Recently, obesity and its metabolic complications have emerged as major health concerns worldwide. Studies have demonstrated that the gut plays a fundamental role in the development of chronic metabolic diseases[1]. Nowadays, besides the sequencing of the gut microbiota profile, the detection of fecal water toxicity towards intestinal epithelial cells can indirectly reflect the state of the intestinal flora and the effect of its metabolites on the host[2]. In the gut, the microbial metabolismis mostly responsible for the complex composition of fecal water. Metabolites in the fecal water produced by the gut microbiota could be absorbed into the bloodstream and, subsequently, impair the gut barrier function or enterohepatic circulation. Some of these gut microbiota-derived metabolites positively affect the host, but others exert toxicity, such as cytotoxins and genotoxins. Increased levels of gut-derived endotoxins would activate Toll-like receptor 4 (TLR4) in colon cells, leading to the induction of pro-inflammatory cytokines, which can cause an imbalance in the intestinal barrier function and ultimately activate inflammatory pathways in peripheral organs, such as adipose and liver tissues.
Bitter melon (Momordica charantia L.) has been used as a folk medicine for disease prevention, particularly for diabetes and obesity. Related mechanistic studies have found that the anti-obesity effect of bitter melon is linked to its anti-inflammation property[3]. However, in the obese, the exact mechanism of action by which bitter melon protects against the development of obesity-associated fatty liver via the inflammatory response remains elusive. Therefore, this study aimed to evaluate the effects of bitter melon powder (BMP) treatment on colonic fecal water toxicity, gut immunity, gut permeability, and metabolic disorders in the liver.
Rats were divided into the following three groups (n= 10 each) and treated as indicated: (a) normal control diet (NCD) and distilled water, (b) HFD and distilled water, and (c) HFD and 400 mg BMP/kg body weight (bw). We conducted oral glucose tolerance tests (OGTT) and measured systemic inflammation indices, triacylglycerol (TG) and total cholesterol (TC) levels in the liver tissues, using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. Harvested colon and liver tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, cut into sections of approximately 4 μm, and stained with hematoxylin and eosin (H & E). The cell counting kit (CCK)-8 assay using tetrazolium-8-[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium] monosodium salt was performed to determine the cytotoxicity of fecal water towards HT-29 cells. Gut permeability was evaluated by determining the lactulose/mannitol (L/M) excretion ratio and mRNA expression levels of tight junction proteins such as zonula occludens-1 (ZO-1) and occludin. The mRNA expression levels of TLR4 and some inflammatory cytokines in the colon mucosa and liver tissues were detected by quantitative real-time polymerase chain reaction (PCR). The primer sequences of all tested genes are listed in Table S1, available in www.besjournal.com.
|
|
Table S1 Primers for Quantitative Real-time PCR |
The data are presented as the means ± standard deviation (SD) and were analyzed using the statistical package for the social sciences (SPSS) IBM Statistics 20 software (SPSS, Inc.). A one-way analysis of variance (ANOVA) with Tukey's range test was performed to identify the differences between independent sample groups. Significant differences were confirmed at P < 0.05 at a 95% confidence limit.
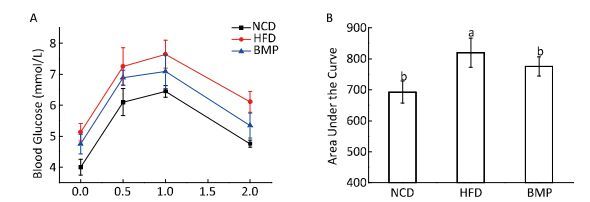
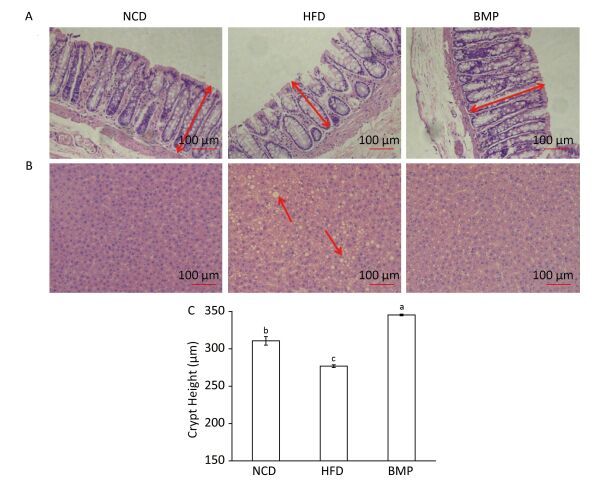
The results indicate that BMP decreased the body weight gain and Lee's index without changing the food intake (Table S2, available in www.besjournal.com). Figure S1 (available in www.besjournal.com) shows that BMP improved oral glucose tolerance levels and significantly decreased the area under the curve (AUC, P < 0.05). BMP exhibited anti-obesity effects and remarkably alleviated glucose tolerance in HFD-fed rats. Regarding the intestinal integrity, we found that BMP treatment increased both the colon length and weight (Table S2, available in www.besjournal.com), suggesting it exerted a trophic effect on the intestinal epithelial cells. Everard[4] found that both the colon weight and length were markedly increased by prebiotic treatment of genetically obese and diet-induced leptin-resistant mice. Moreover, we demonstrated that BMP consumption significantly changed the colonic crypt length, which could be considered beneficial to the digestion and absorption of food (P < 0.05, Figure 1A and 1C).
|
|
Table S2 Tissue Weights and Serum Parameters for Rats in Each Group at the End of Experiment |
|
Download:
|
| Figure S1 Effect of BMP on glucose tolerance in the obese rats. (A) Results of OGTT supplementation with BMP for 8 weeks and (B) significant differences in the area under the curve (AUC) of oral glucose tolerance changes in the HFD and BMP-treated groups. NCD, normal control diet; HFD, high-fat diet; BMP, bitter melon powder. The means with different superscript were considered significantly different (P < 0.05). | |
|
Download:
|
| Figure 1 Microscopy (200 ×) of hematoxylin and eosin (H & E) stained (A) colon (red arrows indicate crypt height measurement) and (B) liver tissues (red arrows indicate typical lipid droplets in the HFD group). (C) Crypt height in the colon. NCD, normal control diet; HFD, high-fat diet; BMP, bitter melon powder. The means with different superscript were considered significantly different (P < 0.05). | |
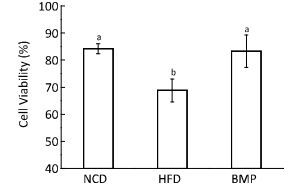
The metabolites in the colon content produced by fermentation induced by the resident microbiota have some levels of toxicity. Fecal water represents the portion of the colonic contents that directly contacts the colonic epithelial cells and is a good tool for assessing the role of dietary intervention in the colon. Research studies have reported that diets that are high in fat but low in dietary fiber increased fecal water toxicity against colonic cells[5], which is consistent with our present study results (Figure 2). Study outcomes have also indicated that dietary intervention with BMP in obese rats significantly reduced fecal water cytotoxicity to levels similar to that in the normal rats (P < 0.05). A considerable number of experiments have shown that different dietary interventions affect the toxicity of fecal water against intestinal epithelial cells. For example, supplementation of a low-fiber diet with konjac glucomannan reduced the toxicity of fecal water and precancerous risk factors for the development of colon cancer, which was associated with changes in β-glucuronidase activities, microflora, and bile acid levels[6]. Studies have mostly shown that the alteration of fecal water toxicity by dietary intervention was linked to the levels of some specific metabolites such as second bile acid, endotoxin, and short-chain fatty acids (SCFAs). We speculated that the changed levels of SCFAs and endotoxin might exert beneficial effects on the fecal water toxicity, based on our previous study[7]. However, the metabolites derived in the gut are extremely complex and therefore, the entire metabolite profile requires further studies.
|
Download:
|
| Figure 2 Effect of Bitter melon powder (BMP) on fecal water cytotoxicity against HT-29 cells in rats, expressed as cell viability. Values are means ± standard deviation (SD, n = 10). Statistical analysis was performed using analysis of variance (ANOVA). The means with different superscript were considered significantly different (P < 0.05). NCD, normal control diet; HFD, high-fat diet; BMP, bitter melon powder. | |
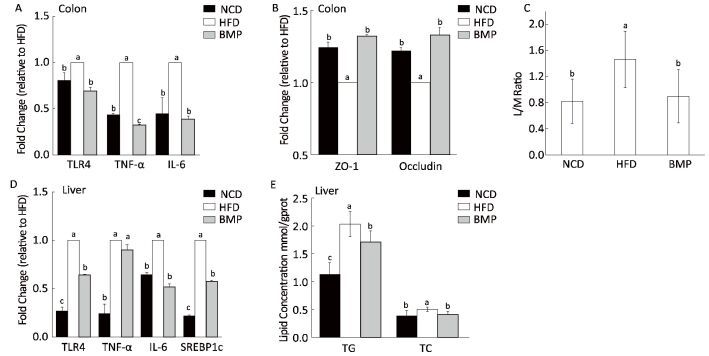
Long-term exposure of the colonic mucosa to fecal water containing toxic metabolites potentially affects epithelial cell metabolism and barrier function. TLR4 plays an important role in the intestinal inflammatory responses, along with the increased expression and release of pro-inflammatory cytokines. The present study revealed that HFD activated colonic TLR4 expression and upregulated the levels of the inflammatory factors, tumor necrosis factor (TNF)-α, and interleukin (IL)-6, and these effects were efficiently inhibited by BMP (Figure 3A). Related studies have reported that bitter melon inhibited bacterial mutagenesis and aberrant crypt focus formation in the rat colon tissue, and methanolic extracts of bitter melon suppressed colon cancer stem cells by affecting energy homeostasis and autophagy[8]. However, few research studies have reported the effects of bitter melon on the colonic inflammation in the obese.
|
Download:
|
| Figure 3 Effect of BMP on (A) Toll-like receptor 4 (TLR4), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 mRNA expression in colon mucosa; (B) zonulaoccludens (ZO-1) and occludin mRNA expression in colon mucosa. (C) Lactulose/mannitol (L/M) ratio; (D) TLR4, TNF-α, and IL-6 mRNA expression in liver tissues; and (E) triacylglycerol (TG) and total cholesterol (TC) levels in liver tissues. Values aremeans ± standard deviation (SD, n = 10). Statistical analysis was performed using analysis of variance (ANOVA). The means with different superscript were considered significantly different (P < 0.05). NCD, normal control diet; HFD, high-fat diet; BMP, bitter melon powder. | |
Study outcomes suggest that TLR4 and some inflammatory factors such as TNF-α and IL-1β could alter tight junctions and increase intestinal permeability. On the other hand, the increased permeability of the colon is postulated to participate in a feedback loop with inflammatory processes. Intercellular tight junctions, including transmembrane proteins (such as occludin and claudins) and junctional complex proteins (such as ZO-1), play an important role in the permeability properties of the gut barrier. Studies suggest that the whole-gut permeability was increased in HFD mice, with a reduction in mRNA expression of tight junction proteins including ZO-1[9]. In this study, we observed that the decrease in expression levels of ZO-1 and occludin in the colon tissue of HFD-fed rats was blunted following BMP supplementation (Figure 3B). In addition to the tight junctions, the L/M ratio could facilitate the quantitative assessment of the gut permeability. As a monosaccharide, mannitol (M) penetrates the transcellular aqueous pores, reflecting the degree of absorption of small molecules, while the disaccharide lactulose (L) passes through the intercellular junction complex, reflecting the permeability of large molecules. In this current study, the L/M ratio was impaired in the HFD group (Figure 3C), similar to what was observed in a clinical research study, which indicated that the L/M ratio was increased in children with non-alcoholic fatty liver disease[10]. Taken together, the increased expression of the tight junctional complex and decreased L/M ratio demonstrate the gut barrier-protectant effects of BMP.
Evidence suggests that the uncontrolled gut-derived inflammatory responses and permeability lead to the conversion of fatty liver to steatohepatitis and cause both local hepatic and systemic inflammation[11], as seen in our study (Figures 1B and 3D and Table S2). Additionally, we found that BMP treatment reduced the liver lipogenesis, as evidenced by the decreased levels of sterol regulatory element-binding protein 1c (SREBP1c) mRNA, TG, and TC (Figure 3E). Liver lipid accumulation is associated with liver and systemic inflammation and therefore, it may be postulated that the decreased inflammatory tone is related to both the lower gut-derived inflammation and liver fat accumulation.
In conclusion, BMP exerts beneficial effects on the colonic morphology and microenvironment, as evidenced by the increased crypt height and reduction of fecal water toxicity, respectively, in obese rats. Following these effects, the colon mucosal inflammation and gut permeability were ameliorated by BMP, accompanied by a mitigation of the systemic inflammatory tone. Ultimately, the pro-inflammatory and fatty liver exhibited an improvement in the pathological signs. Prospectively, BMP might be a potential prebiotic for gut-targeted approaches to clinically managing patients with obesity-associated fatty liver disease.
| 1. | Cani PD, Osto M, Geurts L, et al. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut microbes, 2012, 3: 279–88. doi:10.4161/gmic.19625 |
| 2. | Klewicka E, Nowak A, Zduńczyk Z, et al. Protective effect of lactofermented beetroot juice against aberrant crypt foci formation and genotoxicity of fecal water in rats. Exp Toxicol Pathol, 2012, 64: 599–604. doi:10.1016/j.etp.2010.12.001 |
| 3. | Yang SJ, Choi JM, Park SE, et al. Preventive effects of bitter melon (Momordica charantia) against insulin resistance and diabetes are associated with the inhibition of NF-κB and JNK pathways in high-fat-fed OLETF rats. J Nutr Biochem, 2015, 26: 234–40. doi:10.1016/j.jnutbio.2014.10.010 |
| 4. | Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes, 2011, 60: 2775–86. doi:10.2337/db11-0227 |
| 5. | Rieger MA, Parlesak A, Pool-Zobel BL, et al. A diet high in fat and meat but low in dietary fibre increases the genotoxic potential 'offaecal water'. Carcinogenesis, 1999, 20: 2311–6. doi:10.1093/carcin/20.12.2311 |
| 6. | Wu WT, Cheng HC, Chen HL. Ameliorative effects of konjac glucomannan on human faecal β-glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cells. Br J Nutr, 2011, 105: 593–600. doi:10.1017/S0007114510004009 |
| 7. | Bai J, Zhu Y, Dong Y. Response of gut microbiota and inflam-tory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats. J Ethnopharmacol, 2016, 194: 717–26. doi:10.1016/j.jep.2016.10.043 |
| 8. | Kwatra D, Subramaniam D, Ramamoorthy P, et al. Methanolic extracts of bitter melon inhibit colon cancer stem cells by affecting energy homeostasis and autophagy. Evid Based Complement Alternat Med, 2013, 2013: 702869. |
| 9. | Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes, 2008, 57: 1470–81. doi:10.2337/db07-1403 |
| 10. | Giorgio V, Miele L, Principessa L, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis, 2014, 46: 556–60. doi:10.1016/j.dld.2014.02.010 |
| 11. | Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. J PEN J Parenter Enteral Nutr, 2011, 35: 14S–20S. doi:10.1177/0148607111413772 |
 2017, Vol. 30
2017, Vol. 30


