b. Key Laboratory of Pollution Ecology and Environmental Engineering, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China;
c. Yixian Water Conservancy Affairs Service Center, Yixian, 121100, China
Owing to changes in the world economy and the increase in human activities, the spatial pattern of biological distribution has changed, and some biological populations have been redistributed. The resulting biological invasion has become a global concern (Xue et al., 2021, 2022). Seeds represent the initial stages of the growth cycle of sexually propagated plants. Adaptive change under the influence of external factors is also vital in the transformation of plant populations from the beginning to reality, which is highly significant for individual reproduction, population expansion, and resistance to adverse environments (Baloch et al., 2001; Dashzeveg et al., 2018; VonBank et al., 2018; Jager et al., 2019). Therefore, studying the adaptive strategies of invasive plant seeds in the environment would help clarify the invasion mechanism and implement appropriate prevention and control measures.
The dispersal of seeds by invasive plants into new environments is a means of promoting invasion. The number of new habitats and seeds concerned depends on the transport medium, sustainability, and population characteristics of the invaded area (Nathan and Muller-Landau, 2000). The role of rivers in the dispersal of exotic species has been confirmed. In riparian ecosystems, plant seeds are spread to the soil surface by wind or enter water flow, and then secondary dispersal usually occurs (Nilsson et al., 2010). As dispersal corridors, rivers connect upstream and downstream riparian systems, causing the downward dispersal of alien species along rivers (Maskell et al., 2006). Similar to roads, rivers enhance the directional dispersal of alien species along the flow direction (Von Der Lippe and Kowarik, 2007, 2008). The riparian zone is a unique, dynamic habitat with high species diversity because of its high spatial and temporal variability (Kyle and Leishman, 2009). The riparian habitat of rivers usually favors the colonization of exotic plants (Säumel and Kowarik, 2010), because it has dynamic and diverse natural conditions. The dynamic water flow of the river ecosystem is an effective propagation intermediary (Richardson et al., 2007; Zajěc et al., 2011; Chen et al., 2017).
Riparian species with limited terrestrial dispersal ability can expand their distribution range to a large extent through hydraulic activities. For example, producing many small seeds and having these seeds reach the water body contribute to seed spread (Boedeltje et al., 2003). Knight (1985) found that water flow promoted the spread of Quercus robur seeds, and the number of oak seeds in flooded areas was four times that in unflooded areas. Barsoum (2001) found that non-seasonal floods reduced the number of seeds of Populus nigra and Salix alba, indicating that many seeds would diffuse with water flow. Kowarik and Saumel (2008) studied the potential of seeds and stem fragments of invasive tree Ailanthus altissima to spread through rivers; their results showed that floating in rivers for 3 d could significantly increase the germination rate of seeds, and stem fragments could produce adventitious buds and grow roots within 10 d, indicating that water as a medium may enhance species invasion. In addition to the seeds of woody plants, the seeds of herbaceous plants can be transmitted over long distances by water flow. Jacquemyn et al. (2009) found that the seeds of the exotic plant Sisymbrium austriacum were transmitted over long distances by the Meuse River. S. austriacum is a small plant species with a height between 30 and 60 (sometimes 80) cm and a mean seed weight of 0.3 mg (Jacquemyn et al., 2009). Kobayashi et al. (2012) found that the haplotype gene composition of the Sicyos angulatus population on the lower bank of the Abukuma River was similar to that of the upstream population and considered that the river played an important role in its seed dispersal. Love et al. (2013) found that in river systems, the expansion of Impatiens glandulifera mainly occurred through water flow rather than human factors (Love et al., 2013). Jocienė et al. (2023) posited that Echinocystis lobata spreads seeds through water flow by means of amplified fragment length polymorphism (AFLP).
Research on the expansion of plants by rivers as a transport medium has mainly focused on the population size and genetic diversity characteristics distributed along the river (Khudamrongsawat et al., 2004; Jacquemyn et al., 2009; Belzile et al., 2010; Love et al., 2013; Stabile et al., 2016); few studies have focused on how the seed morphology of invasive alien plants responds to changes in river environmental characteristics (riparian soil nutrients, water quality, riparian zone structure) under temporal and spatial scale dynamics (Cunnings et al., 2016). Environmental factors (water and soil) may exert adaptive differentiation selection pressures on invasive exotic plant populations, promoting the evolution of different adaptive strategies. This adaptive strategy is reflected in the trade-off between plant growth and reproduction and in seed morphological characteristics (Ullah et al., 2022; Ho and Siemann, 2023). For example, the composition and structure of soil nutrients (e.g., carbonate and potassium) and soil structure affect the morphological characteristics of plant seeds. Impatiens capensis seeds grown under high carbonate and alkaline soil conditions are small in size and lightweight (Rewicz et al., 2020). Producing many seeds ensures that the plant has a sufficient population size in barren environments (Malmberg et al., 2023). Changes in the water level fluctuation patterns in rivers can affect seed transport, riparian exposure time and duration, seed deposition, and seedling establishment and survival (Poff et al., 2007). Therefore, exploring adaptation strategies for plant seed morphology along riverbanks will facilitate the understanding of the expansion mechanism of invasive plants and provide a theoretical basis for the restoration and management of riparian vegetation.
Ambrosia trifida was observed in northern China in the 1940s. A. trifida can be transmitted in nature by vehicles, water, and human transport (Xian et al., 2023; Son et al., 2024). Today, A. trifida is now distributed along the Liaohe River, Liaoning Province, China (Wang et al., 2022a). The Liao River is among the seven major rivers in China and runs through the Liaoning Province from south to north. Owing to the dense population, high degree of land development and utilization, and high intensity of economic activities under rapid industrialization and urbanization in the Liao River Basin, its biodiversity is critically affected by invasive plants. The most distributed invasive plant is A. trifida (Meng et al., 2022). In this study, A. trifida and the Liaohe River were used as the study object and area, respectively. The effects of river-related environmental factors on the seed traits of seven A. trifida populations along the Liaohe River were measured and analyzed over three consecutive years. We proposed three hypotheses: 1) The Liaohe River helps spread the seeds of A. trifida over a long distance; 2) differences in seed morphology of A. trifida show regular changes along the Liaohe River; and 3) changes in the seed morphology of A. trifida are affected by the chemical elements, water quality, and coastal soil nutrients of the Liaohe River and the physical properties of riparian zones.
2. Materials and methods 2.1. Overview of study area and plant sample collectionThe Liaohe River Reserve begins at the intersection of the eastern and western Liaohe Rivers (Tieling and Fudedian) and passes through four cities: Tieling, Shenyang, Anshan, and Panjin. The geographical coordinates are 123°55'–121°41' E and 43°02'–40°47' N, covering an area of 1869.2 km2 (Fig. 1; Xu, 2023). The collection points in this study were Shuang anqiao (SAQ, 42°19′38″ N, 123°50'13″ E), Shi fosi (SFS, 41°10′22″ N, 122°4′11″ E), Ma hushan (MHS, 42°08′07″ N, 123°11′18″ E), Ju liuhe (JLH, 42°08′07″ N, 123°11′18″ E), Man duhu (MDH, 41°35′17″N, 122°41′17″ E), Da zhangqiao (DZQ, 41°16′48″ N, 122°31′14″ E), and Pan shanzha (PSZ, 41°16′48″N, 122°31'14″ E). In the autumns of 2011–2013, we selected herbaceous plant communities with A. trifida growth in seven sampling sites along the Liaohe River for 3 consecutive years. The distance between two adjacent plant communities was greater than 20 km. Thirty A. trifida plants were selected from each community, and the spacing between them was greater than 1.5 m. The mature fruits of each plant were collected and naturally dried in the laboratory for later use to determine relevant morphological indicators.
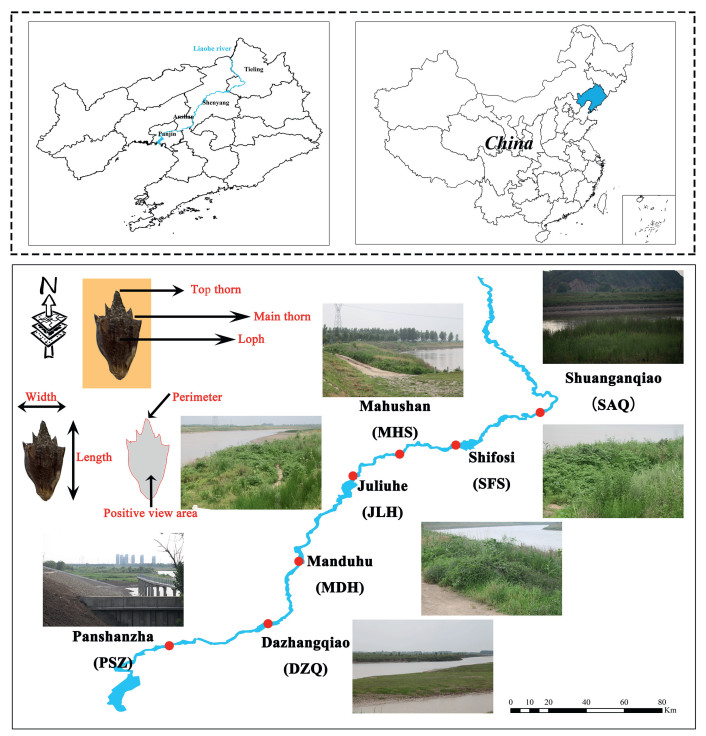
|
| Fig. 1 Overview of sample distribution and seed morphology determination. |
The seed data of 3 consecutive years (2011–2013) were used in triplicate. In 2011, for example, 30 mature seeds from each plant were randomly selected, with 900 seeds in each population, and the average value was calculated as the seed morphological data. The positive view area (PVA), length (LEN, fruit longitudinal axis length), width (WID, fruit transverse axis length), perimeter (PER), number of top thorns (TTN) and main thorns (MTN), and length of top thorns (TTL) and main thorns (MTL) were measured (Fig. 1). The specific measurement methods were based on Wang et al. (2013) and Qu et al. (2015): according to the proportional relationship between pixel density and size, a 1 × 1 cm control rectangle picture was created. The A. trifida seeds and the control rectangle were placed on the same plane, and a single-lens reflex camera (Canon EOS 5D Mark II) was placed above the front of the seeds (60 cm). The seeds were imaged, and the number of image pixels was determined using Adobe Photoshop software (version 21.0.1; Adobe Systems, USA). The results were compared with those of the standard control. Seed parameters, including PVA, PER, and thorn lengths, were calculated. The thousand seed weight (THS) was measured as follows: 100 mature seeds were randomly selected from each plant, with 3000 seeds in each population, and weighed after mixing.
2.3. River water quality indicesWater quality indices of the river were selected based on the indices affecting plant growth and development, and the dissolved oxygen (DO), water total nitrogen (WTN), water total phosphorus (WTP), and water nitrogen-phosphorus ratio (WNP) in the water were determined. In autumn 2011, for example, the Ambrosia trifida seed collection point was the center, and the upper and lower 1 km sections were the measurement area. Three datasets were measured, and the average value was calculated. The DO in the river was measured using a portable multiparameter water quality detector (MDS, China). The WTN and WTP were determined using field sampling and laboratory determination. The 2 L glass container was immersed in water. After the water was collected, the water was preserved in a portable refrigerator and returned to the laboratory for analysis. The total nitrogen content was determined using alkaline potassium persulfate-ultraviolet spectrophotometry (GB11894-89), and the total phosphorus content was determined using ammonium molybdate spectrophotometry (Xu et al., 2023).
2.4. River structure indicesIn this study, the hydrological characteristics and physical structure of the Liaohe River between 2011 and 2013 were used to determine the river structure. The hydrological characteristics included the degree of flow process variation (DFP) and ecological flow satisfaction (ESP). The physical structure primarily included the sediment transport balance index (STE), river bank stability (SRZ; bank slope angle, river bank erosion, river bank vegetation coverage, and longitudinal connectivity), river meander rate (RMR), and river bank shoal deep pool edge index (SDP).
2.4.1. Determination of DFPThe difference between the measured and natural monthly runoff processes in the base year was evaluated under the current development state of river evaluation. This difference was expressed as the average deviation degree between the measured and natural monthly runoffs in the base year and was calculated using Formulas (1-1) and (1-2) (Jin et al., 2017).
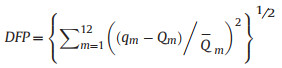
|
(1-1) |
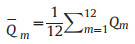
|
(1-2) |
where DFP is the degree of flow process variation, qm is the evaluated monthly runoff in the base year, Qm is the evaluated natural monthly runoff in the base year, and Qm is the evaluated annual average value of the natural monthly runoff in the base year.
2.4.2. Determination of ESPThe monthly water level (discharge volume) changes in the river and lake sections were assessed. The limits were determined using planning or management documents or the status of the historical contemporaneous level. This was calculated using Formula (1-3) (Hu et al., 2021).
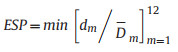
|
(1-3) |
where ESP is the ecological flow satisfaction degree, dm is the water level (discharge) of the month evaluated in the evaluation year of the river and lake section, and Dm is the limit value of the corresponding evaluation month or the historical average value of the same period in the river and lake sections.
2.4.3. Determination of STEFirst, the STE of each month in each year was calculated. Next, the degree of deviation of the average STE from 1 was calculated. STE was calculated using Formula (1-4) (Shi et al., 2009).
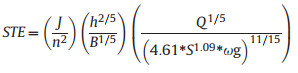
|
(1-4) |
where Q is the water flow, m3/s; S is the sediment concentration, kg/m3; ω is the sediment settling velocity, m/s; h is the average water depth; B is the average river width; J is the sediment transport equilibrium gradient; and n is the river roughness. When STE = 1, the river is in a state of sediment transport equilibrium. The closer STE is to 1, the closer the river sediment transport is to equilibrium. The more STE deviates from 1, the more unbalanced the sediment transport. When STE > 1, the river is in an unsaturated sediment transport state. When STE < 1, the river is in a state of supersaturated sediment transport.
2.4.4. Determination of SRZBased on the current situation of riverbank slope erosion (e.g., occurrence or potential occurrence of riverbank erosion), the evaluation factors included bank slope angle, bank height, matrix characteristics, bank slope vegetation coverage, and slope angle erosion intensity (Table S1). This was calculated using Formula (1-5) (Hu et al., 2021).

|
(1-5) |
Where SAr is the bank slope inclination angle score, SCr is the bank slope coverage score, SMr is the riverbank substrate score, and STr is the slope angle erosion intensity score.
2.4.5. Determination of RMRThe ratio of the length of the river channel to its straight-line distance varies between 1 and 5. A straight river has a bend rate of 1.0–1.2, and a curved river has a bend rate of 1.2–5. This was calculated using Formula (1-6) (Yang, 2012):

|
(1-6) |
The riverbed is distributed with various forms and scales of sediment accumulation below the flat water surface, collectively referred to as shoals. A river section with a large water depth between the shoals is called a deep pool. The sediment accumulation body distributed on the shore is called a side beach and is connected to the riverbank. To calculate this index, we determined the total area of the shoal, deep pool, and side beach in the study section and calculated the ratio of their area to the primary channel area of the study section. This was calculated using Formula (1-7) (Guo, 2019).
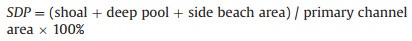
|
(1-7) |
In 2011, the soil was collected from an area where an Ambrosia trifida community was located. Six topsoil samples (0–10 cm) were randomly collected from each sampling point, with a spacing of 2.5 m between adjacent points and approximately 2 kg per bag. The samples were placed in a cool, ventilated area and dried naturally. The stones and residual plants were removed, ground, passed through a 100-mesh sieve, and stored in a self-sealing bag for later use.
Determination of soil nutrient indices was conducted as follows: organic matter (ORM) content, the potassium dichromate dilution heat method (Xiu et al., 2024); soil total nitrogen (STN) content, the semi-micro Kjeldahl method; soil total phosphorus (STP) content, the sodium hydroxide fusion-molybdenum antimony anti-colorimetric method; soil total potassium (STK) content, the alkali fusion flame photometer method; alkali-hydrolyzed nitrogen (AHN) content, the alkali hydrolysis diffusion method. The available phosphorus (AP) was extracted using the sodium bicarbonate antimony anticolorimetric method. The available potassium (AK) was determined using an ammonium acetate extraction flame photometer method (Dan et al., 2024).
2.6. DNA extraction and AFLP-PCR analysis of Ambrosia trifida seedsUsing the seeds from 2011 as the study object, full and mature Ambrosia trifida seeds were first selected, and their hard shells were manually removed. Subsequently, 0.3 g of seeds without hard shells were weighed and ground in a mortar with quartz sand and liquid nitrogen. The ground powder was transferred into a 2-mL centrifuge tube, and 1 mL of modified Cetyltriethylammonium bromide (CATB) and Tris-Glycine-SDS extraction buffers were added. Next, the 2-mL centrifuge tube was placed in a water bath at 65 ℃ for 1 h; the supernatant was centrifuged at 12, 000 g for 10 min at room temperature. Add the same volume of Tris phenol and chloroform: isoamyl alcohol mixture (24:1), shake and mix well, centrifuge at 12, 000 r/min for 10 min at room temperature, and take the supernatant. After the mixture was shaken and mixed, it was centrifuged at 12, 000 g for 10 min at room temperature to remove the supernatant. An equal volume of chloroform: isoamyl alcohol (24:1) was added for efficient mixing, and the mixture was centrifuged at 12, 000 g for 10 min at room temperature to remove the supernatant. This was repeated twice. Furthermore, 2.5 times the volume of pre-cooled anhydrous ethanol was added, and the mixture was refrigerated at −20 ℃ for 30 min and centrifuged at 12, 000 g for 10 min at room temperature to retain the precipitate. In addition, 500 μL of 70% ethanol was added, and the mixture was washed thrice. After drying for 2 h, 100 μL TE buffer was added to dissolve the DNA, and the mixture was refrigerated at −20 ℃. This experiment was repeated thrice. After the DNA samples were qualified, they were stored in TE buffer and stored in a refrigerator at −20 ℃.
The genomic DNA was double digested with restriction enzymes EcoR I and Mse I. The reaction system included EcoRⅠNE buffer (1.5 μL), MseⅠNE buffer (1.5 μL), 100 × BSA (0.2 μL), EcoRⅠ4 U, MseⅠ4 U, water (added to 20 μL DNA digested at 37 ℃ for 3 h), and the restriction endonuclease (inactivated at 65 ℃ for 3 h). Artificial joints were connected, and the reaction system included T4 DNA Ligase buffer (4 μL), MseI adaptor (1 μL), EcoRI adaptor (1 μL), T4 DNA ligase (4 U), 20 μL digestion product, and water (added to 40 μL of the connected joints overnight at 20 ℃). The pre-amplification reaction system included 10 × Taq buffer (2 μL), Mg2+ (25 mmol/L; 1.5 μL), dNTP (10 mmol/L; 0.25 μL), MseIprimer (5 pmol/μL; 2 μL), EcoRIprimer (5 pmol/μL; 2 μL), 10-fold diluted ligation product (5 μL), Taq enzyme (5 U/μL; 0.15 μL), adding water to 20 μL. The reaction conditions were 94 ℃ for 30 s, 56 ℃ for 60 s, 72 ℃ for 60 s, and 24 cycles. Selective amplification reaction system: 10 × Taq buffer (2 μL), Mg2+ (25 mmol/L; 1.5 μL), dNTP (10 mmol/L; 0.4 μL), MseⅠprimer (5 pmol/μL; 2 μL), EcoRⅠprimer (5 pmol/μL; 2 μL), 5 μL of 40-fold diluted pre-amplification product, and 0.2 μL of Taq DNA polymerase (5 U/μL) were used. Reaction conditions were 94 ℃ for 1 min, 65 ℃ (0.7 ℃ lower per cycle) for 1 min, and 72 ℃ for 1 min 30 s after 13 cycles; 94 ℃ for 30 s, 56 ℃ for 1 min, 72 ℃ for 1 min, 23 cycles. Gel analysis: 6% polyacrylamide gel was used, an equal volume of loading buffer was added to the selective amplification product, and electrophoresis was performed at 150 V.
2.7. Statistical and data analysesSPSS software (v.22.0, IBM, USA) was used to compare differences in seed morphology, river water quality, river structure, and soil nutrient content. One-way analysis of variance with least significant difference test. GraphPad Prism 8 (GraphPad Software, USA) and Adobe Photoshop (v.21.0.1; Adobe Systems, USA) were used to draw the diagrams. NTSYS.pc2.10e software was used to calculate the genetic similarity coefficient between different populations, and the unweighted pair-group method with arithmetic means (UPGMA) method was used for cluster analysis. Pearson's correlation analysis was performed based on seed morphology, river water quality and structure indices, and soil nutrient content data (OmicStudio tools at https://www.omicstudio.cn/tool/62; │Pearson's correlation│≥ 0.5; P value < 0.05). Cytoscape 3.8.0 was used to draw the correlation diagram. SPSS software (v.22.0; IBM, USA) and SPSS Amos software (v.24.0.0; IBM, USA) were used for structural equation modeling (SEM) analysis. The reliability (Cronbach α > 0.6) and validity (Kaiser–Meyer–Olkin > 0.5; P value < 0.05) of the four groups of data were tested to verify the reliability of different hypothesis models (Fig. S2). SPSS Amos software (v.24.0.0; IBM, USA) was used to optimize the model several times (the standardized load between latent and observed variables was > 0.5). Adobe Photoshop (v.21.0.1; Adobe Systems, USA) was used for drawing.
3. Results 3.1. Seed morphology differences existed among seven Ambrosia trifida populationsThe differences in fruit morphology among Ambrosia trifida populations distributed along the Liaohe River were as follows: The fruit PVA, PER, LEN, WID, and THS of the PSZ population were significantly higher than those of the other populations (Fig. 2; P < 0.05). The SFS population had the smallest PVA and PER, 0.131 cm2 and 1.502 cm, respectively (Fig. 2; P < 0.05). The seed shape of the MDH population was approximately elliptical, and the top and main spines were not evident. The SFS population had only the main spine structure, and the two populations had significantly different morphologies from those of other populations (Fig. 2). The remaining populations had an evident main thorn structure, and the number was more than four. The MTL of the PSZ population was the longest, with an average of 0.171 cm (Fig. 2; P < 0.05). Except for the MDH population, the number of spikes in the other populations was the same. The spike lengths of SFS, MHS, and JLH were significantly higher than those of the other populations (Fig. 2; P < 0.05), and the spike length of SAQ was the shortest.
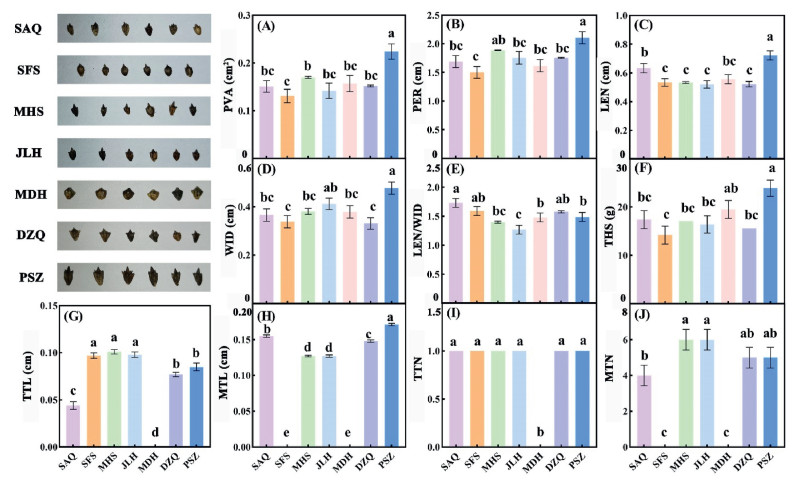
|
| Fig. 2 Seed morphology of seven A.trifida populations in Liaohe River. PVA: positive view area; PER: perimeter; LEN: length; WID: width; LEN/WID: length/width; THS: thousand seed weight; TTL: length of top thorns; MTL: length of main thorns; TTN: number of top thorns; MTN: number of main thorns. SAQ: Shuang anqiao; SFS: Shi fosi; MHS: Ma hushan; JLH: Ju liuhe; MDH: Man duhu; DZQ: Da zhangqiao; PSZ: Pan shanzha. |
The DNA of the seed samples from seven Ambrosia trifida populations was used as a template for AFLP-PCR amplification. Four pairs of primers (Table S2) with clear bands and good polymorphisms were selected from the 64 primer combinations. Furthermore, 206 bands were amplified using four pairs of primers, among which 169 were polymorphic with a polymorphism rate of 82%. The number of bands amplified by each pair of primers ranged from 47 to 58, with an average of 51.5, among which the primer E-ACA: M-CTT had the highest polymorphism rate, 88 %. The percentage of polymorphic bands amplified using four pairs of primers was high (Table S3). The amplification product bands of the seven populations were clear and easy to distinguish, indicating that the selected primer combinations showed good polymorphism (Fig. S1). The PCA of the seed morphology data of the seven A. trifida populations showed that SFS and MDH were significantly clustered together, and the remaining populations also overlapped, indicating a significant correlation among A. trifida populations distributed along the Liaohe River (Fig. 3A). Based on the genetic similarity coefficient, NTSYSpc2.1 software was used to construct a clustering map of seven A. trifida populations by using the UPGMA method (Fig. 3B). These seven populations were divided into two groups for genetic clustering. The JLH, SAQ, MHS, and DZQ populations were classified into one group, and the others were classified into another group. The clustering results showed that the genetic range of the seven A. trifida populations were narrow, the genetic relationships were close, and there was a significant gene exchange among the populations.
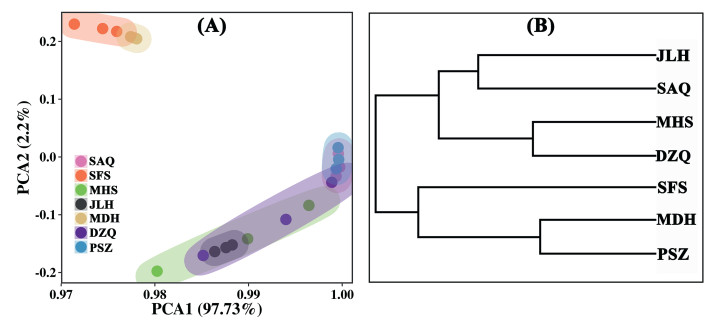
|
| Fig. 3 Cluster analysis of seeds of seven A.trifida populations in Liaohe River. A. Cluster analysis diagram of seed morphology of the populations. B. Population UPGMA unweighted clustering diagram. SAQ: Shuang anqiao; SFS: Shi fosi; MHS: Ma hushan; JLH: Ju liuhe; MDH: Man duhu; DZQ: Da zhangqiao; PSZ: Pan shanzha. |
Along the Liaohe River from top to bottom, most river channel structure indices of the seven A. trifida populations showed an increasing trend (Fig. 4). Between 2011 and 2013, the ESP and SDP of the SFS population were significantly lower than those of the other populations (Fig. 4A–F; P < 0.05). The DFP of the PSZ and SFS populations was significantly higher than that of the other populations (Fig. 4B; P < 0.05). The STE indices of the seven populations showed a phased distribution; the SAQ–SFS–MHS and JLH-MDH-DZQ-PSZ stages showed a significant increasing trend (Fig. 4C; P < 0.05). From SFS to DZQ, SRZ showed a significant downward trend (Fig. 4D; P < 0.05); however, SRZ increased again in PSZ. The RMR values at DZQ and PSZ were significantly lower than those at the other sites (Fig. 4E; P < 0.05). The SDP values of SAQ and SFS in the upper reaches of the Liaohe River were significantly lower than those in the other populations, showing a significant increasing trend from upstream to downstream (Fig. 4F; P < 0.05). There were no significant differences in the DO and N/P ratio among the seven populations (Fig. 4G–J; P < 0.05). Total nitrogen and phosphorus in the reaches where PSZ and DZQ were located were higher than those of the other populations (Fig. 4H and I).
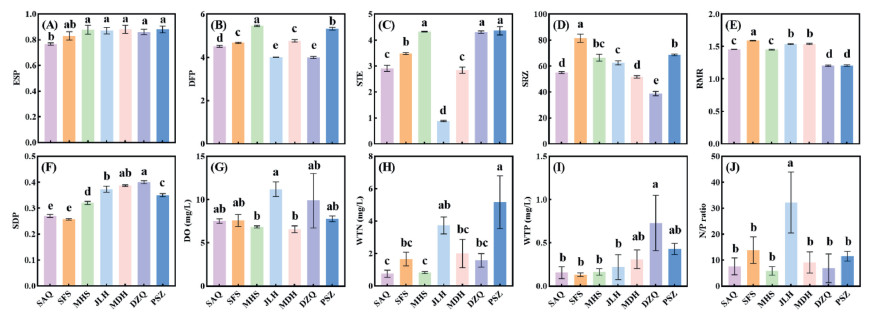
|
| Fig. 4 Water quality and river channel structure indices of seven A.trifida populations in Liaohe River. ESP: ecological flow satisfaction; DFP: degree of flow process variation; STE: sediment transport equilibrium; SRZ: stability of riparian zone; RMR: river meander rate; SDP: shoal, deep pool, and point bar indices; DO: dissolved oxygen; WTN: water total nitrogen; WTP: water total phosphorus; WNP: water N/P ratio. SAQ: Shuang anqiao; SFS: Shi fosi; MHS: Ma hushan; JLH: Ju liuhe; MDH: Man duhu; DZQ: Da zhangqiao; PSZ: Pan shanzha. |
There was a significant change in the soil nutrient content of the seven Ambrosia trifida populations (Fig. 5). Along the Liaohe River, AHN, STN, STK, AK, and ORM in the soil of the seven populations showed a significant increasing trend, and the nutrient content in the soil of the PSZ population was the highest (Fig. 5A–C, E, G; P < 0.05). The AHN (105 mg kg−1) and STN (1.01 g kg−1) contents in the soil of the PSZ population were approximately twice those of the other sites, and AK (218.29 mg kg−1) was almost four times those of the other sites. By contrast, STP and AP showed opposite trends, and the content of the SAQ population in the upper reaches of the Liaohe River was significantly higher than that in the lower reaches (Fig. 5D–F; P < 0.05).
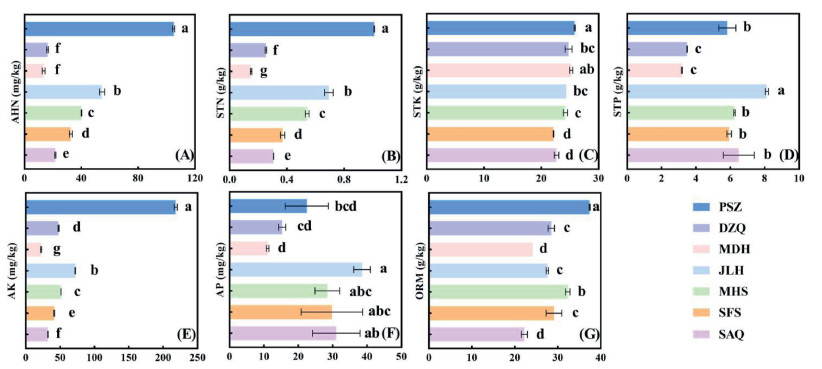
|
| Fig. 5 Soil nutrient contents of seven Ambrosia trifida populations in Liaohe River. AHN: alkali-hydrolyzable nitrogen; STN: soil total nitrogen; STK: soil total potassium; STP: soil total phosphorus; AK: rapidly available potassium; AP: rapidly available phosphorus; ORM: organic matter. SAQ: Shuang anqiao; SFS: Shi fosi; MHS: Ma hushan; JLH: Ju liuhe; MDH: Man duhu; DZQ: Da zhangqiao; PSZ: Pan shanzha. |
Changes in seed morphology along a river are affected by soil nutrients, river structure, and river water quality, and changes in external environmental factors are dynamic. Therefore, exploring the interaction between external factors and plant seed morphology is essential. Cytoscape (v.3.8.2) was used to visualize the seed morphology, river structure, river water quality, and soil nutrient content of seven A. trifida populations along the Liaohe River between 2011 and 2013 (Fig. 6A). There were no close interactions between river water quality, river structure indicators, and A. trifida seed morphology (Fig. 6A). Regarding river water quality indicators, there was a significant positive correlation between DO and seed width (P < 0.05) and a significant positive correlation between total nitrogen and seed PER, WID, and THS (P < 0.05). There were only six pairs of significant correlations between the channel structure indices and seed morphology, and there was a significant negative correlation between RMR and PER, MTL, and MTN (Fig. 6A; P < 0.05). Notably, there were many significant positive correlations between soil nutrient content and seed morphology. There were significant positive correlations between the seed morphology indicators PER, WID, TTL, TTN, and MTN and AHN, STN, AK, and ORM (Fig. 6A; P < 0.05). However, we did not observe a significant relationship between river water quality and channel structure (Fig. 6A; P > 0.05). Furthermore, the degree, betweenness, and closeness centralities showed that the values of river water quality and channel structure indicators were higher than those of soil nutrient content and seed traits, indicating that relying solely on the relationship between indicators does not accurately reflect the actual situation. SEM analysis showed that soil nutrients, river structure, and water quality had direct and indirect effects on seed morphology; however, there were no significant relationships (Fig. 6B; P > 0.05). Only soil nutrients had a significant positive effect on seed morphology (Fig. 6B; P < 0.01). Furthermore, except for seed morphology, there was no significant correlation between the other potential variables (P > 0.05) (Fig. 6A). Soil STN, AK, and ORM had a significant positive effect on seed morphology, with a path coefficient of 0.40 (Fig. 6B; P < 0.01). There was a causal relationship among soil nutrients, seed morphology, and the corresponding observed variables. STN, AK, and ORM had a significant positive correlation with soil nutrients, and the average path coefficient exceeded 0.79 on average (Fig. 6B; P < 0.01). PVA, PER, LEN, and WID had greater effects on seed morphology, with path coefficients of 0.59, 0.61, 0.77, and 0.76, respectively (Fig. 6B; P < 0.01). These results indicate that soil nutrient content has a greater effect on the morphological variation of A. trifida seeds than river factors do.
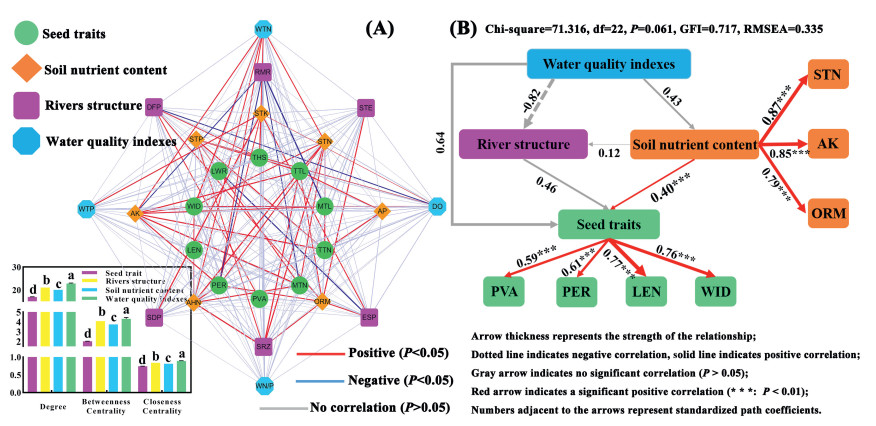
|
| Fig. 6 Interaction diagram of Ambrosia trifida seed morphology, river water quality and channel structure indices, and soil nutrient content. A. Analysis of interaction between indicators. B. Structural equation modeling (SEM) analysis. PVA: positive view area; PER: perimeter; LEN: length; WID: width; LEN/WID: length/width; THS: thousand seed weight; TTL: length of top thorns; MTL: length of main thorns; TTN: number of top thorns; MTN: number of main thorns. ESP: ecological flow satisfaction; DFP: degree of flow process variation; STE: sediment transport equilibrium; SRZ: stability of riparian zone; RMR: river meander rate; SDP: shoal, deep pool, and point bar indices; DO: dissolved oxygen; WTN: water total nitrogen; WTP: water total phosphorus; WNP: water N/P ratio. AHN: alkali-hydrolyzable nitrogen; STN: soil total nitrogen; STK: soil total potassium; STP: soil total phosphorus; AK: Rapidly available potassium; AP: rapidly available phosphorus; ORM: organic matter. |
In this study, we investigated the series of changes in the seed morphology of seven Ambrosia trifida populations along the Liaohe River and whether these changes were closely related to the potential environmental conditions associated with the river. The entire dataset was analyzed to reveal differences in seed area, perimeter, and quality among different populations.
4.1. No direct evidence that Liaohe River is a medium for Ambrosia trifida seed propagationThe AFLP-PCR analysis showed a significant gene exchange among the seven populations, and the clustering results of genetic diversity were consistent with the distribution characteristics. Different population groups were interspersed from upstream to downstream. JLH, SAQ, PSZ, and MDH were adjacent populations in the geographical locations. However, the lack of evidence of directed gene flow along rivers does not rule out the spread of A. trifida seeds by water flow, and we could not directly conclude that A. trifida seeds are transmitted by rivers.
Notably, the potential of Ambrosia trifida seeds to spread along rivers must be considered. Plants often need to fulfill three conditions with the help of water flow as a medium. The first condition is the survival ability and floating duration of seeds in the water flow (Nilsson et al., 2002). If the floating time is too short, seeds will sink to the bottom before reaching the shore. Additionally, there is a positive correlation between the diversity and abundance of species along the river and the floating ability of seeds (Johansson et al., 1996). The second condition is the number of seeds that can be released by plants at one time. For any plant, unpredictable factors in the water flow can cause the plant to lose many seeds (Boedeltje et al., 2003). The third condition is plant height. In the absence of flooding, plant height determines whether the seed can be released into the river. The probability of the tall gramineous plant Arrhenatherum elatius releasing seeds into the river is higher than that of the dwarf gramineous Poa annua (Boedeltje et al., 2003). In this study, from the upstream SAQ to the downstream PSZ, the greater the PVA, the greater the probability of seeds spreading with water (Fig. 6A). Water flow can disperse seeds on the surface and bottom of the riverbank, and the hydraulic fluctuation of the river affects the water level fluctuation, which changes the range, composition, and quantity of seed propagation (Merritt and Wohl, 2002; Murray Hudson et al., 2014). Water dispersal is a complementary means of establishing populations of the invasive plant A. altissima in riparian corridors, for which seed germination rates increase and seedling emergence cycles shorten when floating in rivers (Kowarik and Saumel, 2008). The seeds of the three invasive plants Acer platanoides, A. negundo, and A. altissima were artificially placed in urban rivers, and one quarter of the seeds floated 1200 m within 3 h (Säumel and Kowarik, 2010), demonstrating that rivers are possible carriers of dispersal. A. trifida seeds can float in water for 24 h without sinking. Per the ecological flow satisfaction stability of the river, with an increase in the DFP variation, larger PVA seeds may have better floating and moving abilities. The wide river channel and slow water flow of the Liaohe River can increase the residence time of seeds, and seasonal water flow changes enhance the connection among the upstream, downstream, mainstream, and tributary of the river, further promoting seed spread (Schmiedel and Tackenberg, 2013; Leeuwen et al., 2014; Yan et al., 2015).
Unlike the regular coasts of urban rivers, along natural rivers, there are several types of aquatic plants. Plant debris and artificial floats increase the probability of seed fixations. Under the same conditions, seeds with more barbs and larger perimeters have a higher fixation probability. Studies have confirmed that the spread of A. trifida seeds is closely related to human activities, for example, being physically stuck to vehicles for long-distance dispersal along the direction of traffic roads (Son et al., 2024). In this study, from SAQ to PSZ, the RMR of the river reaches where the seven populations were located showed a downward trend (Fig. 4E), and the RMR of the seven sampling points was > 1.2, all of which belonged to the curved river reach. This helps the seeds to run aground (Ning et al., 2023). The stranded seeds are easily buried by sediment deposits, facilitating subsequent seed germination and seedling establishment (Orth et al., 1994). Furthermore, Ning et al. (2023) found that the geomorphological landscape of tidal rivers may act as a springboard for the invasion of alien invasive plants into the inland. The raised structure in rivers creates a local environment with low fluid disturbance, low bed shear stress, and high reproductive pressure, promoting the establishment and growth of Spartina alterniflora seedlings (Ning et al., 2023). With a decrease in river sinuosity, seeds with larger perimeters, greater number of main thorns, and longer length of main thorns are more likely to remain in the river and successfully colonize. Meandering and changeable rivers increase the complexity of the river and produce complex flow structures, which will retain the seed and facilitate its determination (Nilsson et al., 2010; Cunnings et al., 2016).
In this study, we found no significant correlation between water quality indices (WTN, WTP, water N/P ratio) and dissolved oxygen) and seed morphology and river indices. Only WTN had a significant positive correlation with the soil nutrient (STN, STP, and rapidly available potassium) contents (Fig. 6A; P < 0.05). Nutrient content in water has a significant effect on the growth of plant communities because the soil nutrient content in the riparian zone is easily affected by fluctuations in the river water level. The nutrient content of water may affect the local germination conditions of seeds and change the diversity and structure of plant communities on the bank (Leeuwen et al., 2014). Therefore, the effect of river water quality on seed morphological changes may be indirect and affected by many factors such as river structure, plant population, and climatic conditions. The SEM results showed direct or indirect relationships between river water quality indicators and river structure, soil nutrients, and seed morphology; however, these relationships were not significant (Fig. 6B), indicating that additional potential factors should be considered to reflect the real relationship.
4.2. Soil nutrients are the primary factors regulating morphological adaptation strategies of Ambrosia trifida seedsThe seeds of invasive alien plants have high yield, high tolerance, and multi-morphology and can rapidly adapt to the growth environment and promote colonization and dispersal (Xue et al., 2018). Herbaceous plants have a short growth cycle, and their growth and reproduction are susceptible to external environmental disturbances. Small seed mass may contribute to their long-distance dispersal (Malmberg et al., 2023); that is, the probability of the large-scale release of small seeds scattered farther away is higher than that of larger seeds. In the presence of environmental interference factors, this helps ensure population reproduction and colonization in new habitats (Pausas and Lavorel, 2003; Kyle and Leishman, 2009). In this study, there was a significant positive correlation between seed morphology (PVA, PER, LEN, and WID) and soil nutrients (STN), rapidly available potassium, and organic matter (Fig. 6B). With increasing nutrient content, the seed mass and area of A. trifida gradually increased, and the seed mass and area of the PSZ population were the largest (Fig. 2). Studies have confirmed that different environmental factors have different effects on plant growth and development (Ullah et al., 2022; Wang et al., 2022b). As mentioned in the preface, the adaptive strategies of seed morphology are governed by changes in temperature (Zhou et al., 2021), water (Zhou et al., 2024), soil nutrients (Rewicz et al., 2020), and other factors. A higher nutrient content can promote healthy plant growth and produce more and fuller seeds. The soil nutrient content gradually increased from the SAQ to the PSZ population, which may have been affected by agricultural production and industrialization. Studies have shown that the "percentage of agricultural land" is positively correlated with exotic plant richness. The nutrient content of riparian habitats surrounded by large-scale agricultural areas increased significantly. Similarly, the seasonal planting of agricultural land provides development space for the reproduction of exotic species (Malavasi et al., 2014; Chen et al., 2017). For example, Impatiens parviflora plants growing in ant nests are larger and show higher seed yields than those not growing in ant nests and benefit from high nitrate and AP contents (Holec et al., 2023).
Notably, the role of the maternal effect in plant seed morphology is important. Maternal effect is an adaptive strategy of plants. When offspring experience conditions similar to those of the maternal environment, there are advantages for offspring in the process of growth and development (Galloway and Etterson, 2007; Vivas et al., 2017). Maternal effects can affect the number and size characteristics of plant seeds (Germain et al., 2014; Geshnizjani et al., 2019). The increase in nitrogen content in the environment of Stipa krylovii and Artemisia frigida maternal plants was found to significantly enhance the quality and length of seeds, which aided their dispersion and increased their competitiveness; the offspring of plants experiencing nutrient fluctuations showed better growth in the pot experiments (Li et al., 2017); the quality of Peucedanum oreoselinum seeds grown in woodland was significantly higher than that on the roadside, depending on the nutrient supply in the soil. Larger seeds not only have more development time but also have an advantage in nutrient supply, which contributes to the development of seed growth in the early stage (Kołodziejek, 2017); and the offspring of Suaeda corniculata had higher reproductive allocation. When the offspring was in soil with the same salinity as the female parent, offspring plants allocated more biomass to the reproductive part. This allocation trade-off helped alleviate the harm of reduced seed size and yield (Yang et al., 2015). Suaeda salsa has a dual dynamic of bet-hedging, and maternal effects affect the proportion of different seed coat colors and seed size, which may help plants adapt to heterogeneous environments (Jiang et al., 2019). Furthermore, the maternal effect has a profound impact on the growth and development of offspring, and the environment in which the parents are located provides the offspring with the opportunity to adapt in advance (Lázaro Lobo et al., 2020). When Lupinus angustifolius was planted in water-deficient and water-sufficient soils, the offspring of the former showed significantly higher tolerance to drought than the offspring of the latter (Kalandyk et al., 2017); the offspring of Secale sylvestre, a one-year-old gramineous plant, were weaker than those of the control plants in the early stage of growth and development. This difference was reversed in the later stage, and its biomass and seed yield were higher than those of the control. In dry years, this may help S. sylvestre gain an advantage in sandy grasslands (Mojzes et al., 2021). El Keblawy et al. (2016) and Gan et al. (2024) have conducted similar studies.
The results of this study showed that the contents of STN, rapidly available potassium, and organic matter in the soil gradually increased from the upstream to the downstream along the Liaohe River. Compared with the seeds of the upstream SFS population, the seeds of the downstream PSZ population had larger PVA and PER, which might be significantly affected by the environment in which the A. trifida parent was located. Nutrient-rich soil often produces high-intensity competition. The offspring of A. trifida will increase the biomass and surface size of seeds, increase the competitiveness and diffusivity of seeds, and quickly seize space and nutrient resources in the early growth and development stage. For example, there was a significant positive correlation between fruit length and dispersal distance of Erodium cicutarium, and longer seeds spread farther (Jacobs and Lesmeister, 2012); the relationship between the maternal effect of the invasive plant E. cicutarium grown on serpentine and non-serpentine soils and the seed dispersal distance was found. When the E. cicutarium generation grew in an environment with high soil nutrient consistency, the seed dispersal distance was often farther, which was closely related to fruit length (Jacobs and Lesmeister, 2012). In nutrient-poor soils, the seeds of A. trifida tended to be smaller and more numerous, helping offspring survive in resource-poor environments, ensuring the normal reproduction of the population, and avoiding the extinction of the population due to environmental fluctuations. Different trade-off strategies are of substantial significance for the survival of populations in resource-constrained or highly competitive environments (Ghalambor et al., 2007; Larios et al., 2014). In this study, seven A. trifida populations were distributed along the Liaohe River, and the distance between two adjacent populations was more than 20 km. The SAQ and PSZ populations in the upper and lower reaches of the Liaohe River had a straight distance of nearly 200 km. In addition to soil nutrients, the maternal effects caused by plant community structure, temperature and light changes, human activity frequency, and agricultural development status in different population areas may have some confounding effects on the test results. This study only conducted field population experiments, not conduct greenhouse control experiments. Therefore, this study focused on the role of soil nutrients in the discussion of seed morphology and maternal effects of A. trifida but without considering the potential impact of regional environmental factors on the test results at the large-scale level.
Most of the research objects in studies on maternal effects have been the same species growing in different environments or different phenotypes of the same species. Even if scientists ensure that parental genotypes are consistent by using greenhouse culture, genotype differences remain in wild plants. Awan et al. (2018) used Brassica oleracea with the same genetic background but different alleles as the research object and explored the effect of the interaction between environmental factors and gene differences on seed traits. The results showed that beneficial alleles had a consistent effect on environmental factors. This hedging strategy can minimize the risk of subsequent seed germination (Awan et al., 2018). In this study, the AFLP-PCR analysis of seven A. trifida populations only showed that there was a certain gene exchange between the populations, and the genetic relationship of the populations could not be qualitatively described. Therefore, the interaction between population genetic diversity and seed shape is worthy of further research with rivers as the environmental background, which would help explain the potential influence of gene differences on the maternal effect.
This study has limitations. First, on the spatial scale, this study only focused on seven populations of Ambrosia trifida distributed along the Liaohe River. Additionally, the results of this study were only used to explain the seed characteristics of A. trifida in the Liaohe River; thus, the interaction between river structure characteristics and seed traits needs to be expanded. Second, on the time scale, this study only explored the interaction between seeds and river structure, soil nutrients, and water quality in 2011–2013. Notably, the Liaohe River was closed in 2012 to improve water quality and restore the ecological environment, and the environmental factors of the Liaohe River inevitably underwent major changes before and after the closure. Therefore, the differences between the follow-up related research and the results of this study are worthy of attention and need to be discussed in combination with the specific conditions of the Liaohe River in the past 10 years. Finally, the results of this study emphasize the need to focus on the role of the maternal effect in the change in seed traits. Because the invasion time, invasion location, and even invasion times of A. trifida in Liaohe River cannot be accurately determined, the genetic diversity of A. trifida population might affect the maternal effect.
5. ConclusionsThe continuous invasion of the riverine habitat by invasive plants is a global concern. Changes in the seed traits of invasive plants are crucial in their species distribution and population structure. Exploring the interaction between the seed morphology of invasive plants and river environmental factors will facilitate the understanding of the expansion mechanism of invasive plants. The results of this study showed that compared with river structure and water quality characteristics, soil nutrients are the primary factors regulating morphological adaptation strategies of Ambrosia trifida seeds, and maternal effects may play a critical role. Further research should focus on the interaction between regional environmental factors, genetic diversity of the A. trifida population, and seed morphology on the basis of rivers.
AcknowledgmentsThis research was funded by the National Key Research and Development Program of China (2022YFF1301004), the National Key R & D Program (2023YFC2604500).
CRediT authorship contribution statement
Yufeng Xu: Writing – original draft, Methodology, Formal analysis, Conceptualization. Chenyang Xue: Writing – original draft, Methodology, Conceptualization. Xuezhi Wang: Methodology, Investigation. Lin Meng: Methodology, Investigation. Ying Gao: Methodology, Investigation. Mengyang Yu: Methodology, Investigation. Lin Geng: Methodology, Investigation. Meini Shao: Writing – review & editing, Funding acquisition, Conceptualization. Bo Qu: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.10.005.
Awan, S., Footitt, S., Finch-Savage, W.E., 2018. Interaction of maternal environment and allelic differences in seed vigour genes determines seed performance in Brassica oleracea. Plant J., 94: 1098-1108. DOI:10.1111/tpj.13922 |
Baloch, H.A., Tommaso, A.D., Watson, A.K., 2001. Intrapopulation variation in Abutilon theophrasti seed mass and its relationship to seed germinability. Seed Sci. Res., 11: 335-343. |
Barsoum, N., 2001. Relative contributions of sexual and asexual regeneration strategies in Populus nigra and Salix alba during the first years of establishment on a braided gravel bed river. Evol. Ecol., 15: 255-279. |
Belzile, F.O., Labbe´, J., LeBlanc, M.C., et al., 2010. Seeds contribute strongly to the spread of the invasive genotype of the common reed (Phragmites australis). Biol. Invasions, 12: 2243-2250. DOI:10.1007/s10530-009-9634-x |
Boedeltje, G., Bakker, J.P., Bekker, R.M., et al., 2003. Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J. Ecol., 91: 855-866. |
Chen, C., Wu, S., Meurk, C.D., et al., 2017. Effects of local and landscape factors on exotic vegetation in the riparian zone of a regulated river: implications for reservoir conservation. Landsc. Urban Plan., 157: 45-55. |
Cunnings, A., Johnson, E., Martin, Y., 2016. Fluvial seed dispersal of riparian trees: transport and depositional processes. Earth Surf. Process. Landforms, 41: 615-625. DOI:10.1002/esp.3850 |
Dan, S., Wei, C., Ende, X., et al., 2024. Effect and evaluation of vegetation restoration on soil nutrients in coalmine dump of mine wasteland. J. Shanxi Agric. Univ. (Soc. Sci. Ed.), 44: 1-10. |
Dashzeveg, N., Vornam, B., Nergui, S., et al., 2018. Differentiation of Glycyrrhiza uralensis Fisch. ex DC. populations in the Bulgan River Basin, western Mongolia. Genet. Resour. Crop Evol., 65: 1857-1865. DOI:10.1007/s10722-018-0656-z |
El Keblawy, A., Gairola, S., Bhatt, A., Mahmoud, T., 2016. Effects of maternal salinity on salt tolerance during germination of Suaeda aegyptiaca, a facultative halophyte in the Arab Gulf desert. Plant Spec. Biol., 32: 45-53. |
Galloway, L.F., Etterson, J.R., 2007. Transgenerational plasticity is adaptive in the wild. Science, 318: 1134-1136. DOI:10.1126/science.1148766 |
Gan, Q., Liao, H., Liu, J.Y., et al., 2024. Spatiotemporal interaction of risk-spreading strategies for a seed-dimorphic plant. J. Ecol., 112: 1613-1623. DOI:10.1111/1365-2745.14344 |
Germain, R.M., Gilbert, B., 2014. Hidden responses to environmental variation: maternal effects reveal species niche dimensions. Ecol. Lett., 17: 662-669. DOI:10.1111/ele.12267 |
Geshnizjani, N., Sarikhani Khorami, S., Willems, L.A.J., et al., 2019. The interaction between genotype and maternal nutritional environments affects tomato seed and seedling quality. J. Exp. Bot., 70: 2905-2918. DOI:10.1093/jxb/erz101 |
Ghalambor, C.K., McKay, J.K., Carroll, S.P., et al., 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol., 21: 394-407. DOI:10.1111/j.1365-2435.2007.01283.x |
Guo, F.D., 2019. Analysis of Ecosystem Environmental Characteristics and Health Assessment in Liaohe Reserve. Northeastern University December.
|
Ho, D., Siemann, E., 2023. Fruit provisioning of an invasive tree (Triadica sebifera) varies with environment and among populations. Plant Ecol., 224: 255-265. DOI:10.1007/s11258-023-01293-6 |
Holec, M., Holcová, D., Frouz, J., 2023. Red wood ants (Formica rufa) help propagate invasive small balsam (Impatients parviflora) in accordance with the directed dispersal hypothesis. Appl. Soil Ecol., 191: 105048. |
Hu, Y.C., Lu, G., Zhang, Z., et al., 2021. Guidelines for river and lake health assessment. DB12/T 1058-2021.
|
Jacobs, B.S., Lesmeister, S.A., 2012. Maternal environmental effects on fitness, fruit morphology and ballistic seed dispersal distance in an annual forb. Funct. Ecol., 26: 588-597. DOI:10.1111/j.1365-2435.2012.01964.x |
Jacquemyn, H., Looy, K.V., Breyne, P., et al., 2009. The Meuse river as a corridor for range expansion of the exotic plant species Sisymbrium austriacum: evidence for long-distance seed dispersal. Biol. Invasions, 12: 553-561. |
Jager, M.D., Kaphingst, B., Janse, E.L., et al., 2019. Seed size regulates plant dispersal distances in flowing water. J. Ecol., 107: 307-317. DOI:10.7551/mitpress/11989.003.0021 |
Jiang, L., Wang, L., Baskin, C.C., et al., 2019. Maternal effects on seed heteromorphism: a dual dynamic bet hedging strategy. Seed Sci. Res., 29: 149-153. DOI:10.1017/s0960258519000114 |
Jin, S.Y., Zhang, P., Jiang, X.H., et al., 2017. Analysis of flow process variation degree and influencing factors in Inner Mongolia reach of the yellow river. IOP Conf. Ser.: Earth Environ. Sci., 69: 012015. DOI:10.1088/1755-1315/69/1/012015 |
Jocienė, L., Krokaitė, E., Rekašius, T., et al., 2023. The molecular evidence for invasive climber Echinocystis lobata (Michx.) Torr. & A. Gray in eastern and central europe. Diversity, 15: 1084. DOI:10.3390/d15101084 |
Johansson, M.E., Nilsson, C., Nilsson, E., 1996. Do rivers function as corridors for plant dispersal?. J. Veg. Sci., 7: 593-598. DOI:10.2307/3236309 |
Kalandyk, A., Waligórski, P., Dubert, F., 2017. Role of the maternal effect phenomena in improving water stress tolerance in narrow-leafed lupine (Lupinus angustifolius). Plant Breed., 136: 167-173. DOI:10.1111/pbr.12457 |
Khudamrongsawat, J., Tayyar, R., Holt, J.S., 2004. Genetic diversity of giant reed (Arundo donax) in the Santa Ana River, California. Weed Sci., 52: 395-405. |
Knight, R.S., 1985. A model of episodic, abiotic dispersal for oaks (Quercus robur). S. Afr. J. Bot., 51: 265-269. |
Kobayashi, H., Kurokawa, S., Ikeda, K., 2012. Dairyland populations of bur cucumber (Sicyos angulatus) as a possible seed source for riverbank populations along the Abukuma River, Japan. Weed. Biol. Manag., 12: 147-155. DOI:10.1111/j.1445-6664.2012.00447.x |
Kołodziejek, J., 2017. Effect of seed position and soil nutrients on seed mass, germination and seedling growth in Peucedanum oreoselinum (Apiaceae). Sci. Rep., 7: 1959. |
Kowarik, I., Saumel, I., 2008. Water dispersal as an additional pathway to invasions by the primarily wind-dispersed tree Ailanthus altissima. Plant Ecol., 198: 241-252. DOI:10.1007/s11258-008-9398-x |
Kyle, G., Leishman, M.R., 2009. Plant functional trait variation in relation to riparian geomorphology: the importance of disturbance. Austral Ecol., 34: 793-804. DOI:10.1111/j.1442-9993.2009.01988.x |
Larios, E., Búrquez, A., Becerra, J.X., et al., 2014. Natural selection on seed size through the life cycle of a desert annual plant. Ecology, 95: 3213-3220. DOI:10.1890/13-1965.1 |
Lázaro Lobo, A., Herrera, M., Campos, J.A., et al., 2020. Influence of local adaptations, transgenerational effects and changes in offspring's saline environment on Baccharis halimifolia L. under different salinity and light levels. Environ. Exp. Bot., 177: 104134. |
Leeuwen, C.H.A.V., Sarneel, J.M., Paassen, J.V., et al., 2014. Hydrology, shore morphology and species traits affect seed dispersal, germination and community assembly in shoreline plant communities. J. Ecol., 102: 998-1007. |
Li, Y., Hou, L., Song, B., et al., 2017. Effects of increased nitrogen and phosphorus deposition on offspring performance of two dominant species in a temperate steppe ecosystem. Sci. Rep., 7: 40951. |
Love, H.M., Maggs, C.A., Murray, T.E., et al., 2013. Genetic evidence for predominantly hydrochoric gene flow in the invasive riparian plant Impatiens glandulifera (Himalayan balsam). Ann. Bot., 112: 1743-1750. DOI:10.1093/aob/mct227 |
Malavasi, M., Carboni, M., Cutini, M., et al., 2014. Landscape fragmentation, land-use legacy and propagule pressure promote plant invasion on coastal dunes: a patch-based approach. Landsc. Ecol., 29: 1541-1550. DOI:10.1007/s10980-014-0074-3 |
Malmberg, C., Sheley, R., James, J., 2023. Invasive annual grasses show decrease in seed size but no change in growth or carbon economy following invasion. Biol. Invasions, 25: 1613-1625. DOI:10.1007/s10530-023-02999-4 |
Maskell, L.C., Bullock, J.M., Smart, S.M., et al., 2006. The distribution and habitat associations of non-native plant species in urban riparian habitats. J. Veg. Sci., 17: 499-508. |
Meng, Z., Yuying, S., Yebo, Y., et al., 2022. AFLP analysis of genetic resources of Dendrobium from China. Acta. Hort. Sini., 49: 1339-1350. |
Merritt, D.M., Wohl, E.E., 2002. Processes governing hydrochory along rivers: hydraulics, hydrology, and dispersal phenology. Ecol. Appl., 12: 1071-1087. |
Mojzes, A., Kalapos, T., Kröel-Dulay, G., 2021. Drought in maternal environment boosts offspring performance in a subordinate annual grass. Environ. Exp. Bot., 187: 104472. |
Murray-Hudson, M., Wolski, P., Hudson, F.M., et al., 2014. Disaggregating hydroperiod: components of the seasonal flood pulse as drivers of plant species distribution in floodplains of a tropical wetland. Wetlands, 34: 927-942. DOI:10.1007/s13157-014-0554-x |
Nathan, R., Muller-Landau, H.C., 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol., 15: 278-285. |
Nilsson, C., Andersson, E., Merritt, D.M., et al., 2002. Differences in riparian flora between riverbanks and river lakeshores explained by dispersal traits. Ecology, 83: 2878-2887. |
Nilsson, C., Brown, R.L., Jansson, R., et al., 2010. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev., 85: 837-858. DOI:10.1111/j.1469-185x.2010.00129.x |
Ning, Z., Cui, B., Chen, C., et al., 2023. Tidal channel meanders serve as stepping-stones to facilitate cordgrass landward spread by creating invasion windows. Ecol. Appl., 34: e2813. |
Orth, R.J., Luckenbach, M., Moore, K.A., 1994. Seed dispersal in a marine macrophyte: implications for colonization and restoration. Ecology, 75: 1927-1939. DOI:10.2307/1941597 |
Pausas, J.G., Lavorel, S., 2003. A hierarchical deductive approach for functional types in disturbed ecosystems. J. Veg. Sci., 14: 409-416. |
Poff, N.L., Olden, J.D., Merritt, D.M., et al., 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. U.S.A., 1044: 5732-5737. DOI:10.1073/pnas.0609812104 |
Qu, B., Xun, Z., Xu, Y., 2015. The seed ecological mechanism of Xanthium strumarium invasion. Pratacult. Sci., 32: 1801-1807. |
Rewicz, A., Myśliwy, M., Adamowski, W., et al., 2020. Seed morphology and sculpture of invasive Impatiens capensis Meerb. from different habitats. PeerJ, 8: e10156. DOI:10.7717/peerj.10156 |
Richardson, D.M., Holmes, P.M., Esler, K.J., et al., 2007. Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Divers. Distrib., 13: 126-139. DOI:10.1111/j.1366-9516.2006.00314.x |
Säumel, I., Kowarik, I., 2010. Urban rivers as dispersal corridors for primarily wind-dispersed invasive tree species. Landsc. Urban Plan., 94: 244-249. |
Schmiedel, D., Tackenberg, O., 2013. Hydrochory and water induced germination enhance invasion of Fraxinus pennsylvanica. Forest. Ecol. Manag., 304: 437-443. |
Shi, C.W., Ma, J.M., Tian, J., 2009. Study on existing channel pattern discriminants and their functions for alluvial rivers. J. Sediment. Res., 4: 74-80. |
Son, D., Chu, Y., Lee, H., 2024. Roads as conduits for alien plant introduction and dispersal: the amplifying role of road construction in Ambrosia trifida dispersal. Sci. Total Environ., 912: 169109. |
Stabile, J., Lipus, D., Maceda, L., et al., 2016. Microsatellite DNA analysis of spatial and temporal population structuring of Phragmites australis along the Hudson River estuary. Biol. Invasions, 18: 2517-2529. DOI:10.1007/s10530-016-1157-7 |
Ullah, R., Khan, N., Ali, K., 2022. Which factor explains the life-history of Xanthium strumarium L., an aggressive alien invasive plant species, along its altitudinal gradient?. Plant Direct, 6: 375. |
Vivas, M., Kemler, M., Mphahlele, M.M., et al., 2017. Maternal effects on phenotype, resistance and the structuring of fungal communities in Eucalyptus grandis. Environ. Exp. Bot., 140: 120-127. |
Von Der Lippe, M., Kowarik, I., 2007. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv. Biol., 21: 986-996. DOI:10.1111/j.1523-1739.2007.00722.x |
Von Der Lippe, M., Kowarik, I., 2008. Do cities export biodiversity? Traffic as dispersal vector across urban–rural gradients. Divers. Distrib., 14: 18-25. DOI:10.1111/j.1472-4642.2007.00401.x |
VonBank, J.A., Casper, A.F., Pendleton, J.E., et al., 2018. Water hyacinth (Eichhornia crassipes) invasion and establishment in a temperate river system. River Res. Appl., 34: 1237-1243. DOI:10.1002/rra.3362 |
Wang, A., Baskin, C.C., Baskin, J.M., et al., 2022b. Seed position in spikelet as a contributing factor to the success of the winter annual invasive grass Aegilops tauschii. Front. Plant Sci., 13: 916451. |
Wang, D., Qu, B., Zhou, B., et al., 2022a. Prediction of potential invasive region for Ambrosia trifida based on SDMs-toolbox with climate warming in Liaoning Province. Ecol. Sci., 41: 66-74. |
Wang, X., Ling, X., Zhai, Q., et al., 2013. Fruit morphological diversity of seven giant ragweeds populations in Liaohe River Reserve. Pratacult. Sci., 30: 1808-1813. |
Xian, X., Zhao, H., Wang, R., et al., 2023. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ., 859: 160252. |
Xiu, Y.Z., Jiang, P.C., Cong, W., et al., 2024. Effects of different fertilization patterns on soil improvement and vegetation restoration of desertified grassland in northwest Liaoning Province, China. Chin. J. Appl. Ecol., 35: 55-61. |
Xu, X.L., 2023. China's multi-year provincial administrative division boundary data Resource and Environmental Science Data Registration and Publishing System. http://www.resdc.cn/DOI.
|
Xu, Y., Xie, Z., Wang, H., et al., 2023. Effect of land use on the water quality of rivers flowing into Xingkai Lake and ecological restoration strategies. J. Envi. Engi. Tech., 13: 1997-2005. DOI:10.3390/pr11071997 |
Xue, C., Gao, Y., Qu, B., et al., 2021. Hybridization with an invasive plant of Xanthium strumarium improves the tolerance of its native congener X. sibiricum to cadmium. Front. Plant Sci., 12: 696687. |
Xue, C., Sun, L., Qu, B., et al., 2022. Grafting with an invasive Xanthium strumarium improves tolerance and phytoremediation of native congener X. sibiricum to cadmium/copper/nickel tailings. Chemosphere, 308: 136561. |
Xue, C., Xu, Y., Qu, B., 2018. Comparison of morphology, photosynthesis, and growth among Xanthium strumarium, X. sibiricum and their hybrid under different nitrogen levels. Biodivers. Sci., 26: 554-563. |
Yan, Q., Bi, Y., Deng, Y., et al., 2015. Impacts of the Three Gorges Dam on microbial structure and potential function. Sci. Rep., 5: 8605. |
Yang, F., Yang, X., Baskin, J.M., et al., 2015. Transgenerational plasticity provides ecological diversity for a seed heteromorphic species in response to environmental heterogeneity. Perspect. Plant. Ecol., 17: 201-208. |
Yang, L.P., 2012. Determination and Confirmation of Key Index on River Health Evaluation. Yunnan University.
|
Zając, A., Tokarska-Guzik, B., Zając, M., 2011. The role of rivers and streams in the migration of alien plants into the Polish Carpathians. Biodivers. Res. Conserv., 23: 43-56. DOI:10.2478/v10119-011-0012-z |
Zhou, R., Yang, P., Chen, X., et al., 2024. Simulated climate warming strongly constrains the seedling establishment of alpine cushion Arenaria oreophila. Plant Divers.. DOI:10.1016/j.pld.2023.11.003 |
Zhou, L., Yu, H., Yang, K., et al., 2021. Latitudinal and longitudinal trends of seed traits indicate adaptive strategies of an invasive plant. Front. Plant Sci., 12: 657813. |



