b. CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla 666303, Yunnan, China;
c. Ailaoshan Station of Subtropical Forest Ecosystem Studies, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Jingdong 676209, Yunnan, China
Tropical forests play a crucial role in the global carbon and water cycles (Pan et al., 2011; Matos et al., 2020). Tree growth and their response to climate variability have a substantial impact on the carbon sink capacity of tropical forests (Bonan, 2008; Freidlingstein et al., 2019). However, seasonal droughts severely affect tree growth due to the influence of monsoon climate in Asian tropics (Corlett, 2016; Laurance et al., 2009; Rowland et al., 2015). Nevertheless, our understanding of the seasonal dynamics of tree growth and its relationship with climate change (Dong et al., 2012) in Asian tropical forests, as well as their associations with functional traits, is limited due to insufficient long-term plot data.
Although tropical rainforest ecosystems are known for their high annual precipitation, most tropical karst forests experience seasonal droughts due to monsoonal climate and the presence of karst landforms (Zhu et al., 2003). Tropical karst forest habitats are typically characterized by shallow soil layers, abundant fissures and holes, resulting in low soil water availability and poor water retention (Chen et al., 2015; Fu et al., 2019; Liu et al., 2024), posing challenges for plant growth and survival (Querejeta et al., 2007; Schwinning, 2008). Karst trees primarily rely on the limited water available in the shallow soil and underlying bedrock crevices (Querejeta et al., 2007). Tree species growing in karst environments have thick and small leaves with well-developed, tightly arranged palisade tissue (Cao, 2000), higher sapwood density, smaller vessel diameters, and higher embolism resistance to adapt to the dry karst environment (Zhu et al., 2017). Previous studies have shown strong associations between tree growth and hydraulic efficiency in wet tropical rainforests (Hoeber et al., 2014; Fan et al., 2012; Yan et al., 2020). Other studies found that drought tolerance traits significantly impact tree growth in dry environments (Anderegg et al., 2015; Choat et al., 2018; Liu et al., 2019). Yet, the associations between drought tolerance traits and tree growth rates in Asian tropical karst habitats remain poorly understood.
The leaf economic spectrum and stem hydraulic traits have been found to play a crucial role in plant growth and functional ecology (Hietz et al., 2013). Previous studies revealed positive correlations between tree growth and leaf photosynthetic capacity (Prior et al., 2004), nitrogen content (Markesteijn et al., 2011), and plant hydraulic efficiency (Fan et al., 2012). Drought is a significant environmental stress that negatively impacts plant growth (Kramer and Boyer, 1995) and strongly influences species distributions (Kursar et al., 2009). In diverse tropical rainforests, different tree species exhibit various strategies for drought tolerance and avoidance (Delzon, 2015). In a tropical karst habitat, evergreen and deciduous species differed in their drought tolerance and hydraulic traits (Fu et al., 2012). Evergreen species often possess smaller and denser vessels, smaller leaf size and lower specific leaf area and transpiration rates, allowing them to minimize water loss and remain active under low soil water potentials by reducing xylem embolism (Ackerly, 2004; Kursar et al., 2009; Markesteijn et al., 2011; Hoeber et al., 2014). Evergreen trees employ a conservation strategy to extend their photosynthetic activity beyond the rainy season, characterized by leaf traits such as dense mesophyll layer and thick cell walls, maximizing water use efficiency and avoiding wilt under drought conditions (Aguirre-Gutiérrez et al., 2019; González-M. et al., 2021; Poorter et al., 2009). In contrast, deciduous maintain high photosynthetic rates during wet seasons but avoid drought stress by shedding their leaves during the dry season (Givnish, 2002; Markesteijn and Poorter, 2009; Rodrigues et al., 2022). Compared to trees in lowland rainforests, trees in tropical karst habitats exhibit lower leaf water conductivity and higher resistance to xylem cavitation (Fu et al., 2012). The xylem features of plants are strongly associated with drought tolerance (Tyree et al., 1994; Sperry, 2003). A crucial mechanism that enhances drought tolerance is the presence of a 'safe' hydraulic system consisting of smaller and denser vessels (Baas et al., 2004). For example, xylem vessel diameter generally decreases while vessel density increases in response to reduced precipitation and water availability across different habitats (Carlquist, 1977; Machado et al., 2007). Moreover, hydraulic architecture and foliar nitrogen content may play a more significant role in promoting rapid growth (Hoeber et al., 2014). Different species with varying levels of drought tolerance are likely to respond differently to drought stress (Anderegg et al., 2016). This suggests that drought resistance may confer an advantage for tropical tree growth under low rainfall conditions (Tng et al., 2018).
The coexistence of evergreen and deciduous trees in karst habitats provides an excellent opportunity to unravel their respective growth strategies. Previous studies in karst habitats have primarily focused on investigating the coordination or trade-offs among functional traits of co-occurring tree species (Baker et al., 2003; Wagner et al., 2012; Fu et al., 2012; Linger et al., 2020). Yet, there is limited knowledge about the trait syndromes that contribute to drought tolerance (Zhu et al., 2018) and how they relate to seasonal growth patterns in tropical karst habitats. This study aims to investigate the relationships between tree seasonal growth rates and morphological and physiological traits across evergreen and deciduous tree species in a tropical karst habitat in Xishuangbanna, southwestern China. We measured 17 branch and leaf traits of six evergreen and six deciduous tree species and associated these traits with rainy season diameter growth rate (GR) measured by band-dendrometers over four consecutive years. We asked: (1) Does cross-species variation in seasonal growth correlate with branch and leaf functional traits? We expected that branch and leaf traits associated with drought tolerance would explain the seasonal growth rates of trees in the karst habitat. (2) Are the associations between functional traits and growth rate differ among evergreen and deciduous species? We expected that evergreen trees, as compared with deciduous trees, have a more conservative water strategy with secure xylem structure, ensuring them a higher persistence and a faster growth under lower soil water availability in a tropical karst habitat.
2. Materials and methods 2.1. Study site and speciesThe study site is located in the Xishuangbanna Nature Reserve (21°54′N, 101°46′E, 680 m above sea level), Yunnan Province, southwest China. The region experiences a distinct dry season that lasts from November to April of the following year. The average annual precipitation is 1560 mm, with approximately 87% of the rainfall occurring during the rainy season between May and October. The mean annual temperature is 21.7 ℃, with June being the warmest month and December being the coldest month (Zhu et al., 2017).
The tropical karst forests in the study area are primarily dominated by evergreen broadleaf tree species, such as Cleistanthus sumatranus, Celtis philippensis, and Lasiococca comberi. Meanwhile, there is approximately 33% of deciduous woody species (Wang et al., 2023b). A 0.5-ha permanent plot was established in the tropical karst forest site in 2002, and trees with DBH ≥ 2 cm were tagged, and this plot was expanded to 1-ha in 2010. In the 1-ha plot, metal band dendrometers were installed on 491 healthy trees with DBH ≥ 10 cm at their breast height since early 2016. Tree diameter growth rates were measured monthly at a resolution of 0.01 mm with a vernier caliper. In total, 56 repeated measurements were conducted from May 2016 to December 2020.
We selected twelve co-existing tree species in the karst forest, including six evergreen and six deciduous broadleaved species. Species selection was based on their dominance and important values, the sum of relative density (RD), relative frequency (RF) and relative dominance (RA) (Tables 1 and S1). For each species, we selected five healthy trees and collected a 50 cm long perennial terminal branch from the outer canopy of each tree. In total, we sampled 60 individuals across 12 tree species. Samples were collected during the rainy season in July 2018, after the completion of seasonal shoot growth and leaf expansion.
| Species | Family | Diameter at breast height (DHB, cm) | Phenology |
| Cleistanthus sumatranus | Phyllanthaceae | 41.1 ± 4.01 | Evergreen |
| Celtis philippensis | Ulmaceae | 101.8 ± 44.97 | Evergreen |
| Lasiococca comberi | Euphorbiaceae | 47.2 ± 9.31 | Evergreen |
| Mallotus philippinensis | Euphorbiaceae | 53.4 ± 10.16 | Evergreen |
| Trigonostemon bonianus | Euphorbiaceae | 17.8 ± 0.84 | Evergreen |
| Alphonsea monogyna | Annonaceae | 78.4 ± 26.33 | Evergreen |
| Lagerstroemia tomentosa | Lythraceae | 56.96 ± 2.47 | Deciduous |
| Cipadessa baccifera | Meliaceae | 44.4 ± 13.87 | Deciduous |
| Mayodendron igneum | Bignoniaceae | 53.3 ± 9.73 | Deciduous |
| Croton crassifolius | Euphorbiaceae | 53.1 ± 7.78 | Deciduous |
| Diospyros hasseltii | Ebenaceae | 84.25 ± 19.91 | Deciduous |
| Spondias pinnata | Anacardiaceae | 57 ± 5.61 | Deciduous |
We measured 17 branch and leaf traits for each of the 12 tree species. For individual branch, we cut a 3–5 cm segment to determine sapwood density (WD, g cm−3). Sapwood volume was measured with the water-replace method after removing the bark and pith. The wood samples were then oven-dried at 80 ℃ for at least 48 h until a constant weight was achieved. Sapwood density was calculated as the ratio of their dry mass and volume. Another segment of approximately 5 cm in length was cut from the same branch and fixed in the FAA solution (70% alcohol : acetic acid : formaldehyde = 95% : 5% : 5%). Before sectioning, samples were dehydrated with grading ethanol and d-limonene, and immersed in liquid paraffin, then embedded in paraffin blocks. Transvers sections of around 8–10 μm were cut using a rotary microtome (DM2245, Leica, Germany). These sections were stained with safranin, dehydrated with successive alcohol, and fixed with Canada balsam. Microscopic images were captured at 200× magnification using a digital camera mounted on a microscope (DM2500, Leica, Germany) (Fig. S7). Four images were taken at different locations of each section.
Vessel diameters for their major and minor axes were measured using the ImageJ software (http://rsb.info.nih.gov/ij/). Due to the elliptical shape of the vessel, mean diameter was calculated as D = [32(ab)3/(a2b2)]1/4, where a and b are the major and the minor axis, respectively. Hydraulic weighted vessel lumen diameter (Dh) was calculated as Dh = ∑D5/∑D4. Vessel density (VD, no. μm −2) was calculated as the number of vessels per unit sapwood area. Sapwood theoretical hydraulic conductivity (Ktheo) were calculated according to the Hagen–Poiseuille equation: Ktheo = πρ/(128ηAs)[∑D4], where ρ is the water density (998.2 kg m−3 at 20 ℃) and η is the water viscosity (1.002 × 10−9 MPa s−1 at 20 ℃), As is the sapwood area.
2.2.2. Leaf anatomyFor each sampled branch, we selected ten fully expanded sun-exposed leaves and measured their leaf area (LA) with a leaf area meter (Li-3000A; Li-COR, Nebraska). Leaves were then oven-dried at 80 ℃ for 48 h to determine their dry mass (DM). Specific leaf area (SLA, mm2 g−1) was calculated as leaf area per unit of dry mass.
Additionally, we selected five full-expanded leaves from the same branch and immersed them in the FAA solution for 48 h. Leaf sections without major veins from the middle of leaves were cut and embedded with paraffin. Transverse cross-sections with a thickness of 8–12 μm were made using a rotary microtome (DM2245, Leica, Germany). Cross-sections were mounted on glass slides and then stained with safranin solution. After dehydration (70%-85%-95%-100% ethanol), the sections were fixed with Canada Balsam. Leaf cross-sections were examined under a light microscope (DM 2250, Leica, Germany) and photographic images were captured at 200× magnifications using a digital camera (DFC295, Leica, Germany). Total leaf thickness (LT, μm), palisade thickness (PT, μm), spongy thickness (SP, μm), lower epidermis thickness (LET, μm) and upper epidermis thickness (UET, μm) were measured using the ImageJ software (http://rsb.info.nih.gov/ij/) (Fig. S7). Leaf tissue density (LTD, g cm−3) was calculated from dry mass, leaf area and leaf thickness as: LTD = DM/(LA × LT).
Transparent leaves were prepared by boiling for 5–10 min and then immersed in a 1:1 solution of glacial acetic acid and hydrogen peroxide in a water bath at 70 ℃ for 8–10 h. Then epidermis was separated from the mesophyll cells. The transparent epidermis was stained with safranin and stomatal were photographed under a microscope (Fig. S7). The leaf stomatal size (SS, μm) was represented as the length of the guard cells, and the stomatal density (SD, no. μm −2) was calculated as the number of stomata per unit of area.
2.2.3. Leaf mineral nutrient concentrationOven dried leaves were grounded to powder for nutrient analysis. Leaf mass-based carbon concentrations (C mg g−1) and leaf mass-based nitrogen concentrations (N, mg g−1) were measured using a C–N analyzer (Elemental Analyzer, Vario MAX CN, Germany) (Nieuwenhuise et al., 1994). Leaf mass-based phosphorus concentrations (P, mg g−1) were measured using an atomic-emission spectrometer (IRIS Advantage-ER, Thermo Fisher Scientific USA).
2.2.4. Leaf carbon isotope measurementWe selected healthy leaves and grounded them into powder after drying. By using 100-mesh sieve, 4.000–5.000 mg-powder sample was accurately weighed and wrapped in tinfoil, and then put into the EA automatic sampling tray. It will burn and crack at high temperature to generate CO2. The generated gas was finally separated by adsorption column and carried by carrier gas He into the ion source for ionization for detection. Set 580s to pass CO2 (> 99.999%) as the reference gas for 30s. We obtained the 13C/12C ratio by comparing detection the reference gas and the sample gas. The reaction temperatures were 920 ℃ and 600 ℃, respectively (Isotope Mass Spectrometer, Isoprime100). Using the atmospheric and plant sample δ13C, the carbon isotopic discrimination (Δ13C, ‰) occurring in trees was calculated as, Δ13C = (δ13Cair−δ13Cplant) ∕ (1+δ13Cplant/1000), where δ13Cair and δ13Cplant are the fractional difference in isotopic composition (13C/12C) in the atmospheric CO2 and that of leaf. Carbon isotopic discrimination (Δ13C) is related to intercellular CO2 (Ci) and ambient CO2 (Ca) concentration as, Δ13C = a + (b−a) (Ci∕Ca), where a is fractionation factor during intercellular diffusion (4.4‰), and b is fractionation factor during carboxylation (27‰) (Farquhar and von Caemmerer, 1982). Plant carbon assimilation and water loss are related to stomatal conductance and photosynthetic rate (Lin et al., 2015). Therefore, the carbon isotope ratio can be used to calculate the plant's long-term water use efficiency (iWUE): iWUE = A/g = (Ca−Ci)/1.6 = Ca (b− Δ13C)/1.6(b−a), where A is the CO2 assimilation efficiency and g is the stomatal conductance.
2.3. Tree diameter growth rateThe diameter growth rates of 12 dominant species were determined based on repeated measurements of dendrometer bands from January 2017 to December 2020 in a 1-ha plot of karst forest. For each tree, the average annual diameter growth rate (DGR) over four years was calculated by subtracting the diameter at breast height (DBH) in January from that in December. The DGR2017, DGR2018, DGR2019, and DGR2020 represent growth rates in different years, respectively. Additionally, the growth rate during rainy season (growth rate, GR) was determined by subtracting the DBH in May from that in October. Trees with anomalous growth rates were excluded from the analysis as they were assumed to be caused by measurement errors. To reduce measurement errors, we choose to conduct data measurements around the 15th of each month, ensuring that the same group of people and vernier caliper are used for each measurement. We also maintain a consistent measurement method and promptly inspect and replace any damaged metal band dendrometers.
2.4. Statistical analysesAll analyses were conducted using the R v.4.2.2 statistical software (R Core Team, 2021). We calculated the medians, standard deviations (SD) and coefficients of variation (CV) of all 17 measured traits (Table S2). An ANOVA analysis was performed to assess trait variation among species and individuals within the same species, as well as measurement error. The ANOVA analysis was conducted with the 'lme' function in the 'lme4' package (Fig. 2). The arithmetical means of growth and traits data were estimated for each species. To ensure data were normally distributed for the Pearson's correlation analysis, the original growth rate and trait data were log-transformed (Kerkhoff and Enquist, 2009). We calculated the Pearson's correlations between 17 functional traits and growth rate (GR) during rainy season averaged over four years of the 12 tree species (Fig. 3). Relationships among functional traits were analyzed using the 'cor.tes' function in the R package (R Core Team, 2021, Fig. 3). The contribution of wood density (WD) to the first and second principal components in PCA analysis is minimal, therefore, it was excluded from the PCA analysis. Principal components analysis (PCA) was conducted to analyze the 16 functional traits of 12 studied tree species using log10 transformed average values with the 'FactoMineR' package (Lê et al., 2008) (Figs. 4 and 5(a) (d)).

|
| Fig. 1 (a) Monthly diameter growth rate (DGR, mm month−1) and standard deviation for 12 studied tree species, (b) precipitation (PRE, mm), (c) vapor pressure deficit (VPD, kPa) and soil volumetric water content (d, SVWC, m3 m−3) during the period from January 2017 to December 2020. |

|
| Fig. 2 Variance in functional traits (bars) explained by species (black) and individual (grey) and measurement error (light grey). Trait abbreviations are the same as in Table S2. The traits are ranked according to explained variance by species (n = 12). |

|
| Fig. 3 Correlation matrix among 17 leaf and stem functional traits and yearly averaged (DGR2017, DGR2018, DGR2019, DGR2020) diameter growth rate and four-year averaged rainy season diameter growth rate (GR) of 12 studied tree species in a tropical karst forest. Trait abbreviations are the same as in Table S2. ∗∗∗, p < 0.001; ∗∗, p < 0.01; ∗, p < 0.05. |

|
| Fig. 4 Principal component analyses (PCA) of 16 functional traits and growth rates during rainy season (GR) averaged over four years of 12 studied tree species in a tropical karst forest. Variances explained by the first (35%) and second (27.7%) principal component were also presented in the parentheses. Blue and red dots points represent evergreen and deciduous tree species scores, respectively. Abbreviations are the same as in Table S2. |

|
| Fig. 5 Biplots of principal component analyses (PCA) (a, d), the relationships between stem diameter growth rates and the first (b, e) and second (c, f) principal components. PCs were performs for 16 functional traits of different phonology (a–c: evergreen; d–f: deciduous) of 12 tree species in a tropical karst forest. Values in parentheses in the axis labels are percentages explained by the first and second components, respectively. Blue and red dots represent evergreen and deciduous tree species, respectively. Abbreviations are the same as in Table S2. ∗∗∗, p < 0.001; ∗∗, p < 0.01. |
The relationships between growth rates (GR) and functional traits were evaluated using type II regressions to accommodate errors in both the X and Y variables. Standardized major axis (SMA) regressions were performed for the linear functions Y = α + βX, where Y represents the growth rates, and X represents functional traits. The SMA slope (β) and SMA y-intercept (α) and their respective 95% confidence intervals were estimated utilizing the 'smart' package (Warton et al., 2012). Separate SMAs were fitted for evergreen and deciduous groups, with the statistical differences in slopes and intercepts tested by groups on phenology (Table S4). Linear regressions were used to examine the relationships between a subset of functional traits and growth rates (Fig. 6, Fig. 7, S5 and S6). Linear regressions were also used to examine the relationships between PC scores and growth rates (Figs. 5 and S4). All figures were plotted using the 'ggplot2' package in R.
|
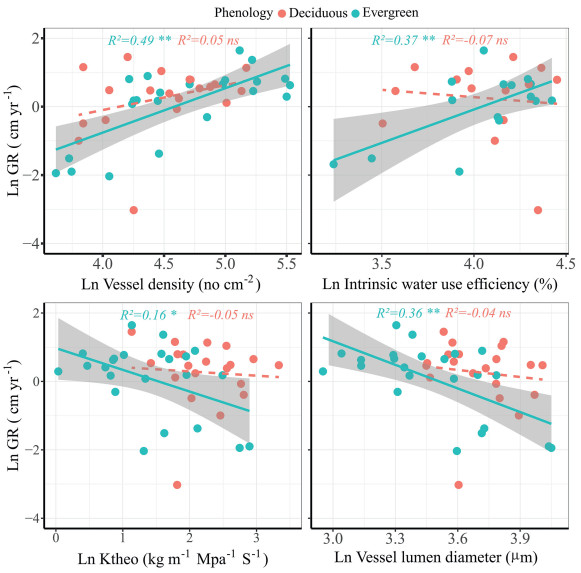
| Fig. 6 Relationships between growth rates (GR) during rainy season averaged over four years and vessel density (VD), intrinsic water use efficiency (iWUE), theoretical hydraulic conductivity (Ktheo) and vessel lumen diameter (Dh) of six evergreen and six deciduous species in a tropical karst forest. Blue and red dots represent evergreen and deciduous tree species, respectively. Note that the growth and trait data was log transformed. Shading areas represent 95% confidence intervals of linear regressions. R2: Explained variance. ∗∗, p < 0.01; ∗, p < 0.05. |

|
| Fig. 7 Relationships between stem growth rates (GR) during rainy season averaged over four years and leaf functional traits of six evergreen and six deciduous species in a tropical karst forest. LT: thickness, LET: lower epidermis thickness, SP: spongy thickness, LTD: leaf tissue density. Blue and red dots represent evergreen and deciduous tree species, respectively. Note that the growth and trait data was log transformed. Shading areas represent 95% confidence intervals of linear regressions. R2: Explained variance, ∗∗∗, p < 0.001; ∗∗, p < 0.01; ∗, p < 0.05. |
The monthly stem growth rate exhibited a distinct seasonal pattern (Fig. 1). In the four study years, the monthly stem growth mainly occurred during the rainy season (May to October) and displayed synchronization with soil volumetric water content (SVWC) and precipitation (PRE), but opposite with vapor pressure deficit (VPD) (Fig. 1). The low growth rates during dry season may occur due to the water-induced stem shrinkage during that period (Fig. 1(a)). There were significant variations in trait values among tree species in this tropical karst forest community. Variance partition analysis for 17 traits revealed that species identity explained an average of 68% of trait variation, ranging from 45% for lower epidermis thickness to 89% for total carbon concentration (Fig. 2). Individual variation accounted for approximately 26% of the trait variation, ranging from 8% for total carbon concentration to 46% for lower epidermis thickness (Fig. 2). Additionally, 29% and 62% of the variances of growth rate (GR) were explained by species and individual, respectively (Fig. 2). Deciduous species showed higher rainy season growth rate (GR) and annual growth rate (DGR) compared with evergreen species (Fig. S3).
3.2. Correlations among functional traitsAll traits exhibited a median coefficient of variation of 35% (range 5%–71%) (Table S2). Wood density (WD) displayed a positive correlation with vessel density (VD) and negative correlations with theoretical hydraulic conductivity (Ktheo) and hydraulic weighted vessel diameter (Dh) (Fig. 3). Dh was positively associated with stomatal density (SD) and negatively correlated with vessel density (VD) and lower epidermis thickness (LET) (Fig. 3). Specific leaf area (SLA) demonstrated a positive correlation with leaf nutrient concentration (TC, TN, TP) and a negative correlation with leaf tissue density (LTD) and tissue thickness (LT, PT, SP, UET) (Fig. 3). LTD correlated with SLA, wood density (WD), vessel density (VD) and stomatal size (SS) (Fig. 3).
The first axis of principal component analyses (PCA) explained 35% of the variance among 16 functional traits and was positively loaded by the leaf thickness (LT), tissue thickness of the different leaf component (UET, SP, PT, LET), iWUE, and negatively by the SLA and leaf nutrient concentration (TC, TN, TP) (Fig. 4). The second axis explained 27.7% of the variance and was loaded by hydraulic traits (Dh, Ktheo, SS, SD, VD) and leaf tissue density (LTD), growth rate (GR) (Fig. 4). Evergreen and deciduous tree species were not clearly separated along the two PCA axes, with deciduous tree species located closer to leaf economic spectrum (SLA, SP, LT, UET and PT), nutrient-use efficiency (TC, TN and TP), whereas evergreen tree species located towards leaf/stem hydraulic traits (Dh, Ktheo, SS, SD, VD, LET, LT, LTD) and GR (Fig. 4). Separate PCA analyses showed similar factor loadings of traits in different phenology (Fig. 5(a), (d)).
3.3. Relationships of stem and leaf traits to diameter growth rateThe correlation analysis revealed a positive association between rainy season growth rate (GR) of tree species and traits related to a safe xylem structure, such as vessel density and stomatal density (Fig. S5). Furthermore, GR exhibited positive relationships with leaf traits that confer resistance to drought and insect attack, including leaf tissue thickness (LT, LET, UET, SP) (Fig. S6). Conversely, GR was found to be negatively correlated with vessel lumen diameter (Dh), stomatal density (SD) and leaf tissue density (LTD) (Figs. S5 and S6). These findings suggest that tree growth rates in the karst habitat are mainly influenced by traits associated with drought tolerance.
Stem diameter growth rates (GR) were positively correlated with the first and second PC scores (Fig. S4), indicating a high growth rate of drought tolerant traits across evergreen and deciduous tree species. However, while tree growth rates were significantly associated with the first and second PC in evergreen species (Fig. 5(b), (c)), no significant correlation was observed between growth rates and the first and second PCs in deciduous species (Fig. 5(e), (f)). The SMA regressions conducted on all 59 individuals revealed that GR were positively (P < 0.05) related to traits associated with drought tolerance (VD, SS, LT, SP, LET and UET), and negatively related to Dh, SD and LTD (Table S4). The SMA slopes were mostly not-significantly different between the evergreen and deciduous groups (Pslope > 0.05), but the SMA intercepts differed significantly (Pintercept < 0.05) for WD, hydraulic traits (Ktheo, Dh), and leaf traits (SLA, LTD, TP) (Table S4).
Compared to deciduous trees, the growth rates of evergreen trees exhibited stronger associations with intrinsic water use efficiency (iWUE), theoretical hydraulic conductivity (Ktheo), vessel lumen diameter (Dh), vessel density (VD), and leaf tissue thickness parameters such as LT, SP, LET and LTD (Table 2; Fig. 6, Fig. 7). In contrast, the growth rates of deciduous tree exhibited minimal correlations with traits in tropical karst forest (Table 2; Fig. 6, Fig. 7). Therefore, it appears that the growth rates of evergreen species are more reliant on branch and leaf traits related to drought tolerance.
|
Traits |
GRevergreen (N = 30) | GRdeciduous (N = 29) | GR (N = 59) | ||
| R | R | R | |||
| WD | 0.236 | −0.192 | −0.063 | ||
| iWUE | 0.644∗ | −0.109 | 0.271 | ||
| Ktheo | −0.448∗ | −0.069 | −0.213 | ||
| Dh | −0.625∗∗ | −0.125 | −0.371∗ | ||
| VD | 0.714∗∗∗ | 0.324 | 0.547∗∗∗ | ||
| SD | −0.276 | −0.456 | −0.346∗ | ||
| SS | 0.406 | 0.675∗∗ | 0.522∗∗ | ||
| SLA | −0.121 | 0.039 | 0.041 | ||
| PT | 0.317 | 0.030 | 0.235 | ||
| LT | 0.611∗∗ | 0.224 | 0.466∗∗ | ||
| SP | 0.647∗∗ | 0.297 | 0.473∗∗ | ||
| LET | 0.699∗∗ | 0.083 | 0.487∗∗ | ||
| UET | 0.443 | 0.138 | 0.369∗ | ||
| LTD | −0.638∗∗ | −0.305 | −0.496∗∗ | ||
| TC | −0.266 | 0.263 | −0.070 | ||
| TN | −0.389 | −0.020 | −0.111 | ||
| TP | −0.114 | −0.158 | −0.062 |
In the tropical karst forest community, there were significant differences in branch and leaf traits among coexisting tree species (Fig. 2). The karst habitats are characterized by large outcrops, shallow and patchy soils, rapid underground drainage, and surface runoff (Zhang et al., 2011; Liu et al., 2024). Due to limited soil water availability, trees in tropical karst forests often experience drought stress, especially during the dry season (Fu et al., 2015). Hydraulic efficiency determines tree growth performance, particularly when trees are under water stress (Ferdous et al., 2023). Previous studies highlighted the trade-off between hydraulic efficiency and safety, that larger vessels enable efficient water transport while numerous smaller vessels serve as a backup system with high resistance to flow (Hacke et al., 2006). The presence of embolized xylem cells reduces hydraulic conductivity and significantly hampers photosynthesis and growth in karst habitat (Fan et al., 2011). Tree species in habitats with distinct rainfall seasonality may optimize their xylem to provide both high safety for dry season persistence through water storage and high efficiency for rapid growth during the wet season (Liu et al., 2021). Compared to tree species found in lowland tropical rainforests, woody plants in tropical karst forests tend to exhibit traits that enhance their tolerance to drought conditions such as higher sapwood density, smaller vessel diameter, and greater cavitation resistance (Figs. S5 and S6). These adaptations allow them to thrive in the arid karst environment (Zhu et al., 2017).
We found that tree diameter growth rate in the tropical karst habitat was positively correlated with stem and leaf traits (Figs. S5 and S6). However, this relationship was observed only in evergreen trees in the tropical karst forest (Fig. 5, Fig. 6, Fig. 7). The growth rate of evergreen trees was positively correlated with vessel density (VD) and intrinsic water use efficiency (iWUE), but negatively correlated with vessel lumen diameter (Dh) and theoretical hydraulic conductivity (Ktheo) (Table 2; Fig. 6). High leaf water use efficiency (iWUE) is considered an adaptive trait to cope with low water availability in the karst habitat (Fu et al., 2019). The evergreen trees can sustain during dry periods through higher iWUE. One important mechanism that facilitates drought tolerance is the presence of a 'safe' hydraulic system characterized by smaller and denser vessels (Baas et al., 2004). Species from dry habitats tend to have narrower vessels, denser wood, and thus greater resistance to drought-induced cavitation but lower hydraulic conductivity (Pockman and Sperry, 2000; Canham et al., 2009). Compared to co-existing deciduous species, evergreen species tend to build xylem with greater resistance to xylem cavitation and lower hydraulic conductance (Choat et al., 2004; Chen et al., 2009). Drought tolerance has been associated with evergreen species because they are thought to possess traits that allow them to be active at low soil water potentials, including adaptations that reduce xylem cavitation (Kursar et al., 2009; Markesteijn et al., 2011). This adaptation allows evergreen trees to maintain water transport even during dry seasons in the karst habitat (Fu et al., 2019), as their smaller vessels can reduce drought-induced cavitation and exhibit more conservative water use strategies (Sperry and Sullivan, 1992; Meinzer et al., 2008).
The co-existing evergreen and deciduous species employ contrasting water-use strategies to adapt to the arid habitat (Fu et al., 2012). In the karst habitat, growth rates of deciduous trees exhibit no significant association with hydraulic traits (Table 2 and Fig. 5). Because of drought avoidance of deciduous species, they may be able to sustain high photosynthetic rates during wet seasons when the conditions are favorable for growth (Givnish 2002; Markesteijn and Poorter 2009). The presence of drought-tolerant traits in evergreen species enables them to have safer xylem structures, which ensure them maintain longer growth periods especially during the dry season in the karst forest. Deciduous species with larger vessels are more vulnerable to hydraulic failure (Apgaua et al., 2015; Islam et al., 2018a). However, deciduous trees shed their leaves in the dry season to prevent water loss. In contrast, evergreens have a high abundance of smaller vessels, allowing their hydraulic system to maintain water conduction even when some vessels experience cavitation induced by water stress (Islam et al., 2018b, 2019a, 2019b). The evergreen trees exhibit higher resistance to xylem cavitation, enabling them to maintain water transport to their leaves even under water-deficient conditions (Fig. 6). These trees possess anatomical structures that are well-suited to water transport in the local tropical karst environment. These findings suggest that evergreen species employing more conservative water-use strategies tend to grow faster in drought-stressed tropical karst forests.
4.2. Associations between leaf traits and diameter growth ratesWe observed a strong positive correlation between plant growth rate and leaf tissue thickness (Table 2 and Fig. S6). Leaf morphological and anatomical traits are expected to influence transpiration and drought tolerance. Thicker upper and lower epidermis can reduce water loss, while larger loose sponge tissue facilitates CO2 diffusion within the leaves for photosynthesis (Liu et al., 2012). The leaves of karst forest trees with greater adaxial and abaxial epidermis thickness exhibit higher drought tolerance compared to non-karst forest trees (Fu et al., 2019). Our findings align with the discovery of positive correlations between leaf upper and lower thicknesses and drought tolerance by Binks et al. (2016). Higher leaf tissue density allows plants to tolerate water stress at the expense of reduced photosynthetic capacity (Niinemets, 2001; Alvarez-Clare and Kitajima, 2007). Our results suggest that leaf anatomy confers significant plasticity in tree growth response to water stress for karst species. Low water availability in the karst habitat may have a greater impact on leaf trait plasticity and their impacts on tree growth. Generally, specific leaf area is considered to be a crucial trait influencing plant growth and is expected to have a positive relationship with growth (Wright et al., 2004). However, stem growth was not correlated with specific leaf area (SLA) in our study. Other studies suggest that SLA may play an important role in the growth of small plants (Pearson et al., 2003), but its significance appears to be less pronounced for large trees (Würth et al., 2005; Yan et al., 2020).
Our results demonstrated distinct differences between evergreen and deciduous trees in the correlations between growth rate and leaf anatomy traits (Table 2 and Fig. 7). Growth rate (GR) in evergreen species was significantly associated with leaf tissue thickness (LT, SP, LET and LTD), while GR in deciduous species was only positively correlated with stomatal size (SS) (Table 2 and Fig. 7). Deciduous and evergreen trees differ in resource acquisition and use strategy (Qi et al., 2020). Generally, evergreen species have thicker leaves, thicker spongy and palisade mesophyll layers, more palisade mesophyll layers, and a thicker lower epidermis (Kröber et al., 2014). The stomata are optimized to balance photosynthetic performance and water use efficiency via regulating the exchange of gas between plants and the external environment (Chaerle et al., 2005). The strategies of water use and carbon gain also differed between evergreen and deciduous trees (Eamus, 1999; Liu et al., 2014). Evergreen species employ conservative water use strategies and exhibit slower life histories, characterized by lower SLA, thicker leaf thickness, and lower leaf nitrogen concentration. These adaptations enable them to sustain photosynthesis even under limited water availability, and prolong their leaf lifespan (Ongole et al., 2021). Evergreen trees tended to have lower photosynthetic capacity but were more conservative in water use, while deciduous trees had higher photosynthetic capacity and transpiration rates (Qi et al., 2020). Evergreen trees allocate more resources to structural defense and leaf protection as part of their conservative resource strategy (Wang et al., 2023a), whereas deciduous trees depend on their higher concentrations of leaf mineral nutrients to achieve an increased photosynthesis rate (Bowman and Prior, 2005; Wang et al., 2023a). In condition of limited water availability, the safety of hydraulic architecture and the nitrogen content of leaves may play a more crucial role in facilitating fast growth.
In order to withstand harsh dry-season conditions, evergreen species have evolved leaves with sclerophyllous characteristics, including thick cell walls that provide resistance against drought and insect attacks (Eamus, 1999; Ávila-Lovera et al., 2019). These species exhibit a relatively low allocation of nitrogen to their leaves, resulting in reduced assimilation rates and an adaptive conservative strategy (Wang et al., 2023a). However, they are capable of continuous carbon fixation throughout the year, which allows for a longer return on investment for the tree (Eamus, 1999; Jin et al., 2018). Evergreen trees have long-lived leaves, and the longevity of leaves is closely associated with leaf maintenance and carbon gain (Russo and Kitajima 2016; Osnas et al., 2018). In tropical karst forests, evergreen trees exhibit year-round carbon fixation and possess leaf traits that enable them to tolerate drought. This extended period of carbon fixation promotes tree growth.
4.3. Why are drought tolerant traits important in the karst habitat?Our results revealed that the growth rate (GR) of trees in karst habitat is positively associated to vessel density, stomatal size, and leaf thickness traits, while negatively correlated with vessel lumen diameter, stomatal density and leaf tissue density (Table 2; Figs. S5 and S6). This is contrast to previous findings that higher growth rates were associated with larger vessels and higher hydraulic conductivity (Fan et al., 2012). The regulation of plant water relations in response to soil water deficit is crucial for adapting to karst forest conditions (Fan et al., 2011; Zhu et al., 2017). Our results suggest that trees growing in the karst habitat employ different strategies for acquiring water resources, striking a balance between hydraulic safety and efficiency. In dry forests, light availability may be less limiting, but low water availability can restrict leaf trait plasticity (Markesteijn et al., 2007). Leaves are often more vulnerable to embolism compared to the stems they are attached to, resulting in a significant reduction in their hydraulic conductance under non-extreme conditions (Brodribb and Holbrook, 2004). Leaf cuticles and epidermises play an important role in reducing water loss through evaporation (Gamage et al., 2003) and protect the photosynthetic tissue from excessive irradiance (Roth, 1984). Thick and dense leaves with small and numerous vessels can be advantageous in water-limited environments where tolerance traits against water stress are crucial.
Compared to deciduous species, the growth rate (GR) of evergreen trees in karst habitat is positively associated to stem and leaf traits (Fig. 5). Evergreen and deciduous woody species coexist in seasonally dry tropical forest, because of strong pressure of seasonal drought (Quigley and Platt 2003; Ishida et al., 2010; Fu et al., 2012; Zhang et al., 2017). Several studies have shown that co-occurring evergreen and deciduous species exhibit clear distinctions in stem hydraulic efficiency and leaf traits (Chen et al., 2009; Fan et al., 2011; Fu et al., 2012). It is crucial for both branches and leaves to possess a sufficient tolerance to water stress in order to maintain their functionality during periods of water deficit (Fu et al., 2012). The growth rates of evergreen trees are more closely linked to traits that associated with hydraulic safety, which contribute to their ability to withstand water stress (Fig. 6). On the other hand, the growth rates of deciduous species are more dependent on traits associated with resource acquisition, such as stomatal size (SS) (Table 2) or photosynthesis traits (Qi et al., 2020). In the karst forest, evergreen trees need to sustain water transport during dry seasons and therefore have smaller vessels that reduce the risk of drought-induced cavitation while conserving water use (Sperry and Sullivan, 1992; Meinzer et al., 2008). Our findings demonstrate a significant relationship between growth rates of evergreen species and vessel lumen diameter (negative), vessel density (positive), and leaf thickness (positive). However, no association was found between deciduous trees growth rates and stem and leaf traits (Fig. 6, Fig. 7), possibility because evergreen and deciduous trees have adopted alternative survival strategies (Tomlinson et al., 2013). Evergreen and deciduous tree species exhibit divergent strategies of drought tolerance, and hydraulic safety under seasonal drought (Zhang et al., 2017, 2019). We attribute this finding to the pronounced seasonal aridity of the karst forest, where the importance of having a safe water transport system becomes even more important compared to more humid conditions. This is further supported by our observation that faster growth of evergreen species is negatively correlated with vessel size (Dh) and leaf tissue density (LTD), indicating that faster growth relies on favorable traits related to drought tolerance during dry periods.
5. ConclusionsWe conducted a systematically collection of four-year growth data using metal band dendrometers in a tropical karst forest. Our results revealed that growth rate of evergreen tree in the tropical karst habitat can be explained by their branch and leaf anatomy traits especially those associated with drought tolerance. We found a strong association between the growth of karst evergreen species and more conservative water use strategies and greater drought tolerance traits, allowing the evergreen trees maintain higher persistence under low soil water availability as compared with deciduous species. Our findings reveal important insights into the water use strategies and drought tolerance of karst evergreen and deciduous tree species. Further research should explore the associations between seasonal growth and other drought tolerance traits, such as leaf turgor loss point and xylem cavitation resistance. Our study provides valuable insights into the seasonal growth patterns of tree species with different water use strategies in tropical karst habitats, which would enhance our understanding of how tree species with different water use strategies coexist and thrive in the unique conditions of tropical karst forests.
AcknowledgmentsWe thank Mr. Hong Ma and Mr. Meiyi Tan for their assistance with the field work. We thank Lijie Wu, Bo Zhou, Tongliang Xu, Xiaolian Wei, Hui Zhang, Limin Chen and others for their help in measuring the dendrometer bands. We thank Zetian Liu for help in review and edit the language. Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies (XSTRFE) of Chinese Ecosystem Research Network (CERN) provided permanent forest plot data. We thank the Biogeochemistry Laboratory at Xishuangbanna Tropical Botanical Garden for the nutrient analysis. This work was financially funded by the National Natural Science Foundation of China (3186113307, 31770533, 31870591) and the West Light Talent Program of the Chinese Academy of Sciences (xbzg-zdsys-202218).
Author contributions
Yu-Mei Yan and Ze-Xin Fan conceived the study; Yu-Mei Yan did field and lab work and wrote the first draft; Yu-Mei Yan, Ze-Xin Fan and Pei-Li Fu analyzed data; Ze-Xin Fan and Pei-Li Fu helped improve language; all authors contributed to the ideas presented in the paper and to revisions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.08.001.
Ackerly, D., 2004. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr., 74: 25-44. DOI:10.1890/03-4022 |
Aguirre-Gutiérrez, J., Oliveras, I., Rifai, S., et al., 2019. Drier tropical forests are susceptible to functional changes in response to a long-term drought. Ecol. Lett., 22: 855-865. DOI:10.1111/ele.13243 |
Alvarez-Clare, S., Kitajima, K., 2007. Physical defense traits enhance seedling survival of neotropical tree species. Funct. Ecol., 21: 1044-1054. DOI:10.1111/j.1365-2435.2007.01320.x |
Anderegg, W.R.L., Flint, A., Huang, C.Y., et al., 2015. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci., 8: 367-371. DOI:10.1038/ngeo2400 |
Anderegg, W.R.L., Klein, T., Bartlett, M., et al., 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U.S.A., 113: 5024-5029. DOI:10.1073/pnas.1525678113 |
Apgaua, D.M.G., Ishida, F.Y., Tng, D.Y.P., et al., 2015. Functional traits and water transport strategies in lowland tropical rainforest trees. PLoS One, 10: e0130799. DOI:10.1371/journal.pone.0130799 |
Ávila-Lovera, E., Urich, R., Coronel, I., Tezara, W., 2019. Seasonal gas exchange and resource-use efficiency in evergreen versus deciduous species from a tropical dry forest. Tree Physiol., 39: 1561-1571. DOI:10.1093/treephys/tpz060 |
Baas, P., Ewers, F.W., Davis, S.D., et al., 2004. Evolution of xylem physiology. In: Hemsley, A.R., Poole, I. (Eds. ), The Evolution of Plant Physiology. Elsevier Academic Press, London, UK, pp. 273-295.
|
Baker, T.R., Burslem, D.F.R.P., Swaine, M.D., 2003. Associations between tree growth, soil fertility and water availability at local and regional scales in Ghanaian tropical rain forest. J. Trop. Ecol., 19: 109-125. |
Binks, O., Meir, P., Rowland, L., et al., 2016. Plasticity in leaf-level water relations of tropical rainforest trees in response to experimental drought. New Phytol., 211: 477-488. DOI:10.1111/nph.13927 |
Bonan, G.B., 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science, 320: 1444-1449. DOI:10.1126/science.1155121 |
Bowman, D., Prior, L.D., 2005. Why do evergreen trees dominate the Australian seasonal tropics?. Aust. J. Bot., 53: 379-399. |
Brodribb, T.J., Holbrook, N.M., 2004. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant Cell Environ., 27: 820-827. |
Canham, C.A., Froend, R.H., Stock, W.D., 2009. Water stress vulnerability of four Banksia species in contrasting ecohydrological habitats on the Gnangara Mound, Western Australia. Plant Cell Environ., 32: 64-72. DOI:10.1111/j.1365-3040.2008.01904.x |
Cao, K.F., 2000. Leaf anatomy and chlorophyll content of 12 woody species in contrasting light conditions in a Bornean heath forest. Can. J. Bot., 78: 1245-1253. |
Carlquist, S., 1977. Ecological factors in wood evolution: a floristic approach. Am. J. Bot., 64: 887-896. |
Chen, J.W., Zhang, Q., Cao, K.F., 2009. Inter-species variation of photosynthetic and xylem hydraulic traits in the deciduous and evergreen Euphorbiaceae tree species from a seasonally tropical forest in southwestern China. Ecol. Res., 24: 65-73. DOI:10.1007/s11284-008-0482-4 |
Chen, Y.J., Bongers, F., Tomlinson, K., et al., 2015. Time lags between crown and basal sap flows in tropical lianas and co-occurring trees. Tree Physiol., 36: 736-747. |
Choat, B., Ball, M.C., Luly, J.G., et al., 2004. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees Struct. Funct., 19: 305-311. |
Chaerle, L., Saibo, N., Straeten, D.V.D., 2005. Tuning the pores: towards engineering plants for improved water use efficiency. Trends Biotechnol., 23: 308-315. |
Choat, B., Brodribb, T.J., Brodersen, C.R., et al., 2018. Triggers of tree mortality under drought. Nature, 558: 531-539. DOI:10.1038/s41586-018-0240-x |
Corlett, R.T., 2016. The impacts of droughts in tropical forests. Trends Plant Sci., 21: 584-593. |
Delzon, S., 2015. New insight into leaf drought tolerance. Funct. Ecol., 29: 1247-1249. DOI:10.1111/1365-2435.12500 |
Dong, S.X., Davies, S.J., Ashton, P.S., et al., 2012. Variability in solar radiation and temperature explains observed patterns and trends in tree growth rates across four tropical forests. P. Roy. Soc. B. Biol. Sci., 279: 3923-3931. DOI:10.1098/rspb.2012.1124 |
Eamus, D., 1999. Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trends Ecol. Evol., 14: 11-16. |
Fan, D.Y., Jie, S.L., Liu, C.C., et al., 2011. The trade-off between safety and efficiency in hydraulic architecture in 31 woody species in a karst area. Tree Physiol., 31: 865-877. DOI:10.1093/treephys/tpr076 |
Fan, Z.X., Zhang, S.B., Hao, G.Y., et al., 2012. Hydraulic conductivity traits predict growth rates and adult stature of 40 Asian tropical tree species better than wood density. J. Ecol., 100: 732-741. DOI:10.1111/j.1365-2745.2011.01939.x |
Farquhar, G.D., von Caemmerer, S., 1982. Modling of phoosynthetic response to environmentl conditions. In: Lange, O.L., Nobel, P.S., Osmond, C.B., et al. (Eds. ), Physiological plant ecology II. Springer-Verlag, Heidelberg, pp. 549-587.
|
Ferdous, J., Islam, M., Rahman, M., 2023. The role of tree size, wood anatomical and leaf stomatal traits in shaping tree hydraulic efficiency and safety in a South Asian tropical moist forest. Glob. Ecol. Conserv., 43: e02453. |
Friedlingstein, P., Jones, M.W., O'Sullivan, M., et al., 2019. Research collection: global carbon budget 2019, Optimal parameter tuning of feedback controllers with application to biomolecular antithetic integral control. Earth Syst. Sci. Data, 11: 1783-1838. DOI:10.5194/essd-11-1783-2019 |
Fu, P.L., Jiang, Y.J., Wang, A.Y., et al., 2012. Stem hydraulic traits and leaf water-stress tolerance are coordinated with the leaf phenology of angiosperm trees in an Asian tropical dry karst forest. Ann. Bot., 110: 189-199. DOI:10.1093/aob/mcs092 |
Fu, P.L., Liu, W.J., Fan, Z.X., et al., 2015. Is fog an important water source for woody plants in an Asian tropical karst forest during the dry season?. Ecohydrology, 9: 964-972. |
Fu, P.L., Zhu, S.D., Zhang, J.L., et al., 2019. The contrasting leaf functional traits between a karst forest and a nearby non-karst forest in south-west China. Funct. Plant Biol., 46: 907-915. DOI:10.1071/fp19103 |
Gamage, H.K., Ashton, M.S., Singakumara, B.M.P., 2003. Leaf structure of Syzygium spp. (Myrtaceae) in relation to site affinity within a tropical rain forest. Bot. J. Linn. Soc., 141: 365-377. DOI:10.1046/j.1095-8339.2003.00138.x |
Givnish, T.J., 2002. Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fenn., 36: 703-743. |
González-M, R., Posada, J.M., Carmona, C.P., et al., 2021. Diverging functional strategies but high sensitivity to an extreme drought in tropical dry forests. Ecol. Lett., 24: 451-463. DOI:10.1111/ele.13659 |
Hacke, U.G., Sperry, J.S., Wheeler, J.K., et al., 2006. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol., 26: 689-701. DOI:10.1093/treephys/26.6.689 |
Hietz, P., Valencia, R., Wright, S.J., 2013. Strong radial variation in wood density follows a uniform pattern in two neotropical rain forests. Funct. Ecol., 27: 684-692. DOI:10.1111/1365-2435.12085 |
Hoeber, S., Leuschner, C., Köhler, L., et al., 2014. The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. For. Ecol. Manage., 330: 126-136. |
Ishida, A., Harayama, H., Yazaki, K., et al., 2010. Seasonal variations of gas exchange and water relations in deciduous and evergreen trees in monsoonal dry forests of Thailand. Tree Physiol., 30: 935-945. DOI:10.1093/treephys/tpq025 |
Islam, M., Rahman, M., Bräuning, A., 2018a. Long-term hydraulic adjustment of three tropical moist forest tree species to changing climate. Front. Plant Sci., 9: 1761. |
Islam, M., Rahman, M., Bräuning, A., 2018b. Xylem anatomical responses of diffuse porous Chukrasia tabularis to climate in a South Asian moist tropical forest. Ecol. Manag., 412: 9-20. DOI:10.1117/12.2305145 |
Islam, M., Rahman, M., Bräuning, A., 2019a. Impact of extreme drought on tree-ring width and vessel anatomical features of Chukrasia tabularis. Dendrochronologia, 53: 63-72. |
Islam, M., Rahman, M., Bräuning, A., 2019b. Long-term wood anatomical time series of two ecologically contrasting tropical tree species reveal differential hydraulic adjustment to climatic stress. Agric. Meteorol., 265: 412-423. |
Jin, Y., Russo, S.E., Yu, M., Gilliam, F., 2018. Effects of light and topography on regeneration and coexistence of evergreen and deciduous tree species in a Chinese subtropical forest. J. Ecol., 106: 1634-1645. DOI:10.1111/1365-2745.12911 |
Kerkhoff, A.J., Enquist, B.J., 2009. Multiplicative by nature: why logarithmic transformation is necessary in allometry. J. Theor. Biol., 257: 519-521. |
Kramer, P.J., Boyer, J.S., 1995. Water Relations of Plants and Soils. Academic Press, San Diego, CA, USA.
|
Kröber, W., Heklau, H., Bruelheide, H., 2014. Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol., 17: 373-383. |
Kursar, T.A., Engelbrecht, B., Burke, A., et al., 2009. Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct. Ecol., 23: 93-102. DOI:10.1111/j.1365-2435.2008.01483.x |
Laurance, S.G., Laurance, W.F., Nascimento, H.E., et al., 2009. Long-term variation in amazon forest dynamics. J. Veg. Sci., 20: 323-333. DOI:10.1111/j.1654-1103.2009.01044.x |
Lê, S., Josse, J., Husson, F., 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw., 25: 1-18. |
Liu, J.Y., Fu, P.L., Wang, Y.J., et al., 2012. Different drought-adaptation strategies as characterized by hydraulic and water-relations traits of evergreen and deciduous figs in a tropical karst forest. Plant Sci. J, 30: 484-493. |
Liu, C., Liu, Y., Guo, K., et al., 2014. Concentrations and resorption patterns of 13 nutrients in different plant functional types in the karst region of south-western China. Ann. Bot., 113: 873-875. DOI:10.1097/00007890-201407151-02978 |
Liu, H., Ye, Q., Gleason, S.M., et al., 2021. Weak tradeoff between xylem hydraulic efficiency and safety: climatic seasonality matters. New Phytol., 229: 1440-1452. DOI:10.1111/nph.16940 |
Liu, J., Shen, Y.X., Zhu, X.A., 2019. Spatial distribution patterns of rock fragments and their underlying mechanism of migration on steep hillslopes in a karst region of Yunnan Province, China. Environ. Sci. Pollut. Res. Int., 26: 24840-24849. DOI:10.1007/s11356-019-05658-1 |
Liu, Y.Y., Chao, L., Li, Z.G., et al., 2024. Water storage capacity is inversely associated with xylem embolism resistance in tropical karst tree species. Tree Physiol., 44: tape017. |
Lin, Y., Medlyn, B.E., Duursma, R.A., et al., 2015. Optimal stomatal behaviour around the world. Nat. Clim. Chang., 5: 459-464. DOI:10.1038/nclimate2550 |
Linger, E., Hogan, J.A., Cao, M., et al., 2020. Precipitation influences on the net primary productivity of a tropical seasonal rainforest in Southwest China: a 9-year case study. For. Ecol. Manage., 467: 118-153. |
Machado, S.R., Rodella, R.A., Angyalossy, V., et al., 2007. Structural variations in root and stem wood of Styrax (Styracaceae) from Brazilian forest and Cerrado. IAWA J., 28: 173-188. DOI:10.1163/22941932-90001632 |
Markesteijn, L., Poorter, L., Bongers, F., 2007. Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am. J. Bot., 94: 515-525. DOI:10.3732/ajb.94.4.515 |
Markesteijn, L., Poorter, L., 2009. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol., 97: 311-325. DOI:10.1111/j.1365-2745.2008.01466.x |
Markesteijn, L., Poorter, L., Paz, H., et al., 2011. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ., 34: 137-148. DOI:10.1111/j.1365-3040.2010.02231.x |
Matos, F.A.R., Magnago, L.F.S., Aquila Chan Miranda, C., et al., 2020. Secondary forest fragments offer important carbon and biodiversity cobenefits. Global Change Biol., 26: 509-522. DOI:10.1111/gcb.14824 |
Meinzer, F.C., Woodruff, D.R., Domec, J.C., et al., 2008. Coordination of leaf and stem water-transport properties in tropical forest trees. Oecologia, 156: 31-41. DOI:10.1007/s00442-008-0974-5 |
Nieuwenhuize, J., Maas, Y.E.M., Middelburg, J.J., 1994. Rapid analysis of organic carbon and nitrogen in particulate materials. Mar. Chem., 45: 217-224. |
Niinemets, Ü., 2001. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology, 82: 453-469. |
Ongole, S., Teegalapalli, K., Byrapoghu, V., 2021. Functional traits predict tree-level phenological strategies in a mesic Indian savanna. Biotropica, 53: 1432-1441. DOI:10.1111/btp.12993 |
Osnas, J.L.D., Katabuchi, M., Kitajima, K., et al., 2018. Divergent drivers of leaf trait variation within species, among species, and among functional groups. Proc. Natl. Acad. Sci. U.S.A., 155: 5480-5485. DOI:10.1073/pnas.1803989115 |
Pan, Y., Birdsey, R.A., Fang, J., Houghton, R., et al., 2011. A large and persistent carbon sink in the world's forests. Science, 333: 988-993. DOI:10.1126/science.1201609 |
Pearson, T.R.H., Burslem, D.F.R.P., Dalling, G.J.W., 2003. Interactions of gap size and herbivory on establishment, growth and survival of three species of neotropical pioneer trees. J. Econ., 91: 785-796. |
Pockman, W.T., Sperry, J.S., 2000. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am. J. Bot., 87: 1287-1299. DOI:10.2307/2656722 |
Poorter, H., Niinemets, Ü., Poorter, L., et al., 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol., 182: 565-588. DOI:10.1111/j.1469-8137.2009.02830.x |
Prior, L.D., Eamus, D., Bowman, D.M.J.S., 2004. Tree growth rates in north Australian savanna habitats: seasonal patterns and correlations with leaf attributes. Aust. J. Bot., 52: 303-314. |
Qi, J.H., Fan, Z.X., Fu, P.L., et al., 2020. Differential determinants of growth rates in subtropical evergreen and deciduous juvenile trees: carbon gain, hydraulics and nutrient-use efficiencies. Tree Physiol., 41: 12-23. |
Quigley, M., Platt, W., 2003. Composition and structure of seasonally deciduous forests in the Americas. Ecol. Monogr., 73: 87-106. DOI:10.1890/0012-9615(2003)073[0087:CASOSD]2.0.CO;2 |
Querejeta, J.I., Estrada-Medina, H., Allen, M.F., et al., 2007. Water source partitioning among trees growing on shallow karst soils in a seasonally dry tropical climate. Oecologia, 152: 26-36. DOI:10.1007/s00442-006-0629-3 |
Rodrigues, R.D., Silva, J.L.A., Trindade, D.N.M., et al., 2022. Leaf habits and their relationship with leaf and wood traits in tropical dry forests. Trees (Berl.), 36: 7-24. DOI:10.55232/1084003.1 |
Roth, I., 1984. Stratification of Tropical Forests as Seen in Leaf Structures. Kluwer, Dordrecht, Netherlands, p. 521.
|
Rowland, L., da Costa, A.C.L., Galbraith, D.R., et al., 2015. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature, 528: 119-122. DOI:10.1038/nature15539 |
R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
|
Russo, S.E., Kitajima, K., 2016. The ecophysiology of leaf lifespan in tropical forests: adaptive and plastic reses to environmental heterogeneity. In: Goldstein, G., Santiago, L. (Eds. ), Tropical Tree Physiology, First ed. Springer, Berlin,
pp. 357-383.
|
Schwinning, S., 2008. The water relations of two evergreen tree species in a karst savanna. Oecologia, 158: 373-383. DOI:10.1007/s00442-008-1147-2 |
Sperry, J.S., 2003. Evolution of water transport and xylem structure. Int. J. Plant Sci., 164: S115-S127. |
Sperry, J.S., Sullivan, J.E.M., 1992. Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol., 100: 605-613. DOI:10.1104/pp.100.2.605 |
Tng, D.Y.P., Apgaua, D.M.G., Ishida, Y.F., et al., 2018. Rainforest trees respond to drought by modifying their hydraulic architecture. Ecol. Evol., 8: 12479-12491. DOI:10.1002/ece3.4601 |
Tomlinson, K.W., Poorter, L.S., Frank, J., et al., 2013. Leaf adaptations of evergreen and deciduous trees of semi-arid and humid savannas on three continents. J. Ecol., 2: 430-440. DOI:10.1111/1365-2745.12056 |
Tyree, M.T., Davis, S.D., Cochard, H., 1994. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction?. IAWA J., 15: 335-360. DOI:10.1163/22941932-90001369 |
Wagner, F., Rossi, V., Stahl, C., et al., 2012. Water availability is the main climate driver of neotropical tree growth. PLoS One, 7: 1-11. DOI:10.1016/s0924-9338(12)74275-x |
Wang, L., He, Y.J., Umer, M., et al., 2023a. Strategic differentiation of subcommunities composed of evergreen and deciduous woody species associated with leaf functional traits in the subtropical mixed forest. Ecol. Indicat., 150: 110281. |
Wang, Y.Q., Song, H.Q., Chen, Y.J., et al., 2023b. Hydraulic determinants of drought-induced tree mortality and changes in tree abundance between two tropical forests with different water availability. Agric. For. Meteorol., 331: 109329. |
Warton, D.I., Duursma, R.A., Falster, D.S., Taskinen, S., 2012. SMATR 3-an R package for estimation and inference about allometric lines. Methods Ecol. Evol., 3: 257-259. |
Wright, I.J., Reich, P.B., Mark, W., et al., 2004. The worldwide leaf economics spectrum. Nature, 428: 821-827. |
Würth, M.K.R., Peláez-Riedl, S., Wright, S.J., et al., 2005. Non-structural carbohydrate pools in a tropical forest. Oecologia, 143: 11-24. DOI:10.1007/s00442-004-1773-2 |
Yan, Y.M., Fan, Z.X., Fu, P.L., et al., 2020. Size dependent associations between tree diameter growth rates and functional traits in an Asian tropical seasonal rainforest. Funct. Plant Biol., 48: 231-240. |
Zhang, J.G., Chen, H.S., Su, Y.R., et al., 2011. Spatial variability and patterns of surface soil moisture in a field plot of karst area in southwest China. Plant Soil Environ., 57: 409-417. |
Zhu, H., Wang, H., Li, B., et al., 2003. Biogeography and floristic affinities of the limestone flora in southern Yunnan, China. Ann. Mo. Bot. Gard., 90: 444-465. DOI:10.2307/3298536 |
Zhang, S.B., Zhang, J.L., Cao, K.F., 2017. Divergent hydraulic safety strategies in three co-occurring Anacardiaceae tree species in a Chinese savanna. Front. Plant Sci., 7: 2075. |
Zhang, S.B., Wen, G.J., Yang, D.X., 2019. Drought-induced mortality is related to hydraulic vulnerability segmentation of tree species in a savanna ecosystem. Forests, 10: 697. DOI:10.3390/f10080697 |
Zhu, S.D., Chen, Y.J., Fu, P.L., et al., 2017. Different hydraulic traits of woody plants from tropical forests with contrasting soil water availability. Tree Physiol., 37: 1469-1477. DOI:10.1093/treephys/tpx094 |
Zhu, S.D., Chen, Y.J., Ye, Q., et al., 2018. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol., 38: 658-663. DOI:10.1093/treephys/tpy013 |



