The latitudinal diversity gradient (i.e., the number of species increasing from the poles to the equator) is among the most striking and consistent biodiversity patterns (Hillebrand, 2004). This trend of species diversity has been recognized for more than two centuries in most studied groups of organisms (Hawkins, 2001; Willig et al., 2003). However, a full explanation of the latitudinal diversity gradient remains elusive, even though much focus has been attributed to the topic (Rosenzweig, 1995; Willig et al., 2003). Species richness is often correlated with climatic variables but in a particular area it is the interplay of the speciation, extinction, and dispersal processes that directly determines its species richness (Ricklefs, 1987). Furthermore, the balance of speciation and extinction rates over time determines diversification rates (Ricklefs, 2007). Thus, the relationships between species richness and climate should be considered as an outcome of the effects of climate on speciation, extinction, and dispersal (Ricklefs, 2006; Wiens and Donoghue, 2004).
Certain climatic conditions might promote faster diversification rates by either promoting speciation rates or reducing extinction rates or both. The latitudinal diversity gradient has frequently been attributed to higher diversification rates in tropical environments, because of either increased speciation rates or decreased extinction rates (Soria-Carrasco and Castresana, 2012). However, previous studies have observed mixed results: some observed higher diversification rates at lower latitudes and higher temperatures (e.g., Cardillo, 1999; Böhm and Mayhew, 2005; Cardillo et al., 2005; Ricklefs, 2006; Wiens, 2007; Jansson and Davies, 2008; Martin and Tewksbury, 2008; Pyron and Wiens, 2013; Rolland et al., 2014), whereas others observed the opposite pattern (e.g., Weir and Schluter, 2007; Wiens et al., 2009; Soria-Carrasco and Castresana, 2012; Igea and Tanentzap, 2020).
Mosses include about 12,000 extant species worldwide (Goffinet and Buck, 2004) and account for 60% of the overall extant species of bryophytes (Patiño and Vanderpoorten, 2018). They are one of the earliest diverging extant land plant lineages. Qian et al. (2024a) showed that species richness of mosses decreases with increasing latitude in both the Northern Hemisphere and the Southern Hemisphere, regardless of whether the globe is considered as a whole or the Old World and the New World are considered separately. Laenen et al. (2014) investigated the variation of diversification rates of mosses, among other groups of plants, across a geological time-scale. Unlike angiosperms for which there are multiple studies investigating geographic patterns and climatic correlates of their diversification rates across the world, there is no study investigating moss diversification rates in different regions across the world.
In this study, I explore diversification rates in mosses across latitude and longitude and across climatic gradients. Specifically, I address the following questions. Do moss species at lower latitudes belong to clades with higher diversification rates? Similarly, do regions with warmer and wetter climates have moss species belonging to clades with higher diversification rates? Of the two major macroclimatic factors shaping large-scale plant distribution, temperature and precipitation, is the former a stronger driver of the geographic variation of diversification rates of mosses, compared with the latter, or vice versa?
2. Materials and methods 2.1. Moss species assemblagesA previous study on global moss species richness (Qian et al., 2024a) divided the world latitudinally into the Northern and Southern Hemispheres, and longitudinally into the New World and the Old World, and further divided the Old World at 75° E longitude into the eastern Old World and western Old World. The combination of the two broad latitudinal sections (i.e., Northern Hemisphere and Southern Hemisphere) and three longitudinal segments (New World, western Old World, and eastern Old World) resulted in six geographic regions. Further, they divided each geographic region into latitudinal bands starting at the equator, each band having 20° in latitude. The above-described processes resulted in six non-overlapped latitudinal gradients with a total number of 21 latitudinal bands—each of the three geographic regions in the Northern Hemisphere included four latitudinal bands (from 0 to 80°N), and each of the three geographic regions in the Southern Hemisphere included three latitudinal bands (from 0 to 60°S). In addition to the 21 latitudinal bands within the six geographic regions, they generated species lists for each 20°-latitudinal band across the world as a whole and across the Old World as a whole. They compiled moss species lists for each latitudinal band within each geographic region based on various data sources (e.g., Geffert et al., 2013; Sanbonmatsu and Spalink, 2022) and standardized species names based on the database Bryophyte Nomenclator (Brinda and Atwood, 2023), using the package U.Taxonstand (Zhang and Qian, 2023). They collapsed all infra-specific names to species. The present study used their global moss database, which included 9164 species in 951 genera.
2.2. Diversification rate estimationDiversification rate may be estimated with either phylogeny-based or non-phylogeny-based approaches. Because there is no phylogeny that includes most of the moss species in the world, a phylogeny-based approach cannot be used to estimate diversification rates for mosses at a global scale. Following many previous studies on various groups of organisms (e.g., Eriksson and Bremer, 1992; Magallón and Sanderson, 2001; Davies et al., 2004; Hughes and Eastwood, 2006; Alfaro et al., 2007; Adams et al., 2009; Rabosky and Adams, 2012; Soria-Carrasco and Castresana, 2012; Rabosky and Matute, 2013; Gómez-Rodríguez et al., 2015; Wiens 2015a, 2015b; Cooney et al., 2016; Marin and Hedges, 2016; Scholl and Wiens, 2016; Tedesco et al., 2017; Boucher et al., 2020; Wu and Wiens, 2022; Yu and Wiens, 2024; Wu et al., 2025), including those on bryophytes (e.g., Laenen et al., 2014; Maul et al., 2025), I used the method-of-moments estimator (Magallón and Sanderson, 2001), which is called the MS approach (Meyer and Wiens, 2018), to estimate moss diversification rates. This approach uses the following formula to estimate a lineage's net diversification rate (r): r = ln[n(1 − ε) + ε]/t, where n is the number of extant species in a lineage, t is the stem age of the lineage, and ε is the relative extinction rate suggested to vary from 0 to 0.9 (Magallón and Sanderson, 2001). Furthermore, I followed previous studies (Soria-Carrasco and Castresana, 2012; Laenen et al., 2014; Maul et al., 2025; Wu and Wiens, 2022) to estimate species diversification rate for each genus.
The megaphylogeny of mosses reported in Sanbonmatsu and Spalink (2022) is the most complete phylogeny of mosses at the present time. I first standardized species names in the phylogeny based on the database Bryophyte Nomenclator (Brinda and Atwood, 2023) and then extracted a genus-level phylogeny from it. The genus-level phylogeny included 85% of the 951 moss genera in the dataset used in this study. The genera that were present in Qian et al. (2024a) but absent from the phylogeny were excluded. As a result, 8930 moss species in 807 genera were included in the present study. I extracted the stem age of each genus (i.e., datum for t in the above-described formula) from the genus-level phylogeny and estimated the number of species in each genus (i.e., datum for n in the above-described formula) based on the database Bryophyte Nomenclator (Brinda and Atwood, 2023). Following previous studies (e.g., Stephens et al., 2025; Wu and Wiens, 2022), I used three values of ε (i.e., 0, 0.5, 0.9) to estimate diversification rates for each genus. Because the three sets of diversification rates estimated using the three values of ε were nearly perfectly correlated with one another (Spearman's rank correlation coefficients ranging from 0.986 to 0.998), suggesting that using different values of ε would have little impact on the results of subsequent analyses on geographic patterns of diversification rates, following previous studies (e.g., Davies et al., 2004; Boucher et al., 2020; Maul et al., 2025), I used the set of estimated diversification rates based on ε being set to zero in subsequent analyses. This setting is appropriate because the extant species richness in each genus is the result of speciation minus extinction (i.e., net diversification) within the genus, which means that n in this study has already accounted for extinction (i.e., n did not include extinct species). Thus, a value of diversification rate calculated with this approach for a genus is the net diversification rate of the genus (Maul et al., 2025).
2.3. Mean diversification rate of mosses in each assemblageFollowing previous studies (e.g., Wu and Wiens, 2022; Maul et al., 2025), each species in an assemblage (i.e., latitudinal band in this study) was assigned the diversification rate of its genus. I calculated the arithmetic mean of diversification rate (MDR) of genera in the latitudinal band, as in Maul et al. (2025). It should be noted that a low or high MDR in a latitudinal band reflects that the species assemblage of the latitudinal band is composed of species from clades with low or high diversification rates, respectively; they should not be taken to indicate that the latitudinal band had a low or high diversification rate, as Stephens et al. (2025) point out.
2.4. Climate dataTemperature and precipitation are the most important climatic factors shaping distributions of plants at a broad spatial scale (Whittaker, 1975). Accordingly, I related the values of MDR in the 21 latitudinal bands to mean annual temperature and annual precipitation. Climatic data were obtained from the CHELSA climate database (https://chelsa-climate.org/bioclim/) at the 30-arc-second resolution. The average of values of each climatic variable within each latitudinal band was used.
2.5. Data analysisEach statistical analysis included the 21 non-overlapped latitudinal bands across the world. I assessed the relationship of MDR with species richness, latitude and each of the two climatic variables using Pearson's correlation analysis. To account for the effect of variation in area among latitudinal bands on species richness, following previous studies (e.g., Qian et al., 2024a), I calculated species density (species richness being divided by log-transformed area). I conducted a variation partitioning analysis (Legendre and Legendre, 2012) to determine the relative importance of mean annual temperature versus annual precipitation on MDR. I used the package SYSTAT v.7 (Wilkinson et al., 1992) for statistical analyses.
3. ResultsMDR was positively correlated with species richness in mosses (Pearson's r = 0.655, P < 0.01). When variation in area among latitudinal bands was accounted for, MDR remained positively correlated with area-corrected species richness (i.e., species density) (r = 0.672, P < 0.01). When the Northern or Southern Hemisphere was considered as a whole, MDR in the latitudinal bands of either hemisphere decreased monotonically with increasing latitude (Fig. 1a). When the New World and Old World were considered separately, MDR decreased monotonically with increasing latitude in both the Northern and Southern Hemispheres and in both the New World and Old World (Fig. 1b and c). The same gradients were observed when the two longitudinal segments of the Old World were considered separately (Fig. 1d and e). Thus, MDR decreased monotonically with increasing latitude along each of the 10 latitudinal gradients shown in Fig. 1.
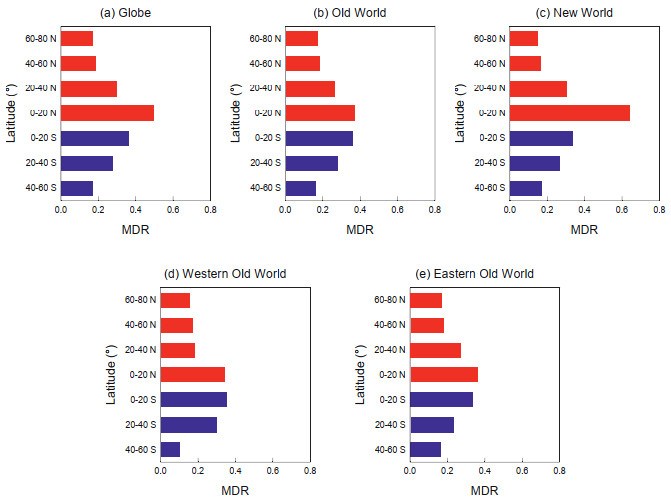
|
| Fig. 1 Mean diversification rate (MDR) of mosses in each of the seven latitudinal bands in the globe (a), in the Old World (b), in the New World (c), in the western Old World (d), and in the eastern Old World (e). Each latitudinal band is 20° in width. Red and blue colors indicated latitudinal bands located in the Northern and Southern Hemispheres, respectively. |
When MDR was related to mean annual temperature and annual precipitation in the 21 latitudinal bands in the six non-overlapped latitudinal gradients (i.e., three longitudinal segments by two hemispheres shown Fig. 1c−e), MDR was positively and significantly correlated with both mean annual temperature and annual precipitation (Pearson's r = 0.71 and 0.46, respectively, P < 0.05 in both cases; Fig. 2). Because no land of the continental Africa is located in latitudes > 40°S, climate data for the latitudinal band between 40° and 60°S in the western Old World were derived from islands, which had very high precipitation (i.e., the datum point with the largest annual precipitation in Fig. 2b; Table S1). Furthermore, the datum point representing the largest value of MDR in the latitudinal band of the New World with both high temperature and high precipitation may represent either a real circumstance or an outlier. With these two datum points being removed, MDR remained positively and significantly correlated with both mean annual temperature and annual precipitation (Pearson's r = 0.82 and 0.53, respectively, P < 0.05 in both cases), indicating that including these two datum points in our data analyses would not qualitatively affect the results of the analyses. When latitudinal bands in each of the six latitudinal gradients were considered, a monotonic trend of increasing MDR with increasing mean annual temperature was observed in each latitudinal gradient (Fig. 2a), but such a consistent trend was not observed in the relationships of MDR with annual precipitation (Fig. 2b).
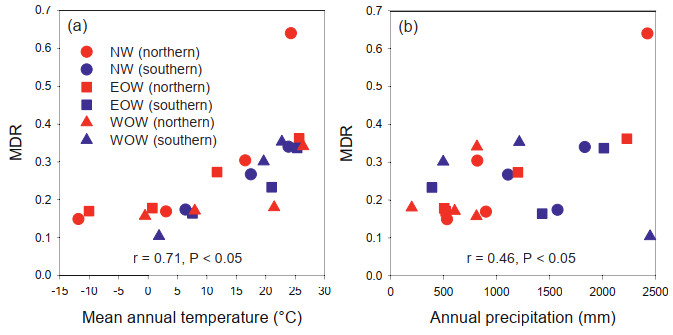
|
| Fig. 2 Relationships between mean diversification rate (MDR) and either mean annual temperature (a) or annual precipitation (b) for mosses in the world. The three longitudinal segments were differentiated by symbol shape (NW, New World, round; EOW, eastern Old World, square; WOW, western Old World, triangle) and the two hemispheres were differentiated by color (northern, red; southern, blue). Pearson's correlation coefficients (r) were derived from all the data in each plot. Data used in this figure were shown in Table S1. |
Mean annual temperature and annual precipitation together explained 57% of the variation in MDR in the 21 latitudinal bands across the world. The variation explained independently by mean annual temperature was six times that explained independently by annual precipitation, and was more than twice as much as that explained jointly by the two climatic variables (Fig. 3).
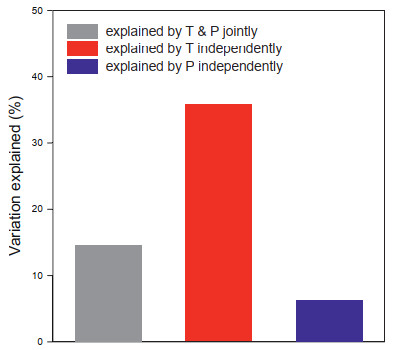
|
| Fig. 3 Variation in mean diversification rate for mosses in the world explained by mean annual temperature (T) and annual precipitation (P) jointly and independently. |
Because a diversification rate in this study was calculated for species within each genus, which may be considered a tip clade of the whole phylogeny of mosses, diversification rates in this study may be considered tip diversification rates. Thus, a mean diversification rate (MDR) in this study reflects a tip mean diversification rate in the genera of each moss assemblage (i.e., mosses in each latitudinal band). I found that MDR is highest at tropical latitudes and in humid and hot environments, and that MDR is influenced by temperature more strongly than by precipitation. I also found that MDR is positively correlated with species richness in mosses.
Previous studies showed mixed results on the relationships of diversification rates and MDR with latitude. Some studies showed higher diversification rates at lower latitudes (Cardillo, 1999; Böhm and Mayhew, 2005; Cardillo et al., 2005; Ricklefs, 2006; Wiens, 2007; Jansson and Davies, 2008; Martin and Tewksbury, 2008; Pyron and Wiens, 2013; Rolland et al., 2014), others showed no significant pattern or even higher diversification at higher latitudes (e.g., Weir and Schluter, 2007; Wiens et al., 2009; Soria-Carrasco and Castresana, 2012; Igea and Tanentzap, 2020). These mixed findings from different studies may result partly from using different methods to estimate diversification rates (e.g., some studies focus on diversification rates of tip branches of a phylogeny whereas others focus on diversification rates of both basal and tip branches of a phylogeny) and partly from differences in places of origin and radiation among different groups of organisms. As a result, it is difficult to directly compare findings among different studies. Nevertheless, the present study provides strong evidence that moss species in assemblages at lower latitudes were assembled from lineages with higher rates of tip diversification.
This study found that MDR in mosses is higher in areas with warmer and wetter climates. This finding is contrary to the findings of a number of previous studies which showed that diversification rates are lower in warmer and wetter climates (e.g., angiosperms: Igea and Tanentzap, 2020; Tietje et al., 2022). Song et al. (2021) found that in China, diversification rate increases with increasing temperature and precipitation in liverworts, and increases with increasing temperature but with decreasing precipitation in mosses, although some of the trends reported in their study are statistically not significant. Maul et al. (2025) found that MDR in liverwort assemblages along elevational gradients at a global scale is positively associated with both mean annual temperature and annual precipitation, consistent with the finding of my study. Laenen et al. (2018) found significantly higher rates of net diversification in tropical liverwort genera than in non-tropical ones. Thus, the finding of my study on mosses that MDR is higher in areas with warmer and wetter climates is consistent with those of previous studies on liverworts. There is a considerable body of data indicating that higher evolutionary rates in the tropics than in temperate regions (Rohde, 1992), supporting the notion that diversification rates are higher in warmer and wetter environments.
This study found that MDR is associated with temperature more strongly than with precipitation, which may suggest that temperature is a more important driver of speciation and extinction, and thus diversification, compared with water availability. Moles et al. (2014) found that temperature is a better predictor of plant traits, compared to precipitation. However, previous studies on species richness and phylogenetic diversity in plants have found mixed results on the relative importance of temperature-related variables versus precipitation-related variables. For example, Qian et al. (2019) found that annual precipitation is correlated with species richness and metrics of phylogenetic structure of angiosperms more strongly than mean annual temperature in China; Qian et al. (2024b) found that annual precipitation explained more variation in phylogenetic metrics of angiosperms in Asia, Northern America, and Australasia, but less variation in Europe and Southern America, compared with temperature-related variables. In ferns, Qian et al. (2023) found that precipitation-related variables explain more variation in phylogenetic metrics at the global scale and in Asia, Africa, and Australasia but less variation in Europe, Northern America, and Southern America, compared to temperature-related variables. In bryophytes, Qian and Chen (2016) found that annual precipitation is a stronger driver of species richness in China, compared with mean annual temperature. However, Qian and Grau (2025) and Qian (2025) found that temperature-related variables explained more variation in phylogenetic metrics in mosses and liverworts, respectively, in Nepal than precipitation-related variables, consistent with the finding of the present study on mean diversification rates of mosses across the world. As poikilohydric organisms that cannot internally regulate their water content, mosses highly depend on environmental conditions for water input from precipitation. Thus, one would expect that precipitation would be a stronger driver of moss diversification rates, compared with temperature. The stronger associations of metrics of phylogenetic structure and diversification rates with temperature may reflect the role of temperature in regulating evaporation and drought stress (Hsiao, 1973).
In conclusion, the present study is the first investigating latitudinal patterns and climate associations of the mean diversification rate of mosses at global, hemispheric, and smaller scales. The study shows that the mean diversification rate of mosses is positively correlated with species richness of mosses, increases with decreasing latitude and increasing temperature and precipitation, and is more strongly associated with temperature than with precipitation. These findings shed light on global variations of species richness in mosses.
AcknowledgementsI thank two anonymous reviewers for their helpful comments.
CRediT authorship contribution statement
Hong Qian: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization.
Data accessibility statement
Data used in this study were published and cited in the manuscript.
Declaration of competing interest
The author has no competing interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2025.07.002.
Adams, D.C., Berns, C.M., Kozak, K.H., et al., 2009. Are rates of species diversification correlated with rates of morphological evolution?. P. Roy. Soc. B-Biol. Sci., 276: 2729-2738. DOI:10.1098/rspb.2009.0543 |
Alfaro, M.E., Santini, F., Brock, C.D., 2007. Do reefs drive diversification in marine teleosts? Evidence from the pufferfishes and their allies (Order Tetraodontiformes). Evolution, 61: 2104-2126. DOI:10.1111/j.1558-5646.2007.00182.x |
Böhm, M., Mayhew, P.J., 2005. Historical biogeography and the evolution of the latitudinal gradient of species richness in the Papionini (Primata: Cercopithecidae). Biol. J. Linn. Soc., 85: 235-246. DOI:10.1111/j.1095-8312.2005.00488.x |
Boucher, F.C., Quatela, A.-S., Ellis, A.G., et al., 2020. Diversification rate vs. diversification density: decoupled consequences of plant height for diversification of Alooideae in time and space. PLoS One, 15: e0233597. DOI:10.1371/journal.pone.0233597 |
Brinda, J.C., Atwood, J.J., 2023. Bryophyte Nomenclator. In: Bánki, O., Roskov, Y., Döring, M., et al. (Eds.), Catalogue of Life Checklist (Jan 2023). https://doi.org/10.48580/dfqt-8zm.
|
Cardillo, M., 1999. Latitude and rates of diversification in birds and butterflies. P. Roy. Soc. B-Biol. Sci., 266: 1221-1225. DOI:10.1098/rspb.1999.0766 |
Cardillo, M., Orme, C.D.L., Owens, I.P.F., 2005. Testing for latitudinal bias in diversification rates: an example using new world birds. Ecology, 86: 2278-2287. DOI:10.1890/05-0112 |
Cooney, C.R., Seddon, N., Tobias, J.A., 2016. Widespread correlations between climatic niche evolution and species diversification in birds. J. Anim. Ecol., 85: 869-878. DOI:10.1111/1365-2656.12530 |
Davies, T.J., Barraclough, T.G., Chase, M.W., et al., 2004. Darwin’s abominable mystery: insights from a supertree of the angiosperms. Proc. Natl. Acad. Sci. U.S.A., 101: 1904-1909. DOI:10.1073/pnas.0308127100 |
Eriksson, O., Bremer, B., 1992. Pollination systems, dispersal modes, life forms, and diversification rates in angiosperm families. Evolution, 46: 258-266. DOI:10.2307/2409820 |
Geffert, J.L., Frahm, J.P., Barthlott, W., et al., 2013. Global moss diversity: spatial and taxonomic patterns of species richness. J. Bryolog., 35: 1-11. DOI:10.1179/1743282012Y.0000000038 |
Goffinet, B., Buck, W.R., 2004. Systematics of the Bryophyta (mosses): from molecules to a revised classification. Mol. Syst. Bryophyt., 98: 205-239. |
Gómez-Rodríguez, C., Baselga, A., Wiens, J.J., 2015. Is climatic niche width related to diversification rate?. Glob. Ecol. Biogeogr., 24: 383-395. DOI:10.1111/geb.12229 |
Hawkins, B.A., 2001. Ecology's oldest pattern?. Trends Ecol. Evol., 16: 470-470. DOI:10.1016/S0169-5347(01)02197-8 |
Hillebrand, H., 2004. On the generality of the latitudinal gradient. Am. Nat., 163: 192-211. DOI:10.1086/381004 |
Hsiao, T.C., 1973. Plant responses to water stress. Annu. Rev. Plant Physiol., 24: 519-570. DOI:10.1146/annurev.pp.24.060173.002511 |
Hughes, C., Eastwood, R., 2006. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl. Acad. Sci. U.S.A., 103: 10334-10339. DOI:10.1073/pnas.0601928103 |
Igea, J., Tanentzap, A.J., 2020. Angiosperm speciation cools down in the tropics. Ecol. Lett., 23: 692-700. DOI:10.1111/ele.13476 |
Jansson, R., Davies, T.J., 2008. Global variation in diversification rates of flowering plants: energy verus climate change. Ecol. Lett., 11: 173-183. DOI:10.1111/j.1461-0248.2007.01138.x |
Laenen, B., Patiño, J., Hagborg, A., et al., 2018. Evolutionary origin of the latitudinal diversity gradient in liverworts. Mol. Phylogenet. Evol., 127: 606-612. DOI:10.1016/j.ympev.2018.06.007 |
Laenen, B., Shaw, B., Schneider, H., et al., 2014. Extant diversity of bryophytes emerged from successive post-Mesozoic diversification bursts. Nat. Commun., 5: 5134. DOI:10.1038/ncomms6134 |
Legendre, P., Legendre, L., 2012. Numerical Ecology. Third ed. Amsterdam: Elsevier.
|
Magallón, S., Sanderson, M.J., 2001. Absolute diversification rates in angiosperm clades. Evolution, 55: 1762-1780. |
Marin, J., Hedges, S.B., 2016. Time best explains global variation in species richness of amphibians, birds and mammals. J. Biogeogr., 43: 1069-1079. DOI:10.1111/jbi.12709 |
Martin, P.R., Tewksbury, J.J., 2008. Latitudinal variation in subspecific diversification of birds. Evolution, 62: 2775-2788. DOI:10.1111/j.1558-5646.2008.00489.x |
Maul, K., Gradstein, S.R., Quandt, D., et al., 2025. Temperature dependence of liverwort diversification reveals a cool origin and hot hotspots. Sci. Rep., 15: 3225. DOI:10.1038/s41598-025-87206-1 |
Meyer, A.L.S., Wiens, J.J., 2018. Estimating diversification rates for higher taxa: BAMM can give problematic estimates of rates and rate shifts. Evolution, 72: 39-53. DOI:10.1111/evo.13378 |
Moles, A.T., Perkins, S.E., Laffan, S.W., et al., 2014. Which is a better predictor of plant traits: temperature or precipitation?. J. Veg. Sci., 25: 1167-1180. DOI:10.1111/jvs.12190 |
Patiño, J., Vanderpoorten, A., 2018. Bryophyte biogeography. Crit. Rev. Plant Sci., 37: 175-209. DOI:10.1080/07352689.2018.1482444 |
Pyron, R.A., Wiens, J.J., 2013. Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. P. Roy. Soc. B-Biol. Sci., 280: 20131622. DOI:10.1098/rspb.2013.1622 |
Qian, H., 2025. Geographic patterns and climatic drivers of phylogenetic structure of liverworts along a long elevational gradient in the central Himalaya. J. Syst. Evol., 63: 62-71. DOI:10.1111/jse.13129 |
Qian, H., Chen, S., 2016. Reinvestigation on species richness and environmental correlates of bryophytes at a regional scale in China. J. Plant Ecol., 9: 734-741. DOI:10.1093/jpe/rtw001 |
Qian, H., Deng, T., Jin, Y., et al., 2019. Phylogenetic dispersion and diversity in regional assemblages of seed plants in China. Proc. Natl. Acad. Sci. U.S.A., 116: 23192-23201. DOI:10.1073/pnas.1822153116 |
Qian, H., Kessler, M., Zhang, J., et al., 2023. Global patterns and climatic determinants of phylogenetic structure of regional fern floras. New Phytol., 239: 415-428. DOI:10.1111/nph.18920 |
Qian, H., Dai, Z., Wang, J., 2024a. Strong evidence for latitudinal diversity gradient in mosses across the world. Plant Divers, 46: 537-541. DOI:10.1016/j.pld.2024.05.004 |
Qian, H., Qian, S., Zhang, J., et al., 2024b. Effects of climate and environmental heterogeneity on the phylogenetic structure of regional angiosperm floras worldwide. Nat. Commun., 15: 1079. DOI:10.1038/s41467-024-45155-9 |
Qian, H., Grau, O., 2025. Geographic patterns and ecological causes of phylogenetic structure in mosses along an elevational gradient in the central Himalaya. Plant Divers., 47: 98-105. DOI:10.1016/j.pld.2024.07.005 |
Rabosky, D.L., Adams, D.C., 2012. Rate of morphological evolution are correlated with species richness in salamanders. Evolution, 66: 1807-1818. DOI:10.1111/j.1558-5646.2011.01557.x |
Rabosky, D.L., Matute, D.R., 2013. Macroevolutionary speciation rates are decoupled from the evolution of intrinsic reproductive isolation in Drosophila and birds. Proc. Natl. Acad. Sci. U.S.A., 110: 15354-15359. DOI:10.1073/pnas.1305529110 |
Ricklefs, R.E., 1987. Community diversity: relative roles of local and regional processes. Science, 235: 167-171. DOI:10.1126/science.235.4785.167 |
Ricklefs, R.E., 2006. Global variation in the diversification rate of passerine birds. Ecology, 87: 2468-2478. DOI:10.1890/0012-9658(2006)87[2468:GVITDR]2.0.CO;2 |
Ricklefs, R.E., 2007. Estimating diversification rates from phylogenetic information. Trends Ecol. Evol., 22: 601-610. DOI:10.1016/j.tree.2007.06.013 |
Rohde, K., 1992. Latitudinal gradients in species diversity: the search for the primary cause. Oikos, 65: 514-527. DOI:10.2307/3545569 |
Rolland, J., Condamine, F.L., Jiguet, F., et al., 2014. Faster speciation and reduced extinction in the tropics contribute to the mammalian latitudinal diversity gradient. PLoS Biology, 12: e1001775. DOI:10.1371/journal.pbio.1001775 |
Rosenzweig, M.L., 1995. Species Diversity in Space and Time. Cambridge: Cambridge University Press.
|
Sanbonmatsu, K.K., Spalink, D., 2022. A global analysis of mosses reveals low phylogenetic endemism and highlights the importance of long-distance dispersal. J. Biogeogr., 49: 654-667. DOI:10.1111/jbi.14333 |
Scholl, J.P., Wiens, J.J., 2016. Diversification rates and species richness across the Tree of Life. P. Roy. Soc. B-Biol. Sci., 283: 20161335. |
Song, X.T., Fang, W.Z., Chi, X.L., et al., 2021. Geographic pattern of bryophyte species richness in China: the influence of environment and evolutionary history. Front. Ecol. Evol., 9: 680318. DOI:10.3389/fevo.2021.680318 |
Soria-Carrasco, V., Castresana, J., 2012. Diversification rates and the latitudinal gradient of diversity in mammals. P. Roy. Soc. B-Biol. Sci., 279: 4148-4155. DOI:10.1098/rspb.2012.1393 |
Stephens, P.R., Farrell, M.J., Davies, T.J., et al., 2025. Global diversity patterns are explained by diversification rates and dispersal at ancient, not shallow, timescales. Syst. Biol.. DOI:10.1093/sysbio/syaf018 |
Tedesco, P.A., Paradis, E., Lévêque, C., et al., 2017. Explaining global-scale diversification patterns in actinopterygian fishes. J. Biogeogr., 44: 773-783. DOI:10.1111/jbi.12905 |
Tietje, M., Antonelli, A., Baker, W.J., et al., 2022. Global variation in diversification rate and species richness are unlinked in plants. Proc. Natl. Acad. Sci. U.S.A., 119: e2120662119. DOI:10.1073/pnas.2120662119 |
Weir, J.T., Schluter, D., 2007. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science, 315: 1574-1576. DOI:10.1126/science.1135590 |
Whittaker, R.H., 1975. Communities and Ecosystems. New York: MacMillan Publishing Company, Inc..
|
Wiens, J.J., 2007. Global patterns of diversification and species richness in amphibians. Am. Nat., 170: S86-S106. DOI:10.1086/519396 |
Wiens, J.J., 2015a. Explaining large-scale patterns of vertebrate diversity. Biol. Lett., 11: 20150506. DOI:10.1098/rsbl.2015.0506 |
Wiens, J.J., 2015b. Faster diversification on land than sea helps explain global biodiversity patterns among habitats and animal phyla. Ecol. Lett., 18: 1234-1241. DOI:10.1111/ele.12503 |
Wiens, J.J., Donoghue, M.J., 2004. Historical biogeography, ecology, and species richness. Trends Ecol. Evol., 19: 639-644. DOI:10.1016/j.tree.2004.09.011 |
Wiens, J.J., Sukumaran, J., Pyron, R.A., et al., 2009. Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution, 63: 1217-1231. DOI:10.1111/j.1558-5646.2009.00610.x |
Wilkinson, L., Hill, M., Welna, J.P., et al., 1992. SYSTAT for Windows: Statistics. Evanston: SYSTAT Inc..
|
Willig, M.R., Kaufman, D.M., Stevens, R.D., 2003. Latitudinal gradients in biodiversity: pattern, process, scale, and synthesis. Ann. Rev. Ecol., Evol. Syst., 34: 273-309. DOI:10.1146/annurev.ecolsys.34.012103.144032 |
Wu, G., Wiens, J.J., 2022. The origins of climate-diversity relationships and richness patterns in Chinese plants. Ecol. Evol., 12: e9607. DOI:10.1002/ece3.9607 |
Wu, G., Ye, Q., Liu, H., et al., 2025. Shaded habitats drive higher rates of fern diversification. J. Ecol., 113: 1200-1208. DOI:10.1111/1365-2745.70026 |
Yu, D., Wiens, J.J., 2024. The causes of species richness patterns among clades. P. Roy. Soc. B-Biol. Sci., 291: 20232436. |
Zhang, J., Qian, H., 2023. U.Taxonstand: an R package for standardizing scientific names of plants and animals. Plant Divers., 45: 1-5. DOI:10.1016/j.pld.2022.09.001 |


