b. Lishui Institute of Agriculture and Forestry Sciences, Lishui, Zhejiang 323000, China
Global environmental change has profoundly influenced biodiversity on earth (Shivanna, 2022). Under global climate changes, unraveling the genetic basis of adaptation to changing environments has attracted widespread attentions from evolutionary geneticists for decades as the capacity to adapt to environmental change affects the long-term survival of species (Lasky et al., 2022). Local adaptation occurs when individuals have higher fitness in their native habitats than elsewhere as various environmental factors exert different selection pressures across a species range, and is commonly found within plants and animals (Kawecki and Ebert, 2004). Despite its prevalence, the genetic underpinnings of local adaptation are still poorly understood (Lascoux et al., 2016). Studying local adaptation is of great importance for understanding how species adapt to environmental change, designing and predicting the effects of environmental change on species distributions (Aguirre-Liguori et al., 2021; Sang et al., 2022).
Traditional approaches, such as reciprocal-transplant experiment and common garden experiment have been used to uncover local adaptation by measuring genetic signals associated with adaptive phenotypes (Capblancq et al., 2023; Wadgymar et al., 2022). However, these methods pose challenges and are usually unfeasible for non-model plants due to long life history and experimental intractability (Sang et al., 2022). Landscape genomics, which integrates landscape ecology and population genomics, offers a relatively new approach to determine the effects of environmental factors shaping adaptive genetic variations and find variants driving local adaptation through genotype-environment associations (GEAs) (Zhang et al., 2024). With the rapid advancement of next-generation sequencing technologies, landscape genomics has greatly enhanced our understanding of local adaptation by focusing on identifying outliers or candidate genes associated with environmental factors, and evaluating current and prospective genotype-environment mismatched scenarios to ascertain the extent of genomic plasticity with regards to climate change (Gugger et al., 2021; Heraghty et al., 2023; Wang et al., 2023; Zhang et al., 2023a).
Previous studies about adaptation to climate are heavily biased towards annual crops and tree species with social and economic benefits (Sang et al., 2022; Yuan et al., 2023). However, as an important component of subtropical and tropical forest ecosystem, perennial lianas (woody vines) were largely ignored for a long time, although they comprise up to 10% of woody individuals and species in subtropical forests and usually have a large impact on restoration and succession in forests (Cai and Song, 2000; Gentry, 1992; Schnitzer and Bongers, 2002). Investigation of adaptive evolution in such taxon will provide additional perspectives into how lianas species response to climate change. Here, we addressed Actinidia eriantha Benth (2n = 2x = 58, Actinidia, Actinidiaceae), a perennial deciduous liana with ecological and economic values (Sun et al., 2015). As an endemic species, A. eriantha is widely distributed in subtropical China with an elevational distribution ranging from 100 to 1500 m (Testolin et al., 2016), and wild individuals show abundant variations in fruit shapes and flower colors (Liao et al., 2021). However, it faces threats from predatory exploitation and climate change now, with its natural habitats rapidly decreasing (Zhang et al., 2023b). Previous phylogeographical study showed that A. eriantha originated from the eastern China with 'oceanic' adaptation and expanded to western China during the glacial periods (Guo et al., 2022). Prior GEA analysis based on restriction site-associated DNA sequencing (RAD-seq) and limited populations demonstrated that local climate factors such as temperature and precipitation imposed strong selection pressures and leaded to adaptation to climatic change for A. eriantha (Zhang et al., 2023b). However, it should be noticed that RAD-seq typically represented only a fraction of the genome and yielded low-density SNPs, which might lose crucial genetic footprints involved in local adaptation (Feng and Du, 2022; Hoffmann et al., 2021). In addition, given that genetic offsets are sensitive to the number of populations especially for species with high genetic differentiation, fewer than 15 populations used to predict maladaptation may compromise the accuracy of genomic offset projections (Aguirre-Liguori et al., 2023). Moreover, other environmental variables such as solar radiations, soil components and metal ion, which could also play a critical role in local adaptation, have not been analyzed in previous study (Zhang et al., 2023b), which hinder a comprehensively understanding of genetic basis of local adaptation in A. eriantha.
Therefore, this study aims to illustrate the genetic basis of adaptation to diverse environments in Actinidia eriantha through whole-genome resequencing and landscape genomics. To this end, we sampled more independent natural populations along environment gradients of A. eriantha and performed GEA analysis to identify genetic variations (single nucleotide polymorphisms and insertions/deletions) associated with environmental variations. Our results highlighted that environment factors driven the evolutionary processes of local adaptation and plants adapted to heterogeneous environments through changes in allele frequencies along with environments in native habitats, which provided an additional insight on how liana species adapted to spatially heterogeneous environments.
2. Materials and methods 2.1. Plant samples and environment factors collectionIn 2021, we collected young fresh leaves of 311 individuals originated from 25 populations covering the distribution range of Actinidia eriantha (Fig. 1a). The fresh leaves were dried with silica gel for DNA extraction and the detailed sampling information was shown in Table S1 and Table S2.
|
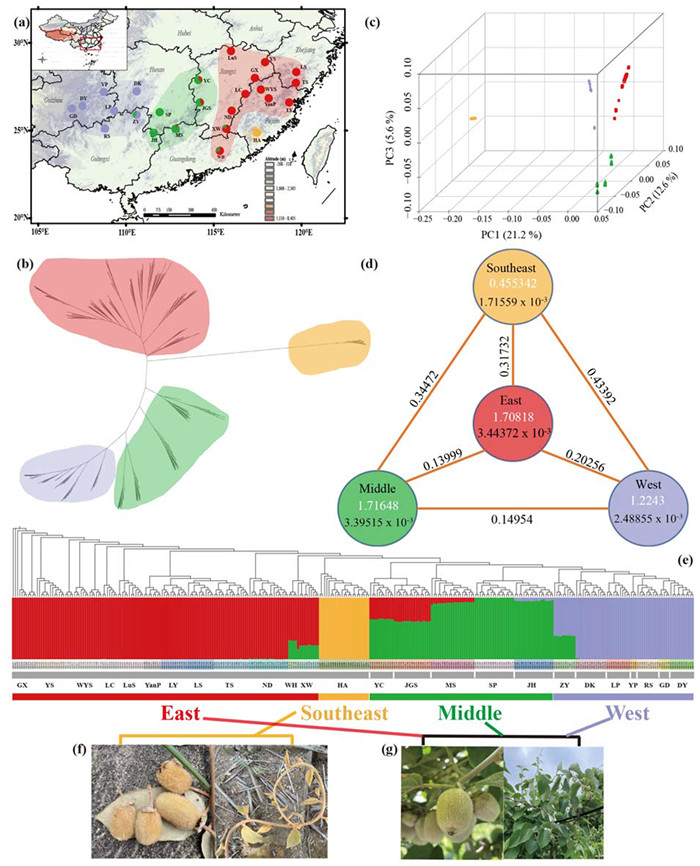
| Fig. 1 The sample distributions and genetic structures of Actinidia eriantha. (a) Geographic distribution map of the samples for resequencing in this study. Each dot represents one population location and the pie charts with mixed color is the ratio of population ancestry proportions of individuals (K = 4). (b) Unrooted Maximum-likelihood phylogenetic tree of 311 individuals of A. eriantha. (c) Three-dimensional (3D) PCA plot. (d) Genetic diversity (π) within clusters and genetic differentiation (FST) among clusters. White numbers are Tajima's D values, black numbers refer to genetic diversity and numbers around the lines are FST between two subpopulations. (e) Genetic structures inferred with Admixture. The different-colored boxes represent the population ancestry proportions for individuals. (f) The morphological characteristics of fruits and leaves of A. eriantha sampled from cluster Southeast (HA population). (g) The morphological characteristics of fruits and leaves of A. eriantha collected from the populations in Middle, East and West clusters, respectively. |
A total of 117 environment factors spanning 40 years (1970–2010) of 25 provenance sites (resolution 2.5 arcmin) were extracted with R package raster (https://CRAN.R-project.org/package=raster). Detailed explanations of environmental factors and raw environment data were provided in Table S3 and Table S4. Among these raw data, six UV-B variables were extracted from glUV database (Beckmann et al., 2014) and eight soil factors were obtained from Soil Sub Center, National Earth System Science Data Center, National Science & Technology Infrastructure (Liu et al., 2022). The remained climate data were downloaded from the World Climate database (WorldClim2) (Fick and Hijmans, 2017).
2.2. DNA extraction, library construction and resequencingGenomic DNA were extracted via a modified cetyltrimethylammonium bromide (CTAB) method (Doyle JJT and Doyle JL, 1990). After DNA extraction, 1 μg genomic DNA was randomly fragmented to an average size of 200–400 bp to construct the libraries. The qualified libraries were sequenced by DNBSEQ platform with a target depth of 10X. The raw sequencing data was processed using SOAPnuke (Chen et al., 2018) with the parameters "-n 0.01 -l 20 –q 0.3 –adaMR 0.25 –ada_trim –polyX 50". Finally, about 7 Gb clean data per sample was obtained.
2.3. Variation calling and annotationAll clean paired-end data were aligned to the reference genome of Actinidia eriantha (Yao et al., 2022) via the algorithm "bwa-mem" implemented in Burrows-Wheeler Aligner (BWA, v.0.7.17-r1198-dirty) (Li and Durbin, 2009) with the additional parameter '−M'. Aligned reads were sorted and converted into BAM format from SAM format using SAMtools v.1.13 (Danecek et al., 2021). Picard v.2.24.0 (https://github.com/broadinstitute/picard) was used to remove the duplicated reads due to PCR amplification with default parameters. The sequencing depths and coverage rates were calculated by SAMtools. The translated BAM files were used for variant calling with Genome Analysis Toolkit v.4.2.0.0 (McKenna et al., 2010).
The SNP detection was performed based on the following pipeline. Firstly, a genomic variant call format (GVCF) file in ERC mode for each sample was obtained using "HaplotypeCaller" program of GATK (DePristo et al., 2011). Then all GVCF files merged into a single file with the tool "CombineGVCFs". Finally, primary SNPs and Indels were detected at the population level by "GenotypeVCFs". To obtain high-quality variants, a hard filtered standard was formulated using "VariantFiltration". For SNPs, the parameters were set as follows: QD < 2.0, MQ < 40.0, FS > 60.0, SOR > 3.0, MQRankSum < −12.5, ReadPosRankSum < −8.0. Indels (shorter than 50 bp) were selected with the recommended parameters: QD < 2.0, FS > 200.0, SOR > 10.0, MQRankSum < −12.5, ReadPosRankSum < −8.0. To improve the accuracy of variations, all variations were further filtered using VCFtools v.0.1.16 (https://vcftools.github.io/man_latest.html) with the following parameters '–maf 0.05 –max-missing 0.9 –min-alleles 2 –max-alleles 2 –minQ 30'. The remained sites were considered as the core subset for subsequent population analysis and annotated by SnpEff v.5.0 (Cingolani et al., 2014).
2.4. Population structure and genetic diversity analysisiTools implement in Reseqtools (He et al., 2013) was employed to delineate the genomic positions of 4DTv (fourfold synonymous transversion) sites. 4DTvs were isolated with BCFtools (https://github.com/samtools/bcftools) based on their positional coordinates and used for building a phylogenetic tree and population structure analysis. For phylogenetic relationship inference, we firstly searched for the best substitution model by ModelFinder algorithm (Kalyaanamoorthy et al., 2017) among 283 DNA models and the best-fit model "GTR + F + R6 + ASC" was chosen according to Bayesian Information Criterion (BIC). The ultimate phylogenetic tree was built with maximum-likelihood (ML) method using IQ-TREE v.1.6.12 (Nguyen et al., 2015) with 1000 bootstrap replicates. Principal component analysis (PCA) was performed via PLINK v.1.90b6.24 (Purcell et al., 2007). Additionally, ADMIXTURE v.1.3.0 (Alexander et al., 2009) was used to conduct the population structure analysis. We performed ten independent runs for each K value from 2 to 10.
We used VCFtools to calculate the nucleotide diversities (π), Tajima's D value and pairwise FST with a 100-Kb window size and a step size of 10 Kb for groups and populations, respectively. To elucidate the relationships between spatial distances and genetic distances (FST/(1-FST)) (isolation by distance, IBD), and between environment distances and genetic divergences (isolation by environment, IBE), a simple Mantel test and a partial Mantel test were performed by vegan v.2.6–4 packages with 9999 permutations, respectively (Dixon, 2003).
2.5. Inference of population demographic historyThe effective population size (Ne) was inferred using SMC++ (Terhorst et al., 2017). To improve reliability, genome regions for each chromosome where reads were not uniquely mapped were masked according to SNPable pipeline (http://lh3lh3.users.sourceforge.net/snpable.shtml). Ten representative and deeply sequenced individuals were selected from each cluster (Table S2). The estimate mutation rate was set to 1 × 10−8 per bp per generation and the generation time was defined as 7 years (Liu et al., 2017).
2.6. Estimation of the importance about environments driving genetic variationsGradient Forest (GF) was employed to assess the most important environmental factor influencing the genetic variations, utilizing nonparametric and machine learning regression trees (Ellis et al., 2012). We ran GF using 20, 000 random SNPs selecting from the whole dataset with five replications to reduce the random error. The parameter listed as following: ntree = 500, maxLevel = log2(0.368n)/2 (n is the number of candidate SNP), and correlation threshold was set to 0.5.
To avoid the multicollinearity of predictors, environmental variables with Pearson correlation coefficients |r| < 0.7 were retained for subsequent analyses. Partial redundancy analysis (pRDA) was performed to estimate the influences of independent environmental factors, geography and population genetic structure on genetic variations. In this study, the first three PCs were sought as natural population structures, while latitudes and longitudes of individuals represented the geography. Each of three categories, named geography, genetic structure and environmental factors, was separately used as explanatory variable, with the remaining two variables analyzed as conditioning variables in partial model. Additionally, all variable regard as explanatory variables in full model. All models were carried out with genotypes as response variables.
2.7. Identification of candidate genetic variations associated with environmentsWe used two approaches, latent factor mixed model (LFMM) and redundancy analysis (RDA), to detect the candidate loci associated with environments using variants without missing data. LFMM introduced population structure as the latent factor to control false positive rate (Frichot et al., 2013). In this study, we adopted the K value inferred by ADMIXTURE. The association value between the frequency of each variant site and each environmental variable was calibrated using genomic inflation factors (GIF). P-value for all sites were transformed into q-values using the R package qvalue (https://github.com/StoreyLab/qvalue), with sites having q-value < 0.01 considered significantly associated with environmental variables. Identified variants were retained if they were correlated with at least one environmental variable.
RDA was considered as a knife with high true-positive and low false-positive rates and widely used in identifying adaptive loci (Capblancq and Forester, 2021). We performed the pRDA with first 3 PC as conditional variants and first three significant RDA axes accounting for most variants were selected to detected candidate loci. Loci with a standard deviation cutoff of 3.5 were considered as outliers associated with environment predictors. Finally, SNPs or Indels identified by two methods were considered as the core adaptive outliers for downstream analysis.
2.8. GO enrichment analysisGO enrichment analysis were performed with R package Clusterprofiler (Wu et al., 2021). The candidate protein sequences were aligned to the local eggnog-MAPPER database (Cantalapiedra et al., 2021) and annotated with default parameters based on the mmseq2 (Steinegger and Soding, 2017). GO database was constructed using AnnotationForge (https://bioconductor.org/packages/AnnotationForge).
2.9. Genetic mismatches between current and future climatesTo predict the potential adaptability and change of Actinidia eriantha populations under future climate accurately, we considered four emission scenarios of the shared socioeconomic pathway (SSP126, SSP245, SSP370 and SSP585) under four different climate models (BCC-CSM2-MR model, ACCESS-CM2 model, CMCC-ESM2 model and GISS-E2-1-G model), respectively. We used nine environmental variables including bio1, bio2, bio3, bio7, bio15, bio18, prec03, prec09, and tmin07 under both current and future conditions and adaptive loci to estimate genomic vulnerability. Besides, all loci were also employed to assess the risk of non-adaptedness (RONA) with Euclidean distances between current and future allele frequencies for each natural population under changing climate scenarios, following the methods outlined by Rellstab et al. (2016).
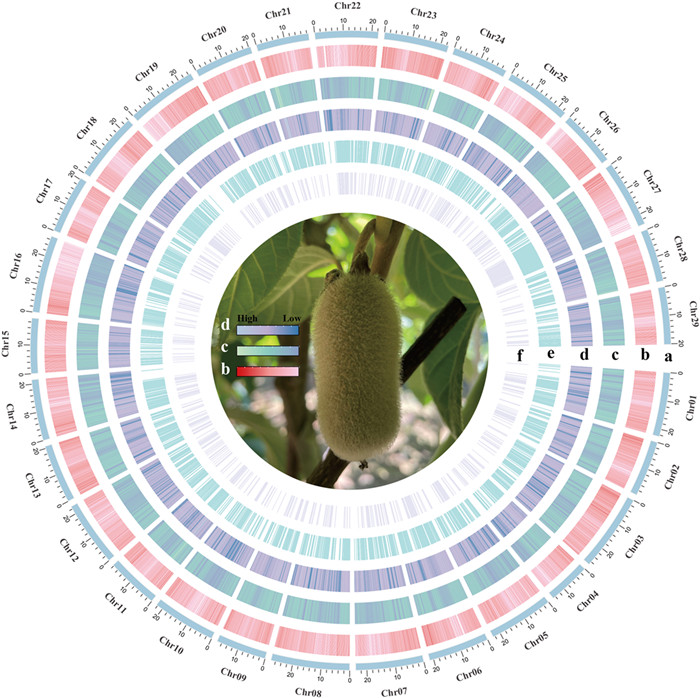
3. Results 3.1. SNP genotyping, population structure analysis and demographic historyResequencing of 311 individuals of Actinidia eriantha yielded a total of 2.2 Tb clean data with an average depth of 11.74X and a mean coverage of 92.22% (Table S2). A core subset of 11, 278, 868 high-confidence SNPs and 1, 420, 355 high-quality Indels corresponding to an average density of 18 SNPs and 2.5 Indels per kilobase were identified, respectively (Fig. 2). A large portion of the SNPs and indels were located in non-coding regions, with 50% of SNPs in intergenic regions and 36% of indels in intron regions. Only a small fraction of SNPs (5%) and indels (3%) were found within the exon regions, respectively (Table S5).
|
| Fig. 2 The variant map displayed genomic variants and adaptive loci associated with environments in Actinidia eriantha. The tracks from the outside to the inside represent Chr number (a), gene density on the whole genome (b), indels density (c) and SNP density (d) identified by genome resequencing of 311 individuals, the adaptation SNPs (e) and indels (f) associated with environments, respectively. |
As inferring the genetic basis of adaptation needs to consider the potential influence of neutral processes that can shape genetic variations, we first elucidated the population structure and demographic history of Actinidia eriantha. Population structure analyses using the fourfold degenerate synonymous sites (4DTvs, = 188, 329) showed that all individuals were divided into four main subgroups denoted as cluster East, Middle, West and Southeast according to the ML tree corresponding to Wuyi Mountains, Luoxiao Mountains and Nanling Mountains, Xuefeng Mountains, and southeastern coastal areas, which is further supported by PCA analysis, with 21.2%, 12.6% and 5.6% of the variation explained by three principal components axes, respectively (Fig. 1a–c). Cluster East predominantly contained natural populations from Zhejiang, Jiangxi, Fujian and Guangdong provinces, while middle group consisted of five populations including two from western of Jiangxi province, one from north of Guangdong province and two populations in Hunan province. West cluster contained seven populations mainly collected from the western China (Guizhou, Guangxi, and Hunan provinces). Notably, seven marginal populations (WH, XW, YC, JGS, MS, JH and ZY populations) within genetic clusters exhibited significant mixtures. In addition, the Southeast cluster consisted of a single population located in Hua'an country in Fujian province that exhibited phenotypic divergence in hair color and strong genetic differentiation from the other clusters (Figs. 1a, e–g and S1). Multiple genetic bottlenecks were observed in these genetic clusters except the East characterized by a gradual reduction in effective population size, followed by a brief recovery and a steady decline (Fig. S2). Specifically, the East, West, and Middle groups experienced a sharp decrease in effective population size around 0.5–1 million years ago. While between 20, 000 and 70, 000 years ago, the population sizes of three groups decreased except for the East group.
3.2. Genetic diversity and genetic differentiationsThe East group had highest genetic diversity, with a coefficient of 3.44 × 10−3, followed by the Middle group and the West group, with coefficients of 3.40 × 10−3 and 2.49 × 10−3, respectively. The Southeast group displayed the lowest genetic diversity, with a coefficient of only 1.72 × 10−3. Tajima's D results were consistent with the patterns of genetic diversity. Besides, Southeast group exhibited significant differentiation from other three groups (FST > 0.3) (Fig. 1d). It was further supported by the clustering analysis of wild individuals, which revealed a greater genetic distance between the Southeast group and the other three groups (Fig. 1b). At the population level, nucleotide diversity ranged from 1.51 × 10−3 (GD) to 3.60 × 10−3 (GX) (Fig. S3). Notably, the genetic diversity was significantly correlated with longitude (Fig. S4).
In addition, the Mantel's test revealed a significant correlation between geographic distance and genetic distance (r = 0.62 and P < 0.001). This was further confirmed by a partial Mantel's test (r = 0.58 and P < 0.001), which accounted for environmental distance. Although a weaker correlation between genetic distance and environmental distance was observed (r = 0.32 and P < 0.001), it was not significant when controlling for geographic distance (r = 0.13 and P = 0.11) (Table S6).
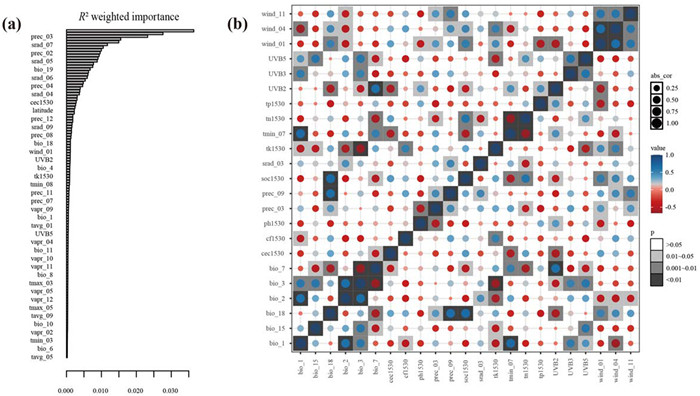
3.3. Primary environmental factors and drivers of genetic variationsTo identify the primary environmental factors influencing genetic variations, we employed GF to rank the importance of environmental variables. Prec_03 emerged as the most crucial environmental driver of allele frequency variations within Actinidia eriantha populations, followed by solar radiation (Figs. 3a and S5). We retained 23 independent environmental factors including five temperature factors (bio_1, bio_2, bio_3, bio_7, tmin_07), four precipitation variables (bio_15, bio_18, prec_03, prec_09), seven soil indexes (cec1530, cf1530, ph1530, soc1530, tk1530, tn1530, tp1530), three UVB factors (UVB2, UVB3, UVB5), three wind elements (wind_01, wind_04, wind_11) and one solar radiation index (srad_03) to characterize the climatic features of A. eriantha for subsequent analysis (Fig. 3b). We further assessed the proportion of variation explained by environmental factors, geography and genetic structure using pRDA to infer the drivers of patterns of genetic diversity. The results demonstrated that three explanatory variables totally explained 65% of the overall genetic variations across A. eriantha (Table 1). Specifically, environment variables explained 19% of variations when controlling for geography and genetic structure, implying that climate was the primary factor driving the patterns of genetic diversity. However, when controlling for another two factors, genetic structure accounted for only 0.4% of total variations. Similarly, the effect of geography explained 0.14% of genotypic variations controlling for climate and genetic structure. In addition, approximately 34.78% variations remained unexplained by climate, geography and genetic structure.
|
| Fig. 3 Importance ranking of environment factors and independent environment variables for adaptation analysis. (a) Overall importance of all environmental factors with 20, 000 selected genomic variations randomly based on GF analysis. (b) 23 independent environment variables for adaptation analysis. The size of the circular point is used to show the size of the correlation coefficient. The color of value is used to show the positive and negative of the correlation coefficient, and a separate square color block is added to show the p-value of the correlation test. |
| Partial RDA models | Inertia | R2 | adj R2 | P (> F) | Proportion of explainable inertia | Proportion of total inertia |
| Full model: F ~ env. + struc. + geog. | 953, 368 | 0.6521616 | 0.6189756 | 0.001 *** | 1 | 0.6521616 |
| Pure environment: F ~ env. | (geog. + struc.) | 288, 002 | 0.1969459 | 0.1726908 | 0.001 *** | 0.30198176 | 0.1969459 |
| Pure geography: F ~ geog. | (env. + struc.) | 2132 | 0.001458 | 0.000249848 | 0.001 *** | 0.0022355 | 0.001458004 |
| Pure ancestry: F ~ struc. | (env. + geog.) | 6266 | 0.00428524 | 0.000648079 | 0.001 *** | 0.0065702 | 0.004285238 |
| Confounded env/geography/structure | 657, 302 | 0.68921254 | 0.449472458 | |||
| Total unexplained | 508, 700 | 0.3478 | ||||
| Total inertia | 1, 462, 000 | 1 |
To reduce the influence of population structure on association analysis, we performed GEA analysis using two high-quality datasets (SNPs and Indels), both with and without HA population. Remarkably, a total of 458, 446 and 82, 470 SNPs associated with at least one environment were identified by LFMM and RDA across all populations, along with 57, 467 and 8708 indels highlighted, respectively (Fig. S6). However, excluding the HA population, we identified a total of 196, 626 SNP sites and 15, 661 Indel sites in the remained populations using the LFMM method. Conversely, the RDA method identified a greater number of SNPs (122, 847) and Indels (11, 159) compared to the analysis included HA population (Fig. S6). In total, we obtained 17, 022 SNPs and 1829 Indels associated with environments identified simultaneously by aforementioned four methods. These loci were associated with 1566 genes including the members of NAC transcription factors (NAC010, NAC102), ERF gene family (ERF3, ERF14 and ERF096, etc.), and the homologs of calcium-dependent protein kinases (CPK5, CPK11, and CPK15) (Table S7). GO analysis suggested these genes were predominantly enriched in processes including positive regulation of long-day photoperiodism and flowering, histone modification, DNA replication (Fig. S7), indicating they might be involved in the adaptation to heterogeneous environments.
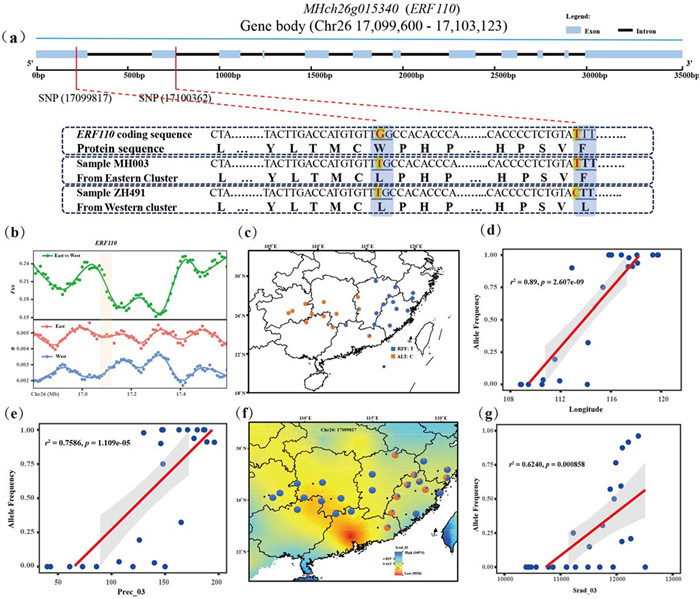
Importantly, we pinpointed two missense mutations (Chr26:17099817 and Chr26:17100362) exhibiting low genetic diversity yet significant genetic differentiation between East and West cluster, which caused the changes of amino acids (Fig. 4a and b). These loci were situated within the coding regions of an environment-associated gene MHch26g015340, which shared homology with the ERF110 gene in Arabidopsis thaliana (Table S7). The allele frequency of locus Chr26:17100362 increased with longitude (Fig. 4d) and showed a similar trend with the precipitation in March (Prec_03), while the other (Chr26:17099817) was significantly associated only with solar radiation (Srad_03) (Fig. 4e–g). In addition, we also noticed that the reference genotype of locus Chr26:17100362 was primarily distributed in low longitude areas (West of China), whereas the alternative allele was prevalent at high longitude areas (East of China) (Fig. 4c). Regarding locus Chr26:17099817, the allele T was mainly distributed in the regions with weak solar radiation, while allele G was almost fixed in areas experiencing high solar radiation (Fig. 4f). Both showed that adaptive loci frequently exhibited environmental and geographic signals.
|
| Fig. 4 Genetic patterns associated with gradient environments. (a) Gene structure of MH26g015340 (ERF110) associated with environments. Two missense mutations (Chr26:17099817 and Chr26:17100362) are highlighted in red line. Black line represents intron and blue rectangle represents exon. The boxes highlight the mutations in the ERF110 and two samples (ZH491 and MH003) show different genotypes and changes of amino acids at two loci. (b) Genetic diversity of each cluster and FST between East and West clusters. (c) Geographic distributions of missense mutation in the exon of ERF110 at locus Chr26:17100362. (d) Correlation between allele frequency of non-synonymous mutation (Chr26:17100362) in ERF110 and longitude for reference genotype (Pearson's r2 and p value calculated with cor.test). (e) Correlation between allele frequency of locus Chr26:17100362 in ERF110 and Prec_03 for reference genotype. (f) Geographic distribution of missense mutation of ERF110 at locus Chr26:17099817. The range of color represents the intensity of Solar radiation in March. (g) Correlation between allele frequency of locus Chr26:17099817 in ERF110 and Srad_03 for reference genotype. |
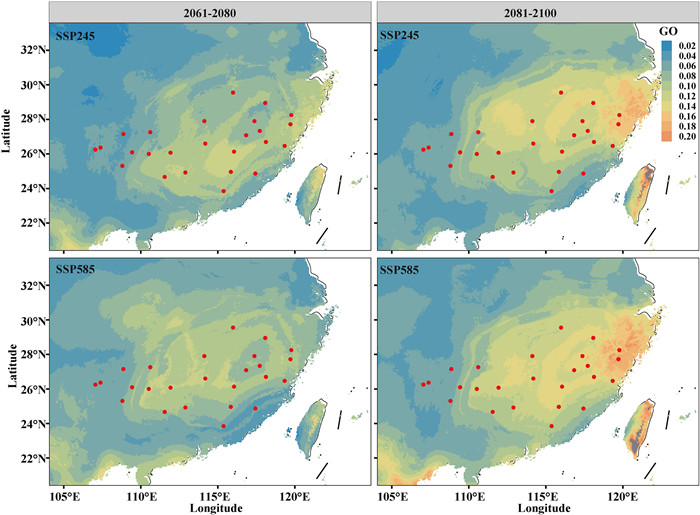
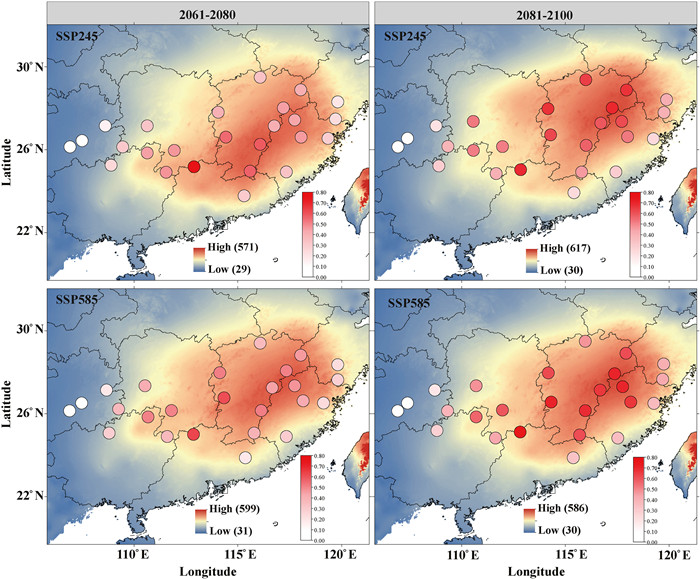
To evaluate whether the adaptive loci identified through GEA analysis were associated with the environmental variables using random variants, we re-ran GF again and precipitation showed the high R2 weight importance, of which prec_03 was still the most important factor (Fig. S8). Taking prec_03 (precipitation) and bio_7 (temperature) as examples to assess the cumulative importance of allelic frequency, the consequences revealed that a faster turnover rate appeared in prec_03 than bio_7, which indicated that precipitation had a greater impact on changes in allele frequencies than temperature (Fig. S9). Meanwhile, we estimated the risk of maladaptation to future climate changes for natural populations and inferred the potential change of geographic distribution using 18, 851 adaptive loci (Fig. S6). We firstly predicted the genetic offsets reflecting the overall influences on allele frequency of environment using GF with five temperature factors and four precipitation variables mentioned in section 3.3 under BCC-CSM2-MR climate model. The results demonstrated that higher genetic offset values appeared in populations from Middle and East clusters, implying they are more vulnerable to future climate changes (Figs. 5 and S10). To improve the accuracy of prediction, we further used other three climate models and reached the similar conclusions (Figs. S11-S13). It suggested an increasing trend over time and with rising emissions among the four future models. Besides, we also calculated the RONA values of nine environment factors under four climate models, and found that the trends of average values of RONA changed under different scenarios were similar with genetic offset analysis (Table S8 and Fig. 6). It was worth mentioning that populations in middle and east clusters showed higher genomic vulnerability especially from 2081 to 2100 under SSP245 and SSP585 scenarios revealed by both methods, which indicated these populations would be not suitable to future environments and need more attentions for a long time. Besides, for the most important environment factor Prec_03, population MS showed the highest RONA value and low adaptive potential under all SSP models in the future (Table S8).
|
| Fig. 5 Genetic offsets under future environment for natural population of Actinidia eriantha under the BCC-CSM2-MR climate model. The genetic offsets were predicted by two typic SSPs (SSP245 and SSP585) in two periods including 2061–2080 (Left) and 2081–2100 (Right) with nine primary climate factors. |
|
| Fig. 6 The Risk of non-adaptedness for each population to response to future climate changes for Pre_03 under the BCC-CSM2-MR climate model. The RONA were calculated by two typic SSPs (SSP245 and SSP585) in two periods including 2061–2080 (Left) and 2081–2100 (Right) with the most important environment predictor. The range of red in the circle indicates the RONA value and the color on the map represents the level of Prec_03. |
Local adaptation promotes genetic divergence of populations, which can enable populations to sustain productivity during climate changes (Wadgymar et al., 2022). Unraveling the adaptive mechanisms is crucial for survivals and reproductions of organisms. Here, we applied landscape genomics to reveal how environments shaped the patterns of genetic variation of Actinidia eriantha and genetic signatures of adaptation to heterogeneous environments. Our results provided novel insights into the adaptation on how plants adapted to their local habitats and offered the guides on applications in conservation efforts.
4.1. Evolutionary lineages shaped by geography and genetic diversity pattern alongside longitudinal gradientsGenetic structure is shaped by multiple factors including gene flow, selection, population demographic history and landscape ecology (Chen et al., 2023). With PCA and clustering analysis, we observed that natural populations of Actinidia eriantha were divided into four main clusters from east to west mirroring their geographic origins (Fig. 1). This pattern of population differentiation had also been observed in other plants such as ginkgo (Zhao et al., 2019), Emmenopterys henryi (Xu et al., 2021), and Phyllostachys edulis (Zhao et al., 2021). In addition, we noted a longitudinal correlation with genetic diversities gradually increasing from west to east, with the highest genetic diversity observed in Cluster East, particularly among populations proximate to Wuyi Mountains, and the lowest in the Southeast group (Figs. S3 and S4). The higher genetic diversity in Cluster East also reflected the demographic history of A. eriantha revealed by chloroplast and microsatellite markers, as the Wuyi Mountains act as a refugium of A. eriantha during the glacial periods (Guo et al., 2022). Intriguingly, we found evidence suggesting a decline in effective population size during the Mid-Pleistocene Transition and the late glacial maximum (10, 000–20, 000 years ago) in all groups except the east cluster (Fig. S2), further supporting the role of the areas surrounding Wuyi Mountains as a historical refuge for A. eriantha (Guo et al., 2022; Huang et al., 2023; Ma et al., 2022).
4.2. Precipitation and solar radiation driven genetic variation and contributed to local adaptationTo our knowledge, natural populations spanning wide geographic ranges are subjected to selection pressures imposed by heterogeneous environments, which often shape the patterns of genetic diversity. For instance, precipitation, one crucial climate factor, exerts contrasting impacts on plants, with appropriate amounts and timing playing pivotal roles in growth, reproduction, and distribution (Li et al., 2019; Xiao et al., 2024). Previous studies have suggested that Actinidia eriantha is sensitive to precipitation, and optimizing water absorption while maintaining an appropriate water balance is crucial for preventing both water deficits and waterlogging due to the species' root system structure and spatial distribution (Xiloyannis et al., 2023). Additionally, Guo et al. (2022) found that the radical expansion of habitats for A. eriantha occurred during the Last Glacial Maximum, while the species faced its most restricted distribution during the Last Interglacial, similar to Taxus cuspidata (Luo et al., 2024). It identified precipitation played a significant role in the distribution and adaptation of kiwifruit to some extent. In our study, the outcome of GF analysis also provided additional support for precipitation as the primary driver of genetic variations (Figs. 3 and S5). Besides, we further identified several homologous genes associated with precipitation or drought stresses, including GBF3, PUBs and ARF1 (Table S7) (Ramegowda et al., 2017; Song et al., 2017; Verma et al., 2022). Among these genes, U-box proteins have been demonstrated to play a significant role in adaptation and are correlated with drought stress in A. eriantha (Zhang et al., 2023b).
Besides precipitation, sunlight is also one of the most important sources of energy, tightly associated with plant growth and development (Dotto et al., 2018). It has been proven that solar radiation serves as a crucial factor influencing genetic variations and adaptation (Bosco et al., 2020; Wang et al., 2008). For example, Triticum dicoccoides between sun and shade habitats exhibited a significant difference of overall variations and showed a non-ignorable genetic differentiation, implying that solar radiation driven natural selections in wheat (Ren et al., 2017). In our study, we not only observed that solar radiation significantly impacts genetic variations as indicated by GF (Figs. 3 and S5), but also found associated homologous genes such as RAD54, Lhcb1.1, and SPL (A.-H.-Mackerness et al., 1998; Hirakawa et al., 2017; Xie et al., 2020). Hence, it is reasonable to infer that precipitation and solar radiation variables are likely to be important factors driving genetic divergence and promoting adaptation within Actinidia eriantha.
4.3. Genomic signatures of adaptation to heterogeneous environmentsNatural selection consistently leads to the genetic and phenotype differentiation due to heterogeneous environments especially for trees with wide distributions, as well as resulted in local adaptation of populations, and numerous genes were involved in this process (Dorman et al., 2009; Morente-Lopez et al., 2022; Nocchi et al., 2023; Yuan et al., 2023). An effective tool to deeply detected adaptation-associated loci along environmental gradients is GEA, which could illustrate the genetic basis of local adaptation. However, we obtained different numbers of variant sites using datasets that include and exclude the HA population (Fig. S6). This significant difference in the number of outlier loci suggested populations at distribution limits influence their adaptive capacity to environmental changes, and high genetic differentiation between them and other populations had great influences on detection effectiveness in GEAs as well (Mendez-Cea et al., 2023).
Totally, we identified 18, 851 outliers linked to the environmental adaptation and uncovered 1, 566 genes (Table S7), some of which played vital roles in abiotic and biotic stresses, phenotypic traits regulation. For instance, AP2/ERF transcript factors were considered as participating in growth, development of plants, and they were also involved in flowering development in Actinidia eriantha (Jiang et al., 2022). We identified multiple members of AP2/ERF family including ERF3, ERF14 and ERF096. Thereinto, ERF096 has been implicated in regulation of Abscisic Acid (ABA) response and selenium tolerance in Arabidopsis thaliana (Jiang et al., 2020; Wang et al., 2015), indicating several ERF genes have similar functions to promote adaptation to stresses in A. eriantha. Besides, we also demonstrated that calcium-dependent protein kinases, including CPK5, CPK11, and CPK15 (Table S7). Their homologous genes, which have been identified in A. thaliana, play crucial roles in plant signal transduction, thereby mediating plant development and environmental responses (Yip Delormel and Boudsocq, 2019).
4.4. Spatial pattern of adaptation loci shaped by environment factorsMore importantly, among these adaptive loci, we discovered clear genetic footprints of divergent selection at two significant loci of AeERF110 which exhibited divergent genotypes between populations of the East and the West. The frequencies of alleles at these loci in the exon were tightly correlated with environmental factors, particularly extremes in solar radiation and precipitation (Fig. 4). This unique distribution pattern of allele frequencies has been identified not only in kiwifruit but also in other plant species, such as Populus koreana (Sang et al., 2022). A similar phenomenon was also reported in Populus cathayana, which showed one adaptive SNP of UVR8 exhibited different alleles in regions with high and weak solar radiation, which played an important role in high-altitude adaptation (Xiang et al., 2024). They also reaffirmed the significance of precipitation and solar radiation in shaping patterns of genetic variation and provided reliable evidence of local adaptation in Actinidia eriantha. Besides, all the cases discussed above indicated that environmental gradients could exert different and strong selective pressures on plant populations, and selections due to heterogeneous environments likely shaped the spatial and temporal distributions of genetic variations across natural populations of plants, potentially driving local adaptation.
It also needs to be pointed out that ERF110 as a crucial gene in flowering time control has been documented in various species, such as chrysanthemum (Huang et al., 2022) and Morusa-tropurpurea (Dai et al., 2023). In angiosperms, flowering time as one of important traits is tightly associated with potentiality of adaptation to local environment and adjusting optimal flowering time based on ambient environments is an important strategy for plants to adapt to climate changes (Price et al., 2020; Shim et al., 2017). Flowering time was associated with many factors, of which rainfall and solar radiation were also sought as important factors (Moore and Lauenroth, 2017). For example, UV-B as a main component of solar radiation can delay flowering time in Arabidopsisthaliana through regulating expressions of MSI1 and CLF (Dotto et al., 2018). Therefore, we speculated precipitation and solar radiation might modulate flowering time through genotype variations, which promoted the adaptation of A. eriantha in local habitats. However, further works including point mutations, transgenic experiment, stress experiments and RNA-seq, etc. are needed to be conducted in the future for illuminating detailed mechanisms about functional differentiation of ERF110 alleles in mediating adaptation to local environments. Besides, in this study, allelic variation in ERF110 may modulate flowering time in A. eriantha, which presents an opportunity to take genome editing technologies such as prime editing in kiwifruit germplasm conservation (Yin et al., 2024; Zhou et al., 2024).
4.5. Genetic mismatches to the changing climates and managements to protect the Actinidia erianthaChanges of local environments would bring challenges for organisms, especially for sessile plants. As climate warming leads to the loss of original habitats, plants are forced to migrate to new areas, such as higher altitudes or latitudes, in search of suitable environments (Aguirre-Liguori et al., 2019; Wu et al., 2022). In the face of potential decrease of individual numbers and distribution areas under environmental change, efforts should be made to explore populations with pre-adaptive genotypes and enhance the gene flow between populations, which may help these risk populations mitigate the impact of future climate change (Kremer et al., 2012). However, more researches now predict the changes based on species distribution models (SDM), which ignore evolution history and genetic change of population and limited in estimated the adaptive potentials of populations (Shen et al., 2022; Thuiller et al., 2019; Wang et al., 2021). To overcome the gaps of knowledge, we used Gradient Forest to value the genomic vulnerability, which was widely used to other species (Bay et al., 2018; Jiang et al., 2021). Results showed that populations in middle and east regions were more vulnerable and sensitive to future environment changes in our study, which was urgent to propose suitable rescue and protection measures. It is important to emphasize that effective biodiversity conservation should firstly build upon traditional methods such as land conservation, species protection, and policies aimed at preventing deforestation. Additionally, it should purposefully integrate local adaptation strategies to manage gene flow, as well as focus on replanting, reintroduction, and restoring resilient populations in areas where local extinctions have occurred. In regions with high risks, particularly in the middle and east populations, utilizing a composite seed source that includes both native (seeds from plants adapted to local environments) and non-native seeds (seeds introduced from foreign areas) can better boost population diversity and resilience. This multi-source approach may effectively enhance diversity in central populations and provide a buffer against climate change, thereby increasing genetic diversity and reducing the risk of inbreeding depression (Aitken and Bemmels, 2016; Meek et al., 2023). Furthermore, through assessment of adaptive capacity, locally-adapted individuals may also be translocated to areas where they can survive in suitable habitat climate conditions (Thurman et al., 2022). These strategies are also applicable to other species in similar circumstances and offer valuable guidance for conservation efforts.
Although genetic offset is an increasingly popular approach to assess genetic maladaptation under changing environments and used to provide implications for biodiversity conservation, it should be also emphasized the limitations and caveats of genetic offset. First, genetic offset analysis does not provide the evidence that outlier loci actually influence the fitness in relation to climate. Besides, it assesses the risk of maladaptation based on the current climate and genetic data, but could not predict how populations respond to climate change in future. In addition, genetic offset analysis assumes that the relationships between environments and genetics remains unchanged from the present to the future (Aguirre-Liguori et al., 2023; Capblancq et al., 2020; Rellstab, 2021; Rellstab et al., 2021). Given these limitations, the results of genetic offsets should be treated cautiously, and further research is needed to validate its assumptions with experiments.
5. ConclusionIn the present study, large-scale genome resequencing was used to shed light on population demographic history, genetic diversity and genetic underpinnings of local adaptation. We constructed a detailed genetic variation map encompassing both SNPs and indels in kiwifruit. In addition, we pointed out that the environmental factors driven the process of local adaptation and genotype-environment associations revealed 1566 genes contributed to the adaptation to various environments. Among them, AeERF110 showed strong geographic and climatic signatures implicated in local adaptation. Moreover, we predicted that populations from east and middle cluster showed higher genetic offsets under future climate changes, which could contribute to designing effective management and conservation strategies. Overall, our study offered a novel understanding of how species adapt to heterogeneous environments and provided the fundamental conservation strategies.
AcknowledgementsThis study was funded by the National Natural Science Foundation of China (grants number 32070377 and 31770374) and Science Fund for Creative Research Groups of the Natural Science Foundation of Hubei Province (2024AFA035).
CRediT authorship contribution statement
Quan Jiang: Writing – review & editing, Writing – original draft. Yufang Shen: Software. Lianhai Wu: Resources. Zhengwang Jiang: Resources. Xiaohong Yao: Writing – review & editing, Conceptualization.
Data availability
Raw sequence reads are deposited in the SRA under BioProject PRJNA916086.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2025.02.003.
A.-H.-Mackerness, S., Liu, L., Thomas, B., et al., 1998. Individual members of the light-harvesting complex Ⅱ chlorophyll a/b-binding protein gene family in pea (Pisum sativum) show differential responses to ultraviolet-B radiation. Physiol. Plantarum, 103: 377-384. DOI:10.1034/j.1399-3054.1998.1030311.x |
Aguirre-Liguori, J.A., Ramirez-Barahona, S., Tiffin, P., et al., 2019. Climate change is predicted to disrupt patterns of local adaptation in wild and cultivated maize. Proc. Roy. Sci. B-Biol. Sci., 286: 20190486. DOI:10.1098/rspb.2019.0486 |
Aguirre-Liguori, J.A., Ramirez-Barahona, S., Gaut, B.S., 2021. The evolutionary genomics of species' responses to climate change. Nat. Ecol. Evol., 5: 1350-1360. DOI:10.1038/s41559-021-01526-9 |
Aguirre-Liguori, J.A., Morales-Cruz, A., Gaut, B.S., et al., 2023. Sampling effect in predicting the evolutionary response of populations to climate change. Mol. Ecol. Resour., 25: e13828. |
Aitken, S.N., Bemmels, J.B., 2016. Time to get moving: assisted gene flow of forest trees. Evol. Appl., 9: 271-290. DOI:10.1111/eva.12293 |
Alexander, D.H., Novembre, J., Lange, K., 2009. Fast model-based estimation of ancestry in unrelated individuals. Genome Res., 19: 1655-1664. DOI:10.1101/gr.094052.109 |
Bay, R.A., Harrigan, R.J., Underwood, V.L., et al., 2018. Genomic signals of selection predict climate-driven population declines in a migratory bird. Science, 359: 83-86. DOI:10.1126/science.aan4380 |
Beckmann, M., Václavík, T., Manceur, A.M., et al., 2014. glUV: a global UV-B radiation data set for macroecological studies. Methods Ecol. Evol., 5: 372-383. DOI:10.1111/2041-210X.12168 |
Bosco, L.C., Bergamaschi, H., Marodin, G.A.B., 2020. Solar radiation effects on growth, anatomy, and physiology of apple trees in a temperate climate of Brazil. Int. J. Biometeorol., 64: 1969-1980. DOI:10.1007/s00484-020-01987-w |
Cai, Y., Song, Y., 2000. The revision of vine life-form system and analysis of it in the subtropical zone of East China. Acta Ecol. Sin., 20: 808-814. |
Cantalapiedra, C.P., Hernandez-Plaza, A., Letunic, I., et al., 2021. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol., 38: 5825-5829. DOI:10.1093/molbev/msab293 |
Capblancq, T., Forester, B.R., 2021. Redundancy analysis: a swiss army knife for landscape genomics. Methods Ecol. Evol., 12: 2298-2309. DOI:10.1111/2041-210x.13722 |
Capblancq, T., Fitzpatrick, M.C., Bay, R.A., et al., 2020. Genomic prediction of (mal)adaptation across current and future climatic landscapes. Annu. Rev. Ecol. Evol. Syst., 51: 245-269. DOI:10.1146/annurev-ecolsys-020720-042553 |
Capblancq, T., Lachmuth, S., Fitzpatrick, M.C., et al., 2023. From common gardens to candidate genes: exploring local adaptation to climate in red spruce. New Phytol., 237: 1590-1605. DOI:10.1111/nph.18465 |
Chen, Y., Chen, Y., Shi, C., et al., 2018. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience, 7: 1-6. |
Chen, T., Xu, J., Wang, L., et al., 2023. Landscape genomics reveals adaptive genetic differentiation driven by multiple environmental variables in naked barley on the Qinghai-Tibetan Plateau. Heredity, 131: 316-326. DOI:10.1038/s41437-023-00647-0 |
Cingolani, P., Platts, A., Wang, L.L., et al., 2014. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly, 6: 80-92. |
Dai, F., Zhuo, X., Luo, G., et al., 2023. Genomic resequencing unravels the genetic basis of domestication, expansion, and trait improvement in Morus atropurpurea
. Adv. Sci., 10: e2300039. DOI:10.1002/advs.202300039 |
Danecek, P., Bonfield, J.K., Liddle, J., et al., 2021. Twelve years of SAMtools and BCFtools. GigaScience, 10: giab008. DOI:10.1093/gigascience/giab008 |
DePristo, M.A., Banks, E., Poplin, R., et al., 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet., 43: 491-498. DOI:10.1038/ng.806 |
Dixon, P., 2003. VEGAN, A package of R functions for community ecology. J. Veg. Sci., 14: 927-930. DOI:10.1111/j.1654-1103.2003.tb02228.x |
Dorman, M., Sapir, Y., Volis, S., 2009. Local adaptation in four Iris species tested in a common-garden experiment. Biol. J. Linn. Soc., 98: 267-277. DOI:10.1111/j.1095-8312.2009.01265.x |
Dotto, M., Gomez, M.S., Soto, M.S., et al., 2018. UV-B radiation delays flowering time through changes in the PRC2 complex activity and miR156 levels in Arabidopsis thaliana
. Plant Cell Environ., 41: 1394-1406. DOI:10.1111/pce.13166 |
Doyle, J.J.T., Doyle, J.L., 1990. Isolation of plant DNA from fresh tissue. Focus, 12: 13-15. |
Ellis, N., Smith, S.J., Pitcher, C.R., 2012. Gradient forests: calculating importance gradients on physical predictors. Ecology, 93: 156-168. DOI:10.1890/11-0252.1 |
Feng, L., Du, F.K., 2022. Landscape Genomics in tree conservation under a changing environment. Front. Plant Sci., 13: 822217. DOI:10.3389/fpls.2022.822217 |
Fick, S.E., Hijmans, R.J., 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol., 37: 4302-4315. DOI:10.1002/joc.5086 |
Frichot, E., Schoville, S.D., Bouchard, G., et al., 2013. Testing for associations between loci and environmental gradients using latent factor mixed models. Mol. Biol. Evol., 30: 1687-1699. DOI:10.1093/molbev/mst063 |
Gentry, A.H., 1992. Tropical forest biodiversity distributional patterns and their conservational significance. Oikos, 63: 10. |
Gugger, P.F., Fitz-Gibbon, S.T., Albarran-Lara, A., et al., 2021. Landscape genomics of Quercus lobata reveals genes involved in local climate adaptation at multiple spatial scales. Mol. Ecol., 30: 406-423. DOI:10.1111/mec.15731 |
Guo, R., Zhang, Y.H., Zhang, H.J., et al., 2022. Molecular phylogeography and species distribution modelling evidence of 'oceanic' adaptation for Actinidia eriantha with a refugium along the oceanic-continental gradient in a biodiversity hotspot. BMC Plant Biol., 22: 89. DOI:10.1186/s12870-022-03464-5 |
He, W., Zhao, S., Liu, X., et al., 2013. ReSeqTools: an integrated toolkit for large-scale next-generation sequencing based resequencing analysis. Genet. Mol. Res., 12: 6275-6283. DOI:10.4238/2013.December.4.15 |
Heraghty, S.D., Jackson, J.M., Lozier, J.D., 2023. Whole genome analyses reveal weak signatures of population structure and environmentally associated local adaptation in an important North American pollinator, the bumble bee Bombus vosnesenskii
. Mol. Ecol., 32: 5479-5497. DOI:10.1111/mec.17125 |
Hirakawa, T., Hasegawa, J., White, C.I., et al., 2017.
RAD54 forms DNA repair foci in response to DNA damage in living plant cells. Plant J., 90: 372-382. DOI:10.1111/tpj.13499 |
Hoffmann, A.A., Weeks, A.R., Sgrò, C.M., 2021. Opportunities and challenges in assessing climate change vulnerability through genomics. Cell, 184: 1420-1425. DOI:10.1016/j.cell.2021.02.006 |
Huang, Y., Xing, X., Tang, Y., et al., 2022. An ethylene-responsive transcription factor and a flowering locus KH domain homologue jointly modulate photoperiodic flowering in chrysanthemum. Plant Cell Environ., 45: 1442-1456. DOI:10.1111/pce.14261 |
Huang, L., Li, S., Huang, W., et al., 2023. Glacial expansion of cold-tolerant species in low latitudes: megafossil evidence and species distribution modelling. Natl. Sci. Rev., 10: nwad038. DOI:10.1093/nsr/nwad038 |
Jiang, L., Yang, J., Liu, C., et al., 2020. Overexpression of ethylene response factor ERF96 gene enhances selenium tolerance in Arabidopsis
. Plant Physiol. Biochem., 149: 294-300. DOI:10.1016/j.plaphy.2020.02.024 |
Jiang, X.L., Su, Z.H., Xu, G.B., et al., 2021. Genomic signals reveal past evolutionary dynamics of Quercus schottkyana and its response to future climate change. J. Syst. Evol., 59: 985-997. DOI:10.1111/jse.12703 |
Jiang, Q., Wang, Z., Hu, G., et al., 2022. Genome-wide identification and characterization of AP2/ERF gene superfamily during flower development in Actinidia eriantha
. BMC Genomics, 23: 650. DOI:10.1186/s12864-022-08871-4 |
Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., et al., 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods, 14: 587-589. DOI:10.1038/nmeth.4285 |
Kawecki, T.J., Ebert, D., 2004. Conceptual issues in local adaptation. Ecol. Lett., 7: 1225-1241. DOI:10.1111/j.1461-0248.2004.00684.x |
Kremer, A., Ronce, O., Robledo-Arnuncio, J.J., et al., 2012. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett., 15: 378-392. DOI:10.1111/j.1461-0248.2012.01746.x |
Lascoux, M., Glémin, S., Savolainen, O., 2016. Local adaptation in plants. eLS: 1-7. DOI:10.1002/9780470015902.a0025270 |
Lasky, J.R., Josephs, E.B., Morris, G.P., 2022. Genotype–environment associations to reveal the molecular basis of environmental adaptation. Plant Cell, 35: 125-138. |
Li, H., Durbin, R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25: 1754-1760. DOI:10.1093/bioinformatics/btp324 |
Li, Y., Hou, L., Song, B., et al., 2019. Seasonal distribution of increased precipitation in maternal environments influences offspring performance of Potentilla tanacetifolia in a temperate steppe ecosystem. J. Plant Ecol., 12: 742-750. DOI:10.1093/jpe/rtz011 |
Liao, G., Xu, X., Huang, C., et al., 2021. Resource evaluation and novel germplasm mining of Actinidia eriantha
. Sci. Hortic., 282: 110037. DOI:10.1016/j.scienta.2021.110037 |
Liu, Y., Li, D., Zhang, Q., et al., 2017. Rapid radiations of both kiwifruit hybrid lineages and their parents shed light on a two-layer mode of species diversification. New Phytol., 215: 877-890. DOI:10.1111/nph.14607 |
Liu, F., Wu, H., Zhao, Y., et al., 2022. Mapping high resolution National soil information Grids of China. Sci. Bull., 67: 328-340. DOI:10.1016/j.scib.2021.10.013 |
Luo, Y., Qin, W., Yan, Y., et al., 2024. Climate change vulnerability and conservation strategies for tertiary relict tree species: insights from landscape genomics of Taxus cuspidata
. Evol. Appl., 17: e13686. DOI:10.1111/eva.13686 |
Ma, Y., Liu, D., Wariss, H.M., et al., 2022. Demographic history and identification of threats revealed by population genomic analysis provide insights into conservation for an endangered maple. Mol. Ecol., 31: 767-779. DOI:10.1111/mec.16289 |
McKenna, A., Hanna, M., Banks, E., et al., 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res., 20: 1297-1303. DOI:10.1101/gr.107524.110 |
Meek, M.H., Beever, E.A., Barbosa, S., et al., 2023. Understanding local adaptation to prepare populations for climate change. BioScience, 73: 36-47. DOI:10.1093/biosci/biac101 |
Mendez-Cea, B., Garcia-Garcia, I., Gazol, A., et al., 2023. Weak genetic differentiation but strong climate-induced selective pressure toward the rear edge of mountain pine in north-eastern Spain. Sci. Total Environ., 858: 159778. DOI:10.1016/j.scitotenv.2022.159778 |
Moore, L.M., Lauenroth, W.K., 2017. Differential effects of temperature and precipitation on early- vs. late-flowering species. Ecosphere, 8: e01819. DOI:10.1002/ecs2.1819 |
Morente-Lopez, J., Kass, J.M., Lara-Romero, C., et al., 2022. Linking ecological niche models and common garden experiments to predict phenotypic differentiation in stressful environments: assessing the adaptive value of marginal populations in an alpine plant. Glob. Change Biol., 28: 4143-4162. DOI:10.1111/gcb.16181 |
Nguyen, L.T., Schmidt, H.A., von Haeseler, A., et al., 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol., 32: 268-274. DOI:10.1093/molbev/msu300 |
Nocchi, G., Wang, J., Yang, L., et al., 2023. Genomic signals of local adaptation and hybridization in Asian white birch. Mol. Ecol., 32: 595-612. DOI:10.1111/mec.16788 |
Price, N., Lopez, L., Platts, A.E., et al., 2020. In the presence of population structure: from genomics to candidate genes underlying local adaptation. Ecol. Evol., 10: 1889-1904. DOI:10.1002/ece3.6002 |
Purcell, S., Neale, B., Todd-Brown, K., et al., 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet., 81: 559-575. DOI:10.1086/519795 |
Ramegowda, V., Gill, U.S., Sivalingam, P.N., et al., 2017. GBF3 transcription factor imparts drought tolerance in Arabidopsis thaliana
. Sci. Rep., 7: 9148. DOI:10.1038/s41598-017-09542-1 |
Rellstab, C., 2021. Genomics helps to predict maladaptation to climate change. Nat. Clim. Change, 11: 85-86. DOI:10.1038/s41558-020-00964-w |
Rellstab, C., Zoller, S., Walthert, L., et al., 2016. Signatures of local adaptation in candidate genes of oaks (Quercus spp.) with respect to present and future climatic conditions. Mol. Ecol., 25: 5907-5924. DOI:10.1111/mec.13889 |
Rellstab, C., Dauphin, B., Exposito-Alonso, M., 2021. Prospects and limitations genomic offset in conservation management. Evol. Appl., 14: 1202-1212. DOI:10.1111/eva.13205 |
Ren, J., Chen, L., Jin, X., et al., 2017. Solar radiation-associated adaptive SNP genetic differentiation in wild emmer wheat, Triticum dicoccoides
. Front. Plant Sci., 8: 258. |
Sang, Y., Long, Z., Dan, X., et al., 2022. Genomic insights into local adaptation and future climate-induced vulnerability of a keystone forest tree in East Asia. Nat. Commun., 13: 6541. DOI:10.1038/s41467-022-34206-8 |
Schnitzer, S.A., Bongers, F., 2002. The ecology of lianas and their role in forests. Trends Ecol. Evol., 17: 223-230. DOI:10.1016/S0169-5347(02)02491-6 |
Shen, Y., Xia, H., Tu, Z., et al., 2022. Genetic divergence and local adaptation of Liriodendron driven by heterogeneous environments. Mol. Ecol., 31: 916-933. DOI:10.1111/mec.16271 |
Shim, J.S., Kubota, A., Imaizumi, T., 2017. Circadian clock and photoperiodic flowering in Arabidopsis: CONSTANS is a hub for signal integration. Plant Physiol., 173: 5-15. DOI:10.1104/pp.16.01327 |
Shivanna, K.R., 2022. Climate change and its impact on biodiversity and human welfare. Proc. Natl. Acad. Sci. U.S.A., 88: 160-171. DOI:10.1007/s43538-022-00073-6 |
Song, J., Mo, X., Yang, H., et al., 2017. The U-box family genes in Medicago truncatula: key elements in response to salt, cold, and drought stresses. PLoS One, 12: e0182402. DOI:10.1371/journal.pone.0182402 |
Steinegger, M., Soding, J., 2017. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol., 35: 1026-1028. DOI:10.1038/nbt.3988 |
Sun, H., Zhang, J., Chen, F., et al., 2015. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym., 121: 388-402. DOI:10.1016/j.carbpol.2014.12.023 |
Terhorst, J., Kamm, J.A., Song, Y.S., 2017. Robust and scalable inference of population history from hundreds of unphased whole genomes. Nat. Genet., 49: 303-309. DOI:10.1038/ng.3748 |
Testolin, R., Huang, H.W., Ferguson, A.R., 2016. The Kiwifruit Genome. Springer International Publishing, Cham, pp. 101-114.
|
Thuiller, W., Gueguen, M., Renaud, J., et al., 2019. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun., 10: 1446. DOI:10.1038/s41467-019-09519-w |
Thurman, L.L., Gross, J.E., Mengelt, C., et al., 2022. Applying assessments of adaptive capacity to inform natural-resource management in a changing climate. Conserv. Biol., 36: e13838. DOI:10.1111/cobi.13838 |
Verma, S., Negi, N.P., Pareek, S., et al., 2022. Auxin response factors in plant adaptation to drought and salinity stress. Physiol. Plant., 174: e13714. DOI:10.1111/ppl.13714 |
Wadgymar, S.M., DeMarche, M.L., Josephs, E.B., et al., 2022. Local adaptation: causal agents of selection and adaptive trait divergence. Annu. Rev. Ecol. Evol. Syst., 53: 87-111. DOI:10.1146/annurev-ecolsys-012722-035231 |
Wang, Y., Qiu, N., Wang, X., et al., 2008. Effects of enhanced UV-B radiation on fitness of an alpine species Cerastium glomeratum Thuill. J. Plant Ecol., 1: 197-202. DOI:10.1093/jpe/rtn018 |
Wang, X., Liu, S., Tian, H., et al., 2015. The small ethylene response factor ERF96 is Involved in the regulation of the abscisic acid response in Arabidopsis
. Front. Plant Sci., 6: 1064. |
Wang, Z., Zhong, C., Li, D., et al., 2021. Cytotype distribution and chloroplast phylogeography of the Actinidia chinensis complex. BMC Plant Biol., 21: 325. DOI:10.1186/s12870-021-03099-y |
Wang, T.R., Meng, H.H., Wang, N., et al., 2023. Adaptive divergence and genetic vulnerability of relict species under climate change: a case study of Pterocarya macroptera
. Ann. Bot., 132: 241-254. DOI:10.1093/aob/mcad083 |
Wu, T., Hu, E., Xu, S., et al., 2021. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation, 2: 100141. |
Wu, L., Zhang, Y., Guo, X., et al., 2022. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol., 7: 1054-1062. DOI:10.1038/s41564-022-01147-3 |
Xiang, X., Zhou, X., Zi, H., et al., 2024.
Populus cathayana genome and population resequencing provide insights into its evolution and adaptation. Hortic. Res., 11: uhad255. DOI:10.1093/hr/uhad255 |
Xiao, S., Li, S., Huang, J., et al., 2024. Influence of climate factors on the global dynamic distribution of Tsuga (Pinaceae). Ecol. Indic., 158: 111533. DOI:10.1016/j.ecolind.2023.111533 |
Xie, Y., Zhou, Q., Zhao, Y., et al., 2020.
FHY3 and FAR1 integrate light signals with the miR156-SPL module-mediated aging pathway to regulate Arabidopsis flowering. Mol. Plant, 13: 483-498. DOI:10.1016/j.molp.2020.01.013 |
Xiloyannis, C., Dichio, B., Mininni, A.N., 2023. Vine nutrition and water requirements. In: Richardson, A.C., Burdon, J.N., Ferguson, R. (Eds.), Kiwifruit Botany, Production and Uses. CABI, Boston, pp. 164-183.
|
Xu, W.Q., Comes, H.P., Feng, Y., et al., 2021. A test of the centre–periphery hypothesis using population genetics in an East Asian Tertiary relict tree. J. Biogeogr., 48: 2853-2864. DOI:10.1111/jbi.14244 |
Yao, X., Wang, S., Wang, Z., et al., 2022. The genome sequencing and comparative analysis of a wild kiwifruit Actinidia eriantha
. Mol. Hortic., 2: 13. DOI:10.1186/s43897-022-00034-z |
Yin, K., Chung, M.Y., Lan, B., et al., 2024. Plant conservation in the age of genome editing: opportunities and challenges. Genome Biol., 25: 1-25. DOI:10.1186/s13059-023-03142-1 |
Yip Delormel, T., Boudsocq, M., 2019. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana
. New Phytol., 224: 585-604. DOI:10.1111/nph.16088 |
Yuan, S., Shi, Y., Zhou, B.F., et al., 2023. Genomic vulnerability to climate change in Quercus acutissima, a dominant tree species in East Asian deciduous forests. Mol. Ecol., 32: 1639-1655. DOI:10.1111/mec.16843 |
Zhang, L.W., Chen, J.Q., Zhao, R.M., et al., 2023a. Genomic insights into local adaptation in the Asiatic toad Bufo gargarizans, and its genomic offset to climate warming. Evol. Appl., 16: 1071-1083. DOI:10.1111/eva.13555 |
Zhang, X., Guo, R., Shen, R.N., et al., 2023b. The genomic and epigenetic footprint of local adaptation to variable climates in kiwifruit. Hortic. Res., 10: uhad031. DOI:10.1093/hr/uhad031 |
Zhang, X., Jiang, Q., Shen, Y.F., et al., 2024. Using landscape genomics to assess local adaptation of fruit trees to current and future climatic conditions. Fruit Res., 4: e003. |
Zhao, Y.P., Fan, G., Yin, P.P., et al., 2019. Resequencing 545 ginkgo genomes across the world reveals the evolutionary history of the living fossil. Nat. Commun., 10: 4201. DOI:10.1038/s41467-019-12133-5 |
Zhao, H., Sun, S., Ding, Y., et al., 2021. Analysis of 427 genomes reveals moso bamboo population structure and genetic basis of property traits. Nat. Commun., 12: 5466. DOI:10.1038/s41467-021-25795-x |
Zhou, S., Cai, L., Wu, H., et al., 2024. Fine-tuning rice heading date through multiplex editing of the regulatory regions of key genes by CRISPR-Cas9. Plant Biotechnol. J., 22: 751-758. DOI:10.1111/pbi.14221 |



