缺血再灌注损伤(ischemia and reperfusion injury,IRI)在临床上比较常见,常见于肾移植手术、肾部分切除术、肾血管成形术等[1, 2]。缺血可导致肾脏急性再灌注损伤,同时缺血再灌注损伤也是导致急性肾功能衰竭的主要原因之一[3]。既往研究已经表明肾缺血再灌注损伤的机制较多,包括氧化应激、炎症反应、细胞凋亡等[4]。有学者研究发现长期的肾缺血再灌注损伤会导致慢性肾纤维化改变[5]。由于引起肾脏缺血再灌注损伤病因机制较多,且再灌注损伤后并发症较多,因此在临床上如何减轻肾缺血缺氧再灌注损伤及随之而来的并发症成为国内外众多学者研究的热点。
臭氧可以通过激活肾小管内皮细胞抗氧化机制,减轻小管细胞氧化还原反应,从而可以减少组织的氧化应激损伤[6]。关于臭氧在肾脏缺血再灌注损伤中的保护作用,也有过学者研究,如臭氧预处理可以通过对TLR4-NF-κB信号通路调节,保护肾缺血再灌注后肾组织的损伤[7];诱导eNOS合成增多、一氧化氮产生增多保护缺血再灌注损伤后的肾组织[8]。
缺血后处理是指在再灌注早期进行多次快速间歇阻断血流,从而机械地改变再灌注的流体动力学[9, 10]。自从Zhao等人[11]第一次报道缺血后处理能够有效保护心脏缺血再灌注损伤后,有许多学者证明缺血后处理在肾脏、大脑等缺血再灌注损伤中分别通过不同的机制同样具有脏器和组织的保护作用[12, 13]。
然而,臭氧后处理联合缺血后处理对大鼠肾脏缺血再灌注损伤引起的肾脏组织损伤及其肾功能的保护作用,目前还尚无相关报道。
1 材料与方法 1.1 模型的建立与分组健康雄性SD大鼠50只,周龄8-10周,体重为250-300 g,由武汉大学医学院实验动物中心提供。随机将大鼠分为以下5组,每组10只:①假手术组:腹腔注射20 g/L戊巴比妥钠(40 mg/kg),麻醉大鼠,然后游离双侧肾脏,切除右肾后缝合腹壁;IRI组:手术步骤与假手术组相同,但在游离双侧肾脏及切除右肾后,用无创伤血管夹夹闭左肾动、静脉45 min进行缺血,然后开放血管夹进行再灌注,肾脏由暗红变色鲜红色代表再灌注成功。缺血后处理组:手术步骤与IRI组相同,但在缺血后、再灌注之前,对肾动静脉进行开放10 s、夹闭10 s的6个循环后,再灌注24 h。臭氧后处理组:手术步骤与IRI组相同,但在术后10 d对该组大鼠经直肠吹入氧气和臭氧的混合气体2.5-3.0 ml[臭氧浓度为50 mg/L,0.5 mg/(kg·d)][14]。缺血后处理+臭氧后处理组:手术步骤与IRI组相同,但在缺血后、再灌注之前,对肾动静脉进行开放10 s、夹闭10 s的6个循环后,再进行臭氧后处理。
1.2 试剂臭氧发生仪由珠海依科医疗器械有限公司提供,聚ADP核糖聚合酶(PARP)-1单克隆抗体购自Santa Cruz公司,BCA蛋白浓度测定试剂盒购自碧云天生物技术研究所。
1.3 样本采集和处理 1.3.1 标本采集分别于IRI 24 h后采集大鼠下腔腹腔静脉血待测,处死各组大鼠获取肾脏组织,一部分迅速置于液氮中保存,另一部分用固定液固定后,常规石蜡包埋。
1.3.2 血清尿素氮(BUN)和肌酐(Cr)的检测将采集的大鼠血液标本常温离心,获取大鼠血清,使用Olympus全自动生化分析仪(Au2700)测定血清Cr和BUN水平。
1.3.3 肾组织病理学检查取大鼠肾组织,用多聚甲醛固定后,脱水,透明,浸蜡,包埋,切片,脱蜡,HE染色,后在400倍光镜下观察其病理改变。
1.3.4 TUNEL法检测肾组织细胞凋亡TUNEL染色后,光镜下观察细胞,细胞核呈棕黄色者为凋亡细胞。
1.3.5 免疫组化检测各组大鼠PARP-1表达肾组织石蜡切片,厚4 μm,按(streptavidin-perosidase,SP)法免疫组化试剂盒进行操作,加入PARP-1(1:100)抗体及相应二抗,DAB显色5 min后封片。细胞核有棕黄色表达者为阳性细胞。
1.3.6 Realtime PCR检测TNF-α、IL-1β和ICAM-1抽提总RNA,核酸蛋白分析仪测定RNA浓度后,取总RNA 1 g,逆转录生成cDNA。TNF-α上游引物:5′-CTTCTCATTCCTGCTCGTGG -3′,下游引物:5′-TCCGCTTGGTGGTTTGCTAC-3′;IL-1β上游引物:5′-ACTATGGCAACTGTCCCTGAAC-3′,下游引物:5′-GTGCTTGGGTCCTCATCCTG-3′;ICAM-1上游引物:5′-GGGATGGTGAAGTCTGTCAA-3′,下游引物:5′-GGCGGTAATAGGTGTAAATGG-3′;内参β-actin上游引物5′-TGCTATGTTGCCCTAGACTTCG-3′,下游引物5′-GTTGGCATAGAGGTCTTTACGG-3′。PCR反应条件均为:94 ℃预变性5 min,94 ℃变性30 s,58 ℃退火30 s,72 ℃延伸1 min,扩增35个循环,最后一次为72 ℃延伸7 min。结果以目的基因与β-actin的比值表示。
1.4 统计学分析采用SPSS 17.0统计软件进行数据处理,所得实验数据以均数±标准差表示,采用方差分析,P<0.05为差异有统计学意义。
2 结果 2.1 各组大鼠血清Cr和BUN水平的比较缺血后处理和臭氧后处理均可以减轻由于缺血再灌注导致的肾功能损伤,差异有统计学意义(P<0.05)。而与缺血后处理组或臭氧后处理组比较,缺血后处理联合臭氧后处理组可以显著改善肾功能水平,差异有统计学意义(P<0.05),见图 1。
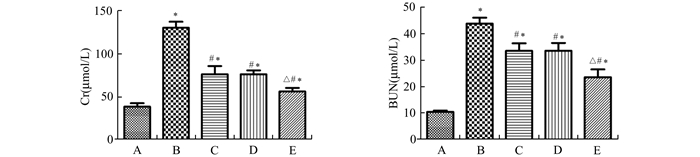
|
图 1 各组大鼠血清Cr、BUN的水平(x±s) A.假手术组;B. IRI组;C.缺血后处理组;D.臭氧后处理组;E.缺血后处理+臭氧后处理组; 与假手术组比较,*P<0.05;与IRI组比较,#P<0.05;与缺血后处理和臭氧后处理组比较,△P<0.05 |
光镜下假手术组无明显改变。IRI组肾小管上皮细胞明显变性坏死,大量炎症细胞浸润,可见管型。而缺血后处理或臭氧后处理组在光镜下可见部分近曲小管细胞轻度水肿样变性,坏死仅累及近端近曲小管,偶见管型。缺血后处理联合臭氧后处理组其细胞水肿、坏死程度明显比缺血后处理或臭氧后处理组减轻。各组肾组织病理学改变见图 2。
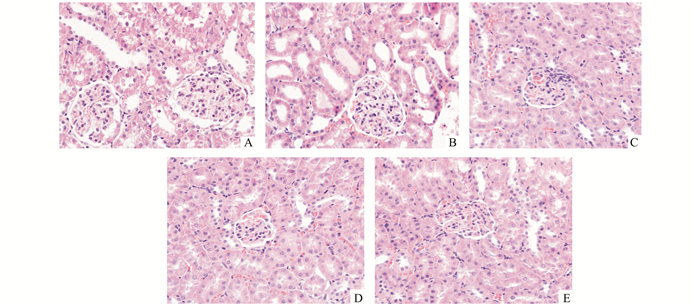
|
图 2 各组大鼠肾组织学检查结果(×400) A.假手术组;B. IRI组;C.缺血后处理组;D.臭氧后处理组;E.缺血后处理+臭氧后处理组 |
缺血后处理和臭氧后处理均可以减轻由于缺血再灌注导致的细胞凋亡。而与缺血后处理组或臭氧后处理组比较,缺血后处理联合臭氧后处理组可以显著降低细胞凋亡水平,见图 3。
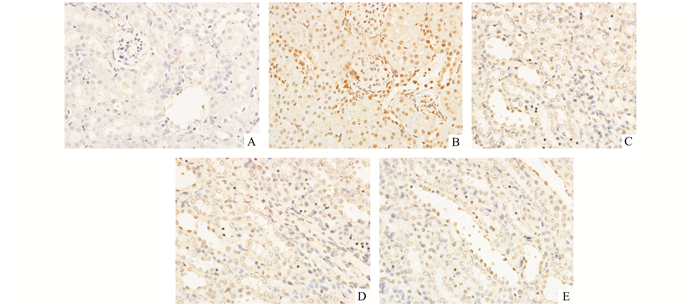
|
图 3 各组大鼠肾组织细胞凋亡情况(×400) A.假手术组;B. IRI组;C.缺血后处理组;D.臭氧后处理组;E.缺血后处理+臭氧后处理组 |
缺血后处理和臭氧后处理均可以抑制由于缺血再灌注损伤导致的PARP-1表达升高。而与缺血后处理组或臭氧后处理组比较,缺血后处理联合臭氧后处理组可以显著降低PARP-1的表达,见图 4。
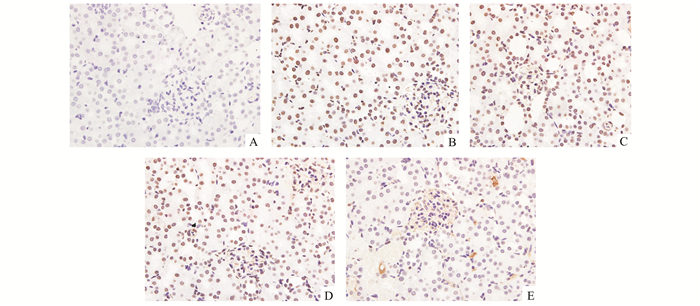
|
图 4 各组大鼠PARP-1表达情况 A.假手术组;B. IRI组;C.缺血后处理组;D.臭氧后处理组;E.缺血后处理+臭氧后处理组 |
与假手术组比较, 缺血再灌注组的TNF-α、IL-1β和ICAM-1的mRNA水平显著增高,差异有统计学意义(P<0.05)。与缺血再灌注组比较,缺血后处理或臭氧后处理组的TNF-α、IL-1β和ICAM-1的mRNA水平明显减低,差异有统计学意义(P<0.05)。而与缺血后处理或臭氧后处理组比较,其联合应用组抑制上述炎性因子mRNA更加明显,见图 5。
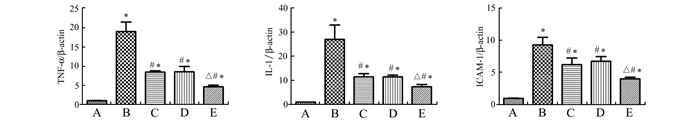
|
图 5 各组大鼠PCR结果 A.假手术组;B. IRI组;C.缺血后处理组;D.臭氧后处理组;E.缺血后处理+臭氧后处理组。与假手术组比较,*P<0.05;与IRI组比较,#P<0.05;与缺血后处理和臭氧后处理组比较,△P<0.05 |
缺血会造成脏器形态和功能的损害,尤其在再灌注时会更严重。再灌注期间通过释放活性氧(reactive oxygen species,ROS), 包括长氧化物、过氧化氢、羟基等被认为是加重肾脏组织损伤的重要因素[15]。当ROS产生过多且没有被抗氧化物质中和时,会引起细胞膜脂质过氧化,和不饱和脂肪酸反应造成脏器结构和功能的进一步改变[16]。此外,有研究表明,ROS可破环肾小球滤过屏障的完整性,损伤其组成部分,如Ⅳ型胶原纤维、蛋白聚糖、唾液蛋白等[17-19]。而臭氧可以激活内源性抗氧化体系,诱导脏器组织适应氧化应激,减轻脏器功能和组织的损伤[20]。有学者在大鼠肝脏缺血再灌注模型中研究中发现,臭氧能够通过上调抗氧化系统,纠正慢性氧化应激反应,从而实现体内氧化还原平衡[21, 22]。
臭氧治疗在医疗领域有着多方面用途,外科系统最初用于创伤性疾病的治疗。有临床研究表明,将臭氧运用于心脏病与糖尿病的治疗可以控制氧化应激,提高抗氧化体系的作用[23, 24]。此外,臭氧已运用于血管病变、整形外科(椎间盘)以及口腔科治疗中[8]。对于臭氧是如何减轻脏器组织的病理损伤以及脏器缺血后再灌注损伤引起的功能障碍,有研究表明可能与臭氧氧化预处理使宿主对ROS产生耐受有关[25]。但其具体机制还有待进一步研究。
自从自从Zhao等人[11]在2003年第一次报道缺血后处理,近年来有不少学者对于缺血后处理在心脏[26]、神经系统[27]、肢体[28]等脏器组织中也有很多相关的研究。在肾脏缺血再灌注损伤的保护研究中,有学者研究发现缺血后处理可以通过下调TLR4(toll-like receptor 4),从而减轻缺血再灌注后的肾脏组织损伤[29]。Gao等人[30]研究发现,缺血后处理通过激活T-LAK细胞来源的蛋白激酶(TOPK)/PTEN/Akt信号通路所介导抗氧化和抗炎症作用,减轻缺血再灌注损伤。因此,缺血后处理在肾脏缺血再灌注损伤中的保护作用也是近年研究的一个热点。
本研究结果表明,臭氧后处理组肾功能较缺血再灌注组有改善, 但联合缺血后处理,肾功能显著改善。同时,缺血后处理或臭氧后处理组在光镜下可见肾组织损伤程度减轻,缺血后处理联合臭氧后处理组其细胞水肿、坏死程度明显比缺血后处理或臭氧后处理组减轻。缺血后处理和臭氧后处理均可以减轻由于缺血再灌注导致的细胞凋亡,缺血后处理联合臭氧后处理组可以显著降低细胞凋亡水平。此外,缺血后处理和臭氧后处理均可以抑制由于缺血再灌注损伤导致的凋亡标志PARP-1表达升高,而缺血后处理联合臭氧后处理组可以显著降低蛋白PARP-1的表达。细胞炎性因子的表达水平也呈现出相同的趋势,即缺血后处理联合臭氧后处理后TNF-α、IL-1β和ICAM-1的mRNA水平也显著下降。这表明,肾脏缺血再灌注损伤后,缺血后处理联合臭氧后处理能够有效保护肾功能,减轻炎症反应和肾组织损伤,从而为临床上缺血再灌注损伤提供了新的治疗思路。
综上所述,缺血后处理联合臭氧后处理可作为有效而又价廉的治疗措施,在减轻缺血再灌注对肾脏组织的损伤、改善肾功能方面具有潜在的应用价值。但其具体分子机制及临床应用前景还有待进一步研究。
| [1] | Sagiroglu T, Torun N, Yagci M, et al. Effects of apelin and leptin on renal functions following renal ischemia/reperfusion: An experimental study[J]. Exp Ther Med, 2012, 3(5): 908-914. DOI: 10.3892/etm.2012.499. |
| [2] | Yun Y, Duan WG, Chen P, et al. Ischemic postconditioning modified renal oxidative stress and lipid peroxidation caused by ischemic reperfusion injury in rats[J]. Transplant Proc, 2009, 41(9): 3597-3602. DOI: 10.1016/j.transproceed.2009.06.203. |
| [3] | Devarajan P. Update on mechanisms of ischemic acute kidney injury[J]. J Am Soc Nephrol, 2006, 17(6): 1503-1520. DOI: 10.1681/ASN.2006010017. |
| [4] | Kusch A, Hoff U, Bubalo G, et al. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury[J]. Acta Physiol (Oxf), 2013, 208(1): 25-40. DOI: 10.1111/apha.2013.208.issue-1. |
| [5] | Wang M, Weng X, Guo J, et al. Metformin alleviated EMT and fibrosis after renal ischemia-reperfusion injury in rats[J]. Ren Fail, 2016, 38(4): 614-621. DOI: 10.3109/0886022X.2016.1149770. |
| [6] | Barber E, Menendez S, Leon OS, et al. Prevention of renal injury after induction of ozone tolerance in rats submitted to warm ischaemia[J]. Mediators Inflamm, 1999, 8(1): 37-41. DOI: 10.1080/09629359990702. |
| [7] | Xing B, Chen H, Wang L, et al. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury via modulation of the TLR4-NF-kappaB pathway[J]. Acta Cir Bras, 2015, 30(1): 60-66. DOI: 10.1590/S0102-86502015001000008. |
| [8] |
邱涛, 周江桥, 陈晖, 等. 臭氧氧化预处理在肾缺血再灌注损伤中对内皮型一氧化氮合酶表达的影响[J].
中华移植杂志:电子版, 2013, 7(2): 83-88.
Qiu T, Zhou JQ, Chen H, et al. Effects of ozone oxidative preconditioning on eNOS in rats with renal ischemia/reperfusion injury[J]. Chinese Journal of Transplantation: Electronic Version, 2013, 7(2): 83-88. |
| [9] | Jiang B, Liu X, Chen H, et al. Ischemic postconditioning attenuates renal ischemic/reperfusion injury in mongrel dogs[J]. Urology, 2010, 76(6): 1511-1519. |
| [10] | Knudsen AR, Kannerup AS, Dich R, et al. Ischemic pre-and postconditioning has pronounced effects on gene expression profiles in the rat liver after ischemia/reperfusion[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 303(4): G482-G489. DOI: 10.1152/ajpgi.00337.2011. |
| [11] | Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning[J]. Am J Physiol Heart Circ Physiol, 2003, 285(2): H579-H588. DOI: 10.1152/ajpheart.01064.2002. |
| [12] | Wever KE, Menting T, Masereeuw R, et al. Local and remote ischemic postconditionings have synergistic protective effects on renal ischemia-reperfusion injury[J]. Transplantation, 2012, 94(1): e1-e2. DOI: 10.1097/TP.0b013e318257ad76. |
| [13] | Wang JY, Shen J, Gao Q, et al. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats[J]. Stroke, 2008, 39(3): 983-990. DOI: 10.1161/STROKEAHA.107.499079. |
| [14] | Fernandez IA, Gonzalez NL, Calunga FJ, et al. Ozone postconditioning in renal ischaemia-reperfusion model. Functional and morphological evidences[J]. Nefrologia, 2011, 31(4): 464-470. |
| [15] | Greene EL, Paller MS. Oxygen free radicals in acute renal failure[J]. Miner Electrolyte Metab, 1991, 17(2): 124-132. |
| [16] | Calunga JL, Trujillo Y, Menendez S, et al. Ozone oxidative post-conditioning in acute renal failure[J]. J Pharm Pharmacol, 2009, 61(2): 221-227. DOI: 10.1211/jpp.61.02.0012. |
| [17] | Calunga JL, Trujillo Y, Menendez S, et al. Ozone oxidative post-conditioning in acute renal failure[J]. J Pharm Pharmacol, 2009, 61(2): 221-227. DOI: 10.1211/jpp.61.02.0012. |
| [18] | Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury[J]. Transplant Rev (Orlando), 2009, 23(1): 1-10. DOI: 10.1016/j.trre.2008.08.003. |
| [19] | Ascon M, Ascon DB, Liu M, et al. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes[J]. Kidney Int, 2009, 75(5): 526-535. DOI: 10.1038/ki.2008.602. |
| [20] | Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful[J]. Mediators Inflamm, 2004, 13(1): 3-11. DOI: 10.1080/0962935062000197083. |
| [21] | Peralta C, Xaus C, Bartrons R, et al. Effect of ozone treatment on reactive oxygen species and adenosine production during hepatic ischemia-reperfusion[J]. Free Radic Res, 2000, 33(5): 595-605. DOI: 10.1080/10715760000301121. |
| [22] | Ajamieh HH, Berlanga J, Merino N, et al. Role of protein synthesis in the protection conferred by ozone-oxidative-preconditioning in hepatic ischaemia/reperfusion[J]. Transpl Int, 2005, 18(5): 604-612. DOI: 10.1111/tri.2005.18.issue-5. |
| [23] | Hernandez F, Menendez S, Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy[J]. Free Radic Biol Med, 1995, 19(1): 115-119. DOI: 10.1016/0891-5849(94)00201-T. |
| [24] | Martinez-Sanchez G, Al-Dalain SM, Menendez S, et al. Therapeutic efficacy of ozone in patients with diabetic foot[J]. Eur J Pharmacol, 2005, 523(1-3): 151-161. DOI: 10.1016/j.ejphar.2005.08.020. |
| [25] | Ajamieh HH, Berlanga J, Merino N, et al. Role of protein synthesis in the protection conferred by ozone-oxidative-preconditioning in hepatic ischaemia/reperfusion[J]. Transpl Int, 2005, 18(5): 604-612. DOI: 10.1111/tri.2005.18.issue-5. |
| [26] | Donato M, Evelson P, Gelpi RJ. Protecting the heart from ischemia/reperfusion injury: an update on remote ischemic preconditioning and postconditioning[J]. Curr Opin Cardiol, 2017, 32(6): 784-790. DOI: 10.1097/HCO.0000000000000447. |
| [27] | Kaur I, Kumar A, Jaggi AS, et al. Evidence for the role of histaminergic pathways in neuroprotective mechanism of ischemic postconditioning in mice[J]. Fundam Clin Pharmacol, 2017, 31(4): 456-470. DOI: 10.1111/fcp.2017.31.issue-4. |
| [28] | Chen G, Ye X, Zhang J, et al. Limb rmote ichemic pstconditioning rduces ichemia-rperfusion ijury by ihibiting NADPH oidase ativation and MyD88-TRAF6-P38MAP-Kinase pthway of nutrophils[J]. Int J Mol Sci, 2016, 17(12): pii:E1971. DOI: 10.3390/ijms17121971. |
| [29] | Jiang BT, Chen QZ, Guo ZH, et al. Ischemic post-conditioning attenuates renal ischemic reperfusion injury via down-regulation of toll-like receptor 4 in diabetic rats[J]. Ren Fail, 2016, 38(9): 1425-1431. DOI: 10.1080/0886022X.2016.1214148. |
| [30] | Gao S, Zhu Y, Li H, et al. Remote ischemic postconditioning protects against renal ischemia/reperfusion injury by activation of T-LAK-cell-originated protein kinase (TOPK)/PTEN/Akt signaling pathway mediated anti-oxidation and anti-inflammation[J]. Int Immunopharmacol, 2016, 38: 395-401. DOI: 10.1016/j.intimp.2016.06.020. |
 2018, Vol. 39
2018, Vol. 39

