2. Second Department of TCM Pulmonary Disease, The Key Institute of State Administration of Traditional Chinese Medicine(pneumonopathy chronic cough and dyspnea), Beijing Key Laboratory(NO. BZ0321), China-Japan Friendship Hospital, Beijing 100029, China;
3. State Key Laboratory for Infectious Diseases Prevention and Control, and National Institute for Communicable Disease Control and Prevention, Beijing 102206, China, Chinese Center for Disease Control and Prevention. Collaborative Innovation Center Diagnosis and Treatment of Infectious Disease, Hangzhou 310003, Zhejiang, China;
4. Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing Key Laboratory of Emerging Infectious Diseases, Beijing 100015, China;
5. Nephrology Department, Aviation General Hospital of China Medical University, Beijing 100012, China;
Objective To investigate whether recuperating lung decoction (RLD) can modulate the composition of gut microbiota in rats during asthma treatment.
Methods Fifteen Sprague-Dawley rats were divided randomly and equally into control group, model group, dexamethasone (DEX) group, RLD medium-dose group, and RLD high-dose group. The asthma model was established in all groups, except for the control group. The rats in the DEX and RLD groups were treated orally with DEX and RLD, respectively. The rats in the control and model groups were treated orally with 0.9% saline. The intestinal bacterial communities were compared among groups using 16S rRNA gene amplification and 454 pyrosequencing.
Results The microbial flora differed between the control and model groups, but the flora in the RLD groups was similar to that in the control group. No significant differences were observed between the RLD high-dose and medium-dose groups. RLD treatment resulted in an increase in the level beneficial bacteria in the gut, such as Lactobacillus and Bifidobacterium spp.
Conclusions Oral administration of RLD increased the number of intestinal lactic acid-producing bacteria, such as Lactobacillus and Bifidobacterium, in asthma model rats.
Asthma is characterized by airway inflammation and high airway reactivity[1]. The inflammatory state makes the body susceptible to various factors that lead to hyperresponsiveness and constriction of the airways. The associated eosinophil (EOS) infiltration of the airway causes clinical changes related to asthma, leading to inflammation and other pathological changes in the airway[2-3]. Although incurable, asthma can be controlled with appropriate drugs, self-management education, and by avoiding exposure to allergens[4].
The microflora hypothesis, proposed by Noverr and Huffnagle[5] in 2005, has been widely accepted as a potential explanation of the relationship between intestinal flora and asthma. Based on this hypothesis, the intestinal flora keeps the body healthy by metabolizing drugs and harmful exogenous compounds, resisting exogenous pathogens and conditioned pathogens inside the body, and regulating the intestinal immune system and metabolism[6]. Long-term antibiotic usage and dietary modifications can disrupt the balance of intestinal flora, possibly leading to the occurrence of allergic diseases. Therefore, it is possible to prevent or even cure existing allergies by probiotic treatment aimed at restoring the normal intestinal flora[7]. The regulation of asthma via immune mechanisms remains unclear; however, oxidized lipids are potential immunomodulatory molecules, and mucosal tolerance can be controlled.
In addition, commensal bacteria can modulate the host innate immune system. Infants that were born by cesarean delivery or were administered large doses of antibiotics are prone to develop asthma or other allergic diseases. This may be because cesarean delivery can potentially alter the normal balance of the intestinal flora in infants[9]. Additionally, infants often develop asthma when their mothers have an imbalance of intestinal flora[8]. Bjrkstén[9] cultured fecal bacterial samples, collected from 62 two-year-old Irish and Swedish children, under anaerobic conditions. He found that the samples from allergic children contained fewer Lactobacilli and anaerobic bacteria and more aerobic bacteria, such as coliform bacteria and Staphylococcus aureus. Bottcher et al.[10] discovered that the bacterial fatty acid profiles differed between allergic and non-allergic Swedish infants, and that allergic infants had higher levels of caproic acid (associated with Clostridium difficile). Furthermore, Penders et al.[11] have reported that the presence of Escherichia coli or Clostridium difficile was associated with an increased risk for eczema and allergic sensitization in two-year-old infants. These data, along with many others, suggest the potential effects of gut microbiota on the adaptive and innate immunity, which could affect the development of asthma.
Currently, glucocorticoids are the most effective drugs for controlling asthma due to their action on multiple aspects of the inflammatory response. Glucocorticoids regulate target gene transcription in the respiratory tract cells, inhibit inflammatory cell activation and inflammatory factor production, and increase airway β-receptor sensitivity; this in turn prevents airway inflammation and respiratory remodeling, and reduces bronchial hyperresponsiveness (BHR). However, long-term use of glucocorticoids may have some side-effects, such as toxicity, dependence, and may lead to financial strain due to the cost of long-term treatment[12-13].
Allergies[14] are considered as the primary pathophysiological basis of development of asthma. In traditional Chinese medicine, it is generally believed that allergies weaken the immune system, and that abnormal functioning of lungs and spleen contribute to the occurrence and development of asthma[15-16]. Clinical practice[17-18] has pr oven that an efficient approach for recovery from asthma is the restoration of normal functioning of the vital organs.
Our study aimed to investigate the effects of recuperating lung decoction (RLD) treatment on the microflora structure in rats with asthma. RLD is a decoction based on a traditional Chinese medicine that includes raw Radix Astragalus, Ramulus Cinnamomi, Suzi, Flos Magnoliae, Rhizoma Zingiberis, Fructus Corni, Fructus Schisandrae Chinensis, Fructus Mune, Flos Inulae, Cortex Magnoliae Offcinalis, Rhizoma Anemarrhenae, and raw Radix Et Rhizoma Glycyrrhizae. Previous studies have demonstrated[19-20] that the lung function of rats with asthma was improved after RLD treatment. The effects include a decreased number of eosinophils in bronchoalveolar lavage fluid (BALF) and reduced serum IgE levels, and improved elimination of oxygen free radicals[21]. RLD use was found to be closely related to changes in seven metabolites (valine, malic ac id, gluconic acid, galactose, pyran glucose, 6-deoxidation mannopyranose and stearic acid) present in asthma rabbit serum[22] and stearic acid in asthma rabbit urine[23]. This study used Sprague-Dawley rats to demonstrate the varying structure and diversity of the intestinal flora in response to various RLD treatments.
Methods Preparation of the RLDAll herbal components of RLD were obtained from Beijing Tong Ren Tang Pieces Company, and all the components followed the standard of the Chinese Pharmacopoeia (2010 edition). The herbs were identified and prepared as a fluid extract according to the standard operating procedure of the School of Chinese Materia Medica, Beijing University of Traditional Chinese Medicine, Beijing, People’s Republic of China.
OVA-induced Asthma Model and TreatmentFour-week-old male Sprague-Dawley rats, obtained from Beijing Hua Fu Kang Biological Technology Ltd., China [License Number: SCXK (jing) 2009-0007], were used after 1 week of acclimation. All experimental procedures were conducted in accordance with the internationally accepted principles for laboratory animal use and care according to the US guidelines (NIH Publication no. 85-23, revised in 1985) and were approved by the Ethics Committee of the China (Japan Friendship Hospital. The rats were divided into five groups: a control group (N), an asthma model group (M), a positive-control group treated with DEX (W), and two RLD-treated groups (Tb and Tc). Rats in the asthma model group were first sensitized by subcutaneous injection of 0.2 mL of a mixture of 10% ovalbumin (OVA)/Al(OH)3 (OVA, A16951, Alfa Aesar, Ward Hill, MS, USA; aluminium hydroxide, A4682, Sigma-Aldrich, St Louis, MO, USA) and 0.0023% whooping cough toxoid (P7208, Sigma-Aldrich) on day 1 to day 8; the injection was administered at five sites (feet, both side of groin, and peritoneum). On days 9-15, after initial sensitization, the rats were challenged with OVA (1%, w/v, in 0.9% saline) for 1 h using an ultrasonic nebulizer (402AI; Yuyue Medical Equipment Co., Jiangsu, China). The rats in the DEX and RLD groups were treated orally with DEX (0.5 g/kg, Sigma-Aldrich) and RLD [3.6 g/kg body weight for the medium-dose (Tb) and 7.26 g/kg bodyweight for the high-dose (Tc) group], respectively, once daily, on days 16-29. The rats in the control and asthma model groups were given 0.9% saline (0.3 mL).
Evaluation of the ModelThe evaluation standard of the model included the following five aspects[24-25]{Kim, 2014 #356}{NOTE:Kim Y, Lee M Y, Kim O S, et al. Acute oral toxicity of Insampaedok-san, a traditional herbal formula, in rats and its protective effects against ovalbumin-induced asthma via anti-inflammatory and antioxidant properties[J]. Bmc Complementary & Alternative Medicine, 2014, 14(1):1-10.}{NOTE:Kim Y, Lee M Y, Kim O S, et al. Acute oral toxicity of Insampaedok-san, a traditional herbal formula, in rats and its protective effects against ovalbumin-induced asthma via anti-inflammatory and antioxidant properties[J]. Bmc Complementary & Alternative Medicine, 2014, 14(1):1-10.}. (1) The general condition of the animals: observing the animals for agitation, nose-scratching, sneezing, shortness of breath, abdominal muscle contraction, lack of luster, etc. (2) After the challenge, their lung function was evaluated using the Flexivent system (Flexivent; SCIREQ, Montreal, QC, Canada) according to the manufacturer’s instructions. The system used forced oscillation to discriminate between airway and lung tissue variables, and measured the flow-volume relationship in the respiratory system. The rats were anesthetized by endotracheal intubation and connected to a computer-controlled small-animal ventilator. To avoid spontaneous breathing, rat breathing was stabilized with an automatic ventilator until the interruption wave disappeared. The lung tissue variables of central airway resistance (Rn) and airway hyperreactivity (I) were measured. (3) Collection of BALF: After determining the lung function, BALF was collected by flushing the lungs twice with normal saline through the trachea. Approximately 1 mL of BALF was recovered. Cells were recovered from BALF by centrifugation at 3500 rpm for 15 min at 4 ℃ and 8 mL of the cellular components were obtained by centrifugal sedimentation. The supernatant was shaken with phosphate-buffered saline (PBS) three times. A smear (0.1 mL) of the sediment was taken, stained with hematoxylin and eosin (H&E), and examined under an optical microscope (400 ×). The eosinophil count was repeated three times, and the average was calculated. (4) Lung tissue histopathology: The right lungs were fixed in 10% neutral buffered formalin. Each tissue specimen was sliced into 4-m sections and stained with H&E stain. Tissue lesions and inflammatory cell infiltration were then evaluated microscopically. With the groups masked, inflammation was assessed from five fields per section, each graded on a scale of 0-3 (0 = no signs of disease; 3 = severe disease) for each of the following parameters: peribronchial infiltration, epithelial damage, alveolar interval, and bronchospasm for a maximum total score of 12. (5) IgE measurements: At 24 hafter the challenge, the rats were anesthetized and dissected. They were then euthanized by aortic exsanguination. The arterial blood was collected in tubes containing either sodium citrate as anticoagulant or a gel procoagulant for different tests. The level of IgE in serum was assessed using a rat enzyme-linked immunoassay kit (MultiSciences Biotech Co., Ltd., Hangzhou, China) according to the manufacturers’ instructions.
Collection and Pretreatment of SpecimensOnce the rats were anesthetized, the abdominal cavity was opened. With sterile scissors, the cecum was cut open and, using sterile plastic loops, approximately 200 mg of cecal matter was transferred directly into cryotubes and flash-frozen in liquid nitrogen.
Microbiological culture, isolation, and identification of species were performed by the China CDC Laboratory of Pathogenic Microbiology, according to standard procedures.
DNA Extraction and 16S rDNA Gene AmplificationDNA was extracted from each cecal specimen using the QIAamp DNA Stool Mini Kit (Qiagen Corp., Valencia, CA, USA) following the manufacturer’s recommended protocol. The extracted total bacterial DNA was used for polymerase chain reaction (PCR) amplification and generation of bacterial 16S rDNA sequences. The 16S rDNA hypervariable region 3 to region 5 (V3, V4, and partial V5, approximately 460 bp) was chosen for PCR, as they have previously been proven to be optimal for use in sequence-based assays species-level classification of clinical bacteria. A relatively small fragment was selected for the 454 pyrosequencing technology. A pair of universal bacterial primers directed against the V3-V4 flanking region was used for library preparation: Bakt_341F (5′-CCTACGGGNGGCWGCAG-3′) and Bakt_805R (5′-GACTACHVGGGTATCTAATCC-3′). The forward and reverse primers were synthesized in conjunction with a specific barcode immediately upstream of each primer, in order to identify the original sample uniquely. The 16S RNA genes were PCR-amplified under the following cycle conditions: initial denaturation at 95 ℃ for 5 min, denaturation at 95 ℃ for 40 s, annealing at 58 ℃ for 40 s, extension at 72 ℃ for 60 s over 30 amplification cycles, and a final elongation step at 72 ℃ for 7 min. Amplification products were identified using 1% agarose gel electrophoresis. The target PCR products were purified using Qiagen Gel Extraction Kit (Qiagen Corp.), quantified on a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA), and then pooled using equimolar amounts of each sample. Sequencing was performed using the Roche/454 GS Junior Titanium Series technology platform (Roche, Indianapolis, IN, USA) at the Chinese Center for Disease Control and Prevention, according to the manufacturer’s recommendations.
Filtering of 454 Pyrosequencing Reads and Microbial Community Diversity EstimatesAll pyrosequencing reads were filtered according to the barcodes. The assigned reads were further screened and filtered for quality and length. Sequences less than 50 bp containing ambiguous characters or mononucleotide repeats of more than six nucleotides were excluded. The remaining high-quality sequences were aligned and clustered into operational taxonomic units (OTUs) based on nucleotide sequence identity, with the threshold of 97%. The Ribosomal Database Project Release 10 classifier with a threshold of 0.8 was used to assign representatives of each OTU to a known taxon at the genera level. Alpha diversity (within-samples) and beta diversity among a collection of samples were estimated to evaluate the community diversity and the relationships among the 56 microbial communities. Rarefaction curves were plotted to describe the relationship between sequencing depth and species richness. Unweighted UniFrac distance metric analyses were performed using the OTUs for each sample. These analyses were performed in QIIME v1.8.0 (http://qiime.org/). The heatmap was generated via the heatmap.2 function of the R package (http://www.r-project.org/).
Statistical AnalysisData were presented as per-group mean±standard error of means (SEM). Statistical analysis was performed using analysis of variance (ANOVA), and data were analyzed using one-factor ANOVA followed by Tukey’s multiple-comparison test. Differences were considered significant at P<0.05 unless otherwise stated. Unless otherwise specified, Student’s t-test with the Benjamini and Hochberg method for significance thresholds of P<0.05 was used for comparing the mean relative abundance of a genus for each pair of treatments. Chi-square tests were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Raw sequence reads for this study are available from NCBI at Sequence Read Archive (http://www.ncbi.nlm. nih.gov/bioproject/), under NCBI Bio Project no. PRJEB5834. Similarity analysis was done using cluster analysis, grouped using the unweighted pair-group method with arithmetic means (UPGMA) computing system[26]. Diversity analysis was performed using richness and biodiversity.
Results General Condition of Rats and Drug Efficacy EvaluationThe rats in the control group were lively and responsive, with smooth breathing and shiny fur. The rats in the asthma group showed scratched facial fur, restless behavior, and other symptoms of allergy. In contrast to the asthma group, the treatment groups (Tb, Tc, and W) showed relief from the symptoms of asthma.However, shortness of breath and dyspnea with mild cyanosis were observed in the treatment groups.
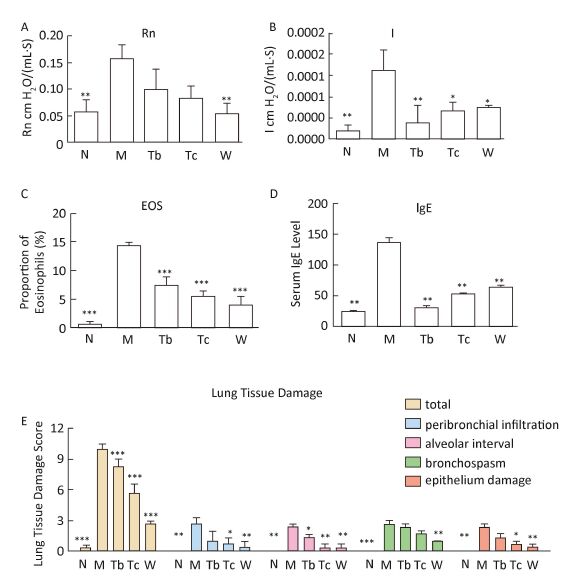
Compared with the control group, the central airway resistance (Rn), airway hyperresponsiveness (I), serum IgE, and eosinophil count in the bronchoalveolar lavage fluid increased in the asthma model group. After medication, the aforementioned indexes had different degrees of improvement. HE staining of the lung tissue in the control group revealed no inflammatory cell infiltration and alveolar expansion, the bronchial mucosa and wall thickness were normal, the epithelial structure of the alveolar and bronchial mucosa was complete and clear, and bronchial spasms were absent. Pathological manifestations, such as infiltration of lung inflammatory cells, disordered arrangement of bronchial mucosal epithelial cells, thickening of the alveolar walls, bronchospasm, and an increase in macrophages were seen in the asthma model group. After treatment (DEX, RLD), these pathological manifestations were reduced, but the change in the RLD medium-dose group was not marked. The asthma model was thus successfully prepared, and drug treatment afforded control of the asthma. The therapeutic effect (high to low) was in the following order: DEX group, RLD high-dose group, RLD medium-dose group. The details are illustrated in Figure 1.
|
Download:
|
| Figure 1 Drug-efficacy evaluation. RLD improved lung function, and reduced lung tissue damage, proportion of eosinophils (in BALF) and serum IgE levels in rats. (A, B) Changes in lung function, (C) changes in the proportion of eosinophils in BALF, (D) changes in serum IgE levels, and (E) changes in lung tissue damage (each group, n=3). Data were presented as mean±SEM. P<0.05, **P<0.01, and ***P<0.001 versus the model group using ANCOVA. Rn, central airway resistance. I, airway hyperresponsivence. | |
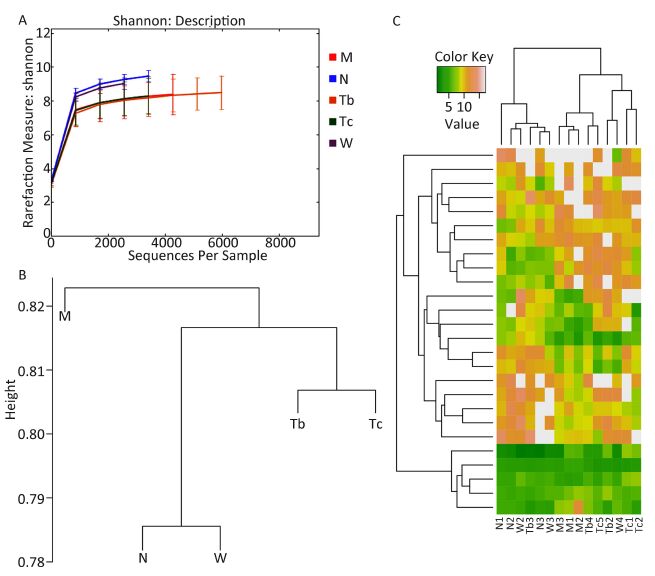
First, the structural changes of the gut microbiota in the five groups were analyzed. Raw sequences were demultiplexed and then quality-filtered following the method described. A total of 138, 958 high-quality 16S rDNA genes covering the V3-V4 regions derived from the 15 samples represented an average length of 419 bps per sample (Table 1). The sequences were clustered into 31, 726 OTUs at a genetic similarity of 97%. Rarefaction and Shannon diversity curves showed a sharp increase in the sampling size of the lower 3000 and gradually reached a plateau, suggesting that most of the diversity was captured at the current sequencing depth (Figure 2A).
|
|
Table 1 Overview of the Distribution of Reads per Samples |
Second, to measure the difference in the gut microbiota structure among the five groups, the UniFrac metric was calculated. Unweighted UniFrac PCoA analysis showed that after treatment, the gut microbiota structure of the three treated groups had significantly deviated from the baseline of the model group and had a smaller difference from the healthy group. This suggested that the structure of the gut microbiota of the treated groups showed a drug-dependent deviation and had a microbial shift toward the control group (Figure 2B).
|
Download:
|
| Figure 2 (A) Shannon index for the M, N, Tb, Tc, and W groups. Values were expressed as mean±SEM. The diversity of microbiota in the drug and control groups was relatively higher, while the diversity in the two RLD and model groups was relatively lower. (B) The UPGMA tree of the five groups. (C) The heatmap graphs prepared using bacteria with a relative abundance that had lead to a significant change in the model and normal groups (t-test, P value<0.2). | |
Finally, cluster analysis was used to test the similarity of the bacterial communities in the five groups. The bacteria that showed a significant change in relative abundance between the model group and the control group were selected (t-test, P<0.2). The heatmap showed that, based on this bacterial microbiota, the model group and the drug-treated groups could be clearly separated, but the three drug-treated groups could not be separated. This indicated that all drug treatments used in this study could improve the gut microbiota, although at the stage of observation, the gut microbiota balance was not yet stable (Figure 2C).
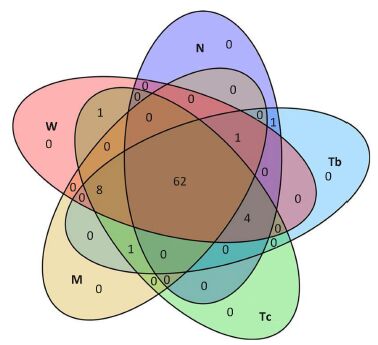
Microbiota Structural Modulation of Asthma Model RatsThe overall structure of the gut microbiota was analyzed to demonstrate changes in the gut microbiota before and after treatment. Of all the tested flora, 74 genera of flora with a level above 0.01% were used for analysis. A total of 62 genera of flora were shared by all groups, which comprised up to 83.9% of all tested types. These genera mainly belonged to the Proteobacteria, Bacteroidetes, and Firmicutes, and could be considered to comprise the microbiota structural modulation of rats with asthma (Figure 3).
|
Download:
|
| Figure 3 Comparison of bacterial community composition. Venn diagram of the microbiota in the five groups. | |
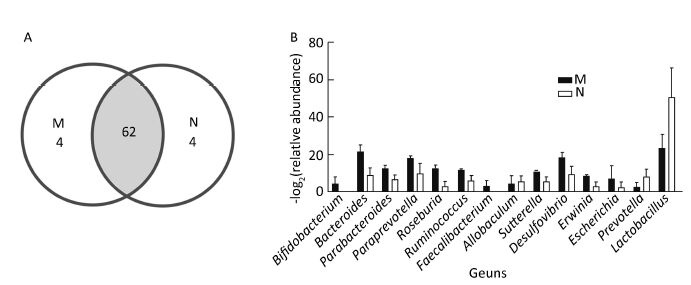
To monitor the structural modulation of gut microbiota before and after induction of asthma, the control and the model groups were analyzed (Figure 4). Alpha diversity analysis showed no difference in the relative abundance and richness of microflora between the two groups. However, the bacterial community compositions of these two groups were distinctive (Figure 4A). Compared with the model group, the relative abundance of Prevotella and Lactobacillus in the control group was higher, while that of Bacteroides, Parabacteroides, Paraprevotella, Roseburia, Ruminococcus, Faecalibacterium, Allobaculum, Sutterella, Desulfovibrio, Erwinia, and Escherichia was lower (Figure 4B).
|
Download:
|
| Figure 4 Comparison of bacterial community composition between the control and model groups. (A) Venn diagram of the microbiota in the two groups. (B) Bar graph of the bacteria that showed a significantly different ratio between the two groups (RatioN/M>2 or RatioN/M<0.5). | |
The microbial community structure of the RLD medium-dose and high-dose groups was compared to determine whether the Chinese herbs had a dose-dependent effect on asthma. The bacterial community structure was considered to be similar in the Tb (medium-dose) and Tc (high-dose) groups, although Aggregatibacter and Akkermansia were unique to Tc. Further, Prevotella was more common in the Tb group (P-value=0.005). The high-dose RLD group showed a higher level of Enterococcus and Proteus and a lower level of Lactobacillus and Bifidobacterium than did the medium-dose RLD group, but no statistically significant dose-dependent effect was found.
There was, however, a pronounced difference between the RLD and DEX groups. Similar to the Tb and Tc groups, the majority of the bacterial community structure was shared between the RLD and DEX groups. However, Adlercreutzia was more prevalent in the RLD groups (P-value=0.036) and Erysipelotrichaceae was more prevalent in the DEX group (P-value=0.038). Compared with the model group, the relative abundance of Lactobacillus was clearly increased in the DEX group, while the relative abundance of Escherichia was markedly increased in the RLD groups. The details of this structural modulation are illustrated in Figure 5.
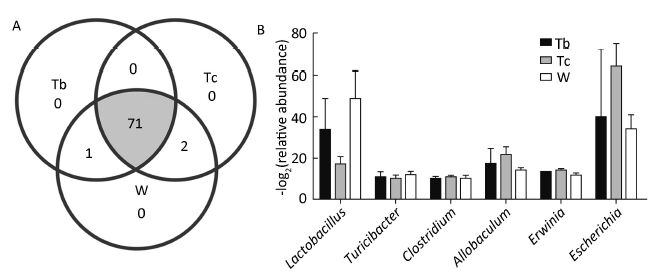
|
Download:
|
| Figure 5 Comparison of bacterial community composition of the two RLD and DEX groups. (A) Venn diagram of microbiota in the three groups. (B) The bar graph of the bacteria that showed a significantly different ratio among the three groups (RatioTb, Tc, W/M>2 or RatioTb, Tc, W/M<0.5). | |
Our study investigate the relationship between intestinal flora diversity and the treatment of asthma with DEX and a traditional Chinese medicine, RLD. Our results suggested that RLD was effective in treating asthma, and that the RLD high-dose group showed more similarities with the DEX group with respect to treatment outcomes. The treatment also changed the diversity of intestinal flora in rats and alleviated their symptoms. The intestinal flora diversity of the control group was higher than that of the model group, and differed in terms of species and abundance, both of which were increased in the treatment group as well(Tb, Tc, and W) compared with that in the model group. This indicated a clear imbalance of intestinal flora in the model group rats.
In recent years, the economy-related change in human dietary patterns and the increased use of antibiotics has resulted in a change in intestinal flora and a higher risk of asthma[8, 27-28]. A reduction in Bacteroidetes, Lactobacilli, and Bifidobacteria has been associated with the asthma phenotype[29]. A recent study on patients with asthma showed that the biodiversity in the patients with airway hyperresponsiveness was higher than that in the healthy subjects[30].
Traditional Chinese medicine holds that meridians link the lungs and the large intestine in an interior-exterior dyad. Based on this theory, facilitating bowel movement while treating asthma can relieve the patient’s symptoms. This could be achieved by improving the microcirculation and reducing pulmonary hypertension, which relieves bronchial smooth muscle spasm, reduces capillary permeability and inflammatory exudate. Additionally, lowering abdominal pressure by promoting bowel movement to release feces and gas, increasing the range of motion of the diaphragm, the resulting impr- oved lung respiratory function may also contribute to this effect. Although the mechanism underlying the pathological changes in asthma is extremely complex with respect to the onset, regulation, and prognosis of the condition, various studies have recently made tremendous progress in understanding this mechanism. This may change the future treatment strategies for asthma.
In recent years, there has been increasing number of reports on the modulatory effect of single herbs or compound herbal preparations on the intestinal flora[31-32]. The effective components in single herbs, such as Astragalus polysaccharide (APS) in Astragalus, have been proven[33] to have a positive effect on the intestinal dysbiosis in rats with ulcerative colitis. For example, reports have shown that, after APS treatment for 7 d, the number of Bifidobacteria and Lactobacilli significantly increased, and that of Enterococci and Enterobacteria decreased. APS has been confirmed[34] to facilitate the growth of harmless bacteria and inhibit the growth of pathogenic bacteria, thus maintaining the intestinal microecological balance. Schisandra has also been proven[35]to have a similar effect on microbial flora and a protective effect on the liver. ShenLingBaiShuSan has been reported[36]to correct intestinal dysbiosis caused by the use of antibiotics in mice; increase in the level of probiotic bacteria in the intestine, and the levels of IgG, endotoxin, and vasoactive intestinal peptide (VIP) in the serum have also been reported.
ConclusionsThis study has provided an explanation for the Chinese theory in which ‘meridians link the lungs and the large intestine’ and has aimed to give a stronger experimental basis for future studies on its cryptic micro-ecology mechanism. The intestina flora structure of rats with asthma was different from that of the control group. After treatment, the intestinal flora structure of the RLD groups was similar to that of the DEX group, and the flora structure of the three treatment groups tended to be similar to that of the control group. Hence, this study demonstrated that oral administration of RLD increased the number of intestinal probiotic bacteria such as Lactobacillus and Bifidobacterium in rats with asthma, but this effect may be due to complicated interactions among various components. It remains unclear whether changes in gut microbiota caused by RLD contribute directly to the treatment outcomes in asthma. The beneficial effect is thought to be associated with an immunoregulatory effect of RLD; this possible association needs to be explored in further studies.
Conflict of interestThe authors declare that they have no actual or potential conflicts of interest.
Author contributionsKong Yan hua, Shi Qi, Zhang Ling, and Li You lin contributed in conducting the study, collecting the data and drafting the manuscript. Han Na and Chen Chen performed the statistical analysis and participated in its design. Gao Tong xin and Zhang Yuan yuan helped in drafting the manuscript. All authors have read and approved the final manuscript.
| 1. | Masoli M, Fabian D, Holt S, et al. , The global burden of asthma:executive summary of the GINA Dissemination Committee report[J]. Allergy , 2004, 59 :469–78. doi:10.1111/all.2004.59.issue-5 |
| 2. | Kay AB. The role of eosinophils in the pathogenesis of asthma[J]. Trends Mol Med , 2005, 11 :148–52. doi:10.1016/j.molmed.2005.02.002 |
| 3. | Kulkarni NS, Hollins F, Sutcliffe A, et al. , Eosinophil protein in airway macrophages:a novel biomarker of eosinophilic inflammation in patients with asthma[J]. J Allergy Clin Immunol , 2010, 126 :61–9. doi:10.1016/j.jaci.2010.03.026 |
| 4. | Kliegman RM, R Behrman, H Jenson, et al. Nelson textbook of pediatrics[J]. Germany:Elsevier Health Sciences , 2007 . |
| 5. | Noverr MC, GB Huffnagle. The ‘microflora hypothesis’ of allergic diseases[J]. Clin Exp Allergy , 2005, 35 :1511–20. doi:10.1111/cea.2005.35.issue-12 |
| 6. | Blaut M, T Clavel. Metabolic diversity of the intestinal microbiota:implications for health and disease[J]. J Nutr , 2007, 137 :751s–5s. |
| 7. | Sun D, Yang X. Progress in the mechanism of action and application of microecological preparations[J]. International journal of pediatrics , 2009, 36 :48–50. |
| 8. | Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma[J]. N Engl J Med , 2011, 364 :701–9. doi:10.1056/NEJMoa1007302 |
| 9. | Björkstén B, Naaber P, Sepp E, et al. The intestinal microflora in allergic Estonian and Swedish 2-year-old children[J]. Clin Exp Allergy , 1999, 29 :342–6. doi:10.1046/j.1365-2222.1999.00560.x |
| 10. | Böttcher M, Nordin EK, Sandin A, et al. Microflora-associated characteristics in faeces from allergic and nonallergic infants[J]. Clin Exp Allergy , 2000, 30 :1591–6. doi:10.1046/j.1365-2222.2000.00982.x |
| 11. | Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy:the KOALA Birth Cohort Study[J]. Gut , 2007, 56 :661–7. doi:10.1136/gut.2006.100164 |
| 12. | Newton R. Molecular mechanisms of glucocorticoid action:what is important?[J]. Thorax , 2000, 55 :603–13. doi:10.1136/thorax.55.7.603 |
| 13. | Lesovaya E, Yemelyanov A, Swart AC, et al. , Discovery of Compound A-a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity[J]. Oncotarget , 2015, 6 :30730–44. |
| 14. | Lee MY, Seo CS, Lee JA, et al. Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1[J]. Food Chem Toxicol , 2011, 49 :829–37. doi:10.1016/j.fct.2010.12.004 |
| 15. | Shi Q, Song Q, Yan Y, et al. Treatment of allerfic diseases from the lung and spleen aspects[J]. China Journal of Traditional Chinese Medicine and Pharmacy , 2013, 28 :3265–8. |
| 16. | Zhang L, Li YL. Experience of treating chronic persistent bronchial asthma based on ‘warming and moisturize lung with supplementing spleen’ method[J]. China Journal of Traditional Chinese medicine and Pharmacy , 2013, 28 :1525–8. |
| 17. | Han J. Efficacy analysis of warming and moisturizing lung with supplementing spleen for the patients suffering from bronchial asthma with allergic rhinitis[J]. Chinese Archives of Traditional Chinese Medicine , 2015, 33 :1341–3. |
| 18. | Yan Y. Impact factors of wenren xuantong method in treating stable chronic obstructive pulmonary diseases and bronchial asthma in chronic persistent period[J]. China Journal of Traditional Chinese medicine and Pharmacy , 2014, 29 :1954–6. |
| 19. | Li SW. Research on the mechanism of bronchial asthma treatment by Qiweilifei Decoction regulating transcription factors activate protein-1(AP-1)[J]. Beijing university of Chinese medicine , 2014 . |
| 20. | Li CL. Research on the antioxidant mechanism of bronchial asthma treatment by Qiweilifei Decoction[J]. Beijing university of Chinese medicine , 2014 . |
| 21. | Li CL. Infuence of Qiwei Lifei Decoction on oxygen free radical scavenging capacity in asthma rats serum[J]. China Journal of Traditional Chinese medicine and Pharmacy , 2015, 30 :1644–7. |
| 22. | Shi Q, Chen XX, Kong YH, et al. Metabolomics study on urine of allergic bronchial asthma rabbits treated by Recuperating Lung Decotion[J]. RSC Adv , 2015, 5 :13768–76. doi:10.1039/C4RA14710C |
| 23. | Shi Q, Kong YH, He B, et al. Metabolomics study on serum of allergic bronchial asthma rabbits treated by Recuperating Lung Decotion[J]. RSC Adv , 2015, 5 :13768–76. doi:10.1039/C4RA14710C |
| 24. | Qin Q, Chen X, Feng J, et al. Low-intensity aerobic exercise training attenuates airway inflammation and remodeling in a rat model of steroid-resistant asthma[J]. Chinese Medical Journal , 2014, 127 :3058–64. |
| 25. | Kim Y, Lee MY, Kim OS, et al. Acute oral toxicity of Insampaedok-san, a traditional herbal formula, in rats and its protective effects against ovalbumin-induced asthma via anti-inflammatory and antioxidant properties[J]. BMC Complement Altern Med , 2014, 14 :365. doi:10.1186/1472-6882-14-365 |
| 26. | Fromin N, Hamelin J, Tarnawski S, et al. Statistical analysis of denaturing gel electrophoresis (DGE) fingerprinting patterns[J]. Environ Microbiol , 2002, 4 :634–43. doi:10.1046/j.1462-2920.2002.00358.x |
| 27. | Riedler J, Braun-Fahrländer C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy:a cross-sectional survey[J]. Lancet , 2001, 358 :1129–33. doi:10.1016/S0140-6736(01)06252-3 |
| 28. | Omland O, Hjort C, Pedersen OF, et al. New-onset asthma and the effect of environment and occupation among farming and nonfarming rural subjects[J]. J Allergy Clin Immunol , 2011, 128 :761–5. doi:10.1016/j.jaci.2011.06.006 |
| 29. | Ouwehand AC, Isolauri E, He F, et al. Differences in Bifidobacterium flora composition in allergic and healthy infants[J]. J Allergy Clin Immunol , 2001, 108 :144–5. doi:10.1067/jmai.2001.116003 |
| 30. | Gollwitzer ES, BJ Marsland. Microbiota abnormalities in inflammatory airway diseases-Potential for therapy[J]. Pharmacol Ther , 2014, 141 :32–9. doi:10.1016/j.pharmthera.2013.08.002 |
| 31. | Yin X, Peng J, Zhao L, et al. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula[J]. Syst Appl Microbiol , 2013, 36 :188–96. doi:10.1016/j.syapm.2012.12.009 |
| 32. | Xu J, Lian F, Zhao L, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula[J]. Isme j , 2015, 9 :552–62. doi:10.1038/ismej.2014.177 |
| 33. | Liang JH, Zheng KW, Sun LQ. Explore the Regulative Action of Astragalus Polysaccharide for Intestinal Dysbacteriosis in Ulcerative Colitis Rat Models[J]. Studies of Trace Elements and Health , 2013, 2 :003. |
| 34. | Zhang Y, Shen Y, Hu XJ, et al. Research on material bases of microecological adjustment of Astragalus polysaccharides[J]. Chinese Journal of Microecology , 2012, 2 :003. |
| 35. | Cheng Y, Wang HH, Yi-Yang HU. Effect of Jianpi Huoxue Recipe on Gut Flora in rats with alcoholic fatty licer induced by LieberDeCarli liquid diet[J]. Chinese Journal of Integrated Traditiona & Western Medicine , 2011, 31 :73–9. |
| 36. | Dong K, Gao Y, Qin N, et al. Effects of shenlinbaizhusan on the dysbacteriosis induced by antbiotics in mices[J]. Chinese Journal of Experimental Traditional Medical Fomulae , 2015, 1 :154–7. |
 2016, Vol. 29
2016, Vol. 29


