b. Research Center for UAV Remote Sensing, Shaanxi Normal University, Xi'an 710119, People's Republic of China;
c. Changqing Teaching & Research Base of Ecology, Shaanxi Normal University, Xi'an 710119, People's Republic of China
Invasive species have a profound impact on biodiversity, ecosystem functions, human health, and the economy (Mack and Lonsdale, 2001; Crowl et al., 2008). Moreover, globalization has accelerated the impact of alien species invasions (Pyšek et al., 2020). Accordingly, it is crucial to understand the factors that drive invasion success in order to predict potential invasive species and mitigate their harm (Qian et al., 2022). One key factor that may predict invasion success is the phylogenetic relationship between alien species and native plants in the invaded region (Lami et al., 2021).
Charles Darwin was one of the first biologists to suggest that invasion success of species is connected to the evolutionary relationship between introduced plants and local plant communities (Li et al., 2015). According to Darwin’s naturalization hypothesis, exotic plants that are distantly related to native flora are more likely to become naturalized due to greater niche differentiation, which reduces competitive pressures (Rejmánek, 1996; Daehler, 2001; Li et al., 2015). Conversely, Darwin’s preadaptation hypothesis suggests that alien plants closely related to native species are more likely to successfully naturalize due to their shared traits and preferences for similar environments (Ricciardi et al., 2013; Park and Potter, 2015). Although these two hypotheses may appear contradictory, they elucidate the complex interactions between alien and native species, often referred to as Darwin's naturalization conundrum (Diez et al., 2008; Thuiller et al., 2010).
Invasion success of alien species proceeds across several stages (e.g., dispersal, establishment, and spread), and at each stage invasion success is determined by distinct factors (Richardson et al., 2000; Blackburn et al., 2011). For instance, during the dispersal stage, the most critical factors for invasion success may be the adaptability of an alien plant, whereas during the establishment and spread stages, the major factors may be phylogenetic relatedness to local species and competitive pressures (Li et al., 2015; Fan et al., 2023; Wang et al., 2023). Thus, the two hypotheses of Darwin’s naturalization conundrum emphasize different dominant factors for plant naturalization in various environmental contexts (Fan et al., 2023). Although these two hypotheses appear contradictory, they share a common premise: the kinship distance and evolutionary history between species can characterize their niche differences (Park and Potter, 2015).
Closely related species often exhibit similar morphological traits and functional attributes, consequently occupying more analogous niches, a phenomenon known as phylogenetic niche conservatism (Crisp and Cook, 2012; Park and Potter, 2015; Qian and Zhang, 2024). Extensive research has supported phylogenetic niche conservatism, indicating that closely related species tend to retain ancestral niche characteristics (Rohr et al., 2010; Guidetti et al., 2011; Santos and Cannatella, 2011). However, the universality of phylogenetic niche conservatism has been contested. Some studies indicate that species with close phylogenetic relationships may exhibit significant functional differences, which could affect their adaptability and invasion potential in new environments (Xu et al., 2022). Furthermore, the strength of phylogenetic niche conservatism may vary across different taxonomic groups. For instance, Cooper et al. (2011) observed significant differences in niche conservation among various clades. Additionally, the niche similarity of closely related species in specific contexts remains a subject of debate (Wiens and Graham, 2005). Therefore, presuming that closely related species have similar niches without empirical testing often leads to biased or erroneous conclusions.
Niche conservatism has been shown to be prevalent among invasive species (Williamson, 2006; Peterson, 2011; Petitpierre et al., 2012; Oliveira et al., 2018; Liu et al., 2020) and is considered critical in predicting invasive potential (Peterson and Vieglais, 2001; Thuiller et al., 2005; Broennimann et al., 2007; Song et al., 2023). However, discrepancies may exist between the niches of invasive species in their native and non-native habitats. New selective pressures in invaded environments can lead to rapid changes in the environmental tolerances of species, thereby inducing niche shifts (Broennimann et al., 2007; Oliveira et al., 2018). These niche shifts are particularly pronounced when there is unoccupied niche space in the non-native areas (Oliveira et al., 2018). For example, factors such as predation, competition, and dispersal limitation allow species to expand their range into areas that are inaccessible in their native environment (Callen and Miller, 2015). These niche shifts have been demonstrated for several invasive plant species (Broennimann et al., 2007; Beaumont et al., 2009; Tingley et al., 2014; Atwater et al., 2018).
Here, we propose three hypotheses: (1) In the early stages of introduction, alien plants show high niche overlap with native plants, and there is a positive correlation between phylogenetic distance and niche overlap; (2) During the expansion process, invasive plants will undergo a niche shift to avoid competition with native species; and (3) At later stages of establishment, invasive plants exhibit a smaller niche overlap with native plants, and there is a negative correlation between phylogenetic distance and niche overlap. We tested these hypotheses on the Asteraceae family, which has become the largest and most detrimental group of invasive plants in China (Weber et al., 2008; Yang et al., 2023a). This research will not only deepen our understanding of the mechanisms that underlie species coexistence but will also provide more reliable theoretical support for predicting the likely invasion success of alien species. Our findings offer new perspectives for advancing invasion ecology theory and lay a critical foundation for formulating effective strategies to manage biological invasions and mitigate their ecological impacts.
2. Materials and methods 2.1. Plant listWe obtained a list of Asteraceae alien plants introduced into China from the “Dataset on Catalogue of Alien Plants in China” (Lin et al., 2022). This dataset categorizes species into five basic states based on the current status of alien plants: Cultivated (C), Escaped (E), Naturalized (N), Invasive (I), or Wild (W). To align with the widely accepted invasion framework that delineates the progression of alien species into introduction, naturalization, and invasion stages (Qian, 2023), we reclassified alien species into three categories. Our first category, introduced species, describes plants that are cultivated or have escaped cultivation but have not established persistent populations. This includes species classified as C, C/E, and E. The second category, naturalized species, refers to plants that have established self-sufficient populations without causing significant ecological or economic impacts. This includes species classified as C/E/N, E/N, and W/E/N. The third category, invasive species, describes plants that not only form self-sustaining populations but also spread rapidly and cause serious ecological or economic harm. This includes species classified as N/I, C/E/N/I, E/N/I, and W/C/E/N/I.
Details on the classification criteria and examples of species within each category are provided in Table S1. We compiled a comprehensive list of native Asteraceae species by using Flora of China (Flora of China Editorial Committee, 2011) to identify Asteraceae species native to China. Alien species listed in the “Dataset on Catalogue of Alien Plants in China” were excluded (Lin et al., 2022). In this study, infraspecific taxa were consolidated with their parent taxa. Species names and spellings were corrected based on The Plant List (http://www.theplantlist.org/) using the R package U.Taxonstand (Zhang and Qian, 2023). Our final list included a total of 2445 Asteraceae plant species, comprising 1879 native and 566 exotic species. Among these, there were 422 introduced, 44 naturalized, and 100 invasive plants.
2.2. Phylogenetic analysesWe use the R package U.PhyloMaker (Jin and Qian, 2022, 2023) to construct phylogenetic relationships for all species under investigation. The giant tree GBOTB.extended.TPL.tre was used as the phylogenetic backbone to establish the phylogenetic relationships of all Asteraceae plants (Jin and Qian, 2019, 2022). GBOTB.extended.TPL.tre represents a refined, corrected, and enlarged amalgamation of clades from the seed plant GBOTB (Smith and Brown, 2018) and the fern phylogeny by Zanne et al. (2014). We pruned the megaphylogeny to produce a phylogenetic tree encompassing solely Asteraceae plant species found in China. Given that all species in this study’s dataset are resolved in GBOTB.extended.TPL.tre, the phylogenetic metrics derived from it are deemed reliable (Qian and Jin, 2021).
To analyze various types of alien species, we calculated the Mean Pairwise Distance (MPD) between each alien species and all native species, as well as the Mean Nearest Neighbor Distance (MNND) between each alien species and its nearest native taxon (Thuiller et al., 2010). This analysis aimed to discern differences among different taxa. Additionally, we computed the Net Relatedness Index (NRI) and Nearest Taxon Index (NTI) for different taxa to assess whether the phylogenetic structure of alien species differed from random expectations. NRI represents a richness-normalized measure of MPD between all pairs of species in a sample, whereas NTI is a richness-normalized version of MNND between an alien species and its closest native relative. NRI tends to be influenced by deeper phylogenetic structures, while NTI is more sensitive to patterns near the phylogenetic tips (Mazel et al., 2016). These metrics allow for the exploration of species assembly patterns across both deep and shallow evolutionary histories. Positive values of NRI and NTI indicate that species within an assemblage are more closely related than expected by chance (Qian, 2023). For example, a positive NRI value indicates that species within a community are more closely related than would be expected under random distribution. This pattern may result from environmental filtering, where only species with specific adaptive traits can survive and coexist in the environment, leading to phylogenetic clustering within the community (Banerjee et al., 2023). Similarly, a positive NTI value suggests that alien species tend to coexist with native species that are closely related to them in the new environment. This may indicate that alien species share a closer evolutionary relationship with native species or possess similar functional traits, allowing them to compete and coexist within similar niches (Qian and Sandel, 2017). All phylogenetic indicators were computed using the R package PhyloMeasures (Tsirogiannis and Sandel, 2016).
2.3. Niche overlapWe obtained global distribution data for all native and alien Asteraceae species in China, using the R package rgbif (Chamberlain, 2017) to access the Global Biodiversity Information Facility database (GBIF, 2022). Subsequently, we employed the R package CoordinateCleaner (Zizka et al., 2019) to examine and organize species distribution data. We excluded species with fewer than five distribution points from our analysis. For the estimation of niches, we employed the conservative method of principal component analysis (PCA) ordination to ensure reliability. In total, 1192 Asteraceae plants were included in our analysis. Of these species, 683 were native plants, 369 introduced species, 43 naturalized species, and 97 invasive species.
In our analysis of the niche overlap between exotic Asteraceae plants and native plants, we considered the influence of environmental factors on their niches. Specifically, we identified five key factors: environmental energy, water resource availability, climate seasonality, and habitat heterogeneity. Environmental energy was represented by the annual average temperature (Bio1), while water resource availability included the drought index (Ai), average annual precipitation (Bio12), and precipitation in the driest quarter (Bio17). Climate seasonality was characterized by the variance of seasonal changes in temperature (Bio4) and precipitation seasonal variation (Bio15). Additionally, habitat heterogeneity was represented by altitude (Alt). Annual average temperature, the variance of seasonal changes in temperature, average annual precipitation, seasonal variation in precipitation, precipitation in the driest quarter, and altitude data were sourced from the WorldClim data center (Fick and Hijmans, 2017), with a resolution of 2.5 arc minutes. The aridity index (Ai) was derived from the CHELSA data center (Karger et al., 2017), initially at a resolution of 30 arc seconds, and was resampled to 2.5 arc minutes using the Resample tool in ArcGIS 10.2 (Environmental Systems Research Institute: Redlands, CA, USA). The Pearson correlation coefficient between all environmental factors was less than 0.8.
We used a PCA ordination approach to estimate the extent to which species share similar environmental conditions and resource use, i.e., niche overlap. This approach applies a kernel density function to species occurrence density and environmental variables in grid space, standardizing the occurrence density between 0 and 1. This standardization mitigates biases in occurrence density caused by incomplete data in the PCA space, thereby preventing underestimation or biased estimation of niche overlap (Barve et al., 2011; Broennimann et al., 2012; Guisan et al., 2014). Consequently, this method reliably assesses niche overlap. Additionally, we employed Schoener’s D (Schoener, 1970) to quantify niche overlap, which ranges from 0 (no overlap) to 1 (complete overlap).
2.4. Niche dynamicsWe conducted a comparative analysis of the niches of 17 invasive Asteraceae species between their native ranges and China, where they have extensively invaded and caused significant harm. These 17 invasive Asteraceae species were identified from the ‘List of Alien Invasive Species in China,’ published by the Ministry of Ecology and Environment of the People’s Republic of China in four installments, specifically in 2003, 2010, 2014, and 2016. Niche mismatches between invaded and native ranges are not always solely attributable to niche shifts. When the niche space occupied by an invasive species in the invaded area is only a subset of its native range, it can lead to a misleading signal of niche mismatch (Petitpierre et al., 2012). For instance, during the initial stages of biological invasion, invasive organisms may not fully exploit all available niches within the invaded area (Václavík and Meentemeyer, 2012; Oliveira et al., 2018). Moreover, invasive species may encounter a bottleneck effect during the invasion process, causing their niche to contract in the new range (Oliveira et al., 2018). To mitigate potential false signals of niche mismatch, we utilized the “COUE” framework (centroid shift, niche overlap, unfilled, expansion) to compare the niche of the invasive species within their native range with their niche after invasion in China (Petitpierre et al., 2012; Guisan et al., 2014). This framework enabled us to assess niche stability, expansion, and unfilling. Specifically, niche stability represents the portion of the niche space that overlaps between the native and invaded ranges, reflecting niche conservatism; niche expansion refers to the part of niche space that is occupied in the invaded range but not utilized in the native range; and niche unfilling indicates the part of suitable environmental conditions in the native range that remain unoccupied in the invaded range (Petitpierre et al., 2012). We quantified niche stability, unfilling, and expansion using gridded environmental data and occurrence density records. Furthermore, to ascertain whether the niche of these invasive plants was altered in China compared to their native habitats, we conducted tests for niche equivalence and similarity between the two regions. These analyses, including niche overlap, niche change, and tests for equivalence and similarity, were performed using the R packages ade4 (Dray and Dufour, 2007) and ecospat (Di Cola et al., 2017).
2.5. Phylogenetic niche conservatism testWe conducted Mantel tests on pairwise phylogenetic distance and niche overlap to identify potential correlations, using 999 random permutations to derive p-values. Assessing pairwise niche overlap among taxa within a clade may be unsuitable due to non-independence arising from phylogenetic relationships and the inclusion of taxa in multiple pairwise comparisons. The Mantel test addresses these issues and is frequently employed for comparing pairwise distances or similarities (Legendre and Fortin, 2010; Diniz et al., 2013).
3. Results 3.1. Composition and characteristics of exotic Asteraceae plantsExotic plants within China’s Asteraceae family are currently, predominantly cultivated species. Of the 566 exotic Asteraceae species, the genus that is most represented is Kleinia, which is characterized by evergreen and succulent plants, making it a popular for extensive planting. Apart from Kleinia, other garden flower varieties that are widely cultivated include Centaurea and Achillea (Table S1). Of the Asteraceae family’s alien plants categorized as invasive species, Erigeron and Bidens stand out for their prolific production of achenes (Table S1). Notably, the majority of exotic Asteraceae species in China originate from the American continent, with over half tracing their origins to the Americas. Among these, 216 species are from North America, while 71 species come from South America (Table S1).
3.2. Phylogenetic patterns of alien plants in AsteraceaeWe conducted a phylogenetic analysis encompassing 566 exotic Asteraceae species and 1879 native Asteraceae species present in China. The phylogenetic tree illustrates the relationships of all Asteraceae species within China, revealing that introduced species typically form distinct clusters, often interspersed among native plant groups. Notably, invasive species are frequently nested within these clusters of exotic species, with a small proportion also nested within native species clusters (Fig. 1).
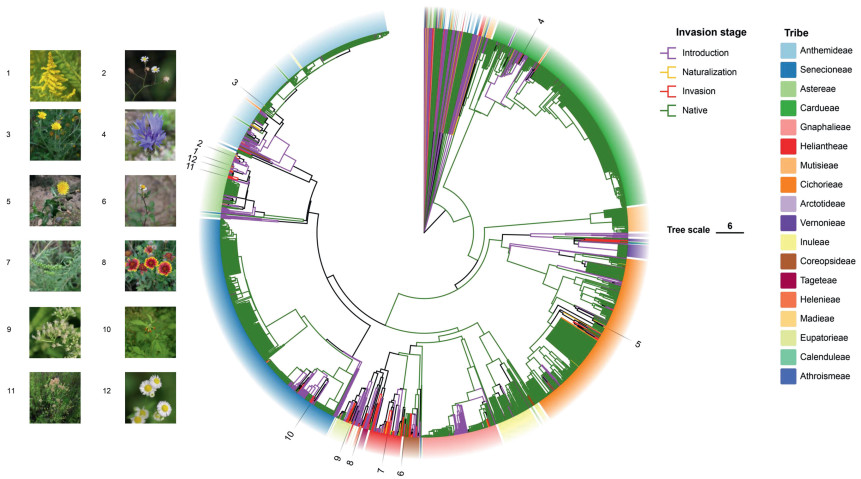
|
| Fig. 1 Phylogeny of Chinese Asteraceae plants and tribes with illustrations of some representative species. Invasive plants: (1) Solidago canadensis; (2) Symphyotrichum subulatum; (4) Centaurea cyanus; (5) Sonchus asper; (6) Bidens pilosa; (7) Ambrosia artemisiifolia; (8) Gaillardia pulchella; (9) Mikania micrantha; (10) Crassocephalum crepidioides; (11) Erigeron canadensis; (12) Erigeron annuus. Naturalized plant: (3) Glebionis coronaria. |
When we compared the mean phylogenetic distances of three distinct types of alien plants with that of their closest related native species, we found that the mean phylogenetic distance (MPD) between invasive Asteraceae species and native Asteraceae species was smaller than that between introduced and naturalized Asteraceae species (Fig. 2a). Furthermore, the mean nearest neighbor distance (MNND) of invasive species was significantly lower than that of species in the introduction phase (Fig. 2b). These findings suggest that invasive species share a closer phylogenetic relationship with native Asteraceae plants than species in the introduction phase and naturalized species.
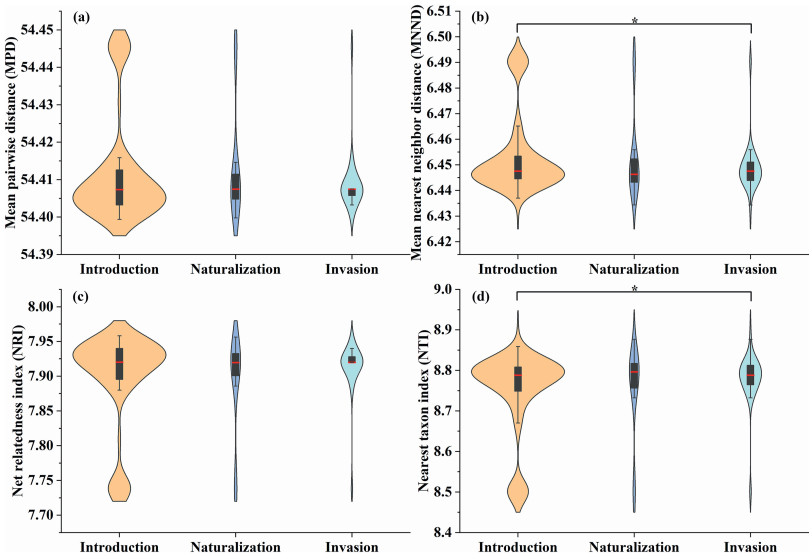
|
| Fig. 2 Violin plots illustrating phylogenetic distance among various types of exotic and native Asteraceae plants in China. The plots include (a) Mean Pairwise Distance (MPD), (b) Mean Nearest Neighbor Distance (MNND), (c) Net Relatedness Index (NRI), and (d) Nearest Taxon Index (NTI). The width of the violin represents the density of the data; wider sections indicate a higher concentration of data points within that numerical interval. The black bar inside the violin denotes the interquartile range (IQR) of the data, while the red line represents the median. Black lines extending from the black bar indicate the lower and upper adjacent values of the data (1.5 times the IQR). An asterisk (∗) denotes a significant difference at the α = 0.05 level. |
We also found that the Net Relatedness Index (NRI) of invasive Asteraceae species was higher than that or introduced and naturalized species (Fig. 2c). Concurrently, the Nearest Taxon Index (NTI) of invasive Asteraceae species was significantly higher than that of introduced species (Fig. 2d). Moreover, regardless of the type of alien plant (introduced, naturalized, or invasive), both the NRI and the NTI were consistently positive. This finding suggests that the presence of alien Asteraceae plants contributes to phylogenetic clustering, supporting Darwin’s preadaptation hypothesis (Qian, 2023).
Many of the alien plants introduced to China are cultivated species that rely on artificial propagation and cannot reproduce independently. After excluding these cultivated species, our analysis showed that the mean phylogenetic distance (MPD) and mean nearest neighbor distance (MNND) of invasive species were significantly lower than those of non-cultivated introduced species (p < 0.05) (Fig. 3a and b). This suggests that cultivated alien plants have closer phylogenetic relationships with native plants.
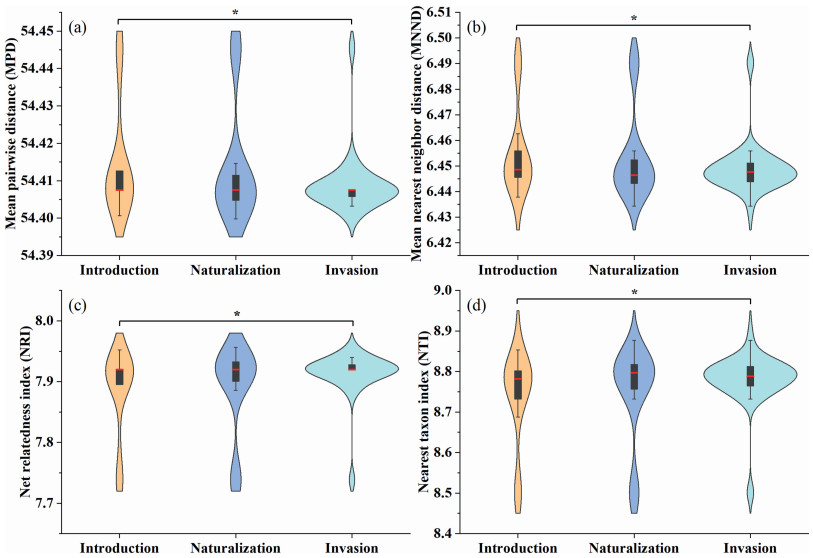
|
| Fig. 3 Violin plots illustrate phylogenetic distance among various types of exotic (excluding cultivated plants) and native Asteraceae plants in China. The plots include (a) Mean Pairwise Distance (MPD), (b) Mean Nearest Neighbor Distance (MNND), (c) Net Relatedness Index (NRI), and (d) Nearest Taxon Index (NTI). The width of the violin represents the density of the data; wider sections indicate a higher concentration of data points within that numerical interval. The black bar inside the violin denotes the interquartile range (IQR) of the data, while the red line represents the median. Black lines extending from the black bar indicate the lower and upper adjacent values of the data (1.5 times the IQR). An asterisk (∗) denotes a significant difference at the α = 0.05 level. |
Niche overlap between the exotic and native Asteraceae was limited, starting with introduced species. Niche overlap increased from introduced to naturalized species, but decreased from naturalized to invasive species (Table 1). This suggests that naturalized species might exhibit slightly greater environmental adaptability compared to introduced and invasive species.
| Type | Mean | SD |
| Introduction | 0.1292 | 0.0593 |
| Naturalization | 0.1398 | 0.0690 |
| Invasion | 0.1316 | 0.0634 |
We conducted a Mantel test to assess the correlation between niche overlap and phylogenetic distance among 509 alien plants and 683 native plants in the Asteraceae family. The results of the Mantel test revealed no significant correlation between niche overlap and phylogeny (Table 2). Moreover, a negative correlation was observed between the phylogenetic distance and niche overlap between the three types of alien plants and native plants.
| Type | Mantel test | |
| R | p | |
| Introduction | −0.00173 | 0.536 |
| Naturalization | −0.06135 | 0.867 |
| Invasion | −0.04144 | 0.921 |
| All exotic plants | −0.02100 | 0.947 |
Comparative analysis of the niches of 17 invasive Asteraceae species that have caused significant harm in China indicated that the niches of these aggressive invaders remained relatively stable during their invasion. The primary variation in niches stems from unfilled niches, with low overlap between the niches of these invasive species in their native habitats and in China (0.008–0.415) (Table 3). Principal component analysis of the niches of these 17 invasive species revealed that the first two principal components, derived from seven climate variables, accounted for 66.92%–76.74% of the niches. The PC1 axis is mainly associated with water resource availability (Ai, Bio12 and Bio17), while the PC2 axis is linked to climate seasonality (Bio4 and Bio15) and environmental heterogeneity (Alt) (Figs. S1–S17). Of these 17 invasive species, only Symphyotrichum subulatum, Bidens frondosa, and Erigeron canadensis have undergone minimal niche expansion (Table 3).
| Species | Unfilling | Stability | Expansion | Overlap/D | Niche equivalence | Niche similarity | |
| Native > China | China > Native | ||||||
| Ageratina adenophora | 0.000 | 1.000 | 0.000 | 0.415 | 0.198 | 0.010 | 0.010 |
| A. conyzoides | 0.172 | 1.000 | 0.000 | 0.026 | 0.376 | 0.248 | 0.158 |
| Ambrosia artemisiifolia | 0.816 | 1.000 | 0.000 | 0.053 | 0.069 | 0.020 | 0.020 |
| A. trifida | 0.677 | 1.000 | 0.000 | 0.085 | 0.475 | 0.020 | 0.020 |
| Bidens frondosa | 0.710 | 0.999 | 0.001 | 0.023 | 0.465 | 0.050 | 0.139 |
| B. pilosa | 0.121 | 1.000 | 0.000 | 0.071 | 0.406 | 0.050 | 0.059 |
| Chromolaena odorata | 0.260 | 1.000 | 0.000 | 0.008 | 0.089 | 0.376 | 0.356 |
| Erigeron annuus | 0.923 | 1.000 | 0.000 | 0.067 | 0.010 | 0.010 | 0.010 |
| E. canadensis | 0.499 | 0.993 | 0.007 | 0.035 | 0.010 | 0.069 | 0.030 |
| E. sumatrensis | 0.182 | 1.000 | 0.000 | 0.032 | 0.554 | 0.050 | 0.089 |
| Flaveria bidentis | 0.081 | 1.000 | 0.000 | 0.05 | 0.228 | 0.079 | 0.099 |
| Mikania micrantha | 0.141 | 1.000 | 0.000 | 0.237 | 0.347 | 0.010 | 0.010 |
| Parthenium hysterophorus | 0.167 | 1.000 | 0.000 | 0.064 | 0.475 | 0.129 | 0.119 |
| Praxelis clematidea | 0.124 | 1.000 | 0.000 | 0.087 | 0.485 | 0.079 | 0.089 |
| Solidago canadensis | 0.805 | 1.000 | 0.000 | 0.039 | 0.129 | 0.069 | 0.050 |
| Symphyotrichum subulatum | 0.426 | 0.977 | 0.023 | 0.042 | 0.158 | 0.059 | 0.089 |
| Xanthium spinosum | 0.070 | 1.000 | 0.000 | 0.056 | 0.416 | 0.089 | 0.109 |
In this study, we conducted a comparative analysis of the phylogenetic distance between alien and native Asteraceae plants. We found that invasive species exhibited a smaller phylogenetic distance from native plants compared to non-invasive species. This suggests that invasive species often share a closer phylogenetic relationship with native plants. Our findings align with the premise of Darwin’s preadaptation hypothesis and are consistent with results reported by previous studies at broader spatial scales (Duncan and Williams, 2002; Diez et al., 2009; Escobedo et al., 2011; Ferreira et al., 2012; Park et al., 2020; Qian et al., 2022; Qian and Sandel, 2022; Qian, 2023).
Darwin’s preadaptation hypothesis posits that alien plants closely related to native species may possess preexisting adaptations to the environment (Park and Potter, 2015). However, by calculating the niche overlap between alien species and native plants, we found that although the niche overlap between naturalized species was slightly stronger than that between other types of alien species and native Asteraceae plants, the overall niche overlap between alien Asteraceae plants and native plants was generally low (Table 1). This result indicates that alien Asteraceae plants do not show a significant pre-adaptation tendency to China’s climatic environment. Compared to species in the introduction stage and invasive species, naturalized species may show stronger environmental adaptability due to a longer period of gradual ecological adjustment (Nota et al., 2024). In contrast, invasive species are more likely to become successful invaders because of their ability to rapidly adapt to new environments, enabling swift population expansion without requiring prolonged adaptation (Mounger et al., 2021). Many species in the introduction stage, often cultivated, may still be in the process of adapting (Lin et al., 2022).
Darwin’s linkage of kinship between alien and native plants to invasion success implicitly suggests that closely related species share more similar niches (Thuiller et al., 2010). Park and Potter’s (2015) investigation into alien plants of the thistle tribe in California revealed a significant correlation between niche distance and evolutionary distance of alien plants. They argued that this correlation could facilitate the pre-adaptation of alien species to the introduced environment, thereby increasing the likelihood of successful invasion. Phylogenetic niche conservatism assumes that closely related species exhibit higher niche similarity than distantly related ones (Losos, 2008; Holt, 2009). As niches reflect species' biological interactions and available habitats (Soberón, 2007), niche similarity between species can be assessed by measuring overlap in environmental variables related to geographical distribution (Broennimann et al., 2012). However, our correlation tests between niche overlap and phylogenetic distance among 509 exotic and native Asteraceae plants produced disparate results. Specifically, we found no correlation between niche overlap and phylogenetic distance in Asteraceae. Previous research found that neither the niches of the two sister species of Prosopis L. (Oliveira et al., 2018), nor the differences between the two subspecies of olive (Olea europaea subsp. europaea and O. europaea subsp. cuspidata), reflected characteristics of phylogenetic niche conservatism (Cornuault et al., 2015). Similarly, studies at a larger geographic scale with a greater number of closely related animal species have failed to uncover evidence supporting phylogenetic niche conservatism (Warren et al., 2008; Peixoto et al., 2017; Calixto-Rojas et al., 2021). These findings suggest that the premise of Darwin's naturalization conundrum, namely, the universality of phylogenetic niche conservatism, may not hold true.
The niche conservatism of invasive species, reflected by the stability of their niche during the invasion process, is crucial for predicting the likelihood and risk of invasion (Thuiller et al., 2005; Aravind et al., 2022; Petrosyan et al., 2023). Our analysis compared the niches of 17 aggressive Asteraceae invasive plants in their native habitats and in China, revealing stability in their niches during the invasion process. However, disparities exist between the niches of these invasive plants in China and their native environments, mainly due to niche unfilling, which refers to suitable environmental conditions in the native range that remain unoccupied or unused in the invaded range (Table 3). Recent studies have shown that niche unfilling is more widespread than niche expansion among exotic plants worldwide (Liu et al., 2023b). Additionally, for many aggressive invasive plants, their invasion niche in China represents a subset of their native niche. This indicates that these species have not yet reached equilibrium with the environment in their introduced range, meaning they have not fully occupied all suitable habitats (Guisan et al., 2014; Zhang et al., 2021; Li et al., 2024). It also suggests that some regions in China, which are at risk of invasion by these aggressive invasive plants, have not yet been invaded. This imbalance between invasive species and their introduced range is driven by multiple factors, including dispersal barriers, biotic resistance (e.g., competition, predation, and pathogens), and environmental constraints (Wilson et al., 2007), which collectively prevent the invaders from achieving their potential regional equilibrium niche (Gallien et al., 2012). Notably, among these species, S. subulatum, B. frondosa, and E. canadensis experienced minimal niche expansion (S5, S6 and S10). This limited expansion primarily occurred along the negative side of PC1 axis, which is associated with the aridity index (Ai), rather than the positive side linked to annual precipitation and precipitation in the driest quarter (Bio12 and Bio17). This pattern suggests that these species have shifted toward habitats with lower water availability in their invasive ranges. Populations of these invasive plants in China exhibit enhanced drought tolerance compared to their native populations, reflecting an improved capacity to exploit drier environments and indicating the potential for further expansion into arid or semi-arid regions. Numerous studies have demonstrated niche differentiation among species during the invasion process (Tingley et al., 2014; Callen and Miller, 2015; Atwater et al., 2018; Oliveira et al., 2018; Zhang et al., 2021; Aravind et al., 2022; Santamarina et al., 2023; Song et al., 2023; Li et al., 2024). Although many studies have highlighted changes in species’ niches upon entering new geographical ranges, the underlying causes of these changes remain unclear. Only a handful of studies have explored the drivers of niche alterations. Potential factors contributing to niche shifts include rapid species adaptation within introduced ranges (Gioria et al., 2023), evolutionary shifts in niches (Manzoor et al., 2020), unfilled suitable habitats in native regions (Perret et al., 2019), selection of predictor variables (Callen and Miller, 2015; Regos et al., 2019; Lo Parrino et al., 2023), and variations in climate conditions within introduced ranges (Aravind et al., 2022; Song et al., 2023). These studies underscore the complexity of niche shift causation.
The findings of this study indicate that the niches of these 17 aggressive invasive plants remained stable during their invasion into China. However, this does not necessarily imply full support for the hypothesis of niche conservatism. For instance, Broennimann et al. (2014) investigated the spatiotemporal dynamics of the niche of Centaurea stoebe in North America along its invasion routes, revealing contrasting patterns. In eastern North America, the invasive niche of C. stoebe gradually expanded over time until reaching a limit akin to its native niche. In contrast, in western North America, the niche initially remained static post-invasion, followed by sudden expansion under new climate conditions. Similarly, studies have demonstrated that ragweed (Ambrosia artemisiifolia) exhibits exceptionally high niche stability in Europe, yet its niche has undergone significant expansion in South America (Song et al., 2023). Furthermore, studies have shown that after entering suitable environments, invasive plants go through a dormant phase before becoming aggressive (Robeck et al., 2024). The existence of this phase makes accurate estimation of their niche more difficult. In summary, the niche dynamics of invasive species during the invasion process are multifaceted. Therefore, investigating niche dynamics during biological invasion processes is crucial, as the use of niche models to predict future invasions relies on the assumption of niche conservatism.
This study primarily utilized niche overlap to assess differences between alien and native plants, elucidating key ecological mechanisms underlying the successful invasion of Asteraceae. However, the study has some inherent limitations. Firstly, it focused exclusively on Asteraceae, and its findings may not be directly applicable to other plant or animal groups. Secondly, China’s unique geographical and climatic conditions may exert region-specific screening effects on alien plants, potentially limiting the generalizability of the results to other regions (Qin et al., 2024). Although this study demonstrated that phylogenetic niche conservatism is not universal, the potential drivers and timing of niche differentiation remain unresolved. Additionally, the successful invasion of alien plants is often driven by the interaction of complex ecological processes (Gioria et al., 2023). This study primarily examined mechanisms at the niche and phylogenetic levels, potentially overlooking other significant factors such as reproductive strategies (Mazzolari et al., 2017), disturbance dynamics (Davis et al., 2000; Liu et al., 2023a), and human activities (Hao and Ma, 2023; Yang et al., 2023b). Future research should address these gaps by integrating niche indicators with phenotypic data to better understand the relationship between ecological and morphological differentiation, ecological variability, and reproductive isolation (Sherpa and Després, 2021). Expanding the scope of research to encompass a wider range of taxa, geographical regions, and additional ecological and environmental drivers will further elucidate the complex dynamics of alien plant invasions, providing more robust theoretical insights for effective management and conservation strategies.
5. ConclusionsPredicting the invasiveness of both alien species and indigenous communities has long been a central and challenging issue in invasion ecology. The findings of this study suggest that the underlying assumption of the Darwinian naturalization conundrum, namely the universality of phylogenetic niche conservatism, does not hold. Alien species are more likely to invade successfully when they are more closely related to native plants, exhibit less niche overlap, and maintain conservative niches during the invasion process than when they are more distantly related to native plants. Although this study revealed important ecological laws in the invasion mechanism of Asteraceae plants, its results may be limited by the study objects and geographical scope, and need to be further verified in other groups and regions. Future research should combine more ecological and environmental factors to fully reveal the complex mechanisms of alien plant invasion and provide theoretical support for management and protection strategies.
AcknowledgementsWe thank Xiao-Yan Chen, Ying-Bo Yang, Wen-Gang Zhang, and Rui-Ling Liu who helped to collect the materials used for the experiments. This study was supported by the National Natural Science Foundation of China (32271584 and 31600445), the Natural Science Basic Research Plan in Shaanxi Province of China (2020JM-286), the Fundamental Research Funds for the Central Universities (GK202103072 and GK202103073), the Key Research and Development Program of Shaanxi Province (2025SF-YBXM-514) and Special Research Project in Philosophy and Social Sciences of Shaanxi Province (2022HZ1795).
CRediT authorship contribution statement
Xing-Jiang Song: Writing – original draft, Visualization, Methodology. Gang Liu: Writing – review & editing, Methodology, Conceptualization. Xin-Di Li: Visualization, Investigation. Yu Chen: Visualization, Investigation. Jia Wang: Visualization, Investigation. Chun-Ling Zhang: Visualization, Investigation. Xin-Ping Ye: Writing – review & editing. Zhi-Hong Zhu: Writing – review & editing.
Consent for publication
All authors have read and approved the paper. The manuscript has not been published previously, nor is it being considered by any other peer-reviewed journal.
Data availability statement
Plant inventory data are available on the eflora website (http://www.efloras.org/florataxon.aspx?flora_id=2on_id=10074) and in Appendix A.
Annual Mean Temperature (Bio1), Temperature Seasonality (Bio4), Annual Precipitation (Bio12), Precipitation Seasonality (Bio15), Precipitation of Driest Quarter (Bio17), and Elevation (Alt) data were sourced from the WorldClim data center (https://www.worldclim.org/data/worldclim21.html).
The aridity index (Ai) originated from the CHELSA data center (https://chelsa-climate.org/downloads/).
Plant occurrence data were from the Global Biodiversity Information Facility (https://doi.org/10.15468/dl.73ap5k).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2025.02.005.
Aravind, N.A., Shaanker, M.U., Bhat, H.N.P., et al., 2022. Niche shift in invasive species: is it a case of “home away from home” or finding a “new home”?. Biodivers. Conserv., 31: 2625-2638. DOI:10.1007/s10531-022-02447-0 |
Atwater, D.Z., Ervine, C., Barney, J.N., 2018. Climatic niche shifts are common in introduced plants. Nat. Ecol. Evol., 2: 34-43. |
Banerjee, A.K., Tan, F.X., Feng, H., et al., 2023. Invasive alien plants are phylogenetically distinct from other alien species across spatial and taxonomic scales in China. Front. Plant Sci., 14: 1075344. DOI:10.3389/fpls.2023.1075344 |
Barve, N., Barve, V., Jimenez-Valverde, A., et al., 2011. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model., 222: 1810-1819. DOI:10.1016/j.ecolmodel.2011.02.011 |
Beaumont, L.J., Gallagher, R.V., Thuiller, W., et al., 2009. Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers. Distrib., 15: 409-420. DOI:10.1111/j.1472-4642.2008.00547.x |
Blackburn, T.M., Pyšek, P., Bacher, S., et al., 2011. A proposed unified framework for biological invasions. Trends Ecol. Evol., 26: 333-339. DOI:10.1016/j.tree.2011.03.023 |
Broennimann, O., Fitzpatrick, M.C., Pearman, P.B., et al., 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecol. Biogeogr., 21: 481-497. DOI:10.1111/j.1466-8238.2011.00698.x |
Broennimann, O., Mráz, P., Petitpierre, B., et al., 2014. Contrasting spatio-temporal climatic niche dynamics during the eastern and western invasions of spotted knapweed in North America. J. Biogeogr., 41: 1126-1136. DOI:10.1111/jbi.12274 |
Broennimann, O., Treier, U.A., Müller-Schärer, H., et al., 2007. Evidence of climatic niche shift during biological invasion. Ecol. Lett., 10: 701-709. DOI:10.1111/j.1461-0248.2007.01060.x |
Calixto-Rojas, M., Lira-Noriega, A., Rubio-Godoy, M., et al., 2021. Phylogenetic relationships and ecological niche conservatism in killifish (Profundulidae) in Mesoamerica. J. Fish. Biol., 99: 396-410. DOI:10.1111/jfb.14727 |
Callen, S.T., Miller, A.J., 2015. Signatures of niche conservatism and niche shift in the North American kudzu (Pueraria montana) invasion. Divers. Distrib., 21: 853-863. DOI:10.1111/ddi.12341 |
Chamberlain, S., 2017. Rgbif: interface to the global biodiversity information facility API. R package version 0.9.8. https://CRAN.R-project.org/package=rgbif.
|
Cooper, N., Freckleton, R.P., Jetz, W., 2011. Phylogenetic conservatism of environmental niches in mammals. Proc. R. Soc. B-Biol. Sci., 278: 2384-2391. DOI:10.1098/rspb.2010.2207 |
Cornuault, J., Khimoun, A., Cuneo, P., et al., 2015. Spatial segregation and realized niche shift during the parallel invasion of two olive subspecies in south-eastern Australia. J. Biogeogr., 42: 1930-1941. DOI:10.1111/jbi.12538 |
Crisp, M.D., Cook, L.G., 2012. Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes?. New Phytol., 196: 681-694. DOI:10.1111/j.1469-8137.2012.04298.x |
Crowl, T.A., Crist, T.O., Parmenter, R.R., et al., 2008. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ., 6: 238-246. DOI:10.1890/070151 |
Daehler, C.C., 2001. Darwin's naturalization hypothesis revisited. Am. Nat., 158: 324-330. DOI:10.1086/321316 |
Davis, M.A., Grime, J.P., Thompson, K., 2000. Fluctuating resources in plant communities: a general theory of invisibility. J. Ecol., 88: 528-534. DOI:10.1046/j.1365-2745.2000.00473.x |
Di Cola, V., Broennimann, O., Petitpierre, B., et al., 2017. Ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography, 40: 774-787. DOI:10.1111/ecog.02671 |
Diez, J.M., Sullivan, J.J., Hulme, P.E., et al., 2008. Darwin's naturalization conundrum: dissecting taxonomic patterns of species invasions. Ecol. Lett., 11: 674-681. DOI:10.1111/j.1461-0248.2008.01178.x |
Diez, J.M., Williams, P.A., Randall, R.P., et al., 2009. Learning from failures: testing broad taxonomic hypotheses about plant naturalization. Ecol. Lett., 12: 1174-1183. DOI:10.1111/j.1461-0248.2009.01376.x |
Diniz, J.A.F., Soares, T.N., Lima, J.S., et al., 2013. Mantel test in population genetics. Genet. Mol. Biol., 36: 475-485. DOI:10.1590/S1415-47572013000400002 |
Dray, S., Dufour, A.-B., 2007. The ade4 package: implementing the duality diagram for ecologists. J. Stat. Softw., 22: 1-20. |
Duncan, R.P., Williams, P.A., 2002. Darwin's naturalization hypothesis challenged. Nature, 417: 608-609. DOI:10.1038/417608a |
Escobedo, V.M., Aranda, J.E., Castro, S.A., 2011. Hipótesis de Naturalización de Darwin evaluada en la flora exótica de Chile continental. Rev. Chil. Hist. Nat., 84: 543-552. DOI:10.4067/S0716-078X2011000400007 |
Fan, S.Y., Yang, Q., Li, S.P., et al., 2023. A latitudinal gradient in Darwin's naturalization conundrum at the global scale for flowering plants. Nat. Commun., 14: 6244. |
Ferreira, R.B., Beard, K.H., Peterson, S.L., et al., 2012. Establishment of introduced reptiles increases with the presence and richness of native congeners. Amphibia-Reptilia, 33: 387-392. DOI:10.1163/15685381-00002841 |
Fick, S.E., Hijmans, R.J., 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol., 37: 4302-4315. DOI:10.1002/joc.5086 |
Flora of China Editorial Committee, 2011. Asteraceae. Flora of China, vols.. Science Press, Beijing, pp. 20-21.
|
Gallien, L., Douzet, R., Pratte, S., et al., 2012. Invasive species distribution models–how violating the equilibrium assumption can create new insights. Global Ecol. Biogeogr., 21: 1126-1136. DOI:10.1111/j.1466-8238.2012.00768.x |
GBIF, 2022. Global biodiversity information facility (GBIF) (26 October 2022) GBIF occurrence download. https://doi.org/10.15468/dl.73ap5k.
|
Gioria, M., Hulme, P.E., Richardson, D.M., et al., 2023. Why are invasive plants successful?. Annu. Rev. Plant Biol., 74: 635-670. DOI:10.1146/annurev-arplant-070522-071021 |
Guidetti, R., Altiero, T., Rebecchi, L., 2011. On dormancy strategies in tardigrades. J. Insect Physiol., 57: 567-576. |
Guisan, A., Petitpierre, B., Broennimann, O., et al., 2014. Unifying niche shift studies: insights from biological invasions. Trends Ecol. Evol., 29: 260-269. |
Hao, Q., Ma, J.S., 2023. Invasive alien plants in China: an update. Plant Divers., 45: 117-121. |
Holt, R.D., 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. U.S.A., 106: 19659-19665. DOI:10.1073/pnas.0905137106 |
Jin, Y., Qian, H., 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography, 42: 1353-1359. DOI:10.1111/ecog.04434 |
Jin, Y., Qian, H., 2022. V.PhyloMaker2: an updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers., 44: 335-339. |
Jin, Y., Qian, H., 2023. U.PhyloMaker: an R package that can generate large phylogenetic trees for plants and animals. Plant Divers., 45: 347-352. |
Karger, D.N., Conrad, O., Böhner, J., et al., 2017. Climatologies at high resolution for the earth's land surface areas. Sci. Data, 4: 1-20. DOI:10.1080/1369118X.2017.1418016 |
Lami, F., Vitti, S., Marini, L., et al., 2021. Habitat type and community age as barriers to alien plant invasions in coastal species-habitat networks. Ecol. Indic., 133: 108450. |
Legendre, P., Fortin, M.J., 2010. Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol. Ecol. Resour, 10: 831-844. DOI:10.1111/j.1755-0998.2010.02866.x |
Li, S.P., Cadotte, M.W., Meiners, S.J., et al., 2015. The effects of phylogenetic relatedness on invasion success and impact: deconstructing Darwin's naturalisation conundrum. Ecol. Lett., 18: 1285-1292. DOI:10.1111/ele.12522 |
Li, X.D., Chen, Y., Zhang, C.L., et al., 2024. Assessing the climatic niche changes and global invasion risk of Solanum elaeagnifolium in relation to human activities. Sci. Total Environ., 954: 176723. |
Lin, Q.W., Xiao, C., Ma, J.S., 2022. A dataset on catalogue of alien plants in China. Biodivers. Sci., 30: 22127. DOI:10.17520/biods.2022127 |
Liu, C.L., Wolter, C., Xian, W.W., et al., 2020. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. U.S.A., 117: 23643-23651. DOI:10.1073/pnas.2004289117 |
Liu, D.J., Semenchuk, P., Essl, F., et al., 2023a. The impact of land use on non-native species incidence and number in local assemblages worldwide. Nat. Commun., 14: 2090. |
Liu, Y.P., Heberling, J.M., Wang, Z.H., et al., 2023b. Niche unfilling dominates the naturalization of species from intercontinentally disjunct genera. Global Ecol. Biogeogr., 32: 1977-1990. DOI:10.1111/geb.13750 |
Lo Parrino, E., Falaschi, M., Manenti, R., et al., 2023. All that changes is not shift: methodological choices influence niche shift detection in freshwater invasive species. Ecography, 2023: e06432. |
Losos, J.B., 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett., 11: 995-1003. DOI:10.1111/j.1461-0248.2008.01229.x |
Mack, R.N., Lonsdale, W.M., 2001. Humans as global plant dispersers: Getting more than we bargained for. BioScience, 51: 95-102. |
Manzoor, S.A., Griffiths, G., Obiakara, M.C., et al., 2020. Evidence of ecological niche shift in Rhododendron ponticum (L.) in Britain: hybridization as a possible cause of rapid niche expansion. Ecol. Evol., 10: 2040-2050. DOI:10.1002/ece3.6036 |
Mazel, F., Davies, T.J., Gallien, L., et al., 2016. Influence of tree shape and evolutionary time-scale on phylogenetic diversity metrics. Ecography, 39: 913-920. DOI:10.1111/ecog.01694 |
Mazzolari, A.C., Marrero, H.J., Vázquez, D.P., 2017. Potential contribution to the invasion process of different reproductive strategies of two invasive roses. Biol. Invasions, 19: 615-623. DOI:10.1007/s10530-016-1315-y |
Mounger, J., Ainouche, M.L., Bossdorf, O., et al., 2021. Epigenetics and the success of invasive plants. Phil. Trans. Biol. Sci., 376: 20200117. DOI:10.1098/rstb.2020.0117 |
Nota, A., Bertolino, S., Tiralongo, F., et al., 2024. Adaptation to bioinvasions: when does it occur?. Glob. Change Biol., 30: e17362. |
Oliveira, B.F., Costa, G.C., Fonseca, C.R., 2018. Niche dynamics of two cryptic Prosopis invading South American drylands. Biol. Invasions, 20: 181-194. |
Park, D.S., Feng, X., Maitner, B.S., et al., 2020. Darwin's naturalization conundrum can be explained by spatial scale. Proc. Natl. Acad. Sci. U.S.A., 117: 10904-10910. DOI:10.1073/pnas.1918100117 |
Park, D.S., Potter, D., 2015. Why close relatives make bad neighbours: phylogenetic conservatism in niche preferences and dispersal disproves Darwin's naturalization hypothesis in the thistle tribe. Mol. Ecol., 24: 3181-3193. DOI:10.1111/mec.13227 |
Peixoto, F.P., Villalobos, F., Cianciaruso, M.V., 2017. Phylogenetic conservatism of climatic niche in bats. Global Ecol. Biogeogr., 26: 1055-1065. DOI:10.1111/geb.12618 |
Perret, D.L., Leslie, A.B., Sax, D.F., 2019. Naturalized distributions show that climatic disequilibrium is structured by niche size in pines (Pinus L.). Global Ecol. Biogeogr., 28: 429-441. DOI:10.1111/geb.12862 |
Peterson, A.T., 2011. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr., 38: 817-827. DOI:10.1111/j.1365-2699.2010.02456.x |
Peterson, A.T., Vieglais, D.A., 2001. Predicting species invasions using ecological niche modeling: new approaches from bioinformatics attack a pressing problem: a new approach to ecological niche modeling, based on new tools drawn from biodiversity informatics, is applied to the challenge of predicting potential species’ invasions. BioScience, 51: 363-371. |
Petitpierre, B., Kueffer, C., Broennimann, O., et al., 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science, 335: 1344-1348. DOI:10.1126/science.1215933 |
Petrosyan, V.G., Osipov, F.A., Feneva, I.Y., et al., 2023. Ecological niches modelling of the top-100 most dangerous invasive species in Russia: testing the hypothesis of ecological niche conservatism. Biol. Bull., 50: S63-S84. DOI:10.1134/s106235902360126x |
Pyšek, P., Hulme, P.E., Simberloff, D., et al., 2020. Scientists' warning on invasive alien species. Biol. Rev., 95: 1511-1534. DOI:10.1111/brv.12627 |
Qian, H., 2023. Patterns of phylogenetic relatedness of non-native plants across the introduction–naturalization-invasion continuum in China. Plant Divers., 45: 169-176. |
Qian, H., Jin, Y., 2021. Are phylogenies resolved at the genus level appropriate for studies on phylogenetic structure of species assemblages?. Plant Divers., 43: 255-263. |
Qian, H., Qian, S.H., Sandel, B., 2022. Phylogenetic structure of alien and native species in regional plant assemblages across China: testing niche conservatism hypothesis versus niche convergence hypothesis. Global Ecol. Biogeogr., 31: 1864-1876. DOI:10.1111/geb.13566 |
Qian, H., Sandel, B., 2017. Phylogenetic relatedness of native and exotic plants along climate gradients in California, USA. Divers. Distrib., 23: 1323-1333. DOI:10.1111/ddi.12620 |
Qian, H., Sandel, B., 2022. Darwin's preadaptation hypothesis and the phylogenetic structure of native and alien regional plant assemblages across North America. Global Ecol. Biogeogr., 31: 531-545. DOI:10.1111/geb.13445 |
Qian, H., Zhang, J., 2024. Phylogenetic diversity and dispersion of angiosperms in plant communities along an elevational gradient in the western United States. J. Biogeogr., 52: 495-504. DOI:10.3390/molecules29020495 |
Qin, F., Han, B.C., Bussmann, R.W., et al., 2024. Present status, future trends, and control strategies of invasive alien plants in China affected by human activities and climate change. Ecography, 2024: e06919. |
Regos, A., Gagne, L., Alcaraz-Segura, D., et al., 2019. Effects of species traits and environmental predictors on performance and transferability of ecological niche models. Sci. Rep., 9: 1-14. |
Rejmánek, M., 1996. A theory of seed plant invasiveness: the first sketch. Biol. Conserv., 78: 171-181. |
Ricciardi, A., Hoopes, M.F., Marchetti, M.P., et al., 2013. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr., 83: 263-282. DOI:10.1890/13-0183.1 |
Richardson, D.M., Pyšek, P., Rejmánek, M., et al., 2000. Naturalization and invasion of alien plants: concepts and definitions. Divers. Distrib., 6: 93-107. |
Robeck, P., Essl, F., van Kleunen, M., et al., 2024. Invading plants remain undetected in a lag phase while they explore suitable climates. Nat. Ecol. Evol., 8: 477-488. DOI:10.1038/s41559-023-02313-4 |
Rohr, R.P., Scherer, H., Kehrli, P., et al., 2010. Modeling food webs: exploring unexplained structure using latent traits. Am. Nat., 176: 170-177. DOI:10.1086/653667 |
Santamarina, S., Mateo, R.G., Alfaro-Saiz, E., et al., 2023. On the importance of invasive species niche dynamics in plant conservation management at large and local scale. Front. Ecol. Evol., 10: 1049142. |
Santos, J.C., Cannatella, D.C., 2011. Phenotypic integration emerges from aposematism and scale in poison frogs. Proc. Natl. Acad. Sci. U.S.A., 108: 6175-6180. DOI:10.1073/pnas.1010952108 |
Schoener, T.W., 1970. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology, 51: 408-418. DOI:10.2307/1935376 |
Sherpa, S., Després, L., 2021. The evolutionary dynamics of biological invasions: a multi-approach perspective. Evol. Appl., 14: 1463-1484. DOI:10.1111/eva.13215 |
Smith, S.A., Brown, J.W., 2018. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot., 105: 302-314. DOI:10.1002/ajb2.1019 |
Soberón, J., 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett., 10: 1115-1123. DOI:10.1111/j.1461-0248.2007.01107.x |
Song, X.J., Liu, G., Qian, Z.Q., et al., 2023. Niche filling dynamics of ragweed (Ambrosia artemisiifolia L.) during global invasion. Plants, 12: 1313. DOI:10.3390/plants12061313 |
Thuiller, W., Gallien, L., Boulangeat, I., et al., 2010. Resolving Darwin's naturalization conundrum: a quest for evidence. Divers. Distrib., 16: 461-475. DOI:10.1111/j.1472-4642.2010.00645.x |
Thuiller, W., Richardson, D.M., Pyšek, P., et al., 2005. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob. Change Biol., 11: 2234-2250. DOI:10.1111/j.1365-2486.2005.001018.x |
Tingley, R., Vallinoto, M., Sequeira, F., et al., 2014. Realized niche shift during a global biological invasion. Proc. Natl. Acad. Sci. U.S.A., 111: 10233-10238. DOI:10.1073/pnas.1405766111 |
Tsirogiannis, C., Sandel, B., 2016. PhyloMeasures: a package for computing phylogenetic biodiversity measures and their statistical moments. Ecography, 39: 709-714. DOI:10.1111/ecog.01814 |
Václavík, T., Meentemeyer, R.K., 2012. Equilibrium or not? Modelling potential distribution of invasive species in different stages of invasion. Divers. Distrib., 18: 73-83. DOI:10.1111/j.1472-4642.2011.00854.x |
Wang, J., Li, S.P., Ge, Y., et al., 2023. Darwin's naturalization conundrum reconciled by changes of species interactions. Ecology, 104: e3850. |
Warren, D.L., Glor, R.E., Turelli, M., 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution, 62: 2868-2883. DOI:10.1111/j.1558-5646.2008.00482.x |
Weber, E., Sun, S.G., Li, B., 2008. Invasive alien plants in China: diversity and ecological insights. Biol. Invasions, 10: 1411-1429. DOI:10.1007/s10530-008-9216-3 |
Wiens, J.J., Graham, C.H., 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst., 36: 519-539. DOI:10.1146/annurev.ecolsys.36.102803.095431 |
Williamson, M., 2006. Explaining and predicting the success of invading species at different stages of invasion. Biol. Invasions, 8: 1561-1568. DOI:10.1007/s10530-005-5849-7 |
Wilson, J.R.U., Richardson, D.M., Rouget, M., et al., 2007. Residence time and potential range: crucial considerations in modelling plant invasions. Divers. Distrib., 13: 11-22. DOI:10.1111/j.1366-9516.2006.00302.x |
Xu, M., Li, S.P., Dick, J.T.A., et al., 2022. Exotic fishes that are phylogenetically close but functionally distant to native fishes are more likely to establish. Glob. Change Biol., 28: 5683-5694. DOI:10.1111/gcb.16360 |
Yang, W.J., Sun, S.X., Wang, N.X., et al., 2023a. Dynamics of the distribution of invasive alien plants (Asteraceae) in China under climate change. Sci. Total Environ., 903: 166260. |
Yang, Y.B., Bian, Z.H., Ren, W.J., et al., 2023b. Spatial patterns and hotspots of plant invasion in China. Global Ecol. Conserv., 43: e02424. |
Zanne, A.E., Tank, D.C., Cornwell, W.K., et al., 2014. Three keys to the radiation of angiosperms into freezing environments. Nature, 506: 89-92. DOI:10.1038/nature12872 |
Zhang, J., Qian, H., 2023. U.Taxonstand: an R package for standardizing scientific names of plants and animals. Plant Divers., 45: 1-5. |
Zhang, W.G., Chen, X.Y., Liu, R.L., et al., 2021. Realized niche shift associated with Galinsoga quadriradiata (Asteraceae) invasion in China. J. Plant Ecol., 15: 538-548. |
Zizka, A., Silvestro, D., Andermann, T., et al., 2019. CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol., 10: 744-751. DOI:10.1111/2041-210x.13152 |



