b. School of Ecological and Environmental Sciences, East China Normal University, Shanghai 200241, China;
c. College of Forestry, Jiangxi Agricultural University, Nanchang 330045, China;
d. Administration of Guanshan National Nature Reserve, Yichun 336300, China;
e. State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, China
Understanding elevational patterns of montane plant diversity and its underlying mechanisms has been a long-standing topic in ecology and biogeography (Körner, 2007). Due to its dramatic environmental changes and extremely rich species diversity, mountains provide an ideal platform to identify the ecological and evolutionary mechanisms of community assembly, and uncover biodiversity changes under the context of global change (Sundqvist et al., 2013).
The crucial role of environment filtering in shaping mountain plant diversity has been documented in many studies (Nottingham et al., 2018; Hamid et al., 2021; Zhang et al., 2021). Temperature has been well documented as a primary driver to limit the growth and reproduction processes of plants (Nottingham et al., 2018). Along elevational gradients in mountains, species’ adaptation to minimum temperature has been the key in limiting species range (Hawkins et al., 2014). Cold tolerance has been reported as the dominant factor for species richness patterns of tropical affinities. In contrast, temperate-affiliated clades are adapted to cool climate and hence may not be strongly limited by winter coldness. For example, Peters et al. (2016) reported that minimum temperature was the main predictor of plant richness in Mt. Kilimanjaro in Africa. However, in the Austrian part of the Alps, Moser et al. (2005) found that high temperature largely determined species diversity, even though low temperature handicapped plant life. These conflicting results suggest that plant diversity patterns along environmental gradients might be shaped by the combined effects of multiple current and historical drivers.
Recent advances in community ecology and macroecology have emphasized niche conservatism mechanism, the tendency of species to retain ancestral ecological traits over evolutionary time, as a key process shaping biodiversity patterns across environmental gradients (Wiens and Donoghue, 2004). Two related hypotheses underlie this framework are useful to explain elevational patterns of plant diversity: biogeographic affinity hypothesis (Harrison and Grace, 2007) and phylogenetic niche conservatism (Wiens and Graham, 2005). The biogeographic affinity hypothesis proposes that temperate-affiliated clades predominate in high-elevation and high-latitude regions due to their evolutionary adaptations to cold conditions, including frost tolerance and deciduous traits, while tropical clades are excluded from these areas because their thermally conservative niches prevent adaptation to colder environments. This hypothesis is one important factor influencing plant diversity and community structure along elevational gradients (González-Caro et al., 2020). Interacted with elevation, biogeographical affinity may produce different patterns of taxonomic diversity via modulating the effects of climatic factors. During the Paleocene (65–55 Mya), the Earth began cooling, and many lineages chose either evolved necessary traits like needle-shaped leaves or deciduousness to adapt temperate zones (Cavender-Bares et al., 2016). Since species have the tendency to maintain ancestral traits, species originated from temperate zones (refer to temperate-affiliated species) have the ability to resist cold environment at higher elevations, but tropical-affiliated species might be prevented by coldness (Li and Feng, 2015). Therefore temperate-affiliated species also show higher fitness in cold areas and lead to low species richness and phylogenetic clustering at high elevations due to habitat filtering (Peters et al., 2016; Nottingham et al., 2018), while tropical-affiliated species may be suited to warmer environment at lower elevations, and the decreasing thermal energy with elevation may filter out tropical genera (Li and Feng, 2015).
The hypothesis of phylogenetic niche conservatism states that environmental filtering leads to coexisting species with similar physiological tolerances, resulting in phylogenetic clustering, while plant competition leads to a community with distantly related species, resulting in phylogenetic overdispersion (Wiens and Graham, 2005; Losos, 2008). Phylogenetic diversity and structure have been widely used in the studies of phylogenetic niche conservatism (Webb et al., 2002; Zhang et al., 2021). Phylogenetic diversity and structure can be inconsistent with taxonomic diversity or even show opposite trends (Culmsee and Leuschner, 2013). For example, the patterns of total species richness in response to elevational gradients on Mt. Taishan and Mt. Laoshan showed a monotonically decreasing trend, while the phylogenetic structure of plant communities on Mt. Laoshan had no significant pattern along elevations, but plant communities on Mt. Taishan exhibited an inverted hump-shaped pattern (Zhang et al., 2016). Therefore, the combination of community phylogenetic structure and biogeographic affinity of species provides insights into the ecological and evolutionary mechanisms underlying montane biodiversity patterns.
In this study, we selected a subtropical mountain in eastern China with typical transitional characteristics, including 49.65% plant genera are temperate-affiliated, and 46.45% genera are tropical-affiliated in this mountain (Liu and Wu 2005). Using woody plant data from 32 subtropical forest plots, we documented the elevational patterns of plant diversity and evaluated the contribution of biogeographic affinity, climate, and soil properties to plant community assembly. We proposed two following predictions to be tested. First, along the elevation, biogeographic affinity could shape plant species composition, leading to increased dominance of temperate-affiliated species. Second, biogeographic affinity constrains the species pool at each elevation belt, while environmental filtering, particularly winter minimum temperature, selects for species persistence. As cold tolerance is phylogenetically conserved, we expect that increasing phylogenetic clustering toward higher elevations will be dominated by temperate-affiliated species.
2. Materials and methods 2.1. Study areaThis study was conducted along an elevational gradient in Mt. Guanshan, which was located in the Guanshan National Nature Reserve in Jiangxi Province, eastern China (28°30′–28°40′N, 114°29′–114°45′E). This reserve belongs to the WWF Global 200 priority ecoregions. The region belongs to the middle subtropical warm and humid climate zone and is significantly influenced by the monsoon. Mean annual temperature is 16.2 ℃, ranging from 26.1 ℃ in summers and below 0 ℃ in winters, and mean annual precipitation is about 1950–2100 mm, as well as the average relative humidity is 85% (Liu and Wu 2005; Wang et al., 2023). Along the elevations, the vegetation types are evergreen broad-leaved forest (< 400 m), evergreen and deciduous broad-leaved mixed forest (400–800 m), deciduous broad-leaved and coniferous mixed forest (800–1100 m), and dwarf forest and shrub (1100–1480 m), respectively.
The region is located at the intersection of the flora of East China and Central China, and also at the intersection of the flora of South China and East China. Therefore, the flora has obvious transitional characteristics (Liu and Wu, 2005), and southern tropical flora and northern temperate flora converging in this region. This area is the northern boundary of many tropical-affiliated species in this province, such as Eurycorymbus cavaleriei and Amentotaxus argotaenia. Among 1915 seed plant species in the reserve, 357 genera (49.65%) are temperate-affiliated, and 334 genera (46.45%) are tropical-affiliated (Liu and Wu, 2005).
2.2. Woody plant surveyWe set up 32 20 m × 20 m permanent sampling plots along an elevational gradient from 300 m to 1360 m (Ma et al., 2022; Wang et al., 2023). Woody plant survey was conducted in 2016–2018. All woody stems with ≥1 cm DBH (diameter at breast height) were measured and identified to species. The Latin names of the species were standardized using the R package U.Taxonstand (Zhang and Qian, 2023; Zhang et al., 2025) based on the World Flora Online (WFO, 2024) and the Flora of China (Wu et al., 1994–2013). Totally, there were 7586 individuals recorded in these plots, belonging to 56 families, 118 genera, and 250 species. Considering huge differences in the evolutionary history of gymnosperms and angiosperms, seven gymnosperm species were excluded for the current analysis.
2.3. The measures of biogeographic affinitiesFollowing the approach of González-Caro et al. (2020) and Culmsee et al. (2010), we categorized each genus into four categories based on the areal-types of Chinese genera of seed plants by Wu (1991) and Wu et al and the geographical distributions of these species in China (Fang et al., 2011). These categories were defined as (1) tropical genera: distributed exclusively in tropical regions, (2) tropical-centered genera: most species occur in tropical regions, with a few extending to subtropical and temperate areas, (3) temperate genera: distributed exclusively in temperate regions, (4) temperate-centered genera: most species occur in temperate regions, with a few extending to (sub)tropical areas. In this study, we merged tropical genera and tropical-centered genera into a single tropical-affiliated category, and similarly combined temperate genera and temperate-centered genera into a temperate-affiliated category. Overall, there are 155 tropical-affiliated species from 82 genera, 44 families, dominated by Lauraceae, Aquifoliaceae, Pentaphylacaceae and Rubiaceae, and 95 temperate-affiliated species from 36 genera, 22 families, dominated by Rosaceae, Fagaceae, Adoxaceae and Sapindaceae (Appendix A).
Biogeographic indexes were used as surrogates for the overall biogeographic affinity of each community and two biogeographic indexes were calculated using the formulas by González-Caro et al. (2020): (1) Biogeographic number index (BNI):
Species richness (SR) and species evenness (SE) of different biogeographic affinities were calculated to quantify the taxonomic α diversity. To measure phylogenetic diversity and structure of plant communities, a species-level phylogenetic tree was constructed first using the R package ‘U.PhyloMaker’ (Jin and Qian, 2023). To reduce the effect of species richness on measuring phylogenetic diversity, we calculated standard effect size of phylogenetic diversity (sesPD), the net relatedness index (NRI) and the nearest taxon index (NTI). NRI reflects phylogenetic relatedness among taxa at both deep and shallow levels within a phylogenetic tree, while NTI measures phylogenetic relatedness among taxa at a shallower level descending from superficial nodes within the phylogenetic tree. A positive NRI or NTI value indicates phylogenetic clustering, while a negative NRI or NTI value indicates phylogenetic evenness or overdispersion.
To consider the effect of species abundance for biodiversity measures, we calculated species richness (SE), phylogenetic species evenness (PSE; Matthew et al., 2007), and NRIab and NTIab taking abundance into account. All the metrics were calculated using R package “picante” (Kembel et al., 2010).
2.5. Environmental variablesWe extracted the air temperature related variables from the CHELSA (Karger et al., 2021) and soil temperature related variables from the SoilTemp database (Lembrechts et al., 2022). Soil pH, total nitrogen (TN), total phosphorous (TP) of each plot were extracted from 90-m resolution National Soil Information Grids of China database (Liu et al., 2022). To evaluate the data quality of these variables from the public database, we analyzed the relations with our collected data for 12 of 32 plots with detailed field survey. We kept only the variables with strong correlations between observed and extracted variables. To reduce potential influence of multicollinearity among these variables, we finally selected minimum temperature of coldest month at soil (MTC), TP and the slope for further analyses.
2.6. Statistical analysesTo reduce the potential influence of spatial autocorrelation, spatial multiple linear regression models (SLM) were used to assess the relative importance of biogeographic affinities and environmental variables in determining plant taxonomic and phylogenetic diversity. For SLM, spatial simultaneous autoregressive error models were used, which allow the inclusion of residual spatial autocorrelation in data (Kissling and Carl, 2008). Akaike's information criterion (AIC) was calculated to select the optimal model by comparing all possible models. The relative importance of each variable was quantified by the summed AIC weights across all possible models (Zhang et al., 2013).
Structure equation models (SEMs) were built to simultaneously evaluate the effects of biogeographic affinities and environmental variables on each metric of plant diversity and phylogenetic structure. All variables were standardized to mean zero and variance one before the analyses. The SEMs were run in R package ‘piecewiseSEM’ (Lefcheck, 2016). All statistical analyses were conducted in R v.4.3.2 (R Core Team, 2023).
3. Results 3.1. Elevational patterns in taxonomic and phylogenetic diversityFor all plant species along elevations (Fig. 1a–d), there was no significant relationship for SR, while SE (R2 = 0.20, P < 0.05), sesPD (R2 = 0.16, P < 0.05) and PSE (R2 = 0.30, P < 0.01) decreased markedly. For species with different biogeographic affinities (Fig. 1), temperate-affiliated species richness had strongly positive trend along elevation (R2 = 0.23, P < 0.01), while negative relation for SE (R2 = 0.26, P < 0.01) and no significant trends for sesPD and PSE. For tropical-affiliated species, only sesPD showed a decreasing pattern along elevations (R2 = 0.16, P < 0.05).
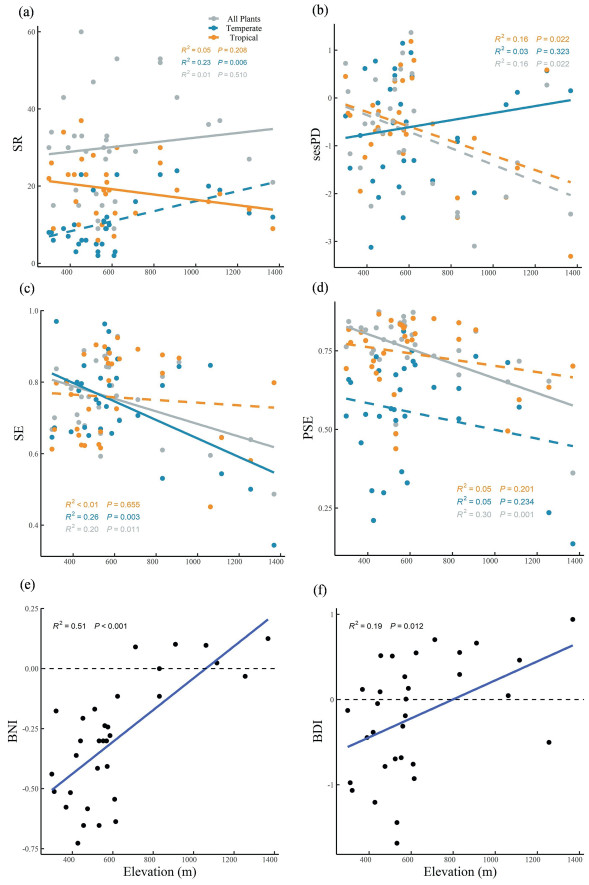
|
| Fig. 1 Elevational patterns of taxonomic and phylogenetic α diversity. (a) SR: species richness; (b) sesPD: standard effect size of phylogenetic diversity; (c) SE: species evenness; (d) PSE: phylogenetic species evenness; (e) BNI: biogeographic number index; (f) BDI: biogeographic diameter index. |
For two measures of biogeographic affinity (Fig. 1e and f), both BNI (R2 = 0.51, P < 0.001) and BDI (R2 = 0.19, P < 0.05) increased obviously along elevations. BNI and BDI were greater than zero when elevations above ~800 m, suggesting that temperate-affiliated species gradually dominated at higher elevations.
For phylogenetic structure (Fig. 2), phylogenetic clustering for both NRI (R2 = 0.12, P = 0.06) and NTI (R2 = 0.16, P < 0.05) along elevations were documented. When taking abundance into consideration, NRIab (R2 = 0.31, P < 0.001) showed strongly positive relationship with elevations, while NTIab had no pattern.
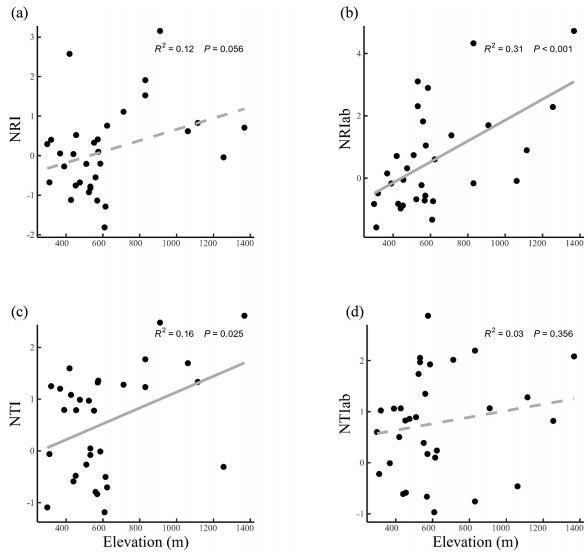
|
| Fig. 2 Elevational patterns of phylogenetic structure. (a) NRI: the net relatedness index; (b) NRIab: the abundance-weighted net relatedness index; (c) NTI: the nearest taxon index; (d) NTIab: the abundance-weighted nearest taxon index. |
The results of both linear models and SEM showed that biogeographic affinity and cold temperature majorly controlled the elevational patterns of taxonomic and phylogenetic diversity (Table 1 and Fig. 3). BNI or BDI were the most important factors for sesPD or SR. MTC was positively correlated with SR, SE and PSE, while slope and soil TP had no significant effect on nearly all diversity measures.
| Variables | SR | SE | PSE | sesPD | |||||||
| Coef. | sumW | Coef. | sumW | Coef. | sumW | Coef. | sumW | ||||
| BNI | 0.356 | 0.428 | 0.423 | −0.629*** | 0.856 | ||||||
| BDI | 0.738*** | 0.998 | 0.336 | 0.268 | 0.561 | 0.537 | |||||
| MTC | 0.223 | 0.614 | 0.458** | 0.962 | 0.684*** | 0.997 | 0.302 | ||||
| Slope | 0.274 | 0.276 | 0.316 | −0.308 | 0.657 | ||||||
| TP | 0.369 | 0.321 | 0.288 | 0.303 | |||||||
| R2 | 0.397 | 0.062 | 0.191 | 0.297 | |||||||
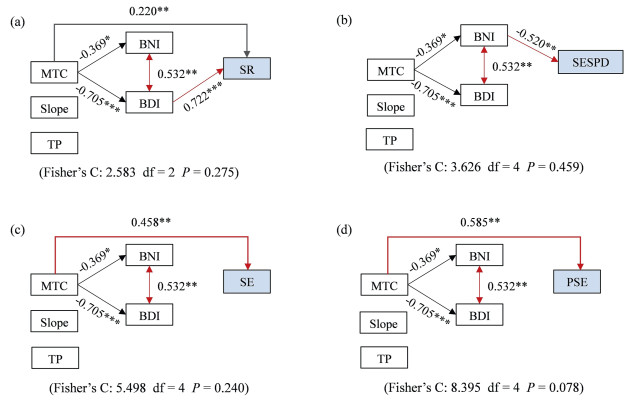
|
| Fig. 3 Structural equation models (SEM) showing the importance of biogeographic affinity and environmental factors on explaining taxonomic and phylogenetic diversity. (a) SR: species richness; (b) SE: species evenness; (c) sesPD: standard effect size of phylogenetic diversity; (d) PSE: phylogenetic species evenness. The standardized path coefficients are shown in the figures. Red and black dashed arrows represent positive and negative relations, respectively. Significance levels are *P < 0.05, **P < 0.01 and ***P < 0.001. |
For phylogenetic structure (Table 2 and Fig. 4), BNI had significantly positive effects on NRI and NTI, but not on NRIab and NTIab. MTC had direct effect only on NRIab, and indirect effect on NRI and NTI through negatively affecting BNI and BDI. The slope and TP had little direct or indirect effect on phylogenetic structure, except for the effects of slope on NRI and TP on NTIab.
| Variables | NRI | NRIab | NTI | NTIab | |||||||
| Coef. | sumW | Coef. | sumW | Coef. | sumW | Coef. | sumW | ||||
| BNI | 0.731** | 0.969 | 0.298 | 0.372* | 0.580 | 0.391 | |||||
| BDI | 0.277 | 0.658 | 0.294 | 0.378 | −0.352 | 0.634 | |||||
| MTC | 0.293 | 0.607 | −0.559*** | 0.970 | 0.496 | −0.248 | 0.450 | ||||
| Slope | 0.280 | 0.750 | 0.396 | 0.392 | −0.388* | 0.782 | |||||
| TP | 0.284 | −0.259 | 0.702 | 0.287 | −0.421* | 0.769 | |||||
| R2 | 0.572 | 0.455 | 0.150 | 0.263 | |||||||
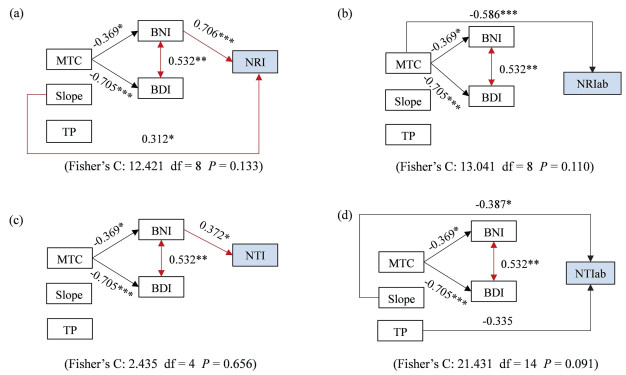
|
| Fig. 4 Structural equation models (SEM) showing the importance of biogeographic affinity and environmental factors on explaining phylogenetic structure. (a) NRI: the net relatedness index; (b) NRIab: the abundance-weighted net relatedness index; (c) NTI: the nearest taxon index; (d) NTIab: the abundance-weighted nearest taxon index. |
In this study, we found the inconsistent patterns between taxonomic and phylogenetic diversity. After considering dependence of species richness, phylogenetic diversity showed strongly declining trends along elevational gradients. This pattern suggests the dominance by younger clades (e.g., the genus Rhododendron) at higher elevations, resulting in greater phylogenetic clustering among terminal taxa in high-elevation communities compared to low-elevation assemblages. The inconsistent patterns are similar with the studies in several subtropical mountains, such as Mt. Tianmushan (Zhang et al., 2021) and Mt. Nanling (Guan et al., 2024). By incorporating the information of species abundance, we found that species abundance provides novel insights into understanding plant community assembly (Zhang et al., 2021). SE and PSE decreased along elevations, suggesting that woody plant communities were gradually dominated by a few genera like Rhododendron and Carpinus that are temperate-affiliated.
Our study showed that the phylogenetic diversity of tropical-affiliated versus temperate-affiliated species showed the opposite trends over the elevational gradient: the proportion of tropical species decreased with elevation, while temperate species increased. These results support our first prediction, showing a clear replacement of temperate-affiliated lineages along the elevations. Specifically, the relative richness and dominance of temperate-affiliated species increased with elevation. This pattern is consistent with the biogeographic affinity hypothesis (Harrison and Grace, 2007). The study was consistent with the contrasting distribution patterns between tropical and temperate genera on Mt. Taishan in northern China (Zhang et al., 2016), and in Mt. Gaoligong of southwestern China (Ma et al., 2024). These observations suggested that biogeographical affinities reflect different tolerances of environments (Peters et al., 2016; Nottingham et al., 2018).
We also found that BNI and BDI increased obviously along elevations. BNI and BDI were greater than zero at elevations above ~800 m, suggesting that temperate-affiliated species gradually dominated at higher elevations in Mt. Guanshan. The significantly increasing species richness of temperate-affiliated species and growing biogeographic affinity indices (BDI and BNI) might reveal that temperate-affiliated species gradually dominate higher elevations like Castanopsis tibetana, Quercus myrsinifolia, and Liquidambar formosana, providing strong supports for biogeographic affinity hypothesis. Woody plant communities at lower elevations (approximately < 750 m) were dominated by tropical-affiliated species like Magnolia chapensis, Camellia caudata, Alniphyllum fortune and Itea omeiensis. The increasing BNI and BDI with elevation suggest a shift in the primary contributors to aboveground biomass from tropical-affiliated species at lower elevations to temperate-affiliated species at higher elevations. In contrast to temperate-affiliated species, tropical-affiliated species exhibited reductions in both size and dominance along the elevational gradient. These differences between the two biogeographic groups reflect distinct ecological strategies and environmental responses. Specifically, tropical species may prioritize rapid growth and resource acquisition in stable lowland environments, whereas temperate species likely adapt stress-tolerant traits suited to harsher high-elevation conditions (Worthy et al., 2019; González-Caro et al., 2020).
For phylogenetic structure of woody plant communities, adding information of abundance indeed altered elevational patterns which supports to consider abundance in phylogeny to display a more real pattern. Increasing trends along elevational gradients for NRI and NTI demonstrated phylogenetic clustering, which has been reported in Mt. Diaoluoshan, Mt. Jianfengling, and Mt. Bawangling of Hainan Island (Liu et al., 2021). According to the hypothesis of phylogenetic niche conservatism, the phylogenetic clustering pattern is commonly explained as a result of environmental filtering (such as winter coldness and soil properties) at higher elevations, because coexisting species are generally ecologically similar, so their ecological traits are phylogenetically conserved. Conversely, the phylogenetic dispersion pattern at lower elevations is commonly explained as a result of interspecific competition, because more closely related species are expected to have widely overlapped niches which would lead to co-occurrence of distantly related species within a local assemblage (Qian et al., 2014).
With regard to the drivers of plant diversity patterns, minimum temperature of coldest month and biogeographic affinity played vital roles in plant community assembly in Mt. Guanshan. The SEM analysis revealed a negatively causal relationship between winter temperature and both BNI and BDI. It has been widely verified that winter temperature is of great importance in shaping community composition and structure (Qian et al., 2020), which can directly impact demographic rates or indirectly via BNI and BDI to filter suitable species with different biogeographic affinities to assemble plant community. The temperature variation from root to peak of Mt. Guanshan can up to 6 ℃ in winter that providing unsuitable habitats for tropical-affiliated species at higher elevations, whereas temperate-affiliated species can adapt well (Wang et al., 2023). For example, Rhododendron, one of the largest flowering plant genera originated at north-eastern Asia since early Miocene (ca. 16.6 Mya) and retained from the Quaternary glacial period, and now contributes a large proportion of abundance and biomass of plant communities in the middle and higher elevations (Zou et al., 2021). Under the combined influence of biogeographic affinity and winter temperature, closely related temperate-affiliated species coexisting at high elevations, resulting the phylogenetic clustering of plant community structure with increasing elevations. That is consistent with the results of the phylogenetic structure of woody plants in East Eurasia (Su et al., 2020). In addition, tropical species at low elevations usually show obvious divergence of traits and plant communities tended to be phylogenetically overdispersed at lower elevations (Zhang et al., 2021). This is because at lower elevations, plants tend to keep diverse traits to respond to increasing competitive exclusion, whereas tropical clades are prevented from expanding to higher elevations. Our findings also provide evidence for the second prediction. The observed increase in phylogenetic clustering at higher elevations suggests that both historical constraints and contemporary environmental filtering act in concert. First, biogeographic affinity limits the species pool size at each elevation belt. Second, minimum winter temperature emerges as the dominant filter determining which lineages persist, consistent with the idea that cold tolerance is phylogenetically conserved (Wiens and Donoghue, 2004; Peters et al., 2016).
Whether or not considering abundance information led to different patterns and altered main predictors, indicating that abundance-weighted community-level measures of phylogenetic diversity are better predictors of ecosystem processes than the unweighted metrics (Cadotte et al., 2010; Zhang et al., 2021). In our study, when disregarding abundance, BNI had significantly positive effects on NRI and NTI, suggesting that biogeographic origins have a fundamental effect on species pool and deciding community composition. However, after taking abundance into account, local environment factors (e.g., minimum temperature of coldest month, slope and soil phosphorous) became the main factors affecting phylogenetic structure, while biogeographic affinity had weak effect on phylogenetic structure, indicating that biogeographic affinity might control whether species can colonize at somewhere while local environment mainly determining their fitness.
In conclusion, we mainly explored the joint effects of biogeographic affinity and environmental variables on woody plant community assembly along a subtropical elevational gradient with typical transitional characteristics. Species with tropical and temperate-affinities dominate the woody plant communities at lower and higher elevations, respectively. Winter temperature can be a barrier for tropical-affiliated species colonizing higher elevations and results in phylogenetic clustering along elevations. Biogeographic affinity mainly regulates which species can access while local environment determines their fitness, and jointly affects elevational patterns of diversity. Our findings suggest that local environment and biogeographic affinity jointly lead to temperate-affiliated species dominated at higher elevations and shape woody plant community assembly in mountains.
AcknowledgmentsWe gratefully acknowledge financial support from the Jiangxi Natural Science Foundation (20242BAB25345) to Z.Z. and the Innovation Program of Shanghai Municipal Education Commission (2023ZKZD36) to J.Z. This work is part of the BEST (Biodiversity along Elevational gradients: Shifts and Transitions) research network (https://BEST-mountains.org).
CRediT authorship contribution statement
Zhaochen Zhang: Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Funding acquisition, Formal analysis. Fang Wang: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization. Xiaoran Wang: Writing – original draft, Visualization, Methodology, Investigation. Mufan Sun: Writing – review & editing, Methodology, Investigation. Pu Zheng: Methodology, Investigation. Jingchao Zhao: Methodology, Investigation. Junhong Chen: Methodology, Investigation. Min Guan: Methodology, Investigation. Pengcheng Liu: Methodology, Investigation. Xiaofan Shang: Methodology, Investigation. Yaoshun Lu: Writing – original draft, Methodology, Investigation. Qingpei Yang: Resources, Methodology, Investigation. Qingni Song: Resources, Methodology, Investigation. Lin Chen: Methodology, Investigation. Quying Zhong: Methodology, Investigation. Jian Zhang: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Conceptualization.
Availability of data and materials
The plant diversity indexes, biogeographic affinity and environmental variables of 32 plant communities used in this research have been shared as Appendix A.
Declaration of competing interest
All the authors have no conflict in interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2025.06.004.
Cadotte, M.W., Jonathan Davies, T., Regetz, J., et al., 2010. Phylogenetic diversity metrics for ecological communities: integrating species richness, abundance and evolutionary history. Ecol. Lett., 13: 96-105. DOI:10.1111/j.1461-0248.2009.01405.x |
Cavender-Bares, J., Ackerly, D.D., Hobbie, S.E., et al., 2016. Evolutionary legacy effects on ecosystems: biogeographic origins, plant traits, and implications for management in the era of global change. Annu. Rev. Ecol. Evol. Syst., 47: 433-462. DOI:10.1146/annurev-ecolsys-121415-032229 |
Culmsee, H., Leuschner, C., 2013. Consistent patterns of elevational change in tree taxonomic and phylogenetic diversity across Malesian mountain forests. J. Biogeogr., 40: 1997-2010. DOI:10.1111/jbi.12138 |
Culmsee, H., Leuschner, C., Moser, G., et al., 2010. Forest aboveground biomass along an elevational transect in Sulawesi, Indonesia, and the role of Fagaceae in tropical montane rain forests. J. Biogeogr., 37: 960-974. DOI:10.1111/j.1365-2699.2009.02269.x |
Fang, J.Y., Wang, Z.H., Tang, Z.Y., 2011. Atlas of Woody Plants in China: Distribution and Climate. Springer and Higher Education Press, Berlin and Beijing.
|
González-Caro, S., Duque, Á., Feeley, K.J., et al., 2020. The legacy of biogeographic history on the composition and structure of Andean forests. Ecology, 101: e03131. DOI:10.1002/ecy.3131 |
Guan, Y.L., Gan, X.H., Yin, Z.Y., et al., 2024. Distribution pattern of plant diversity at different elevations in Nanling nature reserve. Chin. J. Ecol. Environ. Sci., 33: 877-887. |
Hamid, M., Khuroo, A.A., Malik, A.H., et al., 2021. Elevation and aspect determine the differences in soil properties and plant species diversity on Himalayan Mountain summits. Ecol. Res., 36: 340-352. DOI:10.1111/1440-1703.12202 |
Harrison, S., Grace, J.B., 2007. Biogeographic affinity helps explain productivity-richness relationships at regional and local scales. Am. Nat., 170: S5-S15. DOI:10.1086/519010 |
Hawkins, B.A., Rueda, M., Rangel, T.F., et al., 2014. Community phylogenetics at the biogeographical scale: cold tolerance, niche conservatism and the structure of North American forests. J. Biogeogr., 41: 23-38. DOI:10.1111/jbi.12171 |
Jin, Y., Qian, H., 2023. U.PhyloMaker: an R package that can generate large phylogenetic trees for plants and animals. Plant Divers., 45: 347-352. DOI:10.1016/j.pld.2022.12.007 |
Karger, D.N., Wilson, A.M., Mahony, C., et al., 2021. Global daily 1km land surface precipitation based on cloud cover-informed downscaling. Sci. Data, 8: 307. DOI:10.1038/s41597-021-01084-6 |
Kembel, S., Cowan, P., Helmus, M., et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26: 1463-1464. DOI:10.1093/bioinformatics/btq166 |
Kissling, W.D., Carl, G., 2008. Spatial autocorrelation and the selection of simultaneous autoregressive models. Global Ecol. Biogeogr., 17: 59-71. DOI:10.1111/j.1466-8238.2007.00334.x |
Körner, C., 2007. The use of ‘altitude’ in ecological research. Trends Ecol. Evol., 22: 569-574. DOI:10.1016/j.tree.2007.09.006 |
Lefcheck, J.S., 2016. piecewiseSEM: piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol., 7: 573-579. DOI:10.1111/2041-210X.12512 |
Lembrechts, J.J., van den Hoogen, J., Aalto, J., et al., 2022. Global maps of soil temperature. Glob. Change Biol., 28: 3110-3144. DOI:10.1111/gcb.16060 |
Li, M., Feng, J., 2015. Biogeographical interpretation of elevational patterns of genus diversity of seed plants in Nepal. PLoS One, 10: e0140992. DOI:10.1371/journal.pone.0140992 |
Liu, X., Wu, H., 2005. Scientific Survey and Study on the Guanshan Nature Reserve in Jiangxi Province. China Forestry Publishing House, Beijing, China (In Chinese).
|
Liu, H.D., Liu, H., Chen, Y.F., et al., 2021. Identifying the patterns of changes in alpha- and beta-diversity across Dacrydium pectinatum communities in Hainan Island, China. Ecol. Evol., 11: 4616-4630. DOI:10.1002/ece3.7361 |
Liu, F., Wu, H.Y., Zhao, Y.G., et al., 2022. Mapping high resolution national soil information Grids of China. Sci. Bull., 67: 328-340. DOI:10.1016/j.scib.2021.10.013 |
Losos, J.B., 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett., 11: 995-1003. DOI:10.1111/j.1461-0248.2008.01229.x |
Ma, L., Liu, L., Lu, Y.S., et al., 2022. When microclimates meet soil microbes: temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem., 166: 108566. DOI:10.1016/j.soilbio.2022.108566 |
Ma, L.L., Seibold, S., Cadotte, M.W., et al., 2024. Niche convergence and biogeographic history shape elevational tree community assembly in a subtropical mountain forest. Sci. Total Environ., 935: 173343. DOI:10.1016/j.scitotenv.2024.173343 |
Matthew, R.H., Thomas, J.B., Christopher, K.W., et al., 2007. Phylogenetic measures of biodiversity. Am. Nat., 169: E68-E83. DOI:10.1086/511334 |
Moser, D., Dullinger, S., Englisch, T., et al., 2005. Environmental determinants of vascular plant species richness in the Austrian Alps. J. Biogeogr., 32: 1117-1127. DOI:10.1111/j.1365-2699.2005.01265.x |
Nottingham, A.T., Fierer, N., Turner, B.L., et al., 2018. Microbes follow Humboldt: temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology, 99: 2455-2466. DOI:10.1002/ecy.2482 |
Peters, M.K., Hemp, A., Appelhans, T., et al., 2016. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun., 7: 13736. DOI:10.1038/ncomms13736 |
Qian, H., Hao, Z.Q., Zhang, J., 2014. Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in Changbaishan, China. J. Plant Ecol., 7: 154-165. DOI:10.1093/jpe/rtt072 |
Qian, H., Zhang, J., Sandel, B., et al., 2020. Phylogenetic structure of angiosperm trees in local forest communities along latitudinal and elevational gradients in eastern North America. Ecography, 43: 419-430. DOI:10.1111/ecog.04873 |
R Core Team, 2023. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
|
Su, X., Shrestha, N., Xu, X., et al., 2020. Phylogenetic conservatism and biogeographic affinity influence woody plant species richness–climate relationships in eastern Eurasia. Ecography, 43: 1027-1040. DOI:10.1111/ecog.04839 |
Sundqvist, M.K., Sanders, N.J., Wardle, D.A., 2013. Community and ecosystem responses to elevational gradients: processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst., 44: 261-280. DOI:10.1146/annurev-ecolsys-110512-135750 |
Wang, F., Lu, Y.S., Zhang, Z.C., et al., 2023. Altitudinal variations and seasonal dynamics of near-surface and soil temperatures in subtropical forests of Mt. Guanshan, Jiangxi Province, China. Chin. J. Appl. Ecol., 34: 1161-1168. |
Webb, C.O., Ackerly, D.D., McPeek, M.A., et al., 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst., 33: 475-505. DOI:10.1146/annurev.ecolsys.33.010802.150448 |
WFO, 2024. World Flora Online. Version 2023.12. Published on the Internet. http://www.worldfloraonline.org. (Accessed 14 June 2024).
|
Wiens, J.J., Donoghue, M.J., 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol., 19: 639-644. DOI:10.1016/j.tree.2004.09.011 |
Wiens, J.J., Graham, C.H., 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst., 36: 519-539. DOI:10.1146/annurev.ecolsys.36.102803.095431 |
Worthy, S.J., Jiménez Paz, R.A., Pérez, Á.J., et al., 2019. Distribution and community assembly of trees along an andean elevational gradient. Plants, 8: 326. DOI:10.3390/plants8090326 |
Wu, Z.Y., 1991. The areal-types of Chinese genera of seed plants. Acta Bot. Yunnan., 13: 1-139. |
Wu, Z.Y., Raven, P.H., Hong, D.Y., 1994. Flora of China. -2013. Science Press, China (In Chinese).
|
Wu, Z.Y., Zhou, Z., Sun, H., et al., 2006. The Areal-Types of Seed Plants and Their Origin and Differentiation. Yunnan Science and Technology Press, Kunming.
|
Zhang, J., Qian, H., 2023. U.Taxonstand: an R package for standardizing scientific names of plants and animals. Plant Divers., 45: 1-5. DOI:10.1016/j.pld.2022.09.001 |
Zhang, J., Kissling, W.D., He, F., 2013. Local forest structure, climate and human disturbance determine regional distribution of boreal bird species richness in Alberta, Canada. J. Biogeogr., 40: 1131-1142. DOI:10.1111/jbi.12063 |
Zhang, W., Huang, D., Wang, R., et al., 2016. Altitudinal patterns of species diversity and phylogenetic diversity across temperate mountain forests of northern China. PLoS One, 11: e0159995. DOI:10.1371/journal.pone.0159995 |
Zhang, R., Zhang, Z., Shang, K., et al., 2021. A taxonomic and phylogenetic perspective on plant community assembly along an elevational gradient in subtropical forests. J. Plant Ecol., 14: 702-716. DOI:10.1093/jpe/rtab026 |
Zhang, J., Qian, H., Wang, X., 2025. An online version and some updates of R package U.Taxonstand for standardizing scientific names in plant and animal species. Plant Divers., 47: 166-168. DOI:10.1016/j.pld.2024.09.005 |
Zou, J., Luo, Y., Burgess, K.S., et al., 2021. Joint effect of phylogenetic relatedness and trait selection on the elevational distribution of Rhododendron species. J. Syst. Evol., 59: 1244-1255. DOI:10.1111/jse.12690 |



