2. 山东农业大学生命科学学院,泰安 271018
2. College of Life Sciences, Shandong Agricultural University, Tai'an 271018
土壤盐渍化是全球土地退化的主要表现形式之一,目前,全世界至少20%的耕地遭受不同程度的盐渍化威胁[1]。虽然植物能够在盐渍土生境中有一定的产量,但是多数作物和树种耐盐性不高,其中,小麦、玉米、水稻、大麦等主要粮食作物受到盐胁迫减产可达到70%[2]。盐胁迫对植物的生长发育具有多方面的作用,包括复杂的分子、基因、生物化学、生理学和形态学影响[3]。胁迫持续的时间与严重性能够造成植物不同程度的损伤[4-5],如初期的渗透胁迫、氧化胁迫和离子毒性可能导致植物细胞膜结构破坏、营养失衡、活性氧(Reactive oxygen species,ROS)消除能力下降、抗氧化酶活性下降、光合作用减弱等,随着大量的Na+和Cl-的摄入,细胞离子平衡和生理平衡被打破,植物根系吸水能力降低,叶片中含水率下降[6],植物难以吸收K+,逐渐造成植物营养亏缺、生产力下降甚至死亡[4]。与其他胁迫类似,植物通过调控复杂的信号网络来应对盐胁迫,包括胁迫传感蛋白、信号传导因子、转录因子、应激反应基因和代谢物等[7]。
土壤盐渍化降低了植物根际土壤水的能量状态,使土壤溶液渗透压超过了植物细胞液的正常渗透压,导致植物根系长度、密度和结构改变,阻碍了植物对土壤水分和养分的摄取,使植物处于生理干旱状态,生长速度变得缓慢,甚至萎蔫或死亡[8]。渗透胁迫和离子胁迫相互作用产生次级氧化胁迫[9],打破了植物体内活性氧产生与清除间的动态平衡,破坏细胞膜系统的完整性,影响了植物的光合作用[10]。
植物促生菌(Plant growth-promoting bacteria,PGPB)能与植物建立共生关系,并在正常状态下或植物面临胁迫时促进其生长[11]。合理使用PGPB是减轻盐胁迫危害的一种重要途径[12]。不具有耐盐性的PGPB在自然条件下随盐度增加逐渐失去PGP(Plant growth-promoting)特征,开发利用耐盐PGPB是盐胁迫下增加作物产量的可行措施。研究发现PGPB具有产植物激素、铁载体、ACC脱氨酶,溶磷,固氮,解钾,上调抗氧化酶(如SOD、POD等),调节离子转运蛋白(如HKT)表达,分泌胞外多糖(EPS),改变根系结构和形态、导水率和激素状态,释放与胁迫相关的挥发性化合物,积累渗透物(谷氨酸、脯氨酸、肽等),抑杀病原菌等特性,以上机制与提高植物耐盐性、诱导植物产生盐胁迫系统抗性(Induced system tolerance,IST)有关,对促进植物根、茎和叶片的发育及生长、防治病害、调节植物根际微生物群落结构有显著效果[1, 3, 13-23]。此外,蛋白质组学分析显示,PGPB影响参与植物光合作用、抗氧化过程、跨膜转运和发病相关的多种蛋白质的表达[24]。本文综述了PGPB在盐胁迫中提高植物耐盐性的作用机制研究进展,旨在为筛选能够改良盐碱地的耐盐促生菌株奠定理论基础。
1 PGPB产生ACC脱氨酶乙烯是几乎所有植物都会产生的植物生长调节剂和应激激素[25],能够引起植物产生生理、分子水平上的变化。盐胁迫能够激发植物产生过量的乙烯,结节中的乙烯水平升高将降低固氮量,从而抑制植物根及植株的进一步生长,降低胁迫诱导的乙烯水平将减轻作物的胁迫损伤。分泌ACC脱氨酶(ACC deaminase,ACCD)的PGPB通过代谢植物生产乙烯的前体ACC来降低乙烯水平,还可以通过改变编码乙烯合成酶、ACC合酶和ACC氧化酶基因来影响植物乙烯内源稳态,促进根伸长[15, 26]。
近年来,大量研究证明,具有ACC脱氨酶活性的菌株能够在实际生产中改善植物的生长状态。例如,荧光假单胞菌能够使玉米在电导率为9 dS/m的盐碱土中根长增加330%,恶臭假单胞菌能够使玉米在12 dS/m的盐碱土中株高增加230%[27]。巴西固氮螺菌FP2可以使小麦植株ACC氧化酶表达量降低,促进根的生长[28]。耐盐PGPB Micrococcus yunnanensis、Planococcus rifietoensis和Variovorax paradoxus接种到甜菜(Beta vulgaris L.)后,提高了种子发芽率和植株生物量,增强了光合作用,胁迫诱导的乙烯含量也显著降低[29]。以上研究表明,能够产生ACC脱氨酶的PGPB可以减轻盐胁迫对作物的影响,并将乙烯降低到抑制生长的水平以下。多种植物内生菌也被发现具有ACC脱氨酶活性[30],通过对比从各种生境(包括植物的不同组织)中分离菌株的ACC脱氨酶活性,发现内生菌比从根际土以及非根际土等生境分离的PGPB具有更高的ACC脱氨酶的活力[31]。目前,具有ACC脱氨酶活性的PGPB已成为盐土改良的重要生物资源。
2 PGPB调节植物内源激素水平 2.1 PGPB产生IAA通过对受到盐和干旱胁迫的拟南芥和水稻等植物的多种信号传导途径和组分研究,发现植物激素互相协调配合可以提高植物渗透调节水平[32]。植物生长素(auxin,IAA)对植物细胞、组织和新生器官的特异性等有重要作用,作物在受到盐胁迫后体内IAA浓度显著降低,如番茄可降低75%左右[33],IAA信号转导途径与植物幼苗的发育过程关系紧密[34],盐胁迫通过改变植物IAA的积累和再分配重塑了根系结构,影响了植株的生长速度[35]。用IAA处理小麦种子可以减轻盐胁迫对小麦种子萌发和幼苗发育造成的不利影响[36]。
很多PGPB能够产生IAA[37]。研究发现,小麦接种产生IAA的中度嗜盐菌后,根中IAA含量显著提高,其生长量显著增加[38]。Sharma等[39]将产生IAA的菌株Klebsiella、Pseudomonas、Agrobacterium和Ochrobactrum接种到Arthrocnemum indicum后,植物生物量显著增加,耐盐性增强。从盐角草根际分离的产IAA耐盐菌株B. endophyticus、B. tequilensis、Planococcus重新接种到盐角草后,发芽率增加7%-11%,茎长增加13%-22%,根长增加44%-57%,鲜重增加21%-54%[40]。在25 mmol/L NaCl盐胁迫下,茄子幼苗接种产IAA的菌株Xanthobacter autotrophicus BM13、Enterobacter aerogenes BM10和Bacillus brevis FK2后,幼枝鲜重较对照组显著增加12.13%-19.58%,产量也得到提高[41]。以上研究表明,通过PGPB调节盐生植物和甜土植物的IAA生成量可能是增强作物耐盐性的重要因素。
2.2 PGPB产生ABA脱落酸(Abscisic acid,ABA)是一种重要的应激激素,可在植物响应非生物胁迫后合成,并激活抗逆基因的表达[42],是调节植物全生育期活动以及刺激植物产生对广泛非生物胁迫适应性反应的重要信号介质。ABA作为内源信号分子能够通过调节气孔活动影响植物的光合作用,进而减轻盐胁迫对植株的损伤并提高植物的耐盐性和存活率[3, 43-45]。研究发现,许多PGPB能够在盐胁迫下产生ABA,改变植物内源ABA水平,使RAB18、ABA反应元件RD29A和RD29B调节子、脱水反应元件(DRE)等基因表达上调,提高植物耐盐性[21, 46-47]。
豌豆(Pisum sativum)接种产生ABA的争论贪噬菌5C-2降低了根中ABA的水平,并影响韧皮部中芽-根ABA转运的长距离信号传导和木质部中的根-茎ABA转运过程[48]。葡萄与拟南芥接种产ABA的地衣芽孢杆菌、荧光假单胞菌、巴西固氮螺菌后,内源ABA含量增加并且具有更高的渗透胁迫耐受性[49-50]。大量研究表明,PGPB诱导的植物ABA水平变化可能会降低植物对渗透胁迫的敏感性,对提高植物非生物胁迫耐受性具有显著作用。
2.3 PGPB产生GA赤霉素(Gibberellin,GA)参与植物的多个生长发育阶段,如种子萌发、叶片膨大、光形态建成、茎伸长、花器的发育和果实的成熟等,对细胞分裂和伸长,下胚轴、根和叶分生组织大小的调节有积极作用[51-53]。GA3是一种具有生物活性的GA,在低盐胁迫下可以显著提高种子的发芽率和植物的水分利用率[54-55],在高盐胁迫下仍能显著改善植物的生长状况[56]。
PGPB可以影响植物内源GA水平[57]。例如,接种产生GA的蜡状芽孢杆菌MJ-1的辣椒,茎鲜重增加138%,根鲜重增加128%,具有更高的内源GA水平,表现出更好的定殖和促生效果[58]。同样,Leifsonia soli SE134菌株产生的GA对水稻、黄瓜、番茄和萝卜有促生作用[59]。Bacillus sp.和Azospirillum sp.产生的GA增加了植物氮的吸收[60]。接种产GA3的解淀粉芽孢杆菌,水稻内源GA1、GA4、GA7和GA9被上调,水杨酸含量增加,促生效果显著[61]。接种产生GA的PGPB能够通过影响植物内源GA3、水杨酸、脱落酸等激素水平促进作物生长[62]。
2.4 PGPB产生JA和SA茉莉酸(Jasmonic acid,JA)和水杨酸(Salicylic acid,SA)广泛存在于植物体内[63]。JA作为信号分子参与植物对水、盐的响应,也是植物各发育过程和防御反应的重要信号分子[64]。近年来,分子生物学研究证实了JA与植物耐盐性的相关性。SA在植物细胞膜疏水区积累,可以减少细胞内电解质外渗,减轻膜脂过氧化,保护蛋白质及膜结构的完整性,对植物种子萌发、离子吸收与转运、细胞膜透性、光合作用及生长速率等生理生化过程产生影响,进而减轻盐胁迫对植物的伤害[65-66]。
多种能够合成并向植物提供JA、SA的PGPB能够促进植物生长,提高植物耐盐性、抗病能力[67-68]。产生SA的解淀粉芽孢杆菌LJ02接种到黄瓜幼苗后,植株SOD、POX、CAT、PPO、PAL显著提高,叶片中游离SA含量和抗病基因PR-1的表达明显升高,植株抗病能力增强[69]。Burkholderia phytofirmans PsJN作用于葡萄后也使植株内源SA增加,激活并上调了葡萄的防御基因[70]。研究表明,产生JA和SA的PGPB促进了植物内源SA的积累,同时能够提高植物的抗病和抗氧化活性,对植物的耐盐性产生了积极影响。
2.5 PGPB产生CTK嘌呤类化合物参与根愈伤组织分化和芽形成的过程[71]。细胞分裂素(Cytokinins,CTK)主要调节植物细胞分裂、顶端优势、叶绿体生物发生、养分调节、叶片衰老、管组织分化、光信号传导发育、芽分化和花青素的产生等生命活动,参与植物对生物和非生物胁迫抗性的形成[72-73]。多种PGPB能够产生CTK并改变植物内源CTK浓度[37, 74],其中,AHK3、AHK2、CRE1/AHK4/WOL三种受体能够以CTK依赖的方式促进调节因子的表达和信号传导[75-76],对促进植物生长有积极作用[77]。例如侧柏接种产CTK的枯草芽孢杆菌,芽中CTK含量增加,对渗透胁迫的抗性更强[78]。枯草芽孢杆菌接种到莴苣根际,通过调节植株根-芽CTK信号传导来促进作物生长[79]。PGPB合成CTK并改变植物CTK内稳态的能力凸显了PGPB在刺激植物生长和增加植物耐盐性上的重要性。
3 PGPB促进植物营养吸收 3.1 PGPB的溶磷作用磷(P)是植物的主要常量营养素之一。土壤中P含量约0.05%(W/W),其中只有0.1%的P为有效磷,严重限制了P的利用率[80]。盐碱地降低了植物对磷的吸收,抑制了植物生长和代谢,而施用无机磷肥料及盐水灌溉会增加土壤盐分[16]。溶磷菌(Phosphate-solubilizing bacteria,PSB)能够分泌低分子量有机酸,通过螯合、离子交换和酸化等各种机制将不溶性磷酸盐转化为HPO42-和H2PO4-,进而提高植物组织中磷含量,改善植物长势并减轻盐胁迫损伤[81]。
从白骨壤根际筛选鉴定出129种能够溶解磷酸盐岩的细菌菌株,其中,Oceanobacillus picturae能够产生多种有机酸以及酸性、碱性磷酸酶,能够溶解97%磷酸盐岩,增加木质部导管数量,促进根和茎养分吸收,增强光合气体交换,显著促进幼苗根和芽的生长[82]。盐胁迫下,番茄接种Achromobacter piechaudii后植株磷含量和水分利用效率提高[83]。部分植物内生菌,包括Arthrobacter、Bacillus和O. pic-turae等能够溶解Ca3(PO4)2、AlPO4和FePO4,可增加植株高度、干重、侧枝数量和穗大小[82, 84-85]。慢生根瘤菌和假单胞菌通过改变大豆根系结构,促进了氮、磷的吸收,并提高了其耐盐性[86]。综上所述,在盐胁迫环境中,PSB的应用可促进植物磷素吸收而不会加剧土壤盐度。
3.2 PGPB产生铁载体铁(Fe)是植物生长所需的一种微量营养素,是植物呼吸、光合作用等反应中关键酶的组成部分[87]。盐胁迫抑制了土壤中包括Fe在内的大量微量营养素的可利用性,严重抑制了植物生长[88]。铁载体是PGPB分泌的一种水溶性、小分子物质,可以专属、高亲和力地与Fe3+结合成可被吸收的有机螯合物形式[89]。产铁载体的PGPB对铁的亲和力高于真菌病原体,因此,可以有效抑制病原体的增殖。铁载体能够增加铁在根际的供给量,植物则可以利用铁载体螯合的铁来改善植物营养、防止植物缺铁失绿症的发生[90]。目前,已经报道的产铁载体的PGPB有B. thuringiensis、Gracilibacillus saliphilu、Arthrobacter pascens等,能够在盐渍土中促进Arthrocnemum、macrostachyum、Atriplex leucoclada等作物生长[91-92]。盐碱土壤中铁的可用性非常低[88],研究表明,接种产生铁载体的PGPB对提高植物的抗病性和铁元素摄取量具有积极作用[93-94]。
3.3 PGPB的固氮作用氮素(N)是陆地生态系统中最重要、最易匮乏的养分之一,土壤中缺乏有效氮会限制甜土植物及盐生植物的生产力,盐胁迫可以干扰植物氮代谢,抑制铵和硝酸盐的吸收和同化[95],降低植物组织的N含量[96]。过量使用无机肥将增加土壤盐度、破坏土壤结构、改变土壤微生物的组成、干扰植物N吸收,反而降低植物组织的N含量[96-97]。有研究表明,在盐土中施无机氮肥后,盐度增加导致的危害多于氮素增加带来的益处[98]。
固氮菌是盐渍土中植物有效氮的重要来源,同时,固氮PGPB可以通过产生渗透因子来维持植物正常的细胞膨压和新陈代谢[99]。例如,假产碱假单胞菌接种到海蓬子后,植株叶绿素含量和N含量显著增加[100];固氮菌Klebsiella pneumoniae显著促进盐角草早期幼苗生长,发芽率、鲜重、干重和根长均显著高于未接菌处理的植株[101];Jha等[98]研究表明,与对照相比,盐胁迫条件下S. brachiate接种B. saurashtrense和Pseudomonas后生物量显著增加。可见,固氮PGPB对植物及环境的潜在益处突出了其作为盐土农业发展潜在生物资源的重要性。
4 PGPB调节植物抗氧化酶活性盐胁迫会破坏植物体内ROS产生与清除间的动态平衡,引起生物大分子的氧化损伤甚至细胞死亡[102]。过量的自由基一方面生成过量丙二醛等物质造成膜质过氧化,损害生物膜及其功能[103];另一方面造成植物体内负责光合色素合成的特异性酶活性下降,叶绿体基粒片层膨胀松散甚至片层解体,光合系统中的超微结构遭到破坏[104]。PGPB可以通过上调关键酶的活性来激活植物抗氧化防御机制来防止过量ROS导致的膜质变质,所述关键酶包括超氧化物歧化酶、抗坏血酸氧化酶、过氧化物酶、过氧化氢酶和谷胱甘肽还原酶,同时PGPB也可以产生半胱氨酸、谷胱甘肽和抗坏血酸等非酶组分以清除过量的活性氧物质[105]。
5 PGPB调节植物离子平衡 5.1 PGPB产生渗透调节因子维持植物的渗透平衡对提高其抗盐性具有重要作用。PGPB能够促进植物积累相容性溶质以维持细胞内渗透平衡,如氨基酸及其衍生物(如谷氨酸、脯氨酸、肽和N-乙酰化氨基酸等)、季胺(如甘氨酸甜菜碱和脯氨酸甜菜碱等)、糖(如葡萄糖苷、蔗糖和海藻糖等)、四氢嘧啶、羟基四氢嘧啶以及乙酰二氨基酸(乙酰鸟氨酸、乙酰赖氨酸)等[106-107],其表现出的多种与胁迫相关的特性可以使作物在高盐度下受到保护[39]。其中,四氢嘧啶类物质是目前发现的在细菌界分布最广泛的相容性溶质,兼具渗透压调节、诱导渗透压基因表达、抗逆保护、分子伴侣等诸多作用。在盐胁迫下,脯氨酸通过稳定细胞膜和蛋白质等亚细胞结构,清除自由基并缓冲细胞氧化还原电势来减轻植物盐胁迫损伤[108]。甘氨酸甜菜碱(GB)和脯氨酸是植物中积累的两种主要的抵抗盐、干旱胁迫的有机渗透物,两种化合物对酶和膜完整性具有积极作用[109]。萝卜在盐胁迫下接种PGPB后,光合色素、脯氨酸、总游离氨基酸和可溶性蛋白含量增加,同时检测到IAA、GA3、N、P、K+、Ca2+和Mg2+的含量均有提高[110]。Zarea等[111]研究表明,PGPB定殖于小麦根际后可通过增加植物脯氨酸积累促进植物生长。Upadhyay等[112]发现PGPB处理的小麦中脯氨酸和可溶性糖含量的增加是提高其耐盐性的关键。在盐胁迫下,渗透调节物质能够保护生物大分子的结构和功能,防止酶失活,保障光合作用等生理活动进行,增加作物的盐胁迫系统耐受性,对盐土农业利用甚至未来农业发展具有十分重要的意义[113]。
5.2 PGPB产生胞外多糖生物膜建立在植物根和土壤颗粒表面,对提高作物产量、改善土壤理化性质和减少病原体侵染植物根部有重要作用[114-115]。产生胞外多糖(Exopoly saccharides,EPS)的PGPB能够促进生物膜的形成,进而提高土壤肥力、保持种子周围水分充足、促进植物养分吸收和减轻植物Na+损伤。生物膜和EPS的增加有助于土壤颗粒与植物根系的联系[114]。EPS中羟基、巯基、羧基和磷酰基官能团的结合特性会促进土壤颗粒粘结并加强团聚体的形成,提高植物在盐胁迫下生物量积累[116]。目前,报道较多的产EPS的耐盐菌株主要有Aeromonas、hydrophila、caviae、Bacillus sp.、Planococcus rifietoensis、Halomonas variabilis、Burkholderia、Enterobacter、Microbacterium和Paenibacillus等[117-120]。
5.3 PGPB参与转运蛋白调控水通道蛋白是存在于植物细胞的等离子体和细胞内膜中的蛋白,作为水的主要转运蛋白,使植物继续从高盐度土壤中吸收水分,将有助于植物应对干旱和盐胁迫[121],盐胁迫条件下,玉米和豌豆等接种变形菌和巨大芽孢杆菌后,根部PIP2和ZmPIP1-1上调,表明接种PGPB有助于提高植物的根际导水率[14]。
高亲和K+转运载体(HKT)是与植物耐盐性有关的一类Na+或K+转运体或Na+-K+共转运体[122]。研究表明,枯草芽孢杆菌可以通过下调植物根中HKT基因的表达来减少根对Na+的过量吸收,促进芽-根Na+再循环[14, 123]。Islam等[13]的研究表明,盐胁迫下接种PGPB可能通过调节离子转运蛋白的表达来提高植物吸收选择离子的效率以保持高K+/Na+比,减少Na+和Cl-等离子的积累,调节大量营养素和微量营养素的平衡。
6 PGPB诱导植物系统抗性盐胁迫引起的渗透胁迫、离子毒害、氧化胁迫和植物病害等复杂因素能够破坏植物细胞、组织和器官,引起植物生理生化代谢紊乱,甚至造成植物死亡(图 1)。诱导系统抗性(Induced system tolerance,IST)即微生物在植物根际或内部定殖后,植物形成的抵抗广泛的生物与非生物因子的防御能力[124]。研究表明,微生物及其组分,如脂多糖、鞭毛、铁载体、环脂肽、2,4-二乙酰基间苯三酚、高丝氨酸内酯和某些挥发物作为诱导系统抗性的诱导剂保障了植物的健康生长和发育[125],使其能够抵抗多种细菌、真菌和病毒性植物病害[126]。
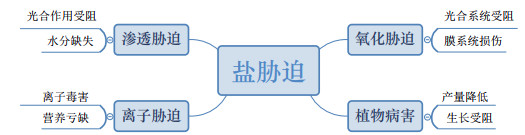
|
| 图 1 盐胁迫对植物的影响 |
挥发性有机化合物(Volatile organic compounds,VOCs)是分子量较低、疏水性、常温常压下容易挥发的化合物,可在大气和土壤中扩散[127]。微生物产生的VOCs和N-acyl-L -homoserine lactones(AHLs)等多种信号在植物形态发生过程中起重要作用[20]。Pseudomonas产生的VOCs可以显著上调营养贮藏蛋白、γ-谷氨酰水解酶和RuBisCo大蛋白,是在盐胁迫下诱导大豆产生系统耐受性的关键[128]。Alcaligenes faecalis JBCS1294通过散发己二酸和丁酸,改变生长素和赤霉素合成途径来提高植物耐盐性[23]。Paraburkholderia phytofirmans PsJN产生的挥发性有机物2-十一酮、7-己醇、3-甲基丁醇和二甲基二硫等也被验证具有促进拟南芥生长并诱导其产生胁迫耐受性的作用[129]。
HCN、吩嗪、硝吡咯菌素、2,4-二乙酰基间苯三酚、藤黄绿脓菌素和张力蛋白等是PGPB能够产生的抗真菌代谢物[23]。PGPB还能够产生真菌细胞壁降解酶,如能降解一些真菌细胞壁脂类的脂肪酶、降解真菌细胞壁碳水化合物的β-1,3-葡聚糖酶、降解真菌细胞壁几丁质的几丁质酶、降解细胞壁蛋白的蛋白酶等[130-131]。Egamberdieva等[132]报道,当用Pseudomonas aeruginosa处理盐碱土中黄瓜和番茄时,可抑制Fusarium solani引起的根腐病。研究证实,即使在盐胁迫下,PGPB仍然能够介导植物产生抵抗多种植物病害的系统抗性[133]。
基于盐渍化土壤的理化性质和盐胁迫下植物的受损机制,结合上述研究成果,我们认为,PGPB能够从多方面通过多种途径改良土壤,依靠不同特性提高植物渗透调节能力、离子转运能力、激素调节能力、抗氧化能力和养分吸收能力(图 2)。
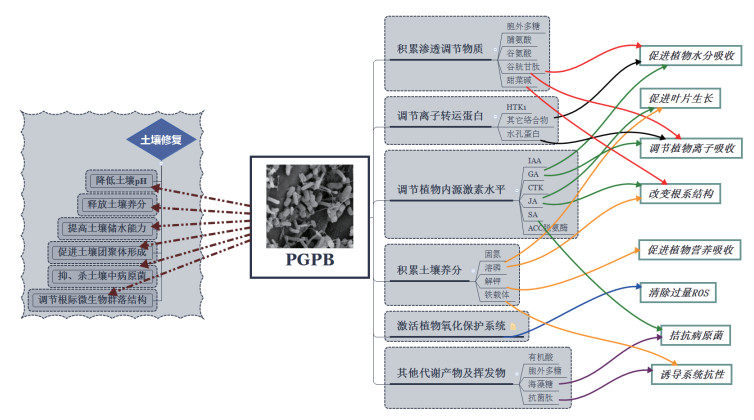
|
| 图 2 盐胁迫环境下PGPB对土壤与植物的作用机制 |
盐胁迫使植物同时面临离子胁迫、渗透胁迫、氧化胁迫和植物病害等威胁,PGPB的多种特性能够协助植物减轻胁迫损伤,其中,调节植物内源激素水平、促进营养吸收等特性对植物生长具有重要促进作用。本课题组依托于黄河下游森林培育国家林业和草原局重点实验室,在研究中证实,耐盐菌株能够在盐胁迫环境中保持更高的生物活性,显著提高小麦和枣树等作物的渗透调节、抗氧化和养分吸收能力,具有改变根系结构,提高生物量积累的作用效果。为了进一步探究植物-土壤-PGPB的作用关系、筛选得到更多具有实效的PGPB菌株、加快高效耐盐PGPB的产业化应用、减少盐渍化土壤快速扩张带来的生产损失,建议在以下方面做进一步研究:
(1)植物激素是植物用于调节各种应激反应的必要因子,分子生物学的突破增加了对植物激素信号通路的认识。盐胁迫使植物产生多种信号传导途径,而IAA、GA、ABA、CTK、JA和SA等在多种信号传导中存在相互促进或抑制的情况[113],不同激素在植株内的协同作用仍有待阐明。此方向的研究有利于更全面地认识PGPB对植物激素的调控机制,可为最终获得可靠的PGPB菌种提供理论依据。
(2)目前,关于盐胁迫下PGPB的研究大多集中在其对宿主植物的促生效应,而PGPB与根际其他微生物对盐渍土的协同作用的研究则鲜有涉及。同时,PGPB能够对植物的根际土、甚至内生菌微生物群落结构、多样性、稳定性产生影响,通过高通量测序等技术手段分析PGPB对其作用规律,找到群落的发生、演替规律,发现稳定存在于根际和作物内部的有益菌群,分析优势菌群的功能特性,了解其对植物生长、耐盐性的影响,将为更好地揭示PGPB在盐渍土改良中的生态学功能提供理论依据。
(3)PGPB对环境条件存在依赖性,部分菌株在胁迫环境中活性降低甚至失活。研究发现,从盐、碱、酸、干旱、水淹、高温及低温等环境分离的PGPB可以对相应农业条件下的作物提供有效保护,因此,盐生植物与盐土中PGPB资源的开发将加快菌剂筛选进程,另外,通过基因工程手段构建生物活力高、试验效果好、活性稳定的菌株也是重要的获得方式。
(4)不同PGPB在不同盐分类型、不同胁迫强度和不同胁迫时间的土壤中定殖能力不同,对不同植物及同一植物不同部位的影响也存在差异。很难用一个标准来界定PGPB对植物的促生效果。若能针对不同盐渍土壤类型、植物种类和组织部位确定适当的评价标准,将更有利于评价PGPB对植物和土壤的影响,也将为筛选高效耐盐的PGPB菌种及其应用提供理论和技术依据。
(5)获得某种具有特定功能的菌株并非难事,但获得生物活力高、可在植物根际或组织内部稳定定殖、发酵性状良好、生物安全性符合要求、分类地位清晰、作用机理明确、大田试验效果好的菌株仍需大量的研究工作。目前,多数研究工作仍局限在人工模拟条件,作物培养方式多为短周期水培、盆栽等,而对在自然生境下土壤与植物间的营养分配和水盐传导规律的认识仍存在不足,植物-土壤-PGPB的作用方式、信号传递途径、干扰因素等研究尚不完善,菌剂在自然环境中最佳的施用条件、适用作物、用法用量等仍需完整的试验验证,该工作的研究要求增加在自然环境和生态系统尺度上的试验。
(6)盐碱土改良对改善植物长势至关重要,综合利用物理、化学、水利、生物等土壤改良措施具有重要意义。目前,通过小规模研究发现PGPB与其他改良剂联合使用能够更好地改善植物长势,增加土壤养分,提高改良水平。科学地结合不同改良剂并创新试验方法对提高盐土改良效果、减少修复成本、维持生态系统稳态具有更大的优势和更高的推广价值。
| [1] |
Zhu F, Qu L, Hong X, et al. Isolation and characterization of a phosphate-solubilizing halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China[J]. Evid Based Complement Alternat Med, 2011, 2011: 615032. |
| [2] |
Acquaah G, Principles of plant genetics and breeding[M]. Blackwell Publishing, 2007.
|
| [3] |
Dodd L, Pérezalfocea F. Microbial amelioration of crop salinity stress[J]. Journal of Experimental Botany, 2012, 63(9): 3415-3428. DOI:10.1093/jxb/ers033 |
| [4] |
James R, Blake C, Byrt C, et al. Major genes for Na+ exclusion, Nax1 and Nax2(wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions[J]. Journal of Experimental Botany, 2011, 62(8): 2939-2947. DOI:10.1093/jxb/err003 |
| [5] |
Rozema J, Flowers T. Crops for a salinized world[J]. Science, 2008, 322(5907): 1478-1480. DOI:10.1126/science.1168572 |
| [6] |
Afrasyab R, Richarda J, Kazem P, et al. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil[J]. Functional Plant Biology, 2010, 37(3): 255-263. DOI:10.1071/FP09148 |
| [7] |
Zhu J. Salt and drought stress signal transduction in plants[J]. Annual Review of Plant Biology, 2002, 53(53): 247-273. |
| [8] |
Evelin H, Kapoor R, Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress:a review[J]. Ann Bot, 2009, 104(7): 1263-1280. DOI:10.1093/aob/mcp251 |
| [9] |
Zhu J. Plant salt tolerance[J]. Trends in Plant Science, 2001, 6(2): 66-71. DOI:10.1016/S1360-1385(00)01838-0 |
| [10] |
Munns R, Tester M. Mechanisms of salinity tolerance[J]. Annual Review of Plant Biology, 2008, 59(1): 651-681. DOI:10.1146/annurev.arplant.59.032607.092911 |
| [11] |
Desgarennes D, Garrido E, Torresgomez M, et al. Diazotrophic potential among bacterial communities associated with wild and cultivated agaves[J]. FEMS Microbiology Ecology, 2015, 90(3): 844-857. |
| [12] |
Bisseling T, Dang J, Schulzelefert P. Next-generation communication[J]. Science, 2009, 324(5928): 691. DOI:10.1126/science.1174404 |
| [13] |
Islam F, Yasmeen T, Arif M, et al. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility[J]. Plant Growth Regulation, 2016, 80(1): 23-36. DOI:10.1007/s10725-015-0142-y |
| [14] |
Yuan Q, Druzhinina I, Pan X, et al. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture[J]. Biotechnology Advances, 2016, 34(7): 1245-1259. DOI:10.1016/j.biotechadv.2016.08.005 |
| [15] |
Etesami H, Beattie G. Plant-Microbe interactions in adaptation of agricultural crops to abiotic stress conditions[M]. //Kumar V, Kumar M, Sharma S, et al. Probiotics and Plant Health. Springer, 2017, 163-200.
|
| [16] |
Etesami H, Beattie G. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops[J]. Frontiers in Microbiology, 2018, 9: 148. DOI:10.3389/fmicb.2018.00148 |
| [17] |
Shukla P, Agarwal P, Jha B. Improved salinity tolerance of Arachis hypogaea(L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria[J]. Journal of Plant Growth Regulation, 2012, 31(2): 195-206. DOI:10.1007/s00344-011-9231-y |
| [18] |
Jordan V, Guilhem D, Marie-Lara B, et al. Plant growth-promoting rhizobacteria and root system functioning[J]. Frontiers in Plant Science, 2013, 4: 356. |
| [19] |
Goswami D, Dhandhukia P, Patel P, et al. Screening of PGPR from saline desert of Kutch:Growth promotion in Arachis hypogea by Bacillus licheniformis A2[J]. Microbiological Research, 2014, 169(1): 66-75. DOI:10.1016/j.micres.2013.07.004 |
| [20] |
Timmusk S, Abd El-Daim I, Copolovici L, et al. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments:enhanced biomass production and reduced emissions of stress volatiles[J]. PLoS One, 2014, 9(5): e96086. DOI:10.1371/journal.pone.0096086 |
| [21] |
Kaushal M, Wani S. Rhizobacterial-plant interactions:Strategies ensuring plant growth promotion under drought and salinity stress[J]. Agriculture Ecosystems & Environment, 2016, 231(1): 68-78. |
| [22] |
Banaei-Asl F, Bandehagh A, Uliaei E, et al. Proteomic analysis of canola root inoculated with bacteria under salt stress[J]. Journal of Proteomics, 2015, 124: 88-111. DOI:10.1016/j.jprot.2015.04.009 |
| [23] |
Bhattacharyya P, Jha D. Plant growth-promoting rhizobacteria (PGPR):emergence in agriculture[J]. World J Microbiol Biotechnol, 2012, 28(4): 1327-1350. |
| [24] |
Cheng Z, Woody O, Mcconkey B, et al. Combined effects of the plant growth-promoting bacterium Pseudomonas putida UW4 and salinity stress on the Brassica napus proteome[J]. Applied Soil Ecology, 2012, 61: 255-263. DOI:10.1016/j.apsoil.2011.10.006 |
| [25] |
Pierik R, Sasidharan R, Voesenek L. Growth control by ethylene:adjusting phenotypes to the environment[J]. Journal of Plant Growth Regulation, 2007, 26(2): 188-200. DOI:10.1007/s00344-006-0124-4 |
| [26] |
Ma W, Penrose D, Glick B. Strategies used by rhizobia to lower plant ethylene levels and increase nodulation[J]. Canadian Journal of Microbiology, 2002, 48(11): 947-54. DOI:10.1139/w02-100 |
| [27] |
Kausar R, Shahzad S. Effect of ACC-deaminase containing rhizobacteria on growth promotion of maize under salinity stress[J]. J Agric Soc Sci, 2006. |
| [28] |
Camilios-Neto D, Bonato P, Wassem R, et al. Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes[J]. BMC Genomics, 2014, 15(1): 378. DOI:10.1186/1471-2164-15-378 |
| [29] |
Zhou N, Zhao S, Tian C. Effect of halotolerant rhizobacteria isolated from halophytes on the growth of sugar beet (Beta vulgaris L.) under salt stress[J]. FEMS Microbiology Letters, 2017, 364(11): fnx091. |
| [30] |
Ali S, Charles T, Glick B. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase[J]. Plant Physiology & Biochemistry, 2014, 80(2): 160-167. |
| [31] |
Bruto M, Prigentcombaret C, Muller D, et al. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria[J]. Sci Rep, 2014, 4: 6261. |
| [32] |
Hadiarto T, Tran L. Progress studies of drought-responsive genes in rice[J]. Plant Cell Reports, 2011, 30(3): 297-310. DOI:10.1007/s00299-010-0956-z |
| [33] |
Dunlap J, Binzel M. NaCI reduces indole-3-acetic acid levels in the roots of tomato plants independent of stress-induced abscisic acid[J]. Plant Physiology, 1996, 112(1): 379-384. |
| [34] |
Steffen L, Gerd J, Ive D. The evolving complexity of the auxin pathway[J]. Plant Cell, 2008, 20(7): 1738-1746. DOI:10.1105/tpc.108.060418 |
| [35] |
Wang Y, Li K, Li X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana[J]. Journal of Plant Physiology, 2009, 166(15): 1637-1645. DOI:10.1016/j.jplph.2009.04.009 |
| [36] |
Gholamali A, Saeed Y. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.)[J]. Pakistan Journal of Biological Sciences, 2007, 10(15): 2557-2561. DOI:10.3923/pjbs.2007.2557.2561 |
| [37] |
Dodd I, Zinovkina N, Safronova V, et al. Rhizobacterial mediation of plant hormone status[J]. Annals of Applied Biology, 2010, 157(3): 361-379. DOI:10.1111/j.1744-7348.2010.00439.x |
| [38] |
Tiwari S, Singh P, Tiwari R, et al. Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth[J]. Biology & Fertility of Soils, 2011, 47(8): 907-916. |
| [39] |
Sharma S, Kulkarni J, Jha B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut[J]. Frontiers in Microbiology, 2016, 7: 1600. |
| [40] |
Zhao S, Zhou N, Zhao Z, et al. Isolation of endophytic plant growth-promoting bacteria associated with the halophyte Salicornia europaea and evaluation of their promoting activity under salt stress[J]. Current Microbiology, 2016, 73(4): 1-8. |
| [41] |
El-Azeem S, Elwan M, Sung J, et al. Alleviation of salt stress in eggplant (Solanum melongena L.) by plant-growth-promoting rhizobacteria[J]. Communications in Soil Science & Plant Analysis, 2012, 43(9): 1303-1315. |
| [42] |
Sah S, Reddy K, Li J. Abscisic acid and abiotic stress tolerance in crop plants[J]. Frontiers in Plant Science, 2016, 7: 571. |
| [43] |
Raghavendra A, Gonugunta V, Christmann A, et al. ABA perception and signalling[J]. Trends in Plant Science, 2010, 15(7): 395-401. DOI:10.1016/j.tplants.2010.04.006 |
| [44] |
He T, Cramer G. Abscisic acid concentrations are correlated with leaf area reductions in two salt-stressed rapid-cycling Brassica species[J]. Plant & Soil, 1996, 179(1): 25-33. |
| [45] |
Cabot C, Sibole J, Barceló J, et al. Abscisic acid decreases leaf Na+ exclusion in salt-treated Phaseolus vulgaris L.[J]. Journal of Plant Growth Regulation, 2009, 28(2): 187-192. DOI:10.1007/s00344-009-9088-5 |
| [46] |
Naz I, Bano A, Ulhassan T. Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L[J]. African Journal of Biotechnology, 2009, 8(21): 5762-5768. DOI:10.5897/AJB09.1176 |
| [47] |
Etesami H, Beattie G. Plant-Microbe interactions in adaptation of agricultural crops to abiotic stress conditions[M]// Probiotics and Plant Health. Springer Singapore, 2017.
|
| [48] |
Jiang F, Chen L, Belimov A, et al. Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum[J]. Journal of Experimental Botany, 2012, 63(18): 6421. DOI:10.1093/jxb/ers301 |
| [49] |
Salomon M, Bottini R, Cohen A, et al. Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, induce abscisic acid accumulation and synthesis of defense-related terpenes in in vitro cultured grapevine[J]. Physiologia Plantarum, 2014, 151(4): 359-374. DOI:10.1111/ppl.12117 |
| [50] |
Cohen A, Bottini R, Pontin M, et al. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels[J]. Physiologia Plantarum, 2015, 153(1): 79-90. DOI:10.1111/ppl.12221 |
| [51] |
Sun T, Gubler F. Molecular mechanism of gibberellins signalling in plants[J]. Annu Rev Plant Biol, 2004, 55(1): 197-223. DOI:10.1146/annurev.arplant.55.031903.141753 |
| [52] |
Davière J, Achard P. Gibberellin signaling in plants[J]. Development, 2013, 140(6): 1147-1151. DOI:10.1242/dev.087650 |
| [53] |
Wang G, Feng Q, Xu Z, et al. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot[J]. BMC Plant Biology, 2015, 15(1): 290. |
| [54] |
Maggio A, Barbieri G, Raimondi G, et al. Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity[J]. Journal of Plant Growth Regulation, 2010, 29(1): 63-72. DOI:10.1007/s00344-009-9114-7 |
| [55] |
牛宋芳, 王利娟, 刘秉儒. 赤霉素对盐胁迫下红砂种子萌发的影响[J]. 草业学报, 2017, 26(6): 89-97. |
| [56] |
Javid N, Sorooshzadeh A, Moradi F, et al. The role of phytohormones in alleviating salt stress in crop plants[J]. Australian Journal of Crop Science, 2011, 32(5): 726. |
| [57] |
Bottini R, Cassán F, Piccoli P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase[J]. Applied Microbiology & Biotechnology, 2004, 65(5): 497-503. |
| [58] |
Giljae J, Youngmog K, Jungtae K, et al. Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers[J]. Journal of Microbiology, 2005, 43(6): 510. |
| [59] |
Kang S, Khan A, You Y, et al. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth[J]. J Microbiol Biotechnol, 2014, 24(1): 106-112. |
| [60] |
Kucey R. Plant growth-altering effects of Azospirillum brasilense and Bacillus C-11-25 on two wheat cultivars[J]. Journal of Applied Microbiology, 2010, 64(3): 187-196. |
| [61] |
Shahzad R, Waqas M, Khan A, et al. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa[J]. Plant Physiology & Biochemistry, 2016, 106: 236-243. |
| [62] |
Kang S, Khan A, Hamayun M, et al. Gibberellin-producing Promicromonospora sp. SE188 improves Solanum lycopersicum plant growth and influences endogenous plant hormones[J]. Journal of Microbiology, 2012, 50(60): 902-909. |
| [63] |
Ahmad P, Rasool S, Gul A, et al. Jasmonates:Multifunctional roles in stress tolerance[J]. Frontiers in Plant Science, 2016, 7(5): 813. |
| [64] |
Kazan K, Manners J. JAZ repressors and the orchestration of phytohormone crosstalk[J]. Trends in Plant Science, 2012, 17(1): 22-31. DOI:10.1016/j.tplants.2011.10.006 |
| [65] |
El-Tayeb M. Response of barley grains to the interactive e. ect of salinity and salicylic acid[J]. Plant Growth Regulation, 2005, 45(3): 215-224. DOI:10.1007/s10725-005-4928-1 |
| [66] |
Pancheva T, Popova L, Uzunova A. Effects of salicylic acid on growth and photosynthesis in barley plants[J]. Journal of Plant Physiology, 1996, 149(S 1-2): 57-63. |
| [67] |
Forchetti G, Masciarelli O, Alemano S, et al. Endophytic bacteria in sunflower (Helianthus annuus L.):isolation, characterization, and production of jasmonates and abscisic acid in culture medium[J]. Applied Microbiology & Biotechnology, 2007, 76(5): 1145-1152. |
| [68] |
Chen Y, Fan J, Du L, et al. The application of phosphate solubilizing endophyte Pantoea dispersa triggers the microbial community in red acidic soil[J]. Applied Soil Ecology, 2014, 84: 235-244. DOI:10.1016/j.apsoil.2014.05.014 |
| [69] |
Li Y, Gu Y, Li J, et al. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew[J]. Frontiers in Microbiology, 2015, 6: 883. |
| [70] |
Bordiec S, Paquis S, Lacroix H, et al. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions[J]. Journal of Experimental Botany, 2011, 62(2): 595-603. DOI:10.1093/jxb/erq291 |
| [71] |
李启任, 王东. 外源生长调节物质对烟草茎髓愈伤组织形成和愈伤组织器官发生的作用[J]. 云南大学学报:自然科学版, 1998(S4): 555-559. |
| [72] |
Hwang I, Sheen J. Two-component circuitry in Arabidopsis cytoki-nin signal transduction[J]. Nature, 2001, 413(6854): 383-389. DOI:10.1038/35096500 |
| [73] |
O'Brien J, Eva B. Cytokinin cross-talking during biotic and abiotic stress responses[J]. Frontiers in Plant Science, 2013, 4(1): 451. |
| [74] |
Salamone I, Hynes R, Nelson L. Role of cytokinins in plant growth promotion by rhizosphere bacteria[J]. Pgpr Biocontrol & Biofertilization, 2005, 173-195. |
| [75] |
Kakimoto T. Perception and signal transduction of cytokinins[J]. Annual Review of Plant Biology, 2003, 54(1): 605-627. DOI:10.1146/annurev.arplant.54.031902.134802 |
| [76] |
Brandstatter I, Kieber J. Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in arabidopsis[J]. The Plant Cell, 1998, 10: 1009-1019. DOI:10.1105/tpc.10.6.1009 |
| [77] |
Arkhipova T, Veselov S, Melentiev A, et al. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants[J]. Plant & Soil, 2005, 272(1/2): 201-209. |
| [78] |
Liu F, Xing S, Ma H, et al. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings[J]. Applied Microbiology & Biotechnology, 2013, 97(20): 9155-9164. |
| [79] |
Arkhipova T, Prinsen E, Veselov S, et al. Cytokinin producing bacteria enhance plant growth in drying soil[J]. Plant & Soil, 2007, 292(1-2): 305-315. |
| [80] |
Goldstein A. Bacterial solubilization of mineral phosphates:Historical perspective and future prospects[J]. American Journal of Alternative Agriculture, 2009, 1(1): 51-57. |
| [81] |
Giri B, Kapoor R, Mukerji K. Improved tolerance of Acacia nilotica to salt stress by Arbuscular Mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues[J]. Microbial Ecology, 2007, 54(4): 753-760. DOI:10.1007/s00248-007-9239-9 |
| [82] |
El-Tarabily K, Youssef T. Enhancement of morphological, anatomical and physiological characteristics of seedlings of the mangrove Avicennia marina inoculated with a native phosphate-solubilizing isolate of Oceanobacillus picturae under greenhouse conditions[J]. Plant & Soil, 2010, 332(1-2): 147-162. |
| [83] |
Mayak S, Tirosh T, Glick B. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress[J]. Plant Physiology & Biochemistry, 2004, 42(6): 565-572. |
| [84] |
Bashan Y, Moreno M, Troyo E. Growth promotion of the seawater-irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp.[J]. Biology & Fertility of Soils, 2000, 32(4): 265-272. |
| [85] |
Banerjee S, Palit R, Sengupta C, et al. Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere[J]. Australian Journal of Crop Science, 2010, 4(6): 378-383. |
| [86] |
Egamberdieva D, Wirth S, Jabborova D, et al. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture[J]. Journal of Plant Interactions, 2017, 12(1): 100-107. |
| [87] |
Kobayashi T, Nishizawa N. Iron uptake, translocation, and regulation in higher plants[J]. Annual Review of Plant Biology, 2012, 63(1): 131. DOI:10.1146/annurev-arplant-042811-105522 |
| [88] |
Abbas G, Saqib M, Akhtar J, et al. Interactive effects of salinity and iron deficiency on different rice genotypes[J]. Journal of Plant Nutrition and Soil Science, 2015, 178(2): 306-311. DOI:10.1002/jpln.201400358 |
| [89] |
贾国东, 钟佐燊. 铁的环境地球化学综述[J]. 环境工程学报, 1999(5): 74-84. |
| [90] |
Masalha J, Kosegarten H, Elmaci, et al. The central role of microbial activity for iron acquisition in maize and sunflower[J]. Biology & Fertility of Soils, 2000, 30(5-6): 433-439. |
| [91] |
Navarro-Torre S, Barcia-Piedras J, Mateos-Naranjo E, et al. Assessing the role of endophytic bacteria in the halophyte Arthrocnemum macrostachyum salt tolerance[J]. Plant Biol, 2017, 19(2): 249-256. DOI:10.1111/plb.12521 |
| [92] |
Ullah S, Bano A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity[J]. Canadian Journal of Microbiology, 2015, 61(4): 307-313. DOI:10.1139/cjm-2014-0668 |
| [93] |
Compant S, Duffy B, Clement C, et al. Use of plant growth-promoting bacteria for biocontrol of plant diseases:principles, mechanisms of action, and future prospects[J]. Applied & Environmental Microbiology, 2005, 71(9): 4951-4959. |
| [94] |
王英丽, 林庆祺, 李宇, 等. 产铁载体根际菌在植物修复重金属污染土壤中的应用潜力[J]. 应用生态学报, 2013, 24(7): 2081-2088. |
| [95] |
Ullrich W. Salinity and nitrogen nutrition[M]. Salinity: Environment-Plants-Molecules. Springer Netherlands, 2004.
|
| [96] |
Naidoo G. Effects of salinity and nitrogen on growth and water relations in the mangrove, Avicennia marina(Forsk.)Vierh[J]. New Phytologist, 2010, 107(2): 317-325. |
| [97] |
Rueda-Puente E, Castellanos-Cervantes T, Díaz J, et al. Bacterial community of rhizosphere associated to the annual halophyte Salicornia bigelovii (Torr.)[J]. Terra Latinoamericana, 2010, 28(4): 345-353. |
| [98] |
Jha B, Gontia I, Hartmann A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential[J]. Plant & Soil, 2012, 356(1-2): 265-277. |
| [99] |
Yan N, Marschner P, Cao W, et al. Influence of salinity and water content on soil microorganisms[J]. International Soil & Water Conservation Research, 2015, 3(4): 316-323. |
| [100] |
Ozawa T, Wu J, Fujii S. Effect of inoculation with a strain of Pseudomonas pseudoalcaligenes isolated from the endorhizosphere of Salicornia europea on salt tolerance of the glasswort[J]. Soil Science & Plant Nutrition, 2007, 53(1): 12-16. |
| [101] |
Ruedapuente E, Castellanos T, Troyodiéguez E, et al. Effects of a nitrogen-fixing indigenous bacterium (Klebsiella pneumoniae) on the growth and development of the halophyte Salicornia bigelovii as a new crop for saline environments[J]. Journal of Agronomy & Crop Science, 2010, 189(5): 323-332. |
| [102] |
张梦如, 杨玉梅, 成蕴秀, 等. 植物活性氧的产生及其作用和危害[J]. 西北植物学报, 2014, 34(9): 1916-1926. |
| [103] |
Miller G, Suzuki N, CiftciYilmaz S, et al. Reactive oxygen species homeostasis and signalling during drought and salinity stresses[J]. Plant, Cell & Environment, 2010, 33(4): 453-467. |
| [104] |
张美月, 陶秀娟, 樊建民, 等. 磷和丛枝菌根真菌对盐胁迫草莓光合作用的影响[J]. 河北农业大学学报, 2009, 32(4): 71-75. DOI:10.3969/j.issn.1000-1573.2009.04.015 |
| [105] |
Jha Y, Subramanian R. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity[J]. Physiology & Molecular Biology of Plants, 2014, 20(2): 201-207. |
| [106] |
Creus C, Sueldo R, Barassi C. Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field[J]. Canadian Journal of Botany, 2004, 82(2): 273-281. |
| [107] |
Antón J. Compatible solute[M]. Springer Berlin Heidelberg, 2011.
|
| [108] |
Kohler J, Hernández J, Caravaca F, et al. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress[J]. Environmental & Experimental Botany, 2009, 65(2): 245-252. |
| [109] |
Ashraf M, Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance[J]. Environmental and Experimental Botany, 2007, 59(2): 206-216. DOI:10.1016/j.envexpbot.2005.12.006 |
| [110] |
Mohamed H, Gomaa E. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress[J]. Photosynthetica, 2012, 50(2): 263-272. |
| [111] |
Zarea M, Hajinia S, Karimi N, et al. Effect of Piriformospora indica and Azospirillum strains from saline or non-saline soil on mitigation of the effects of NaCl[J]. Soil Biology & Biochemistry, 2012, 45(45): 139-146. |
| [112] |
Upadhyay S, Singh J, Saxena A, et al. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions[J]. Plant Biol, 2012, 14(4): 605-611. DOI:10.1111/j.1438-8677.2011.00533.x |
| [113] |
Furusho K, Yoshizawa T, Shoji S. Ectoine alters subcellular localization of inclusions and reduces apoptotic cell death induced by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch[J]. Neurobiology of Disease, 2005, 20(1): 170-178. DOI:10.1016/j.nbd.2005.02.011 |
| [114] |
Muhammad A. Role of exo-polysaccharide producing bacteria in improving fertility of the salt-affected soil[J]. International Journal of Environmental Science & Technology, 2007, 3(1): 43-51. |
| [115] |
Batool R, Hasnain S. Growth stimulatory effects of Enterobacter and Serratia isolated from biofilms on plant growth and soil aggregation[J]. Biotechnology, 2005, 4(4): 347-353. DOI:10.3923/biotech.2005.347.353 |
| [116] |
Nunkaew T, Kantachote D, Nitoda T, et al. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions[J]. Carbohydrate Polymers, 2015, 115: 334-341. DOI:10.1016/j.carbpol.2014.08.099 |
| [117] |
Upadhyay S, Singh J, Singh D. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition[J]. Pedosphere, 2011, 21(2): 214-222. DOI:10.1016/S1002-0160(11)60120-3 |
| [118] |
Qurashi A, Sabri A. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress[J]. Brazilian Journal of Microbiology, 2012, 43(3): 1183-1191. DOI:10.1590/S1517-83822012000300046 |
| [119] |
Ruppel S, Franken P, Witzel K. Properties of the halophyte microbiome and their implications for plant salt tolerance[J]. Functional Plant Biology, 2013, 40(8-9): 3113-3116. |
| [120] |
Khan M, Boër B, Ȫzturk M, et al. Erratum: Sabkha ecosystems volume Ⅴ: The Americas[M]//. Sabkha Ecosystems. Springer International Publishing, 2016.
|
| [121] |
Moshelion M, Halperin O, Wallach R, et al. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants:crop water-use efficiency, growth and yield[J]. Plant Cell & Environment, 2015, 38(9): 1785. |
| [122] |
Mosimann M, Goshima S, Wenzler T, et al. A Trk/HKT-type K+ transporter from Trypanosoma brucei[J]. Eukaryot Cell, 2010, 9(4): 539-546. |
| [123] |
Zhang H, Kim M, Sun Y, et al. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1[J]. Mol Plant Microbe Interact, 2008, 21(6): 737-744. DOI:10.1094/MPMI-21-6-0737 |
| [124] |
Pahm L, Cmj P, van Loon L, et al. Systemic resistance induced by rhizosphere bacteria[J]. Annual Review of Phytopathology, 1998, 36(1): 453-483. DOI:10.1146/annurev.phyto.36.1.453 |
| [125] |
Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria[J]. Annual Review of Microbiology, 2009, 63(1): 541-556. DOI:10.1146/annurev.micro.62.081307.162918 |
| [126] |
Singh R, Jha P. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.)[J]. PLoS One, 2016, 11(6): e0155026. DOI:10.1371/journal.pone.0155026 |
| [127] |
Hung R, Lee S, Bennett J. Fungal volatile organic compounds and their role in ecosystems[J]. Applied Microbiology & Biotechnology, 2015, 99(8): 3395-3405. |
| [128] |
Vaishnav A, Kumari S, Jain S, et al. Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress[J]. Journal of Applied Microbiology, 2015, 119(2): 539-551. DOI:10.1111/jam.12866 |
| [129] |
Ledger T, Rojas S, Timmermann T, et al. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana[J]. Frontiers in Microbiology, 2016, 7(331): 1838. |
| [130] |
Husson E, Hadad C, Huet G, et al. The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies[J]. Green Chemistry, 2017, 19(17): 4122. DOI:10.1039/C7GC01471F |
| [131] |
Vaddepalli P, Fulton L, Wieland J, et al. The cell wall-localized atypical β-1, 3 glucanase ZERZAUST controls tissue morphogenesis in Arabidopsis thaliana[J]. Development, 2017, 144(12): 2259-2269. DOI:10.1242/dev.152231 |
| [132] |
Egamberdieva D. Pseudomonas chlororaphis:a salt-tolerant bacterial inoculant for plant growth stimulation under saline soil conditions[J]. Acta Physiologiae Plantarum, 2012, 34(2): 751-756. |
| [133] |
Paul D, Nair S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils[J]. Journal of Basic Microbiology, 2010, 48(5): 378-384. |




