2. Cytokine Research Laboratory, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston TX 77030, USA;
3. Laboratory of Biomonitoring of the Environment (LR01/ES14), Faculty of Sciences of Bizerte, Zarzouma, Bizerte 7021, Tunisia;
4. Service of Anatomo- Pathology of Menzel Bourguiba, Bizerte 7050, Tunisia;
Objective We evaluate the effects of Thymus algeriensis (TEO) against hydrogen peroxide (H2O2) toxicity on body and testis weight, testis sperm count, testis lipid peroxidation, and antioxidant enzyme activities in rats.
Methods Rats were treated with low (LD) and high dose (HD) of H2O2 (0.1 and 1 mmol/L) in the presence or absence of TEO (150 mg/kg).
Results The results exhibited a significant decrease in body weight and testis weight, in total sperm number decrease (P <0.05), sperm motility and percentage of sperm viability, leading to complete arrest, in sperm flagellar beat frequency by the gavage of 1 mmol/L H2O2 compared to controls. The administration of H2O2 resulted in a significant reduction in testis GSH, GPx, CAT, SOD, and GST activity and significant increase (P <0.05) in MDA concentration compared with the untreated control animals. TEO pre-treatment protected testis from the H2O2 generated oxidative stress. These results were confirmed by histological architecture examinations.
Conclusion H2O2 has the ability to alter the sperm function, characteristics and development of testis. However, TEO is an efficient natural agent, which can prevent the testis from H2O2-induced oxidative damage in rats.
ecently, oxidative stress has become the focus of interest as a potential cause of male infertility. Oxidative stress is induced by reactive oxygen species (ROS) which plays some key roles in the development of testis toxicity. Testis toxicity is a sporadic and challenging issue for pharmaceutical drug development[1]. Some level of oxidative stress are required for sperm capacitation, hyperactivation, and sperm-oocyte fusion. However, extreme levels of ROS can negatively impact sperm quality, motility and increase DNA damage in sperm. Subsequently, sperm-oocytes fusion efficacy decreased too much. Therefore, equilibrium is required between the ROS production and antioxidant scavenging activity in the male reproductive organs[2].
Among a great variety of ROS, hydrogen peroxide (H2O2) plays a crucial role in male infertility. H2O2 is generated from various sources of oxidative stress and oxygen free radicals, which can diffuse freely in and out of cells and tissues[3]. It has also been recognized as the most toxic oxidizing species for human spermatozoa[4]. H2O2 exposure induces lipid peroxidation and decreases activities of antioxidant enzymes; superoxide dismutase (SOD), catalase (CAT) & cellular glutathione (GSH). These scavenger enzymes in semen are considered as the part of first line defence against ROS[5]. Generation of oxygen metabolites induced by the direct addition of H2O2 to sperm was shown to cause a significant increase in DNA fragmentation[6].These effects are counteracted by oxidative defence enzymes and antioxidants including glutathione peroxidase isoforms (GPx1) and (GPx4), glutathione reductase (GR), and reduction in GSH)[7]. Furthermore, GST is responsible for eliminating and detoxifying xenobiotic and their metabolic products[8]. The first defense line against the accumulation of ROS is superoxide dismutase (SOD), which converts the O2− radicals to H2O2[9]. Modification in protein contents as a result of ROS has emerged as a key parameter for measuring oxidative stress[10]. Tissue levels of malo ndialdehyde (MDA) and the activities of SOD, GPx, and CAT are proven indicators of oxidative stress[11].
It has been hypothesized that reduction in oxidative stress through the natural ROS scavengers may be effective to improve the sperm function and treatment of male infertility. As natural resources are the safe and best sources, natural extracts, and essential oils will be good ROS scavengers. The genus Thymus L. (Lamiaceae) comprises more than 250 species growing worldwide and is well known for its medicinal importance including its antioxidant properties. Extensively published literature on genus Thymus revealed that it has many natural compounds with multiple health benefit, such as health tonic, anti-inflammatory, antitussive, antimicrobial, antiparasitic, antiulcer, and carminative properties. It also has the potential to work as an antioxidant, antimicrobial, and antiulcer[12-13]. Essential oil of Thymus algeriensis (Boiss. et Reut.) contains a wide range of monoterpenes among which oxygenated monoterpene hydrocarbon includes borneol, camphor,1,8-cineole, linalool and terpinen-4-ol[12]. An endemic aromatic herb;T. algeriensis, found in Tunisia are largely used in folk medicine for the treatment of respiratory disorders, in digestion and pregnancy complications[14]. However, whether the essential oil of this plant can be used as an antioxidant against oxidative stress for the male infertility has not been studied before. To the best of our knowledge, this is the first study to explain the antioxidant potential of T. algeriensis essential oil against the oxidative stress produced by H2O2 in animal model.
We evaluated the effect of T. algeriensis essential oil against the hydrogen peroxide toxicity on organs weight and histoarchitecture of testis, DNA fragmentation in sperm, alteration in sperm morphology and motility, sperm counts as well as glutathione content (or oxidative status) in testis of rats. On the basis of these findings,T. algeriensis can be suggested to use for the restoration of H2O2 toxicity.
MATERIALS AND METHODS ReagentsThe reagents used for the experiments were of analytical grade. TBA, Reduced glutathione (GSH), bovine serum albumin (BSA), Ellman’s reagent,[5,5’-dithiobis-(2-nitrobenzoic acid)] (DTNB), H2O2, Tris-HCl buffer purchased from Merck chemicals.
Plant Collection and ExtractionThymus sp.algeriensis was collected from the mountain of Orbata, Gafsa (Tunisia). Plants were identified and a specimen was collected by Herbarium of the National Institute of Agronomy of Tunisia (INAT) for future reference. One hundred grams of air dried aerial part of Thymus algeriensis were used for the extraction process. Plant extract was prepared by steam distillation for 6 h, and the appropriate stock solution of the active compound (150 mg/kg) were prepared.
In Vivo Assay of Testis ToxicityAnimals and Experimental Design Adult male Wistar rats (90 days old; 140 to 180 g body weight) obtained from the animal laboratory of Pasteur Institute of Tunis, Tunisia (Ethics No. LNSP/Pro 152012) were housed in a specific pathogen-free environment (in clean polypropylene cages) with 12 h light/dark cycle, relative humidity (55%±10%), and a temperature of 20-25 ℃ with ad libitum access to food and water. All experimental interventions were given daily by oral gavage. The animals were acclimatized 1 week prior to the experiments, and examined on daily basis for 5 min to acclimatize them to human contact and minimize their physiological responses to handling for subsequent protocols. All ethical themes of the studies on animals were considered carefully. After the adaptive period of 1 week, the animals were randomly divided into six groups consisting of six animals each. Group 1 served as normal control receiving saline vehicle through the experimental period. Group 2 rats were administered 0.1 mmol/L H2O2 (LD) orally for 2 weeks without administration of TEO. Group 3 treated with 1 mmol/L H2O2 (HD). Group 4 rats served as drug control group and received TEO [150 mg/(kg·day) dissolved in normal saline] by oral gavage. Group 5 rats received TEO+LD H2O2 [150 mg/(kg·day) and 0.1 mmol/L, respectively] by oral gavage (1 h before treatment with 0.1 mmol/L H2O2). Group 6 rats received TEO+HD H2O2 [150 mg/(kg·day) and 1 mmol/L, respectively]. The rats were sacrificed on day fifteen of the experiment. The testis were carefull y dissected out and quickly fixed in 10% buffered formaldehyde for routine histological study after Haematoxylin & eosin (H&E) method. Sperm count, motility and parameters of specific interest, such as GSH level and antioxidant enzyme activities (SOD, CAT, GSH-Px, and GST) were done in all the groups under investigation.
Reproductive Rrgans Weights Body weights of all experimental rats were accurately recorded daily before, during and after the treatments from day 1 till day 15. All rats were euthanized at the end of the experiment. After animal dissection, the testis were excised out quickly, weighed and the index weight (IW) of this organ were calculated according to Matousek[15] IW=organ weight (g)/100×body weight (g).
Sperm Morphology For the analysis of morphological abnormalities, sperm smears were drawn on clean and grease-free slides, and allowed to air-dry overnight[16]. The slides were stained with 1% eosin-Y/5% nigrosin and examined at 400× for morphological abnormalities in each sample.
Sperm Count and Motility Assessment of sperm motility was done according to WHO laboratory manual protocol for the examination of human semen and sperm-cervical mucus interaction[17]. In brief,10 μL of the sperm suspension was placed on semen analysis chamber. A minimum of five microscopic fields was assessed to evaluate sperm motility on at least 200 sperm for each animal.
Sperm Viability Eosin-nigrosin staining was used to assess sperm viability according to WHO protocol[11].Briefly, eosin and nigrosin was prepared in distilled water. One volume of sperm suspension was mixed with two volume of 1% eosin. After 30 s, an equal volume of nigrosin was added to this mixture. Thin smears were then prepared and observed under a light microscope at 1000× magnification. Viable sperms remained colorless while nonviable sperms stained red.
Histopathological Studies Testis of the different experimental group was removed and quickly fixed in 10% formalin for 24 h, and processed through the conventional paraffin embedding technique, sectioned at 4 μm thick and stained with haematoxylin and eosin (H&E). Photomicrographs of the desired sections were made for further observations[18].
DNA Fragmentation Analysis The integrity of extracted genomic DNA was checked by electrophoresis in 1% agarose gels. The gel was stained with ethidium bromide and visualized under UV light.
In Vitro Assay of Oxidative Stress Marker EnzymesPreparation of Homogenate The testis were weighed and homogenized in a buffer solution of potassium phosphate (pH 7.4) and centrifuged at 3000 rpm/15 min at 4 ℃. The supernatant was used for the enzymatic, non-enzymatic and TBARS assays. All enzyme activity data were normalized by the total protein content in the testis and were expressed as units of activity per mg of protein (U/mg Pr).
Protein Estimation Protein content of the testis tissue was estimated by the method of Lowry[19] using a bovine serum albumin as a standard.
Lipid P<eroxidation (LPO) Lipid peroxide content in testis tissues was determined by thiobarbituric acid reaction as described by Ohkawa[20]. The lipid peroxide concentration was expressed as nmol MDA/mg protein.
Assessment of enzymatic and non-enzymatic antioxidants Catalase (CAT) was assayed as previously mentioned[21]. Superoxide dismutase (SOD) was estimated by the method of Marklund[22]. Glutathione peroxidase (GPx) was determined according to the method of Hafeman[23]. GST was estimated by the method described by Habig[24]. The reaction was initiated with 20 mmol/L 1-chloro-2,4-dinitrobenzene (CDNB) and aethanol solution was added for conjugation of GST with CDNB.
GSH + CDNB → GS-DNB + HCl
Change in OD was read at 340 nm for 3 min at an interval of 30 s. A complete assay mixture without sample was used as control. The GST activity (U) was expressed as nmol CDNB conjugate formed min-1 mg-1 protein using a molar extinction coefficient of 9.6×103mol·L-1·cm-1.
Total reduced glutathione (GSH) was assayed according to the method of Sedlak and Lindsay (1968)[25]. 5,5’-dithiobis-(2-nitrobenzoic acid) is reduced by thiol (SH) group to form 1 mole of 2-nitro-5-mercaptobenzoic acid per mole of SH. The color developed was read at 412 nm against a reagent blank. A standard calibration curve was prepared using reduced glutathione (GSH). The amount of glutathione was expressed as μmol/mg protein.
Statistical AnalysisThe values were reported as mean±S.E.M. The significance (P<0.05) of the results was assessed by one-way analysis of variance (ANOVA), followed by Bonferroni’s test for multiple comparison or Dunnett’s multiple range test.
RESULTS Organs WeightsThe effects of TEO treatment on body weights are shown in Figure 1. All TEO-treated male rats gained weight during the period of treatment. However, the weight gain in treated groups was less than that in the control group except in TEO treated rats. H2O2 induced a decrease in body and testis weight (Figure 2). Administration of H2O2 at only 1 mmol L-1 day-1 resulted in significant decrease in the absolute weights of testis (P<0.05). The average body weight (BW) of 1 mmol/L H2O2 treated was 146±0.71 g. In the present study, reduction in the testis weight after H2O2 treatment was indicative of toxicity. The average testicular weight of treated group with LD and HD of H2O2 was 567±0.37 mg/100 g BW and 538±0.65 mg/100 g BW, respectively.
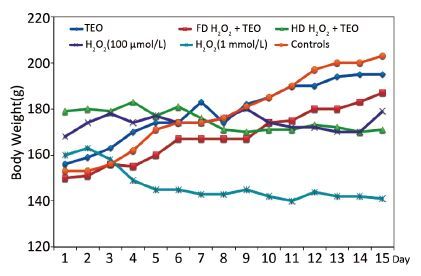
|
Download:
|
| Figure 1 Effects of H2O2 on body weight. | |
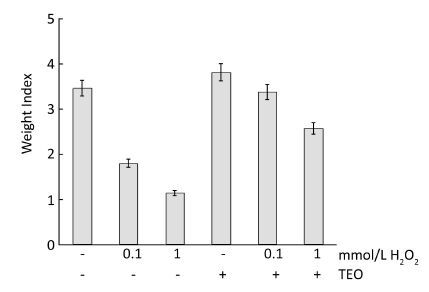
|
Download:
|
| Figure 2 Effect of TEO and H2O2 on index weights of testes. | |
In the present study H2O2, one of the most toxic reactive oxygen species, has the ability to alter sperm function characteristics.
Spermatozoa stained with Hematoxilin & Eosin showed that there was no significant difference between sperm count in TEO group compared to control group (Table 1). However, an opposite trend was encountered when the cells were treated with H2O2. The total numbers of spermatozoa per testis were significantly decreased in dose dependent manner.
The sperm motility was significantly (P<0.05) preserved in TEO group compared to that of the control group (Table 1). The percentage of viable sperm was significantly (P<0.05) preserved in TEO group compared to that of the control group (Table 1).
In the present investigation, reduction in sperm number and motility was associated with an increase of sperm abnormalities in rats exposed to H2O2. As shown in Table 1, it is evident that rat administered with high dose of H2O2 resulted in profound altered sperm morphology. Unlike the control rat, in which 1.2% of the testis spermatozoa exhibited abnormal morphology; low and high dose H2O2 treated rat showed 15.37% and 50.67% abnormal spermatozoa, respectively. These abnormalities included: amorphous head, hookless head, doublet heads, compact head tail with a cytoplasmic droplet, irregular tail and coiled tail. The notable abnormality in high dose H2O2-treated sperms was the appearance of predominant deformities such as the detached and amorphous head with the highly folded tail. We observed significant improvement in morphological abnormalities of sperms by TEO administration.
|
|
Table 1 Effects of TEO and H2O2 on Morphological Abnormalities of Sperm |
A significant decrease in the total protein of testicular tissue was observed in H2O2 treated group (Table 2). While, TEO significantly increased total protein and alleviated the negative effects in H2O2-treated group.
|
|
Table 2 Effect of H2O2 on Oxidative Stress Status |
Mammalian spermatozoa are sensitive to oxygen-induced damages mediated by lipid peroxidation of the cell membrane. Malonaldehydes, markers of lipid free radical peroxidation, were significantly elevated in all testis samples from infertile rats except for the control and TEO subgroups. Testicular cells demonstrated a significant (P<0.01) rise in lipid peroxidation following H2O2 exposure at a 1 mmol/L concentration and above, the MDA level was increased significantly in stressed rats (P<0.01) (25 %)versus the control group whereas that of recovery group rats did not differ from controls (Table 2). However, the effect was not significant at the lowest concentration of H2O2 (0.1 mmol/L). Remarkably TEO (150 mg/kg BW) treatment of rats reduced levels of MDA and as a consequence improvement in GSH level and enzymatic antioxidant activities in testis increased. It should be noted that elevated malonaldehyde values obtained in the studied of infertile rats corresponded with observed SOD activity changes.
The activities of the testicular SOD, CAT, and GST were significantly reduced in stress group as well as in recovery group rats compared to controls (Table 2). After being treated with H2O2, the testis levels of SOD, CAT, GSH-Px, and GST were significantly decreased (P<0.01) in comparison of the corresponding control. Both, superoxide dismutase (SOD) and catalase from treated cells showed a significant (P<0.01) decline in their activities above a 100 μmol/L concentration of H2O2. Interestingly, the decline in the activity of glutathione S-transferase (GST) also revealed that TEO reverses the effect of H2O2 treatment. However, the change in GST activity is lesser than the control one. The activity of GPx was significantly reduced in stress group compared to controls, while the recovery group was significantly higher than stressed rats and did not differ from controls. Total reduced GSH was significantly (P<0.001) decreased (0.093±0.00 μmol/mg protein) in response to H2O2 treatment as compared to non-treated animals. The H2O2-induced alterations in enzymatic activities and GSH level were significantly prevented by TEO pretreatment (Table 2).
To establish the relationship between MDA levels and other sperm parameters, linear correlation coefficients were calculated and results are shown in Table 3. Positive significant (P<0.05) correlations confirmed between proteins concentration (r=-1.00), CAT (r=-0.88), SOD (r=-0.97), GPx (r=-0.96), GSH (r=-0.92), GST (r=-0.84), sperm Count (r=-0.87), Motility (r=-0.86), morphology (r=-0.83), daily body weight gain (r=-0.93), testis weight (r=-0.93) and MDA levels. The results illustrated total lack of correlation between viability (r=-0.78), abnormality (r=-0.70) and MDA levels was detected.
|
|
Table 3 Linear correlation coefficients between MDA concentration, and sperm parameters quality |
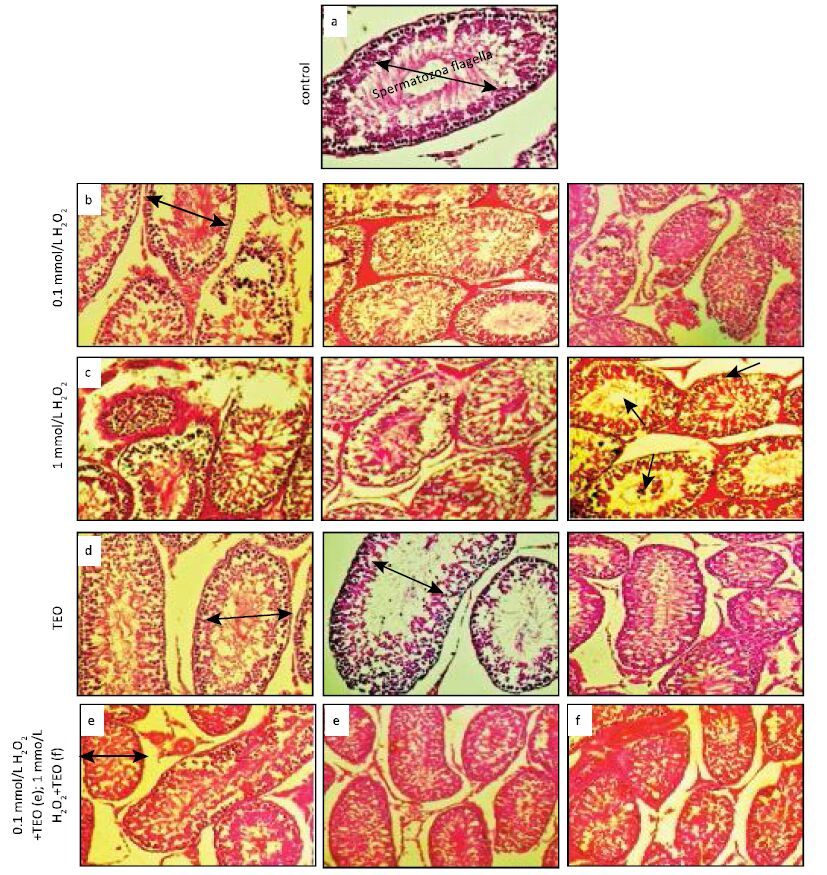
We observed the normal structure of seminiferous tubules (ST), each one is lined with spermatogenic cells and sertoli cells, and swirling movement of sperm in untreated as well as only TEO treated rats. In H2O2-treated rats, many histopathological abnormalities were observed according to treatment dose. At LD H2O2-treated rats, most ST were separated by wide intertubular spaces, few numbers of sertoli cells and degenerated spermatogenic epithelium with incomplete spermatogenesis, devoid of spermatozoa (Figure 3).
|
Download:
|
| Figure 3 Histology of testes stained with hematoxylin-eosin in control rats, rats treated with TEO and different concentrations of H2O2. a) Testis showing the normal structure of the seminiferous tubules in groups of control in male rats, swirling movement of sperm in the seminiferous tubules with complete spermatogenesis and normal interstitial connective tissue in the testes of these rats; b) Testes of male rats intoxicated with single dose of LD H2O2 showing complete testicular necrosis and germinal epithelium of all layers. hemorrhage, shrunken, buckled, disorganized seminiferous tubules, sloughing germinal epithelium (asterisks) in thelumen of the seminiferous tubules of rats who received HD of H2O2; c) Photomicrograph of testis showing degenerative and necrotic seminiferous tubules (white arrow), few spermatozoa (black arrow), vacuolation (small arrows), hemorrhage between seminiferous tubules (arrows) and some of the spermatogenic cells desquamate or vanish (H&E×200) Group 3 (HD H2O2); d) Pretreatment with TEO (Group 4) reversed damage produced by H2O2 suggesting that TEO prevent testicular dysfunction induced by H2O2 and showed the lesser of degenerative and atrophic changes of the tubular epithelium. The testicular histomorphology of rats in the recovery does not resemble that of the stressed rats or control. There was a vacuolization of the seminiferous epithelium (Figure 3c) and the spermatozoa were more abundant than the stressed rats. A marked improvement in spermatogenesis, as evidenced by the presence of spermatids and spermatozoa in the majority of rats treated with Group 4 (TEO) and Group 5 (TEO) + LD H2O2; e) and f) Rats treated with TEO + H2O2 show a significant improvement in all of these deterioration (e), although partial (f). | |
At HD level, vacuolation in the intratubular (ST) (white and black arrows), necrosis (long arrows) and hemorrhage, tunica albugenea contains myoid cells, dark nuclei of spermatogonia, primary spermatocytes, spermatids, spermatozoa, intratubular degeneration in spermatogenic stages; leydig cells, wide intertubular spaces of ST; loss of spermatogenic stages, loss of elongated spermatids, degenerated Leydig cells. Further whether, TEO prevent this LD H2O2-induced morphological alteration was also determined. We examine the effect of LD H2O2 on TEO-pretreated rats and observed that ST, spermatogenic cells and sertoli cells were appeared almost similar to the control. These histological studies also justify the antioxidant potential of TEO against H2O2-induced morphological abnormalities.
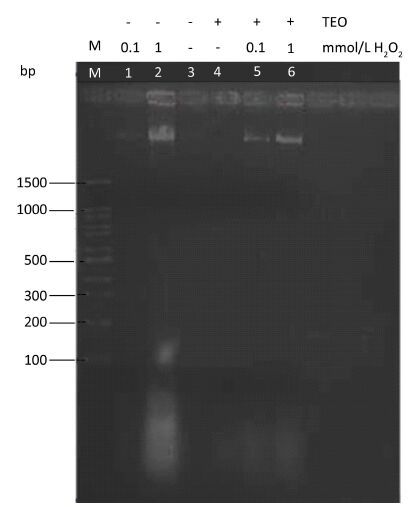
Effect of H2O2 and TEO on DNA FragmentationDNA was extracted from sperms of different untreated and treated groups, and examined by agarose-gel electrophoresis. DNA fragmentation was observed in H2O2 treated testis samples. However, there was no DNA fragmentation in control and TEO treated testis (Figure 4). H2O2 induced DNA damage was evaluated by comparing DNA profiles using 1.5% agarose gel electrophoresis. In 1 mmol/L H2O2 treated rats; a clear DNA fragmentation was observed (Figure 4), where the fragments formed a typical apoptotic ladder in the gel, with most of the DNA accumulated in the lower bands. The degradation of DNA into oligonucleotide fragments confirmed the induction of apoptosis by H2O2 (Figure 4). Treatment with TEO led to significant protection against H2O2 induced DNA fragmentation in testis.
|
Download:
|
| Figure 4 1% agarose gel electrophoresis of DNA extracted from rat testis treated with H2O2 and TEO. Lane M: DNA molecular weight marker. Lane 1: LD H2O2-treated rat. Lane 2: HD H2O2-treated rat. Lane 3: control rat. Lane 4: TEO-treated rat. Lane 5: LD H2O2 with TEO. Lane 6: HD H2O2 with TEO. | |
ROS are supposed to play one of the key roles in the development of testis toxicity, which is a sporadic and challenging issue for pharmaceutical drug development[26]. Since organ weight is a fundamental benchmark for the toxicological studies[27], we determined the weight loss of H2O2 treated testis, which is basically dependent on the mass of the differentiated spermatogenic cells. Weight loss of the testis can occur due to decreased number of germ cells, inhibition of spermatogenesis and steroidogenic enzyme activity[28]. Recently, EL-Kashoury (2009)[29] has shown that the weight of testis was significantly lowered in H2O2 treated male rats. Thus, the observed weight loss of the testis may be due to the adverse effect of H2O2 on the number of germ cells and elongated spermatids. In some reports, the decrease in testicular weight was also the result of reduction of tubule size, spermatogenic arrest and inhibition of steroid biosynthesis of Leydig cells[30]. Other than weight of testis, overall morphology, sperm count, motility should also be analysed to determine semen quality[31]. If the sperm motility is examined only 5% to 10%, sperm viability testing is recommended because low motility can occur due to sperm death or necrospermia[32]. Besides sperm motility and sperm counts, sperm morphology has also been considered as a good indicator of semen quality[33]. A number of morphological alterations occurs due to some abnormal process in sperm morphogenesis and subsequently reduces the fertilizing ability[34]. Oxidative stress has a direct adverse effect on sperm count and motility as excess of ROS induces germ cell apoptosis and loss of mitochondrial membrane potential[35].Progressive sperm motility was also significantly lower in the H2O2 toxicated groups compared to others. The current data concur with the previous studies of Chaki and Misro, who found that 60 μmol/LH2O2 dose showed little effect on motility, the first parameter to be affected at higher concentrations[36]. Administration of H2O2 caused a significant increase in sperm abnormalities and ratio of viable to dead sperm count, and suppression of sperm motility. Our results are supported by a previous study which showed that ROS induced damage of spermatozoa involves an oxidative attack on the lipids of sperm plasma membrane causing spermatozoa to lose their motility[4].Sperms with abnormal morphology were also shown to be less motile. Fadwa et al., found that treatment of drinking water with H2O2 (0.5%) caused a significant (P<0.05) decrease in the percentage of ratio of live to dead sperms with a significant (P<0.05) increase in the p ercentage of morphologically abnormal sperms[37]. Conclusively, the function of a defective sperm is associated with low cell count, impaired motility and abnormal morphology.
As we have examined in our study,Thyme essence helped an early recovery of spermatogenesis. In only TEO rats, the sperm count and motility exceeded the normal mean values. Out results determines that TEO treatment can counteract the deleterious effects of H2O2 on morphological abnormalities observed in rat models. Our result reveals that the protein content was significantly elevated in male reproductive organs due to TEO exposure. A significant increase in the number of ribosome may be occurring due to their increased mobilization and this leads to the augmented protein synthesis[38].Oxidative damage of proteins by H2O2 exposure may lead to the structural alteration and functional inactivation of many enzymes and cell signalling receptors.
Lipid peroxidation (LPO) reaction causes membrane damage which leads to a decrease in sperm motility, presumably by a rapid loss of intracellular ATP, and an increase in sperm morphology defects[39].
Our result reveals that H2O2 treated rats had the highest concentration of MDA in the testicular tissues which indicates the generation of LPO and subsequently loss of membrane, structure, and function. Griveau[40] also reported that H2O2 is involved in the lipid alterations triggered by the xanthine-xanthine oxidase system. As we observed, TEOcould reduce TBARS induced by H2O2 in testis. Our results are thus in agreement with the findings of Rahim[41], who reported an increase in testicular MDA levels in H2O2 treated rats relative to the control group. As shown in numerous studies, increased concentrations of malondialdehyde correlate with maturation arrest and decreased spermatozoa concentration and morphology and, most notably, motility, due to alterations in the membrane[42]. These results suggested that oxidative stress was the main reason of low sperm quality and the etiology of male infertility which can be treated by the TEO. The measure of TBARS could be useful diagnostic tool for estimation of oxidative stress.
As enzymatic defence systems, catalase, and H2O2 scavengers provided a significant protective measure against either heat or oxidative stress induced apoptosis. An appropriate concentration of H2O2 has been shown to induce apoptosis in a variety of cell types including testicular cells[43].H2O2 induced impairment of superoxide dismutase activity has been previously reported[44]. SOD stabilizes the plasmalemma of spermatozoa and so increases motility[45].Cell membranes are protected by GPx from ROS-mediated oxidative damage resulting from the formation of hydrogen peroxide (H2O2) during normal metabolism in the cells mitochondria. The reduction in the activities of CAT and GPx may reflect the inability of testicular mitochondria, microsomes and cytosol to eliminate the H2O2 produced by SOD. This may also be attributed to enzyme inactivation caused by excess ROS production[46].
The testis has high concentration of GSH, which plays an important role in spermatogenesis[47].GSH concentrations may be very less due to oxidative stress, and the major peroxide detoxification enzyme GSH peroxidise[48]. Interestingly, TEOpretreatment attenuated testicular dysfunction and renewed the activities of the antioxidant enzymes and GSH level by down-regulating the ROS level.
H2O2 induced sever testicular toxicity as shown in the histopathological results, which coupled with the marked changes of biochemical results. In the present study, our investigation demonstrated that exposure to H2O2 induces histopathological changes of testis in a concentration-dependent manner. The histomorphological alteration of the testis of recovery group rats did not resemble with the stressed or the control rats. The results showed the cystic degenerative deviations with some vacuolization of seminiferous epithelium, and spermatozoa were more abundant when compared to the control sections.
Rahim[41] presumed that treatment with H2O2 markedly increased MDA in serum and testis homogenates, as well as testicular histopathological alterations. In addition, our findings supported by the other studies confirms that H2O2 at a higher concentration in the medium is toxic and affects cell survival even at physiological concentrations H2O2 (30-50 μmol/L) was shown to induce apoptosis in testicular leydig cells in vitro[43].Histological examination revealed that the number of spermatogonia was dramatically decreased.
Our data exhibited that normal testicular architecture was observed in controls (Figure 3a) and only TEO-administered group (Figure 3d). The H2O2-treated rats show vacuolization in germinal epithelium, lost of germinal line and total reduction of spermatogenesis. Our findings are consistent with Creasy[49], who explained the presence of vacuoles within the seminiferous epithelium, which is sensitive to a variety of toxicants.
DNA fragmentation is not determine in male infertility, but represents an extremely important parameter indicative of infertility and potential outcome of assisted reproduction treatment[50]. Our observations indicated that the direct effect of higher concentrations of H2O2 causes DNA damage which can be seen in the form of a base damage (depurination or depyrimidination), single strand breaks, double strand breaks, chromosomal aberrations, and cross-linking between DNA[51]. Although this would appear to be good evidence for the involvement of an endonuclease, DNA fragmentation was restored in the presence of TEO (Figure 4). In this study we selected histological parameters of the adult rat testis and tested the protective effects exerted by TEO in H2O2 treated rats.
CONCLUSIONSThe present study demonstrated that oxidative stress generated by H2O2 resulted in decline of sperm motility and morphological alterations with the decreased sperm count and induction of DNA damage. The reduction in the level of GSH, disturbed sperm parameters, increased lipid peroxidation and sperm DNA fragmentation revealed an adverse effect of H2O2 on the reproductive system of developing male rats. The eco-friendly, and nontoxic natural compounds such as TEO can prevent these adverse effects. Further preclinical studies and clinical trials in humans are needed to find out a possible place for it in therapies of fertility disturbances.
ACKNOWLEDGMENTS
The authors would like to express their utmost gratitude and appreciation to Mr. Mossadok Ben-Attia for giving us free access to the pet shop, to Dr. Bellamine Houda and the technician Smiti Raowdha who performed histological sections.
| 1. | Sasaki JC, Chapin RE, Hall DG, et al. Incidence and nature of testicular toxicity findings in pharmaceutical development[J]. Birth Defects Res B Dev Reprod Toxicol , 2011, 92 :511–25. doi:10.1002/bdrb.v92.6 |
| 2. | Mehrotra A, Katiyar DK, Agarwal A, et al. Role of total antioxidant capacity and lipid peroxidation in fertile and infertile men[J]. Biomed Res , 2013, 24 :347–52. |
| 3. | Barbouti A, Doulias PT, Nousis L, et al. DNA damage and apoptosis in hydrogen peroxide-exposed Jurkat cells: bolus addition versus continuous generation of H2O2[J]. Free Radical Bio Med , 2002, 33 :691–702. doi:10.1016/S0891-5849(02)00967-X |
| 4. | Aitkin RJ, Harkness D, Buckingham DW. Analysis of lipid peroxidation mechanisms in human spermatozoa[J]. Mol Reprod Dev , 1993, 35 :302–15. doi:10.1002/(ISSN)1098-2795 |
| 5. | Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy[J]. Hum Reprod , 2011, 26 :1628–40. doi:10.1093/humrep/der132 |
| 6. | Aitken RJ, Roman SD. Antioxidant system and oxidative stress in the testes. In: Cheng CY (ed) Molecular mechanism in spermatogenesis. Landes Bioscience and Springer Science+Business media, 2008, 636: 154-71. |
| 7. | Meseguer M, Garrido N, Simo C, et al. Concentration of Glutathione and Expression of Glutathione Peroxidases 1 and 4 in Fresh Sperm Provide a Forecast of the Outcome of Cryopreservation of Human Spermatozoa[J]. J Androl , 2004, 25 :773–80. doi:10.1002/j.1939-4640.2004.tb02855.x |
| 8. | Jokanovic M. Biotransformation of organophosphorus compounds[J]. Toxicology , 2001, 166 :139–60. doi:10.1016/S0300-483X(01)00463-2 |
| 9. | de Carvalho MC. Drought stress and reactive oxygen species: production, scavenging and signaling[J]. Plant Signaling Behav , 2008, 3 :156–65. doi:10.4161/psb.3.3.5536 |
| 10. | Ullah R, Zuberi A, Ullah S, et al. Cypermethrin induced behavioral and biochemical changes in mahseer, Tor putitora[J]. J Toxicol Sci , 2014, 39 :829–36. doi:10.2131/jts.39.829 |
| 11. | Pathak N, Khandelwal S. Oxidative stress and apoptotic changes in murine splenocytes exposed to cadmium[J]. Toxicol , 2006, 220 :26–36. doi:10.1016/j.tox.2005.11.027 |
| 12. | Guesmi F, Ben Farhat M, Mejri M, et al. In vitro assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus sp. algeriensis[J]. Lipids Health Dis , 2014, 13 :114. doi:10.1186/1476-511X-13-114 |
| 13. | Guesmi F, Ben Ali M, Barkaoui T, et al. Effects of Thymus hirtus sp. algeriensis Boiss. et Reut. (Lamiaceae) essential oil on healing gastric ulcers according to sex[J]. Lipids Health Dis , 2014, 13 :138. doi:10.1186/1476-511X-13-138 |
| 14. | Giweli AA, Dzamic AM, Sokovic MD, et al. Chemical composition, antioxidant and antimicrobial activities of essential oil of Thymus algeriensis wild-growing in Libya[J]. Cent Eur J Bot , 2013 :504–11. |
| 15. | Matousek J. Effect on spermatogenesis in guinea pigs, rabbits and sheep after their immunization with sexual fluid of bulls[J]. J Reprod Fertil , 1969, 19 :63–72. doi:10.1530/jrf.0.0190063 |
| 16. | Rezvanfar M, Sadrkhanlou R, Ahmadi A, et al. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress[J]. Hum Exp Toxicol , 2008, 27 :901–10. doi:10.1177/0960327108102046 |
| 17. | World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction[M]. UK: Cambridge University Press , 1999 . |
| 18. | Wang B, Chen D, Guo W, et al. Silica Nanoparticles Suppress Fibronectin-Mediated Adhesion and Migration in Normal Human Keratinocytes[J]. J Nanosci Nanotechnol , 2012, 12 :293–9. doi:10.1166/jnn.2012.5127 |
| 19. | Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with folin-phenol reagent[J]. J Biol Chem , 1951, 193 :265–75. |
| 20. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction[J]. Anal Biochem , 1979, 95 :351–8. doi:10.1016/0003-2697(79)90738-3 |
| 21. | Takahara S, Hamilton HB, Neel JV, et al. Hypocatalasemia: a new genetic carrier state[J]. J Clin Invest , 1960, 39 :610–9. doi:10.1172/JCI104075 |
| 22. | Marklund SL. Pyrogallol autooxidation. In: Greenwald RA, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton[M]. Boca Raton, Florida: CRC Press , 1985 : 243 -7. |
| 23. | Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium and erythrocyte and liver glutathione peroxidise in the rat[J]. J Nutr , 1973, 104 :580–7. |
| 24. | Habig WH, Pabst MJ, Jakoby WB. Glutathione s-transferase: the first enzymatic step in mercapturic acid formation[J]. J Biol Chem , 1974, 249 :7130–9. |
| 25. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent[J]. Anal Biochem , 1968, 25 :192–205. doi:10.1016/0003-2697(68)90092-4 |
| 26. | Jennifer CS, Robert EC, David GH, et al. Incidence and Nature of Testicular Toxicity Findings in Pharmaceutical Development[J]. Birth Defects Res B Dev Reprod Toxicol , 2011, 92 :511–25. doi:10.1002/bdrb.v92.6 |
| 27. | Yavasoglu A, Karaaslan MA, Uyanikgil Y, et al. Toxic effects of anatoxin-a on testes and sperm counts of male mice[J]. Exp Toxicol Pathol , 2008, 60 :391–6. doi:10.1016/j.etp.2008.04.001 |
| 28. | Takahashi O, Oishi S. Testicular toxicity of dietary 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in F344 rat[J]. Arch Toxicol , 2001, 75 :42–51. doi:10.1007/s002040000204 |
| 29. | EL-Kashoury AA. Influence of subchronic exposure of profenofos on biochemical markers and microelements in testicular tissue of rats[J]. J Am , 2009, 5 :19–28. |
| 30. | Sujatatha R, Chitra KC, Latchoumycandane C, et al. Effect of lindane on testicular antioxidant system and steroidogenic enzymes in adult rats[J]. Asian J Androl , 2001, 3 :135–8. |
| 31. | Abbiramy VS, Shanthi V. Spermatozoa Segmentation and Morphological Parameter Analysis Based Detection of Teratozoospermia[J]. Int J Comput Appl , 2010, 3 :19–23. |
| 32. | Rothmann SA, Reese AA. Semen analysis. The test techs love to hate[J]. MLO Med Lab Obs , 2007, 39 :18–20. |
| 33. | Rodriguez-Martinez H. Laboratory semen assessment and prediction of fertility: still utopia?[J]. Reprod Domest Anim, , 2003, 38 :312–8. doi:10.1046/j.1439-0531.2003.00436.x |
| 34. | Jacques A. Assessing human sperm morphology: top models, underdogs or biometrics?[J]. Asian J Androl , 2010, 12 :36–46. doi:10.1038/aja.2009.8 |
| 35. | Baumber J, Ball BA, Gravance CG, et al. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation[J]. J Androl , 2000, 21 :895–902. |
| 36. | Chaki SP, Misro MM. Assessment of human sperm function after hydrogen peroxide exposure: development of a vaginal contraceptive[J]. Contracept , 2002, 66 :187–92. doi:10.1016/S0010-7824(02)00349-9 |
| 37. | Fadwa KhT, Suha MA, Soulaf JK. Effect of Nigella sativa oil treatment on the sex organs and sperm charactors in rats exposed to hydrogen peroxide[J]. Mesopotamia J Agric , 2006, 34 :2–8. |
| 38. | Mukerjee H, Goldfeder A. Release of ribosomes from endoplasmic reticulum (ER) or X-irradiated livers[J]. Radiat Res , 1974, 58 :253–61. doi:10.2307/3573937 |
| 39. | Kistanova E, Marchev Y, Nedeva R, et al. Effect of the Spirulina platensis induced in the main diet on boar sperm quality[J]. Biotech Anim Husbandry , 2009, 25 :547–57. doi:10.2298/BAH0906547K |
| 40. | Griveau JF, Dumont E, Renard P, et al. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa[J]. J Reprod Fertil , 1995, 103 :17–26. doi:10.1530/jrf.0.1030017 |
| 41. | Rahim SM, Taha EM, Mubark ZM, et al. Protective effect of cymbopogon citratus on hydrogen peroxide-induced oxidative stress in the reproductive system of male rats[J]. Syst Biol Reprod Med , 2013, 59 :329–36. doi:10.3109/19396368.2013.827268 |
| 42. | Benedetti S, Tagliamonte MC, Catalani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality[J]. Reprod BioMed Online , 2012, 25 :300–6. doi:10.1016/j.rbmo.2012.05.011 |
| 43. | Gautam DK, Misro MM, Chaki SP, et al. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis[J]. Apoptosis , 2006, 11 :39–46. doi:10.1007/s10495-005-3087-1 |
| 44. | Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: levels of Superoxide dismutase-and catalase-like activities in seminal plasma and spermatozoa[J]. Int J Androl , 1993, 16 :183–8. doi:10.1111/j.1365-2605.1993.tb01177.x |
| 45. | Perumal P, Vupru K, Rajkhowa C. Effect of Addition of Taurine on the Liquid Storage (5 ℃) of Mithun (Bos frontalis) Semen[J]. Vet Med Int , 2011, 2013 :1–7. |
| 46. | Milton SP, Shagirthab K, Muthumani M. The protective role of S-allylmercaptocysteine against cadmium induced changes in sperm characteristics and testicular oxidative damage in rats[J]. J Pharm Res , 2010, 3 :2717–1721. |
| 47. | Sahoo DK. Testicular protection from thyroid hormone mediated oxidative stress[J]. WebmedCentral REPRODUCTION , 2013, 4 :WMC004252. |
| 48. | Nakamura W, Hosoda S, Hayachi K. Purification and properties of rat liver glutathione peroxidase[J]. Biochim Biophys Acta , 1974, 358 :251–61. doi:10.1016/0005-2744(74)90455-0 |
| 49. | Creasy DM. The male reproductive system, In: Turton J, Hooson J, editors. Target Organ Pathology: A Basic Text [M]. London Bristol: Taylor &, Francis , 2005 : 334 -65. |
| 50. | Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility[J]. Reprod BioMed Online , 2014, 28 :684–703. doi:10.1016/j.rbmo.2014.02.004 |
| 51. | Cadet J, Berger M. Radiation-induced decomposition of purine bases within DNA and related model compounds[J]. Int J Rad Biol relat stud Phys Chem Med , 1985, 47 :127–43. |
 2016, Vol. 29
2016, Vol. 29


