捻子素和LPS对IEC-6细胞活力的影响 Figure 1 Effect of α-mangostin and LPS on cell viability of IEC-6 cells |
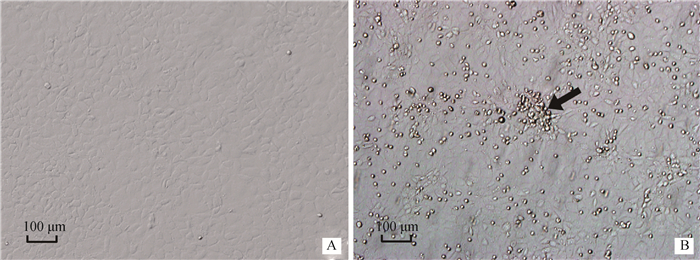
为了检验LPS对IEC-6细胞的毒性作用,5 μg·mL-1的LPS刺激细胞0~24 h,结果发现,随着LPS刺激时间的增加细胞活力持续下降,并且5 μg·mL-1的LPS刺激IEC-6细胞24 h后,细胞的生长有明显的抑制作用(P<0.05)(图 1C);倒置显微镜观察细胞形态,结果显示LPS刺激后细胞排列不规则,细胞皱缩甚至漂浮死亡(图 2B),因此用5 μg·mL-1的LPS刺激IEC-6细胞24 h作为炎症模型。
|
A.对照组;B.模型组 A.Control group; B.Model group 图 2 LPS对IEC-6细胞形态的影响 Figure 2 Effect of LPS on IEC-6 cell morphology |
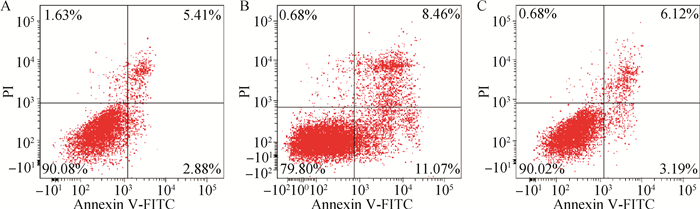
为了检验α-倒捻子素对LPS刺激IEC-6细胞引起细胞凋亡的改变,采用流式细胞术检测细胞凋亡。结果发现与对照组相比,模型组早凋和晚凋细胞数量显著升高(P<0.05)(图 3B),而加药组早凋和晚凋细胞数量显著降低(P<0.05)(图 3C)。
|
A.对照组;B.模型组;C.加药组。左下象限表示正常细胞,右上象限表示晚凋细胞,右下象限表示早凋细胞 A. Control group; B. Model group; C. Dosing group. Left lower quadrant indicate normal cells, right upper quadrant show late withering cells, and right lower quadrant show early withered cells 图 3 α-倒捻子素对LPS刺激IEC-6细胞对细胞凋亡的影响 Figure 3 Effect of α-mangostin on cell apoptosis induced by LPS stimulation in IEC-6 cells |
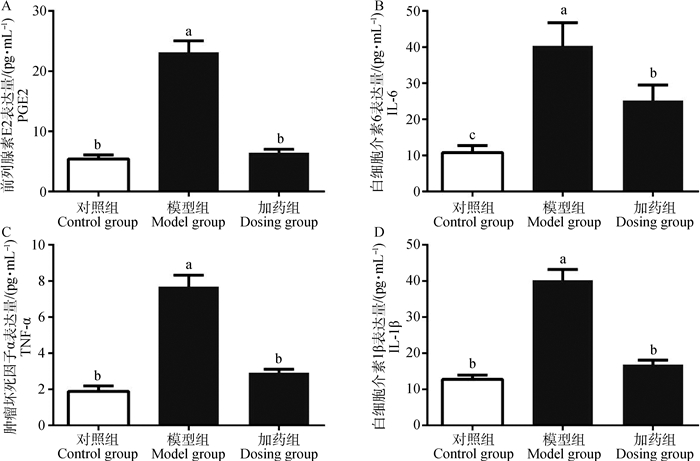
为了检测α-倒捻子素对LPS刺激IEC-6细胞因子的分泌。采用ELISA的方法检测炎症因子。结果发现,与对照组相比,模型组PGE2、IL-6、TNF-α、IL-1β的分泌显著升高(P<0.05),而与模型组相比加药组PGE2、IL-6、TNF-α、IL-1β的分泌显著降低(P<0.05)(图 4)。
|
图 4 α-倒捻子素对LPS刺激IEC-6细胞引起炎性因子表达的影响 Figure 4 The effect of α-mangostin on the expression of inflammatory factors induced by LPS stimulation of IEC-6 cells |
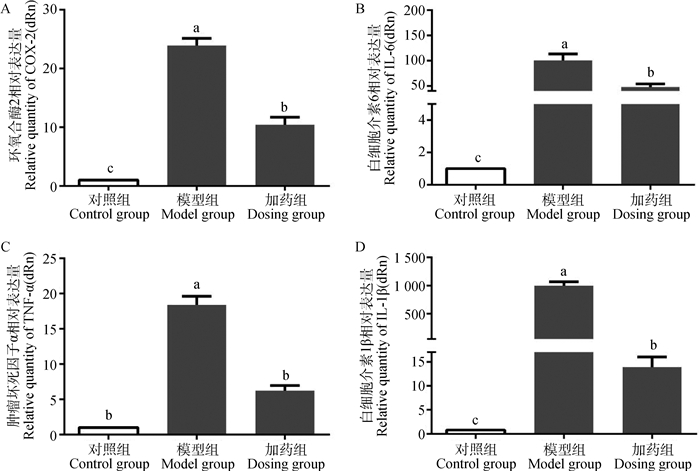
在mRNA水平上检测了α-倒捻子素对LPS诱导的细胞因子的抑制作用,结果发现,模型组COX-2、IL-6、TNF-α、IL-1β mRNA的转录明显升高(P<0.05),而与模型组相比加药组COX-2、IL-6、TNF-α、IL-1β mRNA的转录显著降低(P<0.05)(图 5)。
|
图 5 α-倒捻子素对LPS刺激IEC-6细胞引起炎性因子mRNA表达的影响 Figure 5 α-mangostin influences LPS-stimulated IEC-6 cells to induce inflammatory factor mRNA expression |
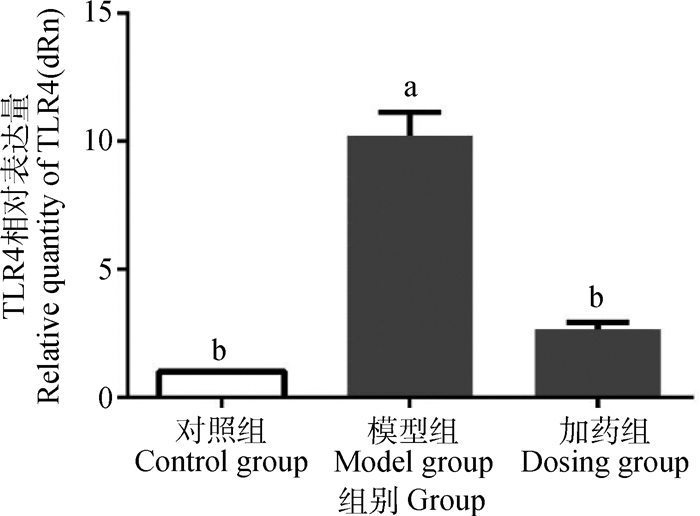
为了探究α-倒捻子素的抗炎机制,检测了细胞表面TLR4 mRNA的转录,结果显示,与对照组相比,模型组TLR4 mRNA转录显著升高(P<0.05),而加药组TLR4 mRNA转录受到抑制(P<0.05)(图 6)。
|
图 6 α-倒捻子素对LPS刺激IEC-6细胞引起TLR4 mRNA的影响 Figure 6 α-mangostin influences LPS-stimulated IEC-6 cells to induce TLR4 mRNA |
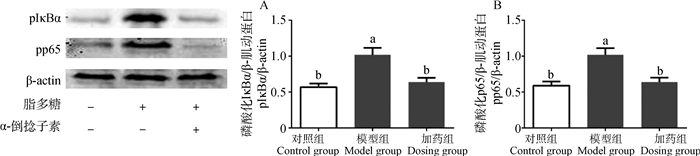
为了进一步探究α-倒捻子素的抗炎机制,笔者检测了NF-κB信号通路中pIκBα和pp65的表达。结果发现,模型组pIκBα和pp65的表达明显升高(P<0.05),而加药组pIκBα和pp65的表达明显降低(P<0.05)(图 7)。
|
A.IκBα磷酸化;B.p65磷酸化 A.IκBα phosphorylation; B.p65 phosphorylation 图 7 α-倒捻子素对LPS刺激IEC-6细胞对NF-κB信号通路蛋白激活的影响 Figure 7 Effects of α-mangostin on LPS-stimulated NF-κB signaling pathway activation in IEC-6 cells |
LPS作为引发炎症的重要因子,在很多研究中经常被用作炎症的刺激物[21]。本研究发现,5 μg·mL-1的LPS刺激IEC-6细胞24 h后细胞变圆甚至死亡、细胞活力显著下降、细胞凋亡增加,因此我们选用5 μg·mL-1的LPS刺激细胞24 h构建炎症模型。本研究表明,CCK-8检测5 μmol·L-1的α-倒捻子素与IEC-6细胞共培养后细胞活力没有显著变化,因此笔者选用5 μmol·L-1的α-倒捻子素来探究其抗炎机制。环氧化酶2(COX-2)为前列腺素E2(PGE2)的限速酶,参与多种病理生理过程,其高表达对炎症、疼痛、心血管疾病起着明显的促进作用,有研究报道倒捻子素可呈浓度依赖性降低LPS引起的COX-2表达升高[22]。IL-6、TNF-α和IL-1β受核转录因子NF-κB调控在LPS引起发炎症模型中表达上升[23-25]。α-倒捻子素作为山竹果皮的主要提取物在抗炎和抗氧化过程中发挥重要作用[10, 26]。研究证明,α-倒捻子素可以延长由亚硝酸钠所致中毒小鼠的存活时间[27];α-倒捻子素可以缓解由丙酸杆菌引发的痤疮炎症,并且由LPS刺激引发的TNF-α和IL-6升高[28];α-倒捻子素对LPS刺激RAW264.7巨噬细胞分泌IL-6和TNF-α有抑制作用[11, 29]。但是,α-倒捻子素对LPS引起的IEC-6细胞炎症的抑制作用及其作用机制尚未见报道。本研究表明,LPS刺激IEC-6细胞后,PGE2、IL-6、TNF-α、IL-1β的分泌量明显升高,而α-倒捻子素可以显著降低其分泌。为了探究炎症因子表达升高的机制,本研究发现LPS刺激后COX-2、IL-6、TNF-α和IL-1β mRNA转录明显上升,而α-倒捻子素可以显著降低其表达,说明α-倒捻子素可以缓解由LPS诱导的炎症,并且可以通过调节炎症因子mRNA的分泌来调节炎症因子的表达。
TLR是免疫系统的重要分子之一,其主要通过先天性免疫反应识别病原体,TLR4是最早被发现且研究最多的受体[12, 30]。TLR4主要通过与LPS结合募集下游髓样分化因子88(MyD88),引起下游信号通路NF-κB激活从而分泌大量的炎症因子引发炎症[31-33]。本研究表明,LPS刺激IEC-6细胞后,TLR4 mRNA的表达明显增加,并且NF-κB信号通路pIκBα和pp65蛋白表达明显增加,说明LPS是通过与TLR4受体结合从而激活NF-κB信号通路使细胞发生炎症反应。与模型组相比,加药组TLR4 mRNA、pIκBα和pp65的表达明显降低,说明α-倒捻子素可以通过TLR4/NF-κB信号通路来缓解炎症。
4 结论α-倒捻子素可以缓解LPS诱导的IEC-6细胞的炎症,并且可以通过TLR4/NF-κB信号通路实现其作用。
| [1] | SANDHIUTAMI N M D, MOORDIANI M, LAKSMITAWATI D R, et al. In vitro assesment of anti-inflammatory activities of coumarin and Indonesian cassia extract in RAW264. 7 murine macrophage cell line[J]. Iran J Basic Med Sci, 2017, 20(1): 99–106. |
| [2] | XU W K, JIANG H, YANG K, et al. Development and in vivo evaluation of self-microemulsion as delivery system for α-mangostin[J]. Kaohsiung J Med Sci, 2017, 33(3): 116–123. DOI: 10.1016/j.kjms.2016.12.003 |
| [3] | ZHAO Y, TANG G S, TANG Q, et al. A Method of effectively improved α-mangostin bioavailability[J]. Eur J Drug Metab Pharmacokinet, 2016, 41(5): 605–613. DOI: 10.1007/s13318-015-0283-4 |
| [4] | VERMA R K, YU W, SHRIVASTAVA A, et al. α-mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (KrasG12D, and KrasG12D/tp53R270H) mice[J]. Sci Rep, 2016, 6: 32743. DOI: 10.1038/srep32743 |
| [5] | NARASIMHAN S, MAHESHWARAN S, ABU-YOUSEF I A, et al. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from Garcinia mangostana[J]. Molecules, 2017, 22(2): 275. DOI: 10.3390/molecules22020275 |
| [6] | LEE W S, SHIN J S, JANG D S, et al. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264. 7 macrophages[J]. Int Immunopharmacol, 2016, 40: 146–155. DOI: 10.1016/j.intimp.2016.08.021 |
| [7] | SHEN Y, LIU B, MAO W, et al. PGE2 downregulates LPS-induced inflammatory responses via the TLR4-NF-κB signaling pathway in bovine endometrial epithelial cells[J]. Prostaglandins Leukot Essent Fatty Acids, 2018, 129: 25–31. DOI: 10.1016/j.plefa.2018.01.004 |
| [8] | FITZGERALD K A, ROWE D C, BARNES B J, et al. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF[J]. J Exp Med, 2003, 198(7): 1043–1055. DOI: 10.1084/jem.20031023 |
| [9] | NAGAI Y, AKASHI S, NAGAFUKU M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution[J]. Nat Immunol, 2002, 3(7): 667–672. DOI: 10.1038/ni809 |
| [10] |
苏途, 方圆, 谢平, 等. α-倒捻子素对过氧化氢诱导的人视网膜色素上皮细胞损伤的保护作用[J]. 国际眼科杂志, 2015, 15(6): 959–962.
SU T, FANG Y, XIE P, et al. Protective effects of α-mangostin on injury of human retinal pigment epithelium cells induced by hydrogen peroxide[J]. International Eye Science, 2015, 15(6): 959–962. (in Chinese) |
| [11] | YANG L, SONG Z, WANG X, et al. Huaier extract enhances the treatment efficacy of paclitaxel in breast cancer cells via the NF-κB/IκBα pathway[J]. Oncol Rep, 2017, 38(6): 3455–3464. |
| [12] | KLEIN M, OBERMAIER B, ANGELE B, et al. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4[J]. J Infect Dis, 2008, 198(7): 1028–1036. DOI: 10.1086/593027 |
| [13] |
黄伟, 李佳, 朱广为. 针刺百会、人中穴对急性脑缺血大鼠模型NF-κB/IκB-α的影响[J]. 中华中医药杂志, 2017, 32(1): 298–302.
HUANG W, LI J, ZHU G W. Effects of acupuncture on Baihui and Renzhong on acute cerebral ischemia model in hippocampal NF-κB, IκB-α expression[J]. China Journal of Traditional Chinese Medicine & Pharmacy, 2017, 32(1): 298–302. (in Chinese) |
| [14] | DOUGLASS J D, DORFMAN M D, FASNACHT R, et al. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation[J]. Mol Metab, 2017, 6(4): 366–373. DOI: 10.1016/j.molmet.2017.01.010 |
| [15] | HE J Y, LI J F, LIU H, et al. Scandoside exerts anti-inflammatory effect via suppressing NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages[J]. Int J Mol Sci, 2018, 19(2): 457. DOI: 10.3390/ijms19020457 |
| [16] |
唐兴梅, 李芳, 刘莉娜, 等. 桂枝茯苓胶囊及其成分对LPS诱导RAW264.7细胞产生炎症介质的影响[J]. 临床医学研究与实践, 2017, 2(20): 6–8.
TANG X M, LI F, LIU L N, et al. Influence of releasing inflammatory mediators in the LPS-induced RAW264. 7 cells by Guizhi Fuling capsule and its ingredients[J]. Clinical Research and Practice, 2017, 2(20): 6–8. (in Chinese) |
| [17] | KUMAR C S V S, REDDY K K, BOOBALAN G, et al. Immunomodulatory effects of Bifidobacterium bifidum 231 on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats[J]. Res Vet Sci, 2017, 110: 40–46. DOI: 10.1016/j.rvsc.2016.10.010 |
| [18] | LÍPEZ-POSADAS R, BECKER C, GVNTHER C, et al. Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation[J]. J Clin Invest, 2016, 126(2): 611–626. DOI: 10.1172/JCI80997 |
| [19] | ZHANG Y Y, SHI Q M, JIA Q H, et al. Effect of Echinacea purpurea polysaccharide on IL-6 m RNA expression level in IEC-6 cell after LPS injury[J]. Agric Sc Technol, 2016, 17(11): 2649–2651. |
| [20] | NARASIMHAN S, MAHESHWARAN S, ABU-YOUSEF I A, et al. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from Garcinia mangostana[J]. Molecules, 2017, 22(2): 275. DOI: 10.3390/molecules22020275 |
| [21] | ZANONI I, OSTUNI R, MAREK L R, et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4[J]. Cell, 2011, 147(4): 868–880. DOI: 10.1016/j.cell.2011.09.051 |
| [22] |
吕红, 方岩雄. α-, β-和γ-倒捻子素药理研究进展[J]. 中药材, 2005, 28(6): 519–523.
LÜ H, FANG Y X. Advances in pharmacological studies of α-, β-and γ-mangostin[J]. Journal of Chinese Medicinal Materials, 2005, 28(6): 519–523. DOI: 10.3321/j.issn:1001-4454.2005.06.033 (in Chinese) |
| [23] |
王丽君, 邓同兴, 张景亮, 等. 白藜芦醇抗炎镇痛作用的COX-2/PGE2信号通路机制研究[J]. 中药药理与临床, 2017, 33(4): 35–38.
WANG L J, DENG T X, ZHANG J L, et al. Resveratrol ameliorates inflammatory pain via inhibiting the COX-2/PGE2 signaling pathway in mice[J]. Pharmacology and Clinics of Chinese Materia Medica, 2017, 33(4): 35–38. (in Chinese) |
| [24] | TANG Q, ZHENG G, FENG Z H, et al. Wogonoside inhibits IL-1β induced catabolism and hypertrophy in mouse chondrocyte and ameliorates murine osteoarthritis[J]. Oncotarget, 2017, 8(37): 61440–61456. |
| [25] | KUMAZAKI M, SHINOHARA H, TANIGUCHI K, et al. Understanding of tolerance in TRAIL-induced apoptosis and cancelation of its machinery by α-mangostin, a xanthone derivative[J]. Oncotarget, 2015, 6(28): 25828–25842. |
| [26] |
方圆, 苏途, 谢平, 等. α-倒捻子素对小鼠视网膜光损伤的保护作用[J]. 国际眼科杂志, 2015, 15(7): 1143–1147.
FANG Y, SU T, XIE P, et al. Protective effect of α-mangostin on retinal light damage in mice[J]. International Eye Science, 2015, 15(7): 1143–1147. (in Chinese) |
| [27] |
王碧琼. α-倒捻子素耐缺氧作用的初步研究[J]. 海峡药学, 2012, 24(1): 30–32.
WANG B Q. Study on anti-anoxia effect of α-mangostin[J]. Strait Pharmaceutical Journal, 2012, 24(1): 30–32. DOI: 10.3969/j.issn.1006-3765.2012.01.010 (in Chinese) |
| [28] | WANG F, MA H X, LIU Z G, et al. α-mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice[J]. Biomed Pharmacother, 2017, 92: 672–680. DOI: 10.1016/j.biopha.2017.05.129 |
| [29] | DONG J, LI J, CUI L, et al. Cortisol modulates inflammatory responses in LPS-stimulated RAW264. 7 cells via the NF-κB and MAPK pathways[J]. BMC Vet Res, 2018, 14(1): 30–40. DOI: 10.1186/s12917-018-1360-0 |
| [30] | MEDZHITOV R, PRESTON-HURLBURT P, JANEWAY C A Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity[J]. Nature, 1997, 388(6640): 394–397. DOI: 10.1038/41131 |
| [31] |
陈超, 杨炼红. TLR4受体抑制剂对脂多糖诱导的小胶质细胞炎症因子的影响[J]. 中华卫生应急电子杂志, 2018, 4(1): 45–49.
CHEN C, YANG L H. Effect of TLR4 inhibitor on the inflammatory cytokines in microglia induced by LPS[J]. Chinese Journal of Hygiene Rescue (Electronic Edition), 2018, 4(1): 45–49. (in Chinese) |
| [32] | ZHU H T, BIAN C, YUAN J C, et al. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury[J]. J Neuroinflammation, 2014, 11: 59. DOI: 10.1186/1742-2094-11-59 |
| [33] | LI L C, VARGHESE Z, MOORHEAD J F, et al. Cross-talk between TLR4-MyD88-NF-κB and SCAP-SREBP2 pathways mediates macrophage foam cell formation[J]. Am J Physiol Heart Circ Physiol, 2013, 304(6): H874–H884. DOI: 10.1152/ajpheart.00096.2012 |



