Toll样受体(toll-like receptors,TLRs)基因家族是机体最早被发现的一类模式识别受体(pattern recognition receptors,PRR),可以准确识别一种或多种病原相关分子模式(pathogen-associated molecular patterns,PAMP),能启动和调节机体的免疫应答,同时也是连接先天性免疫和获得性免疫的关键环节[1-3]。根据脊椎动物TLRs基因家族的胞外结构域可以将其分为TLR1(1、2、6、10)、TLR3、TLR4、TLR5、TLR7(7、8、9)、TLR11、TLR13(13、21)、TLR15八大亚家族[4]。不同物种TLRs基因家族在进化中保持着相似的结构和功能,但其成员组成差别大。果蝇有9种TLRs基因,哺乳动物有13种,其中TLR1~10能在人上特异性表达,小鼠上能表达12种TLRs(TLR1~9、TLR11~13)[5-6],而鸡和鸭均有10种TLRs基因,其中TLR15为禽类特有[7]。禽类TLRs基因家族广泛分布于机体各组织器官中。在鸡不同胚龄组织中,各TLRs基因的表达存在极显著差异(P < 0.01)[8]。TLR3和TLR7能介导鸭对病毒感染的先天免疫过程[9]。TLR4主要参与脂多糖(LPS)的识别,注射LPS能刺激鸡小脑的TLR4极显著上调(P < 0.01)[10-11]。由此可见,分析组织器官中TLRs家族成员的表达变化可以为探究其功能提供依据。
法氏囊作为禽类特有的中枢免疫器官,是影响其发育、功能形成和免疫功能发挥的大量关键基因的聚集地[12]。在鸡5胚龄时法氏囊开始发育,9~28日龄为生长高峰,56日龄后开始退化[13]。而鸭法氏囊在17周龄时开始退化[14]。研究发现,鸡法氏囊中的TLR3和TLR15、TLR2a和TLR4基因分别在识别马立克氏病毒和新城疫病毒中起着关键作用[15-16]。
基因家族各成员一般来源于祖先基因的基因重复以及分子进化[17-18]。基因重复导致了基因多样化发展,而随着新功能和基因缺失的增加,基因家族出现亚功能化,最终导致进化分歧[19-20]。在其他物种上,FoxO基因家族和Coq1基因家族等都在长期的进化过程中呈现出了不同形式的功能分化[21-22]。为探寻鸭TLRs基因家族各成员的功能差异,本研究拟检测TLRs家族各成员mRNA在鸭法氏囊胚后发育各阶段的相对表达模式,并结合分析启动子区和编码区的系统进化树,以探讨TLRs基因家族表达模式、功能分化与基因分子进化的关系,进一步为TLRs基因家族的研究分析提供基础数据。
1 材料与方法 1.1 试验材料选取四川农业大学水禽育种场提供的0、2、4和6周龄的健康农华肉鸭(GF2),每周龄公母各3只(共24只),屠宰后采集法氏囊样品,并用液氮研磨后放入-80 ℃超低温冰箱冻存。
1.2 试验方法 1.2.1 鸭法氏囊TLRs的定量引物设计根据GenBank数据库中鸭TLRs mRNA基因序列,利用Primer Premier 5.0软件设计TLRs基因编码区定量引物(表 1),通过PCR扩增、克隆、测序,用Blast程序将测序结果与GenBank数据库中的已知序列进行比对,检测引物的正确性和特异性,包括鸭TLRs基因家族全部10个成员。
|
|
表 1 鸭TLRs基因和内参基因定量引物信息 Table 1 Information of primers of duck TLRs and reference gene |
采用Trizol法提取鸭法氏囊的总RNA,检测浓度和完整性后,反转录(试剂盒购自TaKaRa公司)合成模板DNA(cDNA),稀释后作为检测模板在荧光定量PCR仪(Bio-Rad)上进行qRT-PCR反应,以β-actin为内参基因,每个样品设3个重复,测定TLRs各成员的相对转录水平。
PCR反应体系为10 μL:模板DNA 1 μL,上下游引物各0.5 μL,PCR Mix 5 μL,ddH2O 3 μL。PCR扩增程序:95 ℃预变性5 min;95 ℃变性30 s,各基因退火温度如表 1所示,退火时间30 s,72 ℃延伸50 s,30个循环;72 ℃最终延伸10 min。
qRT-PCR反应体系为20 μL:SYBR@Premix EX TapTM(2×) 10 μL,上下游引物各0.4 μL,ddH2O 7.2 μL,模板DNA 2 μL。反应程序:95 ℃预变性30 s;95 ℃变性5 s,60 ℃退火30 s,40个循环;65~95 ℃按0.5 ℃增值进行熔解曲线分析。
1.2.3 构建TLRs编码区和启动子区系统进化树TLRs基因序列均下载自NCBI数据库(http://www.ncbi.nlm.nih.gov/)。使用了人(Homo sapiens)、小鼠(Mus musculus)、鸡(Gallus gallus)和鸭(Anas platyrhynchos)的TLRs编码区序列,选取转录起始位点前2 000 bp作为启动子区序列。利用MEGA 7.0软件的邻接法(Neighbor-Joining),Bootstrap分析重复数为1 000,构建TLRs基因家族系统进化树,其余参数默认不变(表 2)。
|
|
表 2 系统进化树参考基因信息 Table 2 The information of reference genes of phylogenetic tree |
qRT-PCR结果用Excel 2016整理,采用2-ΔΔC(T)方法计算,结果使用内参基因β-actin进行标准化校正。用Graphpad Prism 7.0绘图,Mev软件进行聚类分析,SPSS 22.0的ANOVA方法进行显著性比较,P < 0.05表示差异显著。
2 结果 2.1 鸭法氏囊胚后发育过程中TLRs的表达PCR扩增试验结果显示,每个产物均得到单一目的条带,且与预期片段长度一致(图 1),说明定量引物特异性较好。通过PCR扩增试验找到每个TLR定量引物的最适熔解温度(表 1)。从图 2可以看出,TLR2a的表达水平在鸭法氏囊胚后发育的W2和W6时期显著高于W4时期(P < 0.05),其余TLRs各成员在鸭法氏囊胚后发育期的表达差异不显著(P>0.05)。
|
图 1 TLRs的PCR扩增产物电泳结果 Figure 1 The PCR amplification electrophoresis results of TLRs |

|
同一基因在4个时期的表达中,不同小写字母表示差异显著(P < 0.05) Different lowercase letters indicate significant differences of the same gene expression among 4 periods (P < 0.05) 图 2 鸭法氏囊胚后发育中TLRs的表达情况 Figure 2 The expression of TLRs in the postembryonic development stage of duck bursa of Fabricius |
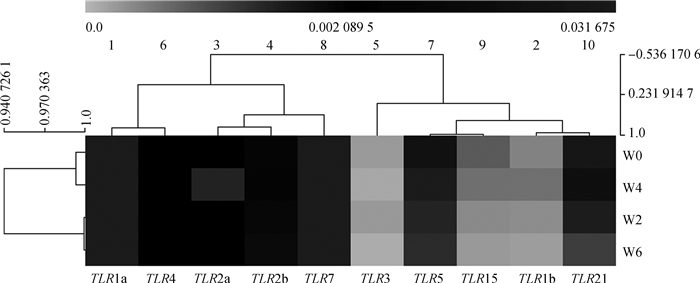
如图 3,TLR1a、TLR2a和TLR2b的表达模式相近,TLR3、TLR5和TLR15也接近,TLR1b和TLR21的表达模式相近,TLRs基因家族的表达模式可以分为两大类:TLR1a、TLR4、TLR2a、TLR2b和TLR7为一类,其余的TLR3、TLR5、TLR15、TLR1b和TLR21归为一类。
|
图 3 鸭法氏囊胚后发育期TLRs表达量热图 Figure 3 The expression heatmap of TLRs in the postembryonic development stage of duck bursa of Fabricius |
分析TLRs编码区进化树(图 4b)可以看出,人、小鼠、鸡和鸭的TLRs基因较为对应的聚在同一分支上。TLR1(1a、1b)和TLR2(2a、2b)聚为一支,与禽类特有的TLR15距离较近;TLR3和TLR5在同一个分支节点上;TLR4和TLR21为单独两分支;鸡和鸭的TLR7与人和小鼠的TLR7、TLR8、TLR9聚在同一支;禽类的TLR21与其他TLRs距离最远。而基于TLRs启动子区构建的系统进化树无明显规律(图 4a)。

|
A为TLRs启动子区进化树;B为TLRs编码区进化树,标记为TLRs各成员所在的亚家族 A is phylogenetic tree based on TLRs promoter sequence; B is phylogenetic tree based on TLRs coding sequence, the TLRs included in each family are also labeled 图 4 TLRs系统进化树 Figure 4 Phylogenetic trees of TLRs |
在机体免疫应答反应中,TLRs基因家族是极为重要的模式识别受体,通过识别脂多糖、鞭毛蛋白、dsRNA等PAMPs在先天性免疫反应中扮演着重要角色[23]。TLRs基因家族的表达与其行使的功能紧密相关。TLR3和TLR7分别参与了鸡法氏囊对传染性法氏囊病毒和H9N2禽流感病毒的感染应答,表明TLRs在禽类免疫信号感知以及法氏囊免疫功能发挥等方面扮演了重要角色[24-25]。然而,最早发现的果蝇Toll基因被认为是一个发育调控蛋白,鸡TLRs基因在10胚龄到2日龄期间存在明显的表达变化,雏鸭免疫器官中TLRs的表达从33胚龄到1日龄呈显著上升趋势[26-28]。这些研究提示,TLRs基因家族的表达与胚胎发育调控和早期免疫功能有关。在胚胎发育阶段,获得性免疫尚未发育完全,先天性免疫系统已经建立了应对早期致病性攻击的免疫准备[29]。
研究发现,TLRs基因在2~21日龄的雏鸡上并不存在时期表达特异性[27]。在10和30日龄的鸡法氏囊上也并无差异表达基因涉及到TLRs信号通路[30]。本研究结果与现有报道基本一致,除TLR2a外,其余TLRs成员在鸭法氏囊发育阶段的表达均不存在显著差异(P>0.05),提示TLRs基因的表达情况基本稳定,可能不参与鸭法氏囊胚后发育调控过程。另外,禽类TLR2a能识别细菌细胞壁成分,功能与哺乳动物的TLR2和TLR4相近[31]。本研究中,TLR2a在4周龄鸭法氏囊的表达水平显著低于2和6周龄(P < 0.05),这可能与该时期鸭法氏囊识别细菌的功能有关,然而仅检测TLRs的表达水平并不能完全代表Toll样受体蛋白的表达情况[32],因此,TLR2a对4周龄鸭法氏囊的发育及功能有何影响还有待进一步研究。
基因家族的进化是由祖先基因通过重复和歧化进化而来,各成员可能具有相同或相关的功能[18]。同时基因的进化也会向着不同物种和种系特异性的方向发展,从而产生特有的基因[33]。Toll基因最初只具有促进机体发育的功能,而由于受到病原体介导的正向选择压力的作用,导致TLRs基因在不同物种的进化中出现差异[19]。本研究中,TLR1a和TLR1b、TLR2a和TLR2b具有较高的同源性,TLR3、TLR5和TLR15的遗传距离较近,TLR21与其余TLRs的遗传关系最远。基因家族中各成员的表达模式比较可以为探究该基因家族的生理生化功能和基因功能分化提供重要信息[34]。如Lopes-Marques等[20]对ACSLs基因家族的研究发现,ACSL1a和1b、ACSL3a和3b、ACSL4a和4b的进化距离较近,其中ACSL3a和3b、ACSL4a和4b功能相似,而ACSL1a和ACSL1b的组织分布表达完全不同,提示其功能存在差异。结合分析TLRs的表达模式与序列进化发现,TLR2a和TLR2b,以及TLR3、TLR5和TLR15具有相似的表达模式和较近的遗传距离,提示其可能具有相似的功能。鸭TLR1a和TLR1b的同源性较高,但表达模式不同,表明可能在基因进化过程中出现了功能分化。
启动子区位于转录起始位点上游,是调控基因表达、决定基因活动的重要结构,TLRs各成员的表达模式差异是否与启动子区的序列进化有关?为此,本研究对TLRs家族各成员启动子区进行了聚类分析,未发现明显规律,这可能是因为基因的表达与调控是一个复杂过程,涉及到了多个基因和多条信号通路,因此TLRs基因家族表达模式差异可能与启动子区进化无关。
4 结论TLRs家族成员在鸭法氏囊中均有表达,且在胚后发育阶段表达变化不明显,提示TLRs家族成员可能不参与鸭法氏囊胚后发育调控过程;TLR2a和TLR2b,以及TLR3、TLR5和TLR15具有相似的表达模式和较近的遗传距离,其功能可能相关;TLR1a和TLR1b虽然具有较高的序列相似性,但表达模式不同,可能出现了功能分化。研究排除了TLRs表达模式与启动子区序列进化的联系。
| [1] | ROACH J C, GLUSMAN G, ROWEN L, et al. The evolution of vertebrate Toll-like receptors[J]. Proc Natl Acad Sci USA, 2005, 102(27): 9577–9582. DOI: 10.1073/pnas.0502272102 |
| [2] | AKIRA S. Toll receptor families:structure and function[J]. Semin Immunol, 2004, 16(1): 1–2. |
| [3] |
郭佳佳, 戴子淳, 陆晨, 等. TLR家族基因在产蛋高峰期和停产期鸭肠道中的表达研究[J]. 畜牧兽医学报, 2016, 47(10): 2012–2019.
GUO J J, DAI Z C, LU C, et al. Expression of toll-like receptors in duck intestinal tract at the peak and end of lay[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(10): 2012–2019. (in Chinese) |
| [4] | WANG J L, ZHANG Z, LIU J, et al. Ectodomain architecture affects sequence and functional evolution of vertebrate toll-like receptors[J]. Sci Rep, 2016, 6: 26705. DOI: 10.1038/srep26705 |
| [5] | TRINCHIERI G, SHER A. Cooperation of Toll-like receptor signals in innate immune defence[J]. Nat Rev Immunol, 2007, 7(3): 179–190. DOI: 10.1038/nri2038 |
| [6] | BUCKLEY K M, RAST J P. Dynamic evolution of toll-like receptor multigene families in echinoderms[J]. Front Immunol, 2012, 3: 136. |
| [7] | JIA H, LI G, LI J, et al. Cloning, expression and bioinformatics analysis of the duck TLR 4 gene[J]. Br Poult Sci, 2012, 53(2): 190–197. DOI: 10.1080/00071668.2012.674208 |
| [8] | KANNAKI T R, REDDY M R, VERMA P C, et al. Differential toll-like receptor (TLR) mRNA expression patterns during chicken embryological development[J]. Anim Biotechnol, 2015, 26(2): 130–135. DOI: 10.1080/10495398.2014.939658 |
| [9] | SONG C P, YU S Q, DUAN Y B, et al. Effect of age on the pathogenesis of DHV-1 in Pekin ducks and on the innate immune responses of ducks to infection[J]. Arch Virol, 2014, 159(5): 905–914. DOI: 10.1007/s00705-013-1900-7 |
| [10] | GONG Y W, FENG S S, LI S Q, et al. Genome-wide characterization of Toll-like receptor gene family in common carp (Cyprinus carpio) and their involvement in host immune response to Aeromonas hydrophila infection[J]. Comp Biochem Physiol Part D Genomics Proteomics, 2017, 24: 89–98. DOI: 10.1016/j.cbd.2017.08.003 |
| [11] | ANSARI A R, WEN L, HUANG H B, et al. Lipopolysaccharide stimulation upregulated toll-like receptor 4 expression in chicken cerebellum[J]. Vet Immunol Immunopathol, 2015, 166(3-4): 145–150. DOI: 10.1016/j.vetimm.2015.05.004 |
| [12] |
刘俊莹.两品种鸭法氏囊差异表达基因、miRNA的富集及验证[D].雅安: 四川农业大学, 2015.
LIU J Y.Enrichment and verification of differentially expressed genes and miRNAs in bursa of Fabricius in two breeds of duck[D].Ya'an: Sichuan Agricultural Uniersity, 2015.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10626-1016049041.htm |
| [13] | SETO F. Early development of the avian immune system[J]. Poult Sci, 1981, 60(9): 1981–1995. DOI: 10.3382/ps.0601981 |
| [14] |
方静, 崔恒敏, 廖婷彬, 等. 胚胎及胚后发育期鸭腔上囊淋巴细胞增殖与凋亡的研究[J]. 畜牧兽医学报, 2008, 39(11): 1581–1587.
FANG J, CUI H M, LIAO T B, et al. Study on lymphocyte proliferation and apoptosis in the embryonic and post embryonic development of the duck bursa of Fabricius[J]. Acta Veterinaria et Zootechnica Sinica, 2008, 39(11): 1581–1587. DOI: 10.3321/j.issn:0366-6964.2008.11.021 (in Chinese) |
| [15] | JIE H, LIAN L, QU L J, et al. Differential expression of Toll-like receptor genes in lymphoid tissues between Marek's disease virus-infected and noninfected chickens[J]. Poult Sci, 2013, 92(3): 645–654. DOI: 10.3382/ps.2012-02747 |
| [16] |
侯巍, 岳华, 汤承. 荧光定量RT-PCR检测雏鸡感染NDV强毒后胸腺和法氏囊中TLR2与TLR4 mRNA的表达[J]. 中国预防兽医学报, 2010, 32(4): 254–258.
HOU W, YUE H, TANG C. Development of a real-time RT-PCR for detection of Toll-like receptor2 and Toll-like receptor4 mRNAs in bursals and thymus of chicken infected with virulent NDV[J]. Chinese Journal of Preventive Veterinary Medicine, 2010, 32(4): 254–258. (in Chinese) |
| [17] | EFSTRATIADIS A, POSAKONY J W, MANIATIS T, et al. The structure and evolution of the human β-globin gene family[J]. Cell, 1980, 21(3): 653–668. DOI: 10.1016/0092-8674(80)90429-8 |
| [18] |
李萌.脊椎动物MAPK家族的进化研究和三角河蚌防御素基因(Defensins)的克隆与分析[D].镇江: 江苏大学, 2012.
LI M.The evolution of vertebrate MAPK family and the Triangle Mussel defensin gene (Defensins) cloning and analysis[D].Zhenjiang: Jiangsu University, 2012.(in Chinese) http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y2092481 |
| [19] | DARFOUR-ODURO K A, MEGENS H J, ROCA A L, et al. Adaptive evolution of toll-like receptors (TLRs) in the family suidae[J]. PLoS One, 2015, 10(4): e124069. |
| [20] | LOPES-MARQUES M, CUNHA I, REIS-HENRIQUES M A, et al. Diversity and history of the long-chain acyl-CoA synthetase (Acsl) gene family in vertebrates[J]. BMC Evol Biol, 2013, 13: 271. DOI: 10.1186/1471-2148-13-271 |
| [21] | WANG M H, ZHANG X Z, ZHAO H B, et al. FoxO gene family evolution in vertebrates[J]. BMC Evol Biol, 2009, 9: 222. DOI: 10.1186/1471-2148-9-222 |
| [22] | ZHAO M, YING X X, SUN J, et al. Molecular evolution of Coq1 gene family in eukaryotes[J]. J Syst Evol, 2017, 55(5): 417–425. DOI: 10.1111/jse.v55.5 |
| [23] | LAN D L, LIN B S, XIONG X R, et al. Identification and characteristics analysis of toll-like receptors family genes in yak[J]. Genes Genomics, 2016, 38(5): 429–438. DOI: 10.1007/s13258-016-0390-x |
| [24] |
赵霞, 张瑞莉, 王海彬, 等. IBDV感染SPF雏鸡法氏囊中TLR3表达的动态变化[J]. 黑龙江畜牧兽医, 2016(5): 168–170.
ZHAO X, ZHANG R L, WANG H B, et al. Dynamic changes in the expression of TLR3 in the bursa of Fabricius of chicks infected with IBDV[J]. Heilongjiang Animal Science and Veterinary Medicine, 2016(5): 168–170. (in Chinese) |
| [25] |
王建琳, 曹志伟, 王冬冬, 等. 不同致病性H9N2亚型禽流感病毒诱导鸡TLR-7和Mx基因mRNA转录水平的差异[J]. 畜牧兽医学报, 2017, 48(5): 907–913.
WANG J L, CAO Z W, WANG D D, et al. Transcriptional responses of TLR-7 and Mx mRNA in 3 chicken host systems infected with H9N2 influenza viruses with different pathogenicities[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(5): 907–913. (in Chinese) |
| [26] | ZHANG A G, XU J H, LAI H Z, et al. Age-related changes and distribution of T cell markers (CD3 and CD4) and toll-like receptors(TLR2, TLR3, TLR4 and TLR7) in the duck lymphoid organs[J]. Immunobiology, 2017, 222(7): 857–864. DOI: 10.1016/j.imbio.2017.01.004 |
| [27] | LIU X D, ZHANG F B, SHAN H, et al. mRNA expression in different developmental stages of the chicken bursa of Fabricius[J]. Poult Sci, 2016, 95(8): 1787–1794. DOI: 10.3382/ps/pew102 |
| [28] | HASHIMOTO C, HUDSON K L, ANDERSON K V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein[J]. Cell, 1988, 52(2): 269–279. DOI: 10.1016/0092-8674(88)90516-8 |
| [29] | MEADE K G, HIGGS R, LLOYD A T, et al. Differential antimicrobial peptide gene expression patterns during early chicken embryological development[J]. Dev Comp Immunol, 2009, 33(4): 516–524. DOI: 10.1016/j.dci.2008.10.003 |
| [30] |
杭柏林, 桑建君, 钱琨, 等. 利用基因芯片分析鸡法氏囊差异表达基因[J]. 畜牧兽医学报, 2014, 45(7): 1162–1169.
HANG B L, SANG J J, QIAN Q, et al. Analysis of differential expression genes in chicken bursa of Fabricius with gene expression array[J]. Acta Veterinaria et Zootechnica Sinica, 2014, 45(7): 1162–1169. (in Chinese) |
| [31] | FULUI A, INOUE N, MATSUMOTO M, et al. Molecular cloning and functional characterization of chicken toll-like receptors.A single chicken toll covers multiple molecular patterns[J]. J Biol Chem, 2001, 276(50): 47143–47149. DOI: 10.1074/jbc.M103902200 |
| [32] | TIRUMURUGAAN K G, DHANASEKARAN S, RAJ G D, et al. Differential expression of toll-like receptor mRNA in selected tissues of goat (Capra hircus)[J]. Vet Immunol Immunopathol, 2010, 133(2-4): 296–301. DOI: 10.1016/j.vetimm.2009.08.015 |
| [33] | TEMPERLEY N D, BERLIN S, PATON I R, et al. Evolution of the chicken toll-like receptor gene family:a story of gene gain and gene loss[J]. BMC Genomics, 2008, 9: 62. DOI: 10.1186/1471-2164-9-62 |
| [34] |
汤龙军, 李鹏程, 朱璐, 等. 天冬氨酸蛋白酶在拟南芥和水稻中的分子进化、表达模式以及在花药发育中的功能分析[J]. 植物生理学报, 2015, 51(3): 323–336.
TANG L J, LI P C, ZHU L, et al. Identification, evolutionary and expression profile analysis of the aspartic protease gene superfamily in arabidopsis thaliana and rice[J]. Plant Physiology Journal, 2015, 51(3): 323–336. (in Chinese) |



