b. State Key Laboratory of Plant Diversity and Specialty Crops, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China;
c. Sino-Africa Joint Research Center, Chinese Academy of Sciences, Wuhan 430074, China;
d. Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Ministry of Education) & Guangxi Key Laboratory of Landscape Resources Conservation and Sustainable Utilization in Lijiang River Basin, Guangxi Normal University, Guilin 541004, China;
e. Environment Institute, School of Biological Sciences, The University of Adelaide, SA 5005, Australia;
f. Honorary Research Associate, Singapore Botanic Gardens, National Parks Board, 1 Cluny Road, 259569 Singapore;
g. Departamento de Biodiversidade, Instituto de Biociências, Universidade Estadual Paulista "Júlio de Mesquita Filho", Av. 24 A 1515, Bela Vista, P.O. Box 199, 13506-900 Rio Claro, SP, Brazil;
h. Key Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China, Ministry of Education, Southwest Forestry University, Kunming 650224, China;
i. Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences & Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China;
j. Yunnan International Joint Laboratory of Southeast Asia Biodiversity Conservation & Yunnan Key Laboratory for Conservation of Tropical Rainforests and Asian Elephants, Mengla 666303, China;
k. Institute of Leisure Agriculture, Shandong Academy of Agricultural Sciences, Jinan 250100, China;
l. Research Centre for Ecosystem Resilience, Australian Institute of Botanical Science, Royal Botanic Gardens and Domain Trust, Mrs Macquaries Road, Sydney, NSW 2000, Australia;
m. Missouri Botanical Garden, 4344 Shaw Boulevard, St. Louis, Missouri 63110, U.S.A;
n. Co-Innovation Center for Sustainable Forestry in Southern China, College of Life Sciences, Nanjing Forestry University, Nanjing 210037, China;
o. Institute of Plant Science and Microbiology, Universität Hamburg, Ohnhorststr. 18, D-22609 Hamburg, Germany
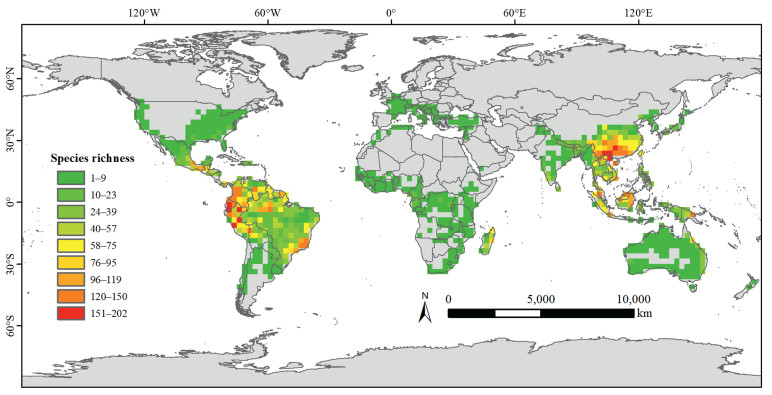
In this review, we give an overview of recent advances in our understanding of Lauraceae, the largest (almost) entirely woody family in the subclass Magnoliidae. POWO (https://powo.science.kew.org) lists 3384 accepted species in 58 genera, with only the approximately 20 species of Cassytha L. being neither trees nor shrubs, but hemiparasitic twiners. The vast majority of Lauraceae are distributed in tropical and subtropical regions of the world, where they are usually among the 10 most abundant tree families in moist to wet forests (Fig. 1). The main centers of diversity are tropical to subtropical America and Asia, with only a few species growing in temperate zones, such as the Asian Lindera obtusiloba Blume and North American Sassafras albidum (Nutt.) Nees. Members of this family are used as fruit trees (avocado, Persea americana Mill.), spices (e.g., true cinnamon, Cinnamomum verum J. Presl; Saigon cinnamon, 肉桂= rou-gui, C. aromaticum Nees; Indian bay leaf, C. tamala (Buch.-Ham.) T. Nees & C.H. Eberm.; bay laurel, Laurus nobilis L.; and ishpingo de olor, Mespilodaphne quixos (Lam.) Rohwer.), medicine (e.g., 乌药= wu-yao, Lindera aggregata (Sims) Kosterm.; laurel dodder, Cassytha filiformis L.), for extraction of aromatic compounds (e.g., camphor, Camphora officinarum Boerh. ex Fabr.; Brazilian rosewood, Aniba rosodora Ducke), seed fats (山鸡椒= shan-ji-jiao Litsea cubeba (Lour.) Pers.), or as timber trees (e.g., 楠木= nan-mu, Phoebe spp.; greenheart, Chlorocardium rodiei (R.H. Schomb.) Rohwer, H.G. Richt. & van der Werff; Imbuia, Ocotea porosa (Nees & Mart.) Barroso; Medang, Alseodaphne insignis Gamble; and Queensland walnut, Endiandra palmerstonii (F.M. Bailey) C. White & W.D. Francis).
|
| Fig. 1 Species richness of georeferenced records in Lauraceae, based on data from GBIF (GBIF.org, 2024). |
Traditional morphological classifications of Lauraceae were based on a relatively limited set of inflorescence, flower, and fruit characters, which were often combined almost randomly (e.g., Kostermans, 1957; Rohwer, 1993a). This resulted in conflicting classifications based on the relative importance attributed to these characters by different authors (van der Werff and Richter, 1996), with some of the most contentious problems in the family being infrafamilial and generic classification. Since the start of the new millennium, molecular systematic studies have elucidated at least the basic relationships within the family and are starting to converge toward a consensus, especially the infrafamilial classification into nine tribes. Nevertheless, numerous problems remain within several groups of closely related species at both intergeneric and intrageneric levels and it has become evident that "easy" characters such as merosity (trimerous vs. dimerous), number of fertile stamens (2, 3, 4, 6, 9, or more numerous), or number of pollen sacs per anther (2 vs. 4) are constant in some evolutionary lineages but highly variable in others. This makes it challenging, if not almost impossible, to re-circumscribe some genera in such a way that the resulting units are both monophyletic and recognizable morphologically.
Six new genera have been described since the last major overview of the family (Rohwer, 1993a): Alseodaphnopsis H.W. Li & J. Li (Mo et al., 2017), Andea van der Werff (2022), Kuloa Trofimov and Rohwer (2020), Sextonia van der Werff (1997 publ. 1998), Sinopora J. Li, N.H. Xia & H.W. Li (Li et al., 2008a) and Yasunia van der Werff (van der Werff and Nishida, 2010). An additional five genera have also been reinstated: Camphora Fabr. (Yang et al., 2022), Clinostemon Kuhlm. & Samp. (Alves and Souza, 2013), Damburneya Raf. (Trofimov et al., 2016), Mespilodaphne Nees (Trofimov et al., 2019), and Tamala Raf. (Weakley et al., 2023). However, the process of taxonomic rearrangement is far from complete. In addition to molecular systematic studies, much taxonomic progress has also been made over the last two decades (see Section 3, "Taxonomy"), mainly focused on the most diverse regions of the family, e.g., tropical Asia, tropical America, and Africa (Madagascar).
Based on these molecular studies, large-scale biogeographic patterns of Lauraceae tribes and genera have been studied further (e.g., Chanderbali et al., 2001; Li et al., 2011, 2016; Huang et al., 2016; Song et al., 2023). Generally, different lineages within the family are sorted into two main geographic groups, largely consistent with Gondwanan and Laurasian histories, respectively. The biogeographic patterns for the Laurasian lineages may result mainly from the disruption of boreotropical flora caused by global climatic cooling, whereas those of Gondwanan lineages may be the results of multiple long-distance dispersal events. Nevertheless, the complicated biogeographic patterns of Lauraceae are far from well explained, as the phylogenetic relationships among many closely related groups remain unresolved.
The development of high-throughput and long-read sequencing technologies have led to a rapid accumulation of genetic resources for Lauraceae, with plastid and nuclear genomes offering valuable information about the evolutionary history of the family. Plastomes provide robust and significantly supported relationships among deep lineages of Lauraceae (e.g., Song et al., 2020; Liu et al., 2021; Yang et al., 2023). Whole genomic analysis has revealed the genome evolution, as well as key genes involved in the synthesis of terpenoids, flavonoids, aromatic compounds, and D-borneol of some economically important Lauraceae species, helping us understand the genomic evolution, metabolic diversity, and adaptive strategies of the family (e.g., Chaw et al., 2019; Rendón-Anaya et al., 2019; Chen et al., 2020a, 2020b; Li et al., 2022; Wang et al., 2022; Xiong et al., 2022; Zhang et al., 2022; Schmitt et al., 2024; Tao et al., 2024).
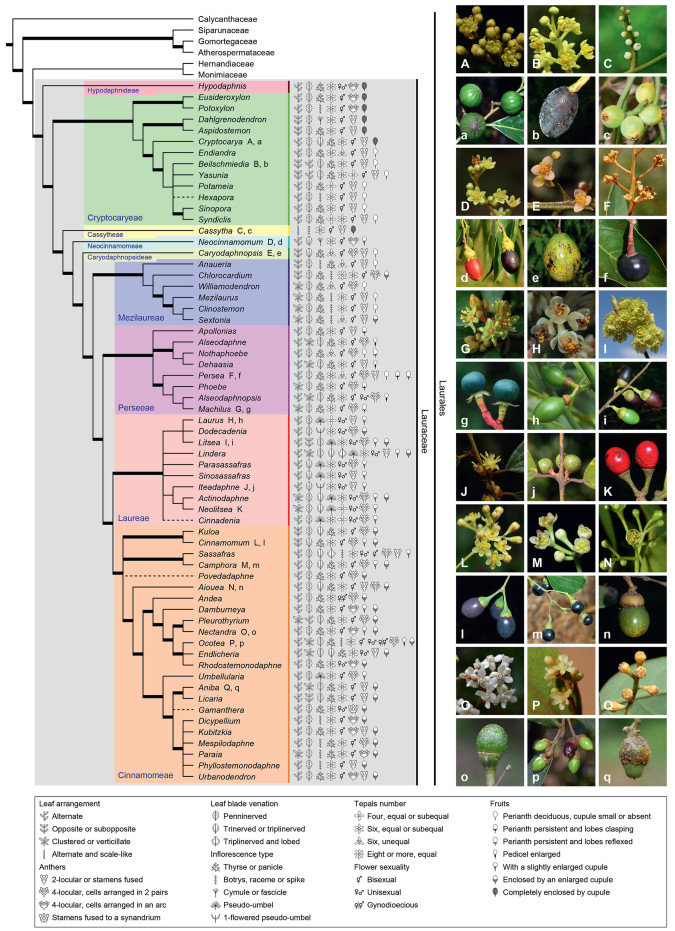
2. Phylogeny 2.1. Backbone phylogeny of LauraceaeThe main phylogenetic lineages within Lauraceae have become increasingly clear and well established over the past 25 years (Fig. 2). Recent results by Song et al. (2020), Liu et al. (2021) and Yang et al. (2023), based on plastid DNA, as well as PAFTOL (Zuntini et al., 2024; https://treeoflife.kew.org/tree-of-life), based on nuclear DNA, are largely congruent, and confirm most of the evolutionary lineages already recognized in the pioneering studies of Rohwer (2000), Chanderbali et al. (2001), and Rohwer and Rudolph (2005). The result of the most recent study (Helmstetter et al., 2025), based on the Angiosperms353 probe set developed by Johnson et al. (2019), is largely congruent as well, except for a few taxa "that tended to be in different positions in the different analyses". Both the phylogenetic analyses of Song et al. (2020) and Yang et al. (2023) showed that Lauraceae were divided into nine high-supported clades. Hypodaphnis Stapf was confirmed to be the first genus of Lauraceae to diverge. Then a Beilschmiedia–Cryptocarya clade, Cassytha, Neocinnamomum H. Liu and Caryodaphnopsis Airy Shaw diverged in turn. The remaining core group of Lauraceae comprises four clades: Chlorocardium–Mezilaurus, Machilus–Persea, Laurus–Neolitsea, and Cinnamomum–Ocotea. The Chlorocardium–Mezilaurus clade is basal within the core group, with the Machilus–Persea clade sister to the Laurus–Neolitsea clade and the Cinnamomum–Ocotea clade terminal pair. In this review, these nine clades are treated as tribes: Hypodaphnideae, Cryptocaryeae, Cassytheae, Neocinnamomeae, Caryodaphnopsideae, Mezilaureae, Perseeae, Laureae and Cinnamomeae (Fig. 2). An updated phylogenetic classification of Lauraceae is provided in Appendix A.
|
| Fig. 2 Phylogenetic relationships among all recognized genera and tribes in Lauraceae. This tree summarizes results from several molecular phylogenetic investigations (Li et al., 2008c, 2011, 2020; Alves and Souza, 2013; Rohwer et al., 2014; Rohde et al., 2017; Song et al., 2020; Liu et al., 2021; Trofimov and Rohwer, 2020; Penagos Zuluaga et al., 2021; Yang et al., 2022, 2023). For those non-monophyletic genera, only one node is displayed, which includes the type species of each genus. Nodes resolved with 90%–100% bootstrap support or Bayesian posterior probabilities of 0.95–1.00 are shown with bold lines. Genera without molecular sampling are temporarily placed in possible positions and represented by dashed lines. Morphological characters are shown as diagrams following each tip, with explanation provided below. Representative photos are shown on the right: A. Cryptocarya; B. Beilschmiedia; C. Cassytha; D. Neocinnamomum; E. Caryodaphnopsis; F. Persea; G. Machilus; H. Laurus; I. Litsea; J. Iteadaphne; K. Neolitsea; L. Camphora; M. Cinnamomum; N. Aiouea; O. Nectandra; P. Ocotea; Q. Aniba. Photos credit: A–E and G–M by B. Liu (刘冰); F and N–Q by J.G. Rohwer. |
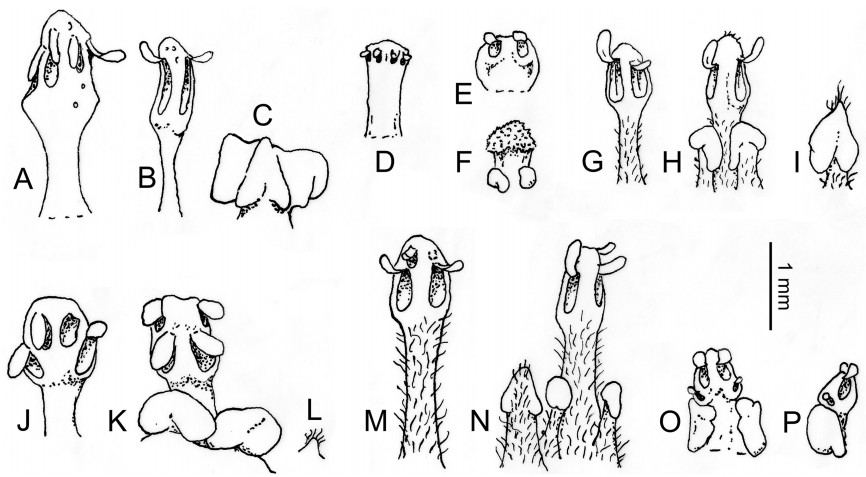
It is noteworthy that the early diverging lineages in Lauraceae include predominantly taxa in which the ovary is (semi-) inferior (Hypodaphnis, Potoxylon Kosterm., Eusideroxylon Teijsm. & Binn.) and/or at least enclosed in fruit by a deep hypanthium (Cryptocaryeae except the Beilschmiedia complex; Cassytha). Superior ovaries surrounded by a shallow or almost no hypanthium have originated at least twice: in the Beilschmiedia complex and again above the separation of Cassytha from the remaining taxa in the family. The anthers of the early diverging taxa may be either 2-locular or 4-locular, but when 4-locular, the pollen sacs are arranged (almost) collaterally (Fig. 3A–F). In 2-locular anthers of Cryptocaryeae there is often a longitudinal septum recognizable in each locule, showing that they are derived by lateral fusion of the pollen sacs of each theca (Fig. 3G and H). Above Cassytha there is a tendency for the more central pollen sacs (i.e., those closer to the connective) to shift to a position higher on the anther than the more lateral pollen sacs (Fig. 3J and K). Pollen sacs arranged in two almost completely superposed pairs have developed in Chlorocardium Rohwer, H.G. Richt. & van der Werff, Sextonia, and Williamodendron Kubitzki & H.G. Richt. of the Mezilaureae, and appear to be a synapomorphy of the Cinnamomeae–Laureae–Perseeae clade (= Core Lauraceae according to Rohwer and Rudolph, 2005), but with several reversals in the Cinnamomeae. When taxa of the Core Lauraceae have 2-locular anthers, they were derived not by lateral fusion, but instead by reduction of either the upper or the lower pair of pollen sacs (Fig. 3M–P).
|
| Fig. 3 Examples of stamens and staminodes. A–C Hypodaphnis zenkeri (Engl.) Stapf; A. Stamen of whorl 1, adaxial side; B. Stamen of whorl 3, adaxial side; C. Gland complex alternating with the stamens of whorl 3, interpreted as staminode of whorl 4 fused to adjacent glands. D. Eusideroxylon zwageri Teijsm. & Binn., stamen of whorl 3, abaxial side; glands hardly discernible. E, F Aspidostemon glandulosus Rohwer; E. Stamen of whorl 1, adaxial side; F. Staminode of whorl 3, abaxial side, with attached glands. G–I Cryptocarya botelhensis P.L.R. Moraes; G. Stamen of whorl 2, with longitudinal septum inside the locules; H. Stamen of whorl 3, with stalked glands, abaxial side; I. Staminode of whorl 4 with cordate glandular head, adaxial side. J–L Ocotea tonduzii Standl.; J. Stamen of whorl 1, adaxial side; K. Stamen of whorl 3, abaxial side, with glands; L. Minute remnant of staminode of whorl 4. M, N Persea alpigena Spreng. var. harrisii (Mez) L.E. Kopp; M. Stamen of whorl 2, adaxial side, upper locules reduced or absent; N. Staminode of whorl 4 (left) and stamen of whorl 3 (right), abaxial side, with stalked glands. O, P Urbanodendron verrucosum (Nees) Mez; O. Stamen of whorl 2, adaxial side, lower locules reduced (or absent), filament with glands; P. Stamen of whorl 3, lateral view, lower locules reduced (or absent), filament with glands. Scale bar = 1 mm. Camera lucida drawings from herbarium material by Jens G. Rohwer [A–C Leeuwenberg 5557 (HBG); D Endert 15E2P680 (L); E, F Service Forestier 16534 (P); G–I Moraes 2329 (HBG); J–L Tonduz 1739 (B); M, N Harris 5335 (NY); O, P Kuhlmann s.n., RB91278 (RB)]. |
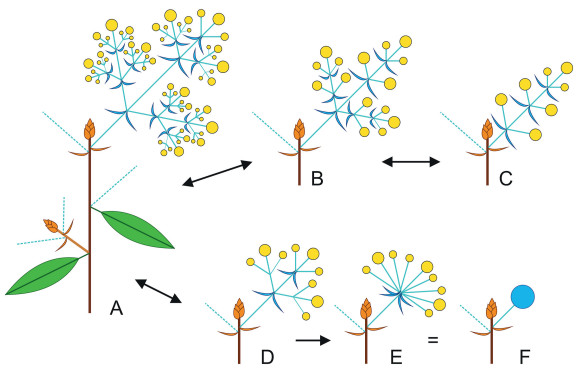
The inflorescences of early divergent Lauraceae are rather diverse, most frequently irregularly paniculate, but also spicate in some Cassytha species, fasciculate in Neocinnamomum and (di-)botryoid in several Mezilaureae. Thyrsoid inflorescences with strictly opposite lateral flowers or cymes in the higher orders of branching appear to be a synapomorphy of the Core Lauraceae. Studies on inflorescence development suggest that the umbellate, involucrate inflorescences of Laureae may have been derived from such thyrsoid inflorescences (Fig. 4) (Ruge, 2000; Heintz, 2007).
|
| Fig. 4 Generalized inflorescence diagrams. A. Branch with thyrsoid inflorescence, i.e., proximally with one to several order(s) of racemose branching, distally with one to several orders of cymose (dichasial) branching. Inflorescences may be arranged in the axils of cataphylls below the terminal vegetative bud (top), and/or in the axils of foliage leaves (middle, right), and/or on axillary brachyblasts (lower left). Bracts within the inflorescence (blue) may or may not be present in mature inflorescences; B. Dibotryoid inflorescence, i.e., determinate double raceme, cymose part not developed; C. Botryoid inflorescence (determinate raceme); D. Inflorescence consisting of three flower triads; E. Pseudo-umbel, i.e., internodes in the inflorescence not elongating, except peduncle and pedicels; F. Same pseudo-umbel in bud, enclosed by its involucral bracts (externally looking like a flower bud). |
Hypodaphnideae consists of the monotypic genus Hypodaphnis (Table 1), endemic to tropical Africa (Cameroon, Gabon, Nigeria, Rep. Congo). Early molecular studies suggested that Hypodaphnis appears to be sister to all other extant Lauraceae (Rohwer, 2000; Chanderbali et al., 2001; Rohwer and Rudolph, 2005). Its basal position in Lauraceae was confirmed further by recent phylogenomic studies (Song et al., 2020; Yang et al., 2023).
| Tribes | Kostermans (1957) | Rohwer (1993a) | van der Werff and Richter (1996) |
| Hypodaphnideae Kosterm. ex Reveal (2012) | Subfam. A. Lauroideae | Tribe 1. Perseeae | Subfam. Lauroideae |
| Hypodaphnis | Tribe Ⅰ Perseeae | Cryptocarya group | Tribe 1. Laureae |
| Cryptocaryeae Nees (1836) | Subtrib. a. Perseineae | Cassytha subgroup | Actinodaphne, Iteadaphne, Laurus, |
| Aspidostemon, Beilschmiedia, Cryptocarya, | Persea, Phoebe | Cassytha | Lindera, Litsea, Neolitsea, Sassafras, |
| Dahlgrenodendron, Endiandra, Eusideroxylon, | Subtrib. b. Beilschmiediineae | Cryptocarya subgroup | Umbellularia |
| Hexapora, Potameia, Potoxylon, Sinopora, | Apollonias, Dehaasia, Beilschmiedia, | Cryptocarya, Ravensara | Tribe 2. Perseeae |
| Syndiclis, Yasunia | Endiandra, Mezilaurus, Hexapora, | Aspidostemon subgroup | Aiouea, Aniba, Alseodaphne, |
| Cassytheae Dumort. (1829) | Potameia | Aspidostemon | Aspidostemon, Chlorocardium, |
| Cassytha | Tribe Ⅱ Cinnamomeae | Eusideroxylon subgroup | Cinnamomum, Dehaasia, |
| Neocinnamomeae Yu Song, W.B. Yu & | Subtrib. a. Cinnamomineae | Eusideroxylon, Potoxylon | Dicypellium, Endlicheria, Licaria, |
| Y.H. Tan (2020) | Actinodaphne, Cinnamomum, Ocotea, | Hypodaphnis subgroup | Nectandra, Nothaphoebe, Ocotea, |
| Neocinnamomum | Sassafras, Umbellularia, Dicypellium | Hypodaphnis | Persea, Phoebe, Pleurothyrium, |
| Caryodaphnopsideae Yu Song, W.B. Yu & Y.H. | Subtrib. b. Anibineae | Beilschmiedia group | Systemonodaphne, Urbanodendron |
| Tan (2020) | Aiouea, Aniba, Endlicheria, Licaria, | Beilschmiedia, Brassiodendron, | Tribe 3. Cryptocaryeae |
| Caryodaphnopsis | Phyllostemonodaphne, | Endiandra, Hexapora, Potameia | Beilschmiedia, Caryodaphnopsis, |
| Mezilaureae Rohwer, trib. nov. | Systemonodaphne, Urbanodendron | Ocotea group | Cryptocarya, Endiandra, |
| Anaueria, Chlorocardium, Mezilaurus, | Tribe Ⅲ Litseeae | Persea subgroup | Eusideroxylon, Hypodaphnis, |
| Clinostemon, Sextonia, Williamodendron | Subtrib. a. Litseineae | Alseodaphne, Apollonias, | Potameia, Potoxylon, Ravensara, |
| Perseeae Nees (1836) | Litsea, Neolitsea subtrib. b. Lauriineae | Caryodaphnopsis, Dehaasia, | Triadodaphne |
| Alseodaphne, Alseodaphnopsis, Apollonias, | Lindera, Laurus | Nothaphoebe, Persea, Phoebe | Subfam. Cassythoideae |
| Dehaasia, Machilus, Nothaphoebe, Persea, | Tribe Ⅳ Cryptocaryeae | Ocotea subgroup | Cassytha |
| Phoebe | Subtrib. a. Eusideroxylineae | Aiouea, Cinnamomum, Endlicheria, | |
| Laureae Le Maout & Decne. (1868) | Eusideroxylon | Nectandra, Neocinnamomum, Ocotea, | |
| Actinodaphne, Cinnadenia, Dodecadenia, | Subtrib. b. Cryptocaryineae | Pleurothyrium, Rhodostemonodaphne | |
| Iteadaphne, Laurus, Lindera, Litsea, Neolitsea, | Cryptocarya, Ravensara | Aniba subgroup | |
| Parasassafras, Sinosassafras | Tribe Ⅴ Hypodaphneae | Aniba, Dicypellium, Gamanthera, Licaria, | |
| Cinnamomeae Nees (1836) | Hypodaphnis | Paraia, Phyllostemonodaphne, | |
| Aiouea, Andea, Aniba, Camphora, Cinnamomum, | Subfam. B. Cassythoideae | Systemonodaphne, Urbanodendron | |
| Damburneya, Dicypellium, Endlicheria, | Cassytha | Mezilaurus subgroup | |
| Gamanthera, Kubitzkia, Kuloa, Licaria, | Anaueria, Mezilaurus, Povedadaphne, | ||
| Mespilodaphne, Nectandra, Ocotea, Paraia, | Williamodendron | ||
| Phyllostemonodaphne, Pleurothyrium, | Tribe 2. Laureae | ||
| Povedadaphne, Rhodostemonodaphne, | Actinodaphne, Dodecadenia, Iteadaphne, | ||
| Sassafras, Umbellularia, Urbanodendron | Laurus, Lindera, Litsea, Neolitsea, Parasassafras, Sassafras, Umbellularia |
The flowers of Hypodaphnis are (usually) trimerous as in other Lauraceae, but otherwise show some unusual characters. The number of pollen sacs per anther is highly variable, but in most flowers the anthers in whorls Ⅰ and Ⅱ have (3–) 4 collateral pollen sacs, whereas those of whorl Ⅲ frequently have (1–) 2 pollen sacs (Rohwer, pers. observ.; Fig. 3A and B). In the outer whorls, the more central pollen sacs are introrse and tend to be somewhat smaller than the latrorse lateral pollen sacs. The pollen sacs in whorl Ⅲ are latrorse-introrse. In contrast to most other Lauraceae, the nectar glands look as if they are not associated with the stamens of whorl Ⅲ. Instead, there are three large gland complexes in front of the stamens of whorl Ⅱ. Seen from the adaxial side, these gland complexes have a triangular structure in the middle (Fig. 3C), suggesting that they may be interpreted as glands of adjacent whorl Ⅲ stamens fused via a likewise glandular whorl Ⅳ staminode. The ovary is almost inferior. The flowers of H. zenkeri (Engl.) Stapf are unisexual, as originally described by Stapf (1909) and only a minute ovule, if any, can be found in flowers with fertile stamens. Female flowers are rarely described and apparently poorly represented in herbaria.
2.2.2. CryptocaryeaeAs currently recognized, Cryptocaryeae consist of 12 genera (Table 1), including a total of about 800 species distributed in tropical and subtropical regions worldwide. Three genera are rich in species: Beilschmiedia Nees (~270 spp.), Cryptocarya R. Br. (~360 spp.), and Endiandra R. Br. (~130 spp.). The present circumscription of Cryptocaryeae largely corresponds to Rohwer's (1993a) Beilschmiedia and Cryptocarya groups and van der Werff and Richter's (1996) tribe Cryptocaryeae (Table 1). Cryptocaryeae have pinninerved leaves (rarely triplinerved), thyrsoid-paniculate inflorescences whose ultimate branches and flowers are not quite opposite, with small bracts (not forming an involucre), 2-locular anthers (rarely 4-locular), and fruits either completely enveloped by the receptacle at maturity or free on their pedicel, with or without minute remnants of tepals (Rohwer, 1993a; van der Werff and Richter, 1996).
Phylogenetic relationships of the early diverging genera of the tribe have been elucidated by Rohwer et al. (2014) and confirmed by Song et al. (2023). Eusideroxylon and Potoxylon have anthers with four separate collateral pollen sacs, whereas the remaining genera have two-locular anthers, in Beilschmiedia, Endiandra and Cryptocarya often with a vestigial longitudinal septum in each locule. Eusideroxylon and Potoxylon also differ from the remaining genera by having a semi-inferior ovary, and from each other by having three vs. nine fertile stamens. In Aspidostemon Rohwer & H.G. Richt., Cryptocarya and Dahlgrenodendron J.J.M. van der Merwe & A.E. van Wyk, the ovary is enclosed in a deep receptacular tube, whereas the receptacle is shallow to cup-shaped in the Beilschmiedia complex.
In early diverging Cryptocaryeae lineages the fruit is seemingly inferior, with the receptacle completely enclosing the fruiting carpel and often crowned by remains of floral parts. In the Beilschmiedia complex, on the other hand, it is unenclosed on its pedicel, with remains of floral parts (if any) below the berry. Aspidostemon and the genera of the Beilschmiedia complex share the spinulose pollen grains that are typical for most Lauraceae, whereas Dahlgrenodendron, Cryptocarya, Eusideroxylon and Potoxylon show deviating pollen sculptures (van der Merwe et al., 1988, 1990; Rohwer, 2018). Aspidostemon and Dahlgrenodendron also share opposite leaves; a character otherwise found rarely in Cryptocarya and some taxa of the Beilschmiedia complex.
Problems remain in the delimitation and morphological definition of some genera and subgeneric taxa within the Beilschmiedia complex, because the current phylogenies have low species sampling, resulting in poorly-supported trees. For example, in Rohwer et al. (2014), Endiandra (incl. Brassiodendron C.K. Allen and Triadodaphne Kosterm.), Potameia Thouars, Sinopora and Yasunia appear to be nested within Beilschmiedia, although often with negligible support and Syndiclis was not included. In Song et al. (2020), although including a smaller number of species, Syndiclis and Endiandra appear as successive sister groups of Beilschmiedia. In Yang et al. (2023), including a single species of each genus, Sinopora and Syndiclis Hook. f. are sister taxa.
In the most comprehensive analysis to date (Song et al., 2023), Endiandra appears monophyletic and nested within Beilschmiedia among a clade of Australian and Zealandian species, Yasunia is nested among South American Beilschmiedia species, and Potameia among Malagasy species, supporting Rohwer et al. (2014). Sinopora from Hong Kong (China) is nested in Syndiclis, and should be included in the latter. Sinopora may also be identical with Hexapora Hook.f. from Penang (Malaysia), but the latter has not been collected for more than 120 years (de Kok, 2016a). However, if that latter assertion is correct, then Hexapora also needs to be included in Syndiclis. Furthermore, Song et al. (2023) found that Syndiclis appears to be nested among Central American and Asian species of Beilschmiedia.
2.2.3. Cassytheae, Neocinnamomeae and CaryodaphnopsideaeCassytheae, Neocinnamomeae, and Caryodaphnopsideae each consist of a single genus respectively: Cassytha, Neocinnamomum, and Caryodaphnopsis (Table 1), all of which are monophyletic in several recent molecular studies (Li et al., 2016; Song et al., 2017a, 2020; Liu et al., 2021). Cassytha is the only genus of hemiparasitic vines in Lauraceae and includes a total of ~20 species centered in Australia, but with representatives in Africa and tropical Asia (Weber, 1981, 2007). Only one species, C. filiformis, is cosmopolitan, but occurs mainly in the tropics. Neocinnamomum contains seven species found in tropical and subtropical Asia (Kostermans, 1974a; Li et al., 1982, 2008b), while Caryodaphnopsis includes more than 20 species distributed disjunctly in tropical Asia and tropical America (Kostermans, 1974b; Li et al., 1982, 2008b, 2016; van der Werff and Richter, 1985; Li and Li, 1991; Zhang et al., 2024a). The placement of these genera as isolated lineages within Lauraceae is supported by several morphological traits: the parasitic habit and reduced leaves in Cassytha; condensed few-flowered thyrses, alternate, triplinerved leaves and enlarged cupules with persistent tepals in Neocinnamomum; and opposite or subopposite, trinerved or triplinerved (rarely pinninerved) leaves, unequal, deciduous tepals and fruits lacking a cupule in Caryodaphnopsis.
Morphological and molecular investigations of the infrageneric phylogeny of Neocinnamomum indicate that inflorescence morphology and ontogeny may help resolve the evolution and classification of the genus (Wang et al., 2010; Cao et al., 2023; Li et al., 2023). Studies have suggested that there are two isolated clades of Caryodaphnopsis, one Asian and one American (Li et al., 2016), with eight American and 12 Asian species, including three new Asian species (Zhang et al., 2024a). However, species delimitation and genetic diversity of Asian Caryodaphnopsis requires further investigation (Cao et al., 2024; Yang et al., 2024).
The infrageneric phylogeny and species boundaries in Cassytha have not been studied comprehensively but are the subject of active ongoing research. Several recent molecular studies, mainly focused on Cassytha species distributed in China and Japan, have indicated that combining molecular phylogeny, morphology, and distribution patterns may help to improve taxon definition and provide insights into the biodiversity, phylogeography and conservation of Cassytha (Kokubugata et al., 2012; Yu et al., 2023; Liu et al., 2024).
2.2.4. MezilaureaeA surprising result of the molecular systematic studies has been the recognition of the entirely Neotropical tribe Mezilaureae with six genera from Costa Rica to Peru, Bolivia and SE Brazil that were often considered "aberrant" before the availability of DNA data (Table 1). Mezilaureae have pinninerved leaves that are either opposite or aggregated at the tips of the branches, botryoid or dibotryoid inflorescences, flowers with three (more or less columnar, 2- or 4-locular), or six (triangular, 2-locular), or 8–20 (tongue-shaped, papillose, 4-locular) stamens, and fruits with small plate-like or well-developed to almost urceolate cupules. Three of these genera, Clinostemon, Mezilaurus Kuntze ex Taub., and Williamodendron, have three fertile stamens, representing the third androecial whorl, small, plate-like cupules and leaves clustered at the tips of the branches and had been considered either to be closely related, or treated as a single genus (Mezilaurus). The remaining genera have more stamens, with six in Anaueria Kosterm. (whorls Ⅰ and Ⅱ), nine in Sextonia, and nine or 8–20 in Chlorocardium.
A relatively close relationship between Clinostemon, Mezilaurus and/or Williamodendron has been confirmed in all molecular systematic studies where they were included (Rohwer, 2000; Chanderbali et al., 2001; Rohwer and Rudolph, 2005; Alves and Souza, 2013; Song et al., 2020). However, most of these studies placed Sextonia as sister to either Clinostemon (Alves and Souza, 2013; Chanderbali et al., 2001; Song et al., 2020, in the latter two publications as Mezilaurus triunca van der Werff) or Williamodendron (Rohwer and Rudolph, 2005, Clinostemon not included). Sextonia is similar to both these genera and to Mezilaurus in having leaves clustered at the apex of the branches but differs in having tongue-shaped papillose stamens, like Chlorocardium. Additionally, Sextonia differs from Mezilaurus, Clinostemon and Williamodendron in having nine stamens and fruits seated in well-developed cupules. Anaueria and Chlorocardium share opposite leaves. These taxa were also retrieved as a clade in the studies of Chanderbali et al. (2001) and Rohwer and Rudolph (2005), but formed separate clades in the studies of Alves and Souza (2013; Chlorocardium basal) and Song et al. (2020; Anaueria basal), albeit in both cases with negligible node support. Thus, questions remain about the positions of these genera relative to one another, as well as morphological character evolution within the tribe.
2.2.5. PerseeaeAs currently recognized, Perseeae (the Persea group) consists of eight genera, Alseodaphne Nees, Alseodaphnopsis, Apollonias Nees, Dehaasia Blume, Machilus Rumph. ex Nees, Nothaphoebe Blume, Persea Mill., and Phoebe Nees (Table 1), with ~400 species distributed in tropical to subtropical Asia and warm-temperate to tropical regions of the New World. Based on morphological evidence, Rohwer (1993a) largely recognized their present circumscription as the Persea subgroup. In the circumscription adopted here, Perseeae largely corresponds to Kostermans' (1957) subtribe Perseineae of tribe Perseeae and includes all the non-cupulate genera of van der Werff and Richter's (1996) tribe Perseeae (Table 1). Perseeae usually have pinninerved leaves (some leaves subtriplinerved in Persea ekmanii O.C. Schmidt), thyrsoid inflorescences (consisting of cymes whose lateral flowers are opposite), undeveloped cupules (sometimes with enlarged pedicels), and the staminodes of the fourth androecial whorl are distinct in most species, often with a glandular head (Kostermans, 1957; Rohwer, 1993a; van der Werff and Richter, 1996).
The monophyly of Perseeae was confirmed by several recent molecular studies (e.g., Rohwer, 2000; Chanderbali et al., 2001; Rohwer and Rudolph, 2005; Rohwer et al., 2009; Li et al., 2011; Mo et al., 2017; Song et al., 2020; Liu et al., 2021; Xiao et al., 2022; Yang et al., 2023), with several traditional genera or subgenera also confirmed as monophyletic, viz., Machilus, Phoebe, Persea subgen. Eriodaphne, and P. subgen. Persea (Rohwer et al., 2009; Li et al., 2011; Song et al., 2020; Liu et al., 2021). Alseodaphnopsis is likewise monophyletic, having been separated recently from Alseodaphne Nees by Mo et al. (2017), based on both morphological and molecular evidence. The recognition of Alseodaphnopsis was confirmed further by Song et al. (2020). However, Alseodaphnopsis appeared non-monophyletic in some plastid-based studies (Liu et al., 2021; Xiao et al., 2022), suggesting that further research is needed. In addition, van der Werff (2019) found that the type species of Alseodaphnopsis and three other species had unisexual flowers, making Alseodaphnopsis the only genus in Perseeae with unisexual flowers.
Machilus is by far the most homogeneous group within Perseeae (Rohwer et al., 2009; Li et al., 2011; Mo et al., 2017; Song et al., 2020; Liu et al., 2021; Xiao et al., 2022) and its persistent, not or scarcely indurate and spreading to reflexed tepals in fruit are important morphological characters for its generic delimitation (Li et al., 2011). However, the sections or subsections of Machilus accepted by Li et al. (1982) are questionable, with traditional morphological features, such as the presence or absence of hairs on the outside of the tepals at anthesis and the shape and size of the fruit deemed insufficient for infrageneric delimitation within Machilus (Rohwer et al., 2009; Li et al., 2011). Phoebe, like Machilus, is also monophyletic and its persistent, thickened, leathery to woody tepals, which clasp the base of the fruit are important characters for its generic delimitation from other Perseeae (Li et al., 2011; Mo et al., 2017; Song et al., 2020; Liu et al., 2021; Xiao et al., 2022).
Persea, as presently circumscribed, is polyphyletic, but the two subgenera accepted by Kopp (1966): Persea subgen. Eriodaphne and P. subgen. Persea, are monophyletic and well supported (Rohwer et al., 2009; Li et al., 2011). Nevertheless, some species do not fit these traditional subgenera (e.g., P. nudigemma van der Werff and P. sphaerocarpa (H.J.P. Winkl.) Kosterm.) and appear instead as separate clades closer to Alseodaphne or Phoebe. Weakley et al. (2023) reinstated the genus Tamala for the three North American species of Persea, viz., P. borbonia (L.) Spreng., P. humilis Nash and P. palustris (Raf.) Sarg. However, P. borbonia and P. palustris were nested among species of Persea subgen. Eriodaphne in Rohwer et al. (2009) and Li et al. (2011). Therefore, we prefer not to recognize Tamala until a full revision of Persea has been undertaken.
The affinities of the two Macaronesian species, Persea indica (L.) Spreng. and Apollonias barbujana (Cav.) Bornm., are clearly American rather than Asian (Rohwer et al., 2009; Li et al., 2011; Mo et al., 2017; Song et al., 2020) and the latter was transferred to Persea by Mabberley and Nieto Feliner (2017), as its most important diagnostic character (disporangiate anthers) also occurs in Neotropical Persea (Li et al., 2011). In contrast, Rohwer et al. (2009) predicted that Apollonias arnottii Nees from India should group Asian Perseeae such as Machilus or Phoebe. Yang et al. (2025) recently resolved this species as sister to a species of Phoebe, confirming the prior prediction.
Both Alseodaphne (currently circumscribed as including only species with tetrasporangiate anthers) and Dehaasia (separated from Alseodaphne for having disporangiate anthers, see van der Werff, 2001a) are polyphyletic, with the Alseodaphne (excluding Alseodaphnopsis), Dehaasia and Nothaphoebe species investigated so far forming a single, well-supported but intermixed clade in molecular studies (Rohwer et al., 2009; Li et al., 2011; Song et al., 2020; Xiao et al., 2022). It has long been known that Dehaasia and Alseodaphne are very closely related and insufficiently separated (e.g., Kostermans, 1973a, b; van der Werff, 2001a), the only difference being the number of anther locules (2 vs. 4), a feature not correlated with vegetative characters. Furthermore, the delimitation between Nothaphoebe and Alseodaphne is similarly vague (e.g., tepals unequal vs. equal, or persistent vs. deciduous in fruit), with van der Werff (2001) suggesting that Nothaphoebe should be placed within Alseodaphne. Thus, both Dehaasia and Nothaphoebe apparently belong in Alseodaphne, but a major revision is needed before dozens of new combinations are made, as current taxon sampling is too incomplete to confirm the relationships of these three genera definitively (Nishida and van der Werff, 2014).
2.2.6. LaureaeAs currently recognized, Laureae (the Litsea complex) consists of four large genera (Actinodaphne Nees, Lindera Thunb., Litsea Lam., and Neolitsea (Benth.) Merr.), each with over 100 species, and six monotypic or oligotypic genera (Cinnadenia Kosterm., Dodecadenia Nees, Iteadaphne Blume, Laurus L., Parasassafras D.G. Long, and Sinosassafras H.W. Li) (Table 1). The tribe includes ~700 species mainly distributed in tropical and subtropical Asia, but also with representatives from North America to subtropical South America and in the Mediterranean region, Macaronesia, Australia, and the Pacific islands. The present circumscription of Laureae largely corresponds to Kostermans' (1957) tribe Litseae, Rohwer's (1993a) tribe Laureae, and van der Werff and Richter's (1996) tribe Laureae (Table 1). Laureae are characterized by possessing pseudo-umbellate inflorescences bearing involucral bracts, unisexual flowers, and mainly introrse anthers in the third whorl (Rohwer, 1993a; van der Werff and Richter, 1996; Li and Christophel, 2000).
The monophyly of Laureae has been confirmed by several recent molecular studies (e.g., Chanderbali et al., 2001; Li et al., 2008c; Song et al., 2020; Liu et al., 2021; Qin et al., 2023), but a major disparity exists between these molecular results and traditional morphology-based classifications. The use of two-versus four-locular anthers for Laureae generic delimitation has resulted in polyphyletic genera, and the character of dimerous versus trimerous flowers is of only limited phylogenetic value in this group. In contrast, several major lineages have been supported by inflorescence morphology and ontogeny (Li et al., 2008c), traits important for improving classification within Laureae and understanding its evolution. Of the large genera, only Neolitsea is confirmed monophyletic, whereas Actinodaphne, Lindera, and Litsea are all polyphyletic (Li et al., 2004, 2006, 2007, 2008c; Fijridiyanto and Murakami, 2009; Song et al., 2020; Liu et al., 2021; Qin et al., 2023).
Despite previous morphological studies suggesting that Neolitsea is closest to Litsea (Kostermans, 1957; Hyland, 1989; Rohwer, 1993a), several recent molecular phylogenies indicate that Neolitsea is instead closely related to Actinodaphne (Li et al., 2006, 2007, 2008c; Fijridiyanto and Murakami, 2009; Song et al., 2020; Liu et al., 2021; Qin et al., 2023). The Neolitsea–Actinodaphne clade is supported by the synapomorphies of pseudo-verticillate or subverticillate leaves and sessile involucrate pseudo-umbels in the leaf axils, lacking vegetative buds among the involucra, and not arranged along a discernible short-shoot. Further, Neolitsea is well defined within Laureae by the possession of triplinerved (rarely pinninerved or subtriplinerved), alternate or pseudo-verticillate, leaves crowded at branch apices, decussate persistent bracts, and dimerous flowers. The inflorescences of Neolitsea and many Actinodaphne species are different from those of Laurus, Lindera, and Litsea species, as the latter possess mostly stalked pseudo-umbels arranged along short-shoots ending in vegetative buds, each pseudo-umbel enclosed by decussate, tardily deciduous involucral bracts.
Previous studies have suggested that the pseudo-umbels arranged along leafless short-shoots seen in Laurus, Lindera, and Litsea may result from shortening of brachyblasts (or short-shoots), with the peduncles of these pseudo-umbels shortening sequentially (e.g., Li, 1985; Tsui, 1987; Li et al., 2004). In contrast, the sessile pseudo-umbels of Neolitsea and many Actinodaphne species may arise directly from thyrsoid inflorescences by shortening the main axes and secondary peduncles of the cymes to form the clustered or fasciculate pseudo-umbels, as suggested by Li et al. (2006, 2007, 2008c).
However, another scenario seems more plausible, that is, the inflorescences of Neolitsea and these Actinodaphne species are derived conditions of the forms seen in genera such as Laurus, Lindera, and Litsea by the aborting of vegetative buds during development, with failure of the short-shoots to elongate, so that the pseudo-umbels remain sessile in the leaf axils (Fig. 5). In addition, the phylogeny of Laureae suggests that the inflorescences of Neolitsea and these Actinodaphne species are more likely derived from those of Laurus, Lindera, and Litsea, because Neolitsea and these Actinodaphne species do not form the sister group to the remaining Laureae and are instead deeply nested within them (e.g., Li et al., 2008c; Fijridiyanto and Murakami, 2009; Song et al., 2020; Liu et al., 2021; Qin et al., 2023). In Iteadaphne and Dodecadenia, the number of flowers per involucre or pseudo-umbel is reduced to one, representing the most reduced Laureae and suggesting that the pattern is convergent in this group (Li et al., 2008c).
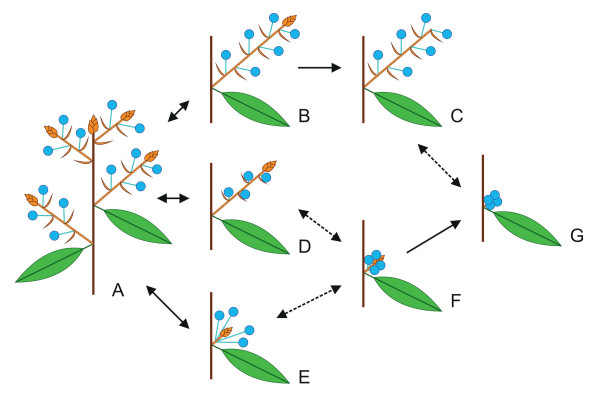
|
| Fig. 5 Inflorescence structures in the Laureae. Pseudo-umbels enclosed by their involucra represented by blue circles. A. Basic pattern; B. Brachyblast more elongate, with terminal bud; C. Brachyblast looking like a raceme of umbels; terminal bud aborted during development; D. Peduncles scarcely elongating, pseudo-umbels (sub)sessile. E. Fascicle of stalked pseudo-umbels, brachyblast scarcely elongating; F. Cluster of (sub)sessile pseudo-umbels around a vegetative bud, both brachyblast and peduncles scarcely elongating; G. Cluster of (sub)sessile pseudo-umbels, vegetative bud aborted during development. |
Previous morphology-based studies recognized considerable variability in both Litsea and Lindera and variously subdivided them into sections (Bentham, 1880; Hooker, 1890; Li et al., 1982; Tsui, 1987). Although traditional intrageneric delimitations are not supported by recent molecular studies, several subclades do correspond partly to previously recognized sections and help to explain their relationships and character evolution in the tribe (Li et al., 2008c; Fijridiyanto and Murakami, 2009; Tian et al., 2019; Song et al., 2020; Liu et al., 2022a; Qin et al., 2023). The reticulate evolution and homoplasy of diagnostic morphological characters in Laureae make classification extremely complicated. For example, characteristics such as habit, leaf venation, inflorescence, and floral structure all appear to be the result of convergent and/or parallel evolution and, therefore, may not be indicative of evolutionary affinity or useful for taxon delimitation at higher levels. Since the number of taxa so far examined in the molecular studies is limited, it seems premature to propose a new classification for Laureae and major, densely-sampled revisions of Actinodaphne, Lindera, and Litsea are needed. Such studies will then provide the foundation for a future revised phylogeny-based classification of Laureae.
2.2.7. CinnamomeaeCinnamomeae are the largest tribe within Lauraceae, and estimated to include about 1300 species in 23 genera (Table 1). They are distributed mainly in the tropical and subtropical regions of Asia, America, and (to a lesser extent) Africa, but with representatives in Australia and the western Pacific islands. The present circumscription of Cinnamomeae largely corresponds to Kostermans' (1957) tribe Cinnamomeae and Rohwer's (1993a) Ocotea and Aniba subgroups (Table 1). They usually have thyrsoid-paniculate inflorescences without an involucre, the ultimate cymes and flowers usually strictly opposite (pseudo-umbellate and involucrate in Umbellularia Nutt.), and fruits seated on, or in well-developed cupules (Kostermans, 1957; Rohwer, 1993a).
Recent molecular systematic studies of Cinnamomeae resulted in the largest number of generic rearrangements of Lauraceae to date. For example, Trofimov et al. (2016) found that the Nectandra coriacea Griseb. species group was excluded from the clade containing the type of Nectandra Rol. ex Rottb., leading to the reinstatement of Damburneya. Similarly, Rohde et al. (2017) found that the American species of Phoebe transferred to Cinnamomum Schaeff. by Kostermans (1961) belonged to neither of these Asian genera and transferred them to the American genus Aiouea Aubl. In the original circumscription, the number of pollen sacs (two vs. four) was the only difference between Aiouea and American "Cinnamomum", but this character is variable in multiple Lauraceae genera. Even Cinnamomum in Asia is not monophyletic (Huang et al., 2016; Trofimov and Rohwer, 2020; Yang et al., 2022), with section Cinnamomum found to be sister to a small group of African species previously included in Ocotea Aubl. now reclassified as the new genus Kuloa by Trofimov and Rohwer (2020). Section Camphora (Fabr.) Meisn., on the other hand, appears to be sister to Sassafras J. Presl, which includes two Asian and one American species. Most of these relationships were already seen in the nrITS data of Chanderbali et al. (2001).
Zeng et al. (2021) presented anatomical evidence separating Cinnamomum sect. Cinnamomum from sect. Camphora, with Yang et al. (2022), reinstating Camphora as a separate genus. However, there is incomplete agreement between the morphological and molecular delimitations of Cinnamomum and Camphora and Xiao and Ge (2022) found that the two genera were inter-mixed in different well-supported clades, suggesting further investigation is needed. In addition, alternative relationships were retrieved in different analyses for the Cinnamomum–Kuloa and Camphora–Sassafras clades to each other and with the clade including Aiouea as sister to the Ocotea complex (Rohde et al., 2017; Yang et al., 2022).
The Ocotea complex, first recognized by Chanderbali et al. (2001), consists of species currently placed in Ocotea s.l. and several other taxa nested among them (Chanderbali et al., 2001; Trofimov et al., 2016, 2019; Trofimov and Rohwer, 2020; Penagos Zuluaga et al., 2021). All of these taxa (Andea, Aniba Aubl., Damburneya, Dicypellium Nees & Mart., Endlicheria Nees, Gamanthera van der Werff, Kubitzkia van der Werff, Licaria Aubl., Mespilodaphne, Nectandra, Paraia Rohwer, H.G. Richt. & van der Werff, Phyllostemonodaphne Kosterm., Pleurothyrium Nees, Povedadaphne W.C. Burger, Rhodostemonodaphne Rohwer & Kubitzki, Umbellularia, and Urbanodendron Mez) are restricted to America, whereas Ocotea, with more than 450 Neotropical species, also includes 42 species from Africa and neighboring islands (34 in Madagascar). Furthermore, Penagos Zuluaga et al. (2021) found that Aniba and Licaria, although well-characterized morphologically, were not monophyletic. On the other hand, several of Rohwer's (1986) Ocotea species groups were confirmed by molecular analyses, but most of them have not yet been defined by diagnostic characters. Nevertheless, Mespilodaphne was reinstated by Trofimov et al. (2019) for the Ocotea dendrodaphne group and Andea was created by van der Werff (2022) for the Ocotea smithiana group. Andea and at least some species of its sister clade (the Ocotea minarum group) are gynodioecious, with either bisexual or female flowers seen on different individuals (Penagos Zuluaga et al., 2020). The dioecious species of the Ocotea complex (including the type species Ocotea guianensis Aubl.) were also monophyletic in all analyses, but with species of Endlicheria (with two pollen sacs per anther), Ocotea (with four pollen sacs per anther in two superposed pairs) and Rhodostemonodaphne (with four collateral pollen sacs per anther) all inter-mixed in several subclades. In contrast to the unisexual flowers of Laureae, vestigial anther locules are still recognizable in staminodes of female flowers in the Cinnamomeae (Fig. 6). Thus, further studies are needed to resolve character evolution within the Ocotea complex.
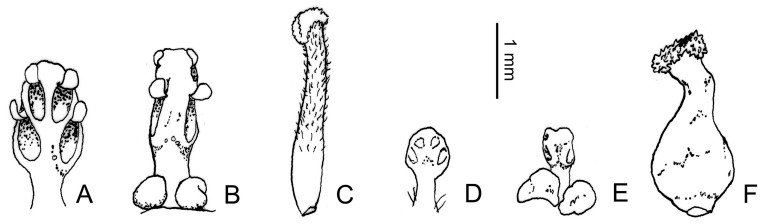
|
| Fig. 6 Floral parts of unisexual flowers of Ocotea lancifolia (Schott) Mez. A–C Staminate flower. A. Stamen whorl 1, adaxial side; B. Stamen of whorl 3, abaxial side; C. Pistillode. D–F Pistillate flower. D. Staminode of whorl 1, adaxial side; E. Staminode of whorl 3, abaxial side; F. Pistil. Scale bar = 1 mm. Camera lucida drawings from herbarium material by Jens G. Rohwer [A–C Glaziou 6666 (C); D–F Martius 1112 (M)]. |
Cytonuclear discordance refers to the differing phylogenetic signals obtained from nuclear and organellar (chloroplast or mitochondrial) genes or genomes (Rieseberg and Soltis, 1991; Xu et al., 2021). Various factors can potentially lead to incongruence between phylogenetic trees based on different genes or genomes data, including convergence (Soltis and Soltis, 2020), hybridization and/or introgression (Rieseberg and Soltis, 1991; Kremer and Hipp, 2020; Soltis and Soltis, 2020), incomplete lineage sorting (Doyle, 1992; Rivas-González et al., 2023), heterogeneity of evolutionary rates (Chen et al., 2015; Liu et al., 2024), sampling error (Liu et al., 2017, 2022b), and taxon sampling density (Chase et al., 1993; Soltis and Kuzoff, 1995).
The phylogenetic relationships in Lauraceae still show many poly- or paraphyletic genera, mainly distributed in Core Lauraceae, such as Alseodaphne and Persea in the Persea group (Perseeae); Actinodaphne, Lindera, and Litsea in the Litsea complex (Laureae); and Endlicheria and Ocotea from the Ocotea complex (Cinnamomeae) (Rohwer, 2000; Chanderbali et al., 2001; Li et al., 2007, 2008c, 2011; Rohwer et al., 2009; Penagos-Zuluaga et al., 2021; Rohde et al., 2017; Trofimov et al., 2016, 2019; Trofimov and Rohwer, 2020). These poly- or paraphyletic genera potentially result from the limited discrimination of nuclear and organelle markers, the incongruence between biparentally inherited nuclear DNA and maternally inherited chloroplast, and the complex history of morphological character interpretations. Rohde et al. (2017), using psbA–trnH, trnG–trnS, and ITS sequence data, reported such incongruence in Cinnamomeae; Liu et al. (2021) reconstructed the relationships using nrDNA sequences in contrast with the phylogenetic tree derived from plastomes, showing that relationships between early diverging Lauraceae, such as Cryptocaryeae and Cassytheae, were consistent with either data source. However, tribes such as Laureae, Cinnamomeae, Perseae, Caryodaphnopsideae, and Neocinnamomeae, show conflicts between nuclear or chloroplast gene trees (Liu et al., 2021). The incongruence between Caryodaphnopsideae and Neocinnamomeae suggests that historical reticulation or other complex processes may have shaped the early evolutionary history of these groups. Similarly, the incongruences seen in Laureae, Perseeae, and Cinnamomeae emphasize the significance of hybridization and/or introgression in these groups (Liu et al., 2021). Accordingly, researchers should avoid inferring Lauraceae relationships by using nuclear or chloroplast genes or genomes in isolation, instead using as comprehensive a data set as possible.
3. Taxonomy 3.1. Taxonomic treatments (mainly on the generic level) based on morphological and molecular evidenceThe most recent infrafamilial classification of Lauraceae was proposed by van der Werff and Richter (1996) and, as with traditional treatments, divided Lauraceae into two subfamilies: Cassythoideae and Lauroideae. However, molecular evidence indicates that the parasitic climbing genus Cassytha is not sister to the remainder of Lauraceae (Rohwer, 2000; Chanderbali et al., 2001; Rohwer and Rudolph, 2005; Song et al., 2020). Song et al. (2020) proposed a tribal classification based on phylogenetic relationships treating the Hypodaphnis clade as Hypodaphnideae, the Beilschmiedia–Cryptocarya clade as Cryptocaryeae, and the Cassytha clade as Cassytheae, with two new tribes, Neocinnamomeae and Caryodaphnopsideae, for the Neocinnamomum and Caryodaphnopsis clades respectively. Although Song et al. (2020) treated the other four Lauraceae clades (Chlorocardium–Mezilaurus, Machilus–Persea, Laurus–Neolitsea, and Cinnamomum–Ocotea) as Laureae s.l., we prefer here to recognize them at the tribal level as Mezilaureae, Perseeae, Laureae, and Cinnamomeae. The novel tribal name Mezilaureae is therefore validated here:
Mezilaureae Rohwer, trib. nov. – Type: Mezilaurus Kuntze ex Taub.
Diagnosis: Leaves pinninerved, either opposite or crowded at the tips of the branches; inflorescences botryoid or dibotryoid; stamens either 3, more or less columnar, 2- or 4-locular, or 6, triangular, 2-locular, or 8–20, tongue-shaped, papillose, 4-locular.
Distribution: Neotropics (from Costa Rica to Peru, Bolivia and SE Brazil).
Included genera: Anaueria Kosterm., Chlorocardium Rohwer, H.G. Richt. & van der Werff, Clinostemon Kuhlm. & A. Samp., Mezilaurus Kuntze ex Taub., Sextonia van der Werff, and Williamodendron Kubitzki & H.G. Richt.
Within Cryptocaryeae, two new genera have also been published in the last two decades, with Sinopora separated from Syndiclis by its trimerous flowers and instead related morphologically to Hexapora (Li et al., 2008a). Similarly, Yasunia was established for possessing conspicuous, pubescent staminodia, exserted stamens and reduced number of stamens, and considered to be closely related to Beilschmiedia (van der Werff and Nishida, 2010).
In addition, Clinostemon was reinstated, as Mezilaurus was not monophyletic, with M. mahuba (A. Samp.) van der Werff and M. triunca being placed instead as sister taxa to Sextonia (Alves and Souza, 2013). Furthermore, Clinostemon has stamens with downward recurved anthers and one pair of glands at the base of the filaments, while Mezilaurus has erect anthers, and glands are absent (Alves and Souza, 2013).
Within Perseeae, at least Alseodaphne and Persea were polyphyletic in recent molecular studies (Rohwer et al., 2009; Li et al., 2011; Song et al., 2020). Consequently, the Alseodaphne species mainly distributed on the border between SW China and the Indochina Peninsula were separated as a new genus Alseodaphnopsis (Mo et al., 2017). Similarly, Persea subgen. Eriodaphne was not sister to subgen. Persea (Rohwer et al., 2009; Li et al., 2011), suggesting that they need to be split into separate genera. In addition, several other Persea species do not fit morphologically into either subgenus and appear to be closer to Alseodaphne and Phoebe (Rohwer et al., 2009; Li et al., 2011). Thus, resolution of relationships in Persea requires further study with more detailed taxon sampling, and a full revision of the genus is needed.
Molecular analyses indicate that most of the larger genera of Laureae are not monophyletic (Li et al., 2008c; Song et al., 2020), with traditional characters used for delimitation of genera in the family such as the merosity of flowers and number of pollen sacs per anther proving insufficient to characterize them. However, to date no full taxonomic revision and reassessment of the tribe exists.
Cinnamomeae, the largest tribe in Lauraceae, has undergone the most taxonomic changes within the family. Ocotea is widely distributed in Africa and America and was shown to be polyphyletic, leading to the creation of a new genus Andea for the Ocotea smithiana group (van der Werff, 2022). In addition, three African species were excluded from Ocotea and described as a new genus Kuloa (Trofimov and Rohwer, 2020). Mespilodaphne was reinstated based on the Ocotea dendrodaphne group (Trofimov et al., 2019), while Damburneya was reinstated based on the Nectandra coriacea group (Trofimov et al., 2016). Cinnamomum was previously considered to be distributed in tropical Asia and tropical America. However, molecular studies show that the genus is polyphyletic (Huang et al., 2016; Liu et al., 2021), resulting in the American members of Cinnamomum being transferred to Aiouea (Rohde et al., 2017), and the Asian C. sect. Camphora reinstated as the genus Camphora (Yang et al., 2022).
3.2. Morphological studiesOver the past two decades, molecular systematic studies have become predominant in Lauraceae. Nevertheless, morphological studies remain important for the taxonomy of this family.
Morphological studies involving leaf venation, epidermal anatomy, wood and bark anatomy, palynology, and embryology have contributed significantly to advancing the taxonomy of Lauraceae. Leaf venation patterns are important for taxonomic delimitation among genera and species, and can also help identify fossil leaf impressions and herbarium specimens (Klucking, 1987; Christophel and Rowett, 1996). Epidermal features are useful for the intergeneric and infrageneric recognition of taxa, such as genera and species groups (Christophel and Rowett, 1996; Nishida and van der Werff, 2007, 2011, 2014; Yang et al., 2012; Zeng et al., 2014, 2021; Nishida et al., 2016; Trofimov and Rohwer, 2018, 2020). However, due to the limited number of character states, they are rarely sufficient by themselves to define genera or clades found in molecular phylogenies, nor can they be used reliably for the identification of specimens of uncertain affinity (Nishida and van der Werff, 2011). Many studies on wood and bark anatomy have been conducted by Richter and collaborators (Richter, 1981a, 1981b, 1985, 1990; Richter and van Wyk, 1990). Based on characters of wood and bark anatomy and inflorescence structure, van der Werff and Richter (1996) proposed an improved classification, dividing Lauraceae into two subfamilies: one consisting of Cassytha and the other including all other genera. This latter group was further divided into three tribes: the Laureae, Perseeae, and Cryptocaryeae (Table 1). Moreover, palynological studies have suggested that pollen morphology could serve as a potential source of taxonomic evidence mainly at the generic level (Raj and van der Werff, 1988; van der Merwe et al., 1990; Rohwer, 2018). Embryological studies have also been incorporated into the suprageneric classification of the family (Heo et al., 1998).
3.3. Regional taxonomic studiesLauraceae are an almost cosmopolitan family, being absent only from the coldest and driest regions of the planet. In the Americas, they extend from southernmost Canada to central Chile; in the Old World from southern Europe to South Africa and across southern Asia south of the Himalayas to Japan and Indonesia; and in the Pacific region from Australia to Hawaii and New Zealand. Their main centers of diversity are tropical to subtropical America and Asia, with more than 1000 species in each of these regions, but they are also diverse in tropical Africa (mainly Beilschmiedia), Madagascar, and Australia.
3.3.1. Tropical AsiaTaxonomic studies of Lauraceae in Tropical Asia can be divided into three periods. Much work was done in the 18th and 19th Centuries (Nees von Esenbeck, 1831, 1836; Blume, 1825–1826, 1849–1851; Miquel, 1858, 1861) culminating in several regional floras in the early 20th Century: Indo-China by Lecomte (1914), Indian and Peninsular Malaysia by Gamble (1910a, b, c, d), and northeastern New Guinea (Teschner, 1923).
The second period comprises most of the 20th Century, with progress mainly resulting in numerous new species accounts (e.g., Liu, 1932 publ. 1934; Merrill, 1917; Allen, 1938, 1939, 1941, 1942a, 1942b), but very few detailed accounts of genera (Das, 1937). An exception is the economically important genus Cinnamomum, with the first revisions done by the Nees von Esenbeck brothers (1823, 1836), while Blume (1836) and Miquel (1864) reviewed some Asian species. Cammerloher (1925) revised the genus for Indonesia, Backer et al. (1963) for Java, Indonesia and Liu and Ou (1969) for Taiwan, China. Kostermans planned to publish a set of five regional accounts for the genus; however, only three of these were published: South India (Kostermans, 1985), the eastern Malesian Islands (Kostermans, 1986), and Sri Lanka (Kostermans, 1995). The two remaining planned treatments (Western Malesia and China) were never published, but a treatment of Myanmar Cinnamomum species was published (Kostermans, 1998). More recently regional accounts have been published for Borneo (Soh, 2011) and Peninsular Malaysia (de Kok, 2019), as well as an annotated checklist for India (Geethakumary et al., 2021). In 2022, the genus was divided into a widespread Cinnamomum and a mainly continental Asian Camphora that also has one species native to Sumatra, Java, and Borneo (Yang et al., 2022).
In the latter 20th Century, Kostermans continued to dominate research in the family, writing papers on Alseodaphne (Kostermans, 1973a); Caryodaphnopsis (Kostermans, 1974b); Dehaasia (Kostermans, 1973b), Neocinnamomum (Kostermans, 1974a) and many other papers dealing with new taxa. Progress accelerated in the later 20th and early 21st Century, with many national and regional family accounts produced in Asia: South India (Robi, 2014), Sri Lanka (Kostermans, 1995), Pakistan (Kostermans, 1978a), Nepal (Pendry, 2011), Bhutan (Long, 1984), China (Liao, 1996; Li et al., 2008b), Vietnam (Hô, 1991, 1999; Nguyễn, 2017), Indo-China (de Kok, 2025a), Thailand (de Kok and Middleton, 2025), Peninsular Malaysia (Kochummen, 1989; de Kok, 2025b), and Singapore (de Kok and Thomas, 2025).
In the Pacific, most of the larger islands have been covered: New Caledonia (Kostermans, 1974c), Fiji (Smith, 1981), Hawaii (Wagner et al., 1990), and Samoa (Christophersen, 1935). In addition, generic identification in the region became easier due to the key published by van der Werff (2001). Checklists also became available for India and the Andaman and Nicobar Islands (Chakrabarty et al., 2010), Bangladesh (Ara et al., 2007), and Myanmar (Kress et al., 2003). In contrast, the Malesian Archipelago does not yet have a comprehensive account for Lauraceae, apart from Java (Backer and Bakhuizen van den Brink, 1963). However, genus-level accounts for Tropical Asia are now available for multiple taxa, including Actinodaphne (Julia, 2005; Tanaros et al., 2010), Alseodaphne (Julia et al., 2009), Alseodaphnopsis (Mo et al., 2017; van der Werff, 2019), Beilschmiedia (Tetsana, 2005; Nishida, 2008; de Kok, 2016b, 2021a), Caryodaphnopsis (Zhang et al., 2024a), Cryptocarya (Gangopadhyay and Chakrabarty, 2005; Ng, 2005a; de Kok, 2015, 2016c), Dehaasia (Julia et al., 2009; Fijridiyanto et al., 2020; Chakrabarty et al., 2022), Endiandra (Arifiani, 2001), Iteadaphne (Ng, 2005b), Lindera (Ng, 2005b), Litsea (Li, 2001; Ng, 2005b; Bhuinya et al., 2009, 2010; Ngernsaengsaruay et al., 2011; de Kok, 2021b), Machilus (Mase et al., 2020), Neolitsea (Ng, 2005b), Nothaphoebe (Julia et al., 2009), and Phoebe (Chakrabarty et al., 2023). Detailed checklists and/or generic accounts are also available for the Philippines (Co, 2024) and New Guinea (de Kok and Utteridge, 2021).
Tropical Asia has numerous endemic genera, many of which are common and widespread, but a few small genera are restricted to the northern part of the region, such as Alseodaphnopsis, Dodecadenia, Parasassafras, Sinopora, and Syndiclis. In addition, Cinnadenia is a small genus with three rarely collected species, which are endemic to either north-east India and Bhutan, Peninsular Malaysia, and Vietnam and South China respectively (de Kok and Sengun, 2020). Similarly, Triadodaphne (included in Endiandra by Rohwer et al., 2014) consists of three species from Sarawak, New Guinea, and the Solomon Islands, respectively. The most enigmatic Asian endemic is the monotypic genus Hexapora, which is only known from a few, pre-1901, specimens from the island of Penang, Peninsular Malaysia, none of which has fully mature fruits. It may be closely related to Sinopora from Hong Kong, China (Li et al., 2008a; see also de Kok, 2016a), but more research and complete material is needed to determine its relationships. Two other unusual small genera are the Ironwood timber genus Eusideroxylon from Sumatra and Borneo (Irawan, 2004) and its close relative Potoxylon from Borneo (Kostermans, 1978b). The former one formed extensive monospecific stands in lowland rainforest, but most have now been cleared for oil palm production.
There are also several introduced species/genera from outside the region and only known from cultivation: the fruit tree Persea americana (Avocado) which was introduced from Central America and Laurus nobilis (Bay leaf) from the Mediterranean, the leaves of which are used as a culinary seasoning.
3.3.2. Tropical AmericaSince the beginning of the 1980's morphological studies by Kubitzki and his students (HBG), van der Werff and his students (MO), Madriñán (ANDES), Moraes (HRCB), and Lorea-Hernández (XAL) have all greatly improved our knowledge of Neotropical Lauraceae. By the end of 1980's, the number of species in the New World was estimated at 700–800 in 31 genera (van der Werff, 1988). However, Rohwer et al. (1991) estimated that 25–35% of Neotropical Lauraceae were still undescribed. Currently, Lauraceae are represented in Tropical America by 29 genera and more than 1200 species (Appendix A), although several hundred new species have been described since the 1990s, a Neotropical inventory of the family is still incomplete.
Despite 499 new species having been described from the Americas since the 1980's, many from the Andes, Mesoamerica and the Caribbean, Brazil is still the most diverse region for the family, with 477 species (39.2%), 255 of them endemic (21% of the total, 53.4% of the Brazilian taxa). Revisions for most genera have now been published, including Aiouea and Aniba (Kostermans, 1938a, 1938b; Kubitzki and Renner, 1982), Beilschmiedia (Kostermans, 1938b; Nishida, 1999), Neotropical "Cinnamomum" (= Aiouea with 4-locular anthers: Lorea-Hernández, 1996), Cryptocarya (Kostermans, 1937, 1938a; Moraes, 2007), Endlicheria (Kostermans, 1937; Chanderbali, 2004), Licaria (Kostermans, 1937, 1938a; Kurz, 2000), Mezilaurus (Kostermans, 1938a; van der Werff, 1987), Nectandra (Rohwer, 1993b), Pleurothyrium (van der Werff, 1993), and Rhodostemonodaphne (Madriñán, 2004), as well as several small genera (Dicypellium, Phyllostemonodaphne, Systemonodaphne Mez = Kubitzkia van der Werff and Urbanodendron) by Rohwer (1988). An older revision of Persea by Kopp (1966) is also available. There is no full revision of the large genus Ocotea with about 466 species, but several regional treatments are available. Several new, mostly small genera have also been published, Andea (van der Werff, 2022), Chlorocardium (Rohwer et al., 1991), Gamanthera (van der Werff and Endress, 1991), Mocinnodaphne Lorea-Hern. (Lorea-Hernández, 1995) = Aiouea, Paraia (Rohwer et al., 1991), Povedadaphne (Burger, 1988), Sextonia (van der Werff, 1997 publ. 1998), Williamodendron (Kubitzki and Richter, 1987), and Yasunia (van der Werff and Nishida, 2010). Two older genera (Damburneya and Mespilodaphne) have also been resurrected (Trofimov et al., 2016, 2019).
An updated list of accepted Lauraceae species recorded in Tropical America (Appendix A) has been compiled here from binomials available at POWO, Tropicos and online Brazilian databases (i.e., Reflora, Jabot, CNCFlora and speciesLink). Updated lists of Lauraceae recorded in several Brazilian Protected Areas are also available from the Catálogo de Plantas das Unidades de Conservação do Brasil (https://catalogo-ucs-brasil.jbrj.gov.br) and corollary data. However, these databases also reveal how little we still know about these floras and highlight the necessity of supporting floristic surveys in poorly known areas, especially those remaining as forest remnants. The same scenario can be extrapolated to other Neotropical regions.
Several regional treatments for Lauraceae are also available, including the Flora of the Pico das Almas, Brazil (van der Werff, 1995), Flora del Bajio, Mexico (van der Werff and Lorea-Hernández, 1997), Flora of the Venezuelan Guayana (van der Werff and Rohwer, 1999), Flora of Reserva Ducke, Manaus, Brazil (Vicentini et al., 1999), Flora of Nicaragua (van der Werff, 2001b), Lauraceae en el Sur de Mexico (Lorea-Hernández, 2002), Flora of Central French Guiana (van der Werff, 2002), Florula of Reserva Ecológica de Macaé de Cima, Nova Friburgo, Rio de Janeiro, Brazil (Quinet and Andreata, 2002), Flora of the São Paulo State, Brazil (Baitello et al., 2003), Flora of Costa Rica (González and Poveda, 2007), Flora of Goiás and Tocantins States, Brazil (Moraes and Oliveira, 2007), Flora of Río Cenepa, Peru (van der Werff, 2010), Florula of Santa Teresa, Espírito Santo, Brazil (Barbosa et al., 2012), Flora of Cuba (Rohwer, 2014), Flora Argentina (van der Werff et al., 2015), Flora of the canga of the Serra dos Carajás, Pará, Brazil (Moraes, 2018), Florula of the Reserva Natural Vale, Linhares, Espírito Santo, Brazil (Moraes and Vergne, 2019a, 2019b), and Flora Mesoamericana (van der Werff, 2025).
Molecular phylogenetic analyses have improved our understanding of relationships among Neotropical Lauraceae, resulting in the recognition of Damburneya, Mespilodaphne and Andea (Trofimov et al., 2016, 2019; Penagos Zuluaga et al., 2021; van der Werff, 2022). Such studies also showed that large, morphologically variable genera such as Ocotea and Persea are not monophyletic. For example, the most complete phylogeny of the Ocotea complex resulted in the recognition of 11 clades of which only two (Andea and Mespilodaphne) have been accepted to date as distinct genera with corresponding morphological support. Penagos Zuluaga et al. (2021) found that the medium-sized genera Aniba and Licaria are also probably not monophyletic.
Continued field work will very likely result in the further discovery of new species and there is already evidence of undescribed species from collections amassed in many herbaria. Many of these specimens, particularly those with fruiting material but no corresponding flowering parts, await further collection and study for proper identification and description. More urgent is the need for a new classification with genera that have both morphological and molecular support. Such a classification can only be achieved through taxon dense studies using improved techniques of DNA extraction, as functional DNA cannot be extracted currently from alcohol-preserved specimens and nearly all recent collections have been preserved in alcohol to reduce mold growth on the specimens. The number of collections currently available for DNA-based research is insufficient, so intensive field work is needed to collect DNA samples from reliably identified and vouchered specimens.
3.3.3. AfricaLauraceae are less diverse in African forests than they are in American or SE Asian forests and as a result, there have been relatively fewer changes in the taxonomy of African Lauraceae. The largest genus by far is Beilschmiedia, for which a regional treatment is available for Madagascar (van der Werff, 2003). In continental Africa, notable taxonomic changes at the generic level include the recognition of the monotypic Dahlgrenodendron from South Africa (van der Merwe et al., 1988) and the small genus Kuloa from Central Africa (Trofimov and Rohwer 2020); the latter now including the only continental African species described in Ocotea over the past 30 years (van der Werff, 1996). Recently, Wieringa and Simons (2024) revised African Cassytha and recognized five species, including a new species from Gabon (C. graminicola Wieringa & E.L.A.N. Simons) and C. schliebenii Robyns & R. Wilczek resurrected to species level.
Over the last twenty years intensive collecting efforts on Madagascar have resulted in a revision of Aspidostemon following its separation from Cryptocarya, and the merging of Ravensara with Cryptocarya (van der Werff, 2006, 2013a, 2017). Currently five native genera are accepted in Madagascar, of which three have been revised: Aspidostemon, Beilschmiedia and Ocotea (van der Werff, 2003, 2006, 2013b, 2017). Revisions of Cryptocarya and Potameia are still needed. Aspidostemon and Potameia are endemic in Madagascar, whereas the other genera have wider distributions. Forty-three new species have been described, mostly in Aspidostemon (19 species) and Ocotea (11 species). Of the endemic genera, Aspidostemon is closely related to the South African Dahlgrenodendron and Potameia is closely related to Beilschmiedia, from which the latter differs in its dimerous flowers (Rohwer et al., 2014; Song et al., 2023). Future field work is expected to yield more new species, but no changes at the generic level are expected.
3.3.4. Australia, New Zealand and New CaledoniaAustralian Lauraceae consist of 10 genera and 141 species, including ~115 endemic species (Le Cussan et al., 2007; Weber and Forster, 2021), mostly occurring in warmer rainforests along the eastern coast, but with Cassytha also widespread in drier forests and heathlands across the continent (Weber, 2007). Of these, the majority are represented by the Beilschmiedia/Endiandra group (50 spp.), Cryptocarya (47 spp.), and Cassytha (19+ spp.).
Although fossil evidence suggests some Australian Laureae and Cryptocaryeae represent paleo-Gondwanan elements (Vadala and Greenwood, 2001), the assumption of widespread ancient vicariance across the family has been challenged by more recent molecular studies (Huang et al., 2016; van der Merwe et al., 2016). Similarly, the fossil Lauraceae flora of the now submerged Ninetyeast Ridge in the Indian Ocean has also been considered the result of ancient long-distance dispersal from the Australian region and/or vicariance from the Kerguelen Plateau region (Carpenter et al., 2010).
There are five genera and at least ~50 species of Lauraceae on New Caledonia, with all except Cassytha filiformis endemic (Endemia, 2001 onwards). A molecular study by Carter (2017) suggested that more taxa needed recognition, with Munzinger et al. (2022, 2024) adding several new Endiandra species, as well as synonymizing Adenodaphne S. Moore into Litsea. The recent discovery of abundant Miocene-aged fossil Cryptocarya-like leaves resembling extant New Caledonian species suggests that Lauraceae were a major component of some of the rainforests present at that time (Garrouste et al., 2021). However, Carter (2017) found that although the biogeographic relationships of modern taxa were unresolved, New Caledonian Beilschmiedia and Endiandra were apparently each the result of single colonizations followed by radiation, whereas extant Cryptocarya species were probably derived from multiple colonization events (Munzinger and Gemmill, 2025).
Lauraceae in New Zealand are known back to at least the Late Cretaceous (Pole and Douglas, 1999; Kennedy, 2003; Pole and Vajda, 2009; Cantrill et al., 2011). Although now restricted to only five native species in four genera, there are abundant macrofossils throughout the Cenozoic, occurring as leaves, dispersed cuticles, and occasional flowers and fruits with affinities to Beilschmiedia, Cryptocarya, and Litsea, indicating a much more diverse fossil record (e.g., Pole, 2007, 2012; Bannister et al., 2012 and references therein).
4. Biogeography 4.1. PhytopaleontologyLauraceae diverged from other basal angiosperms by at least the Early Cretaceous if not Late Jurassic (Xiao et al., 2022; Song et al., 2023; Zuntini et al., 2024) and have an extensive fossil record starting in the late Early Cretaceous, followed by significant radiation in the early Cenozoic (Taylor et al., 2009; Friis et al., 2011), although some older reports of fossils assigned to the family are unreliable and in need of further evaluation (Rohwer, 1993a; Friis et al., 2011).
Macrofossil evidence for Lauraceae is abundant and includes evidence from leaves (e.g., Pole, 2007; Carpenter et al., 2007; Wu et al., 2008; Bannister et al., 2012; Shi et al., 2014; Wang et al., 2017, 2019; Cevallos-Ferriz et al., 2021; Maccracken et al., 2022), wood (Poole et al., 2000; Dupéron-Laudoueneix and Dupéron, 2005; Franco et al., 2015; Huang and Li, 2018; Zhang et al., 2024b), and various reproductive structures (e.g., Drinnan et al., 1990; Upchurch and Dilcher, 1990; Herendeen et al., 1994; Eklund and Kvaček, 1998; Eklund, 2000; Poole et al., 2000; von Balthazar et al., 2007; Little et al., 2009; Chambers et al., 2011, 2012; Beurel et al., 2024; Takahashi et al., 2014; Atkinson et al., 2015; Moreau et al., 2016; Friis et al., 2011; Wang et al., 2019 and references therein).
Lauraceae flowers of Potomacanthus lobatus Balthazar, K.R. Pedersen, P.R. Crane, Stampan. & E.M. Friis occur as early as the Early Cretaceous of North America (von Balthazar et al., 2007) and Mauldinia Drinnan, P.R. Crane, E.M. Friis & K.R. Pedersen flowers are known from across the Northern Hemisphere by the mid-Cretaceous (Friis et al., 2011 and references therein).
In contrast, unlike most other angiosperm plant families, reliable fossil Lauraceae pollen records are very rare (Herendeen et al., 1994; Atkinson et al., 2015), partly due to generally poor preservation of the grains (Macphail, 1980), combined with difficulty of distinguishing dispersed Lauraceae pollen from other basal angiosperms and some monocots (Raine et al., 2011).
These fossils suggest that the family diversified globally in tandem with the development and worldwide expansion of tropical rainforests (Kvaček et al., 2020, 2024; Coiffard et al., 2023) during Late Cretaceous and early Cenozoic warm periods. Studies mainly using fossil leaves and some importantly disjunct wood fossils, such as Caryodaphnopsoxylon richteri H. Gottwald from the Late Eocene of Germany (Gottwald, 1992), show that Lauraceae were able to colonize all continents and many islands. Evidence for past diversity has been found in both the Northern (e.g., Taylor, 1988; Denk et al., 2005; Friis et al., 2011; Manchester, 2014) and Southern Hemispheres (Pole, 1993, 2007, 2012; Pole and Douglas, 1999; Vadala and Greenwood, 2001; Carpenter et al., 2007, 2010; Cantrill et al., 2011; Bannister et al., 2012; Conran et al., 2016; Hill, 2017, and references therein; Pujana et al., 2024), even at high latitudes. For example, the Late Cretaceous fossil wood taxon Sassafrasoxylon gottwaldii I. Poole, H.G. Richt. & J.E. Francis from Antarctica (Poole et al., 2000; Tosolini et al., 2021) was thought to reveal links to the Northern Hemisphere (Poole et al., 2000), although some of these apparent anatomical similarities might be the result of convergence to strong seasonality responses. Further studies are needed to determine the phylogenetic utility of some wood anatomical characteristics, such as ring-porous wood.
4.2. Historical biogeographyLauraceae are one of the most basal angiosperm families, with fossil records dating back to the Mid-Cretaceous and the family is distributed across tropical and subtropical latitudes globally (Chanderbali et al., 2001; Kvaček et al., 2020, 2024; Coiffard et al., 2023). The widespread distribution and long geological history reflect the incredible evolutionary success of Lauraceae. However, uncertain phylogenetic relationships within Lauraceae make it difficult to explain why the family accounts for such a large proportion of Earth's biodiversity and how the complex biogeographical patterns of the family were established.
Lauraceae lineages can be sorted into two main biogeographic groups—Gondwanan and Laurasian—that date back to the Late Cretaceous (Chanderbali et al., 2001; Friis et al., 2011; Kvaček et al., 2020, 2024; Coiffard et al., 2023). However, large scale biogeographic patterns of Lauraceae remain unclear. Attempts at reconstructing the historical biogeography of Lauraceae have mostly focused on certain genera and groups (Fig. 7), such as, Caryodaphnopsis, the Persea group (Perseeae), Cinnamomum group, Beilschmiedia group, and Cryptocaryeae (Li et al., 2011, 2016, 2020; Huang et al., 2016; Song et al., 2023).
|
| Fig. 7 Major migration and dispersal events of Lauraeae lineages in Cenozoic, summarized from studies of Chanderbali et al. (2001), Li et al. (2011, 2016), Huang et al. (2016), and Song et al. (2023). Green dotted lines: Laurasian lineages; Yellow dotted lines: Gondwanan lineages. Paleogeographic maps were modified based on Scotese (2014a, b). |
The broad distribution of Lauraceae spans several continents in a biogeographic pattern that indicates intercontinental disjunctions, however, evidence for intercontinental disjunctions is not generally clear. For example, amphi-Pacific tropical and subtropical disjunctions have been explained to have arisen from boreotropical paleofloras (Fig. 7B). Specifically, the early Eocene Cenozoic Thermal Maximum allowed boreotropical paleoflora to spread to high latitudes in the Northern Hemisphere, such that Lauraceae could have spread easily between Eurasia and North America along high-latitude land bridges such as Beringia or the North Atlantic land bridge (Reid and Chandler, 1933; Chandler, 1964; Collinson et al., 1981; Miller et al., 1987; Wolfe, 1975, 1997; Meng et al., 2014, 2015). However, the subsequent cooling events resulted in the retreat of boreotropical flora, leading to fragmentation and thus the current disjunct patterns inferred for the Persea group (Li et al., 2011), Cinnamomum group (Huang et al., 2016), and Caryodaphnopsis (Li et al., 2016). Eastern Asian–North American disjunctions are less well studied. One study suggested that Sassafras has a relict distribution in the Northern Hemisphere without a Gondwanan link (Nie et al., 2007).
Several genera and multiple species groups show disjunctions between Asia, Africa, America, and/or Australia, with the numerous islands between the Asian and Australian plates potentially serving as steppingstones for dispersal (Morley, 2003). One process that may explain how modern biotas became distributed over vast ranges, especially those with intercontinental disjunctions, is long-distance dispersal (Wilkinson, 1997; Meng et al., 2014). Although Lauraceae long-distance dispersal events may involve several media, (e.g., ocean flow, wind, and birds), birds are particularly important for dispersing Lauraceae (Snow, 1981; Wilkinson, 1997) and have been suggested as long-distance dispersal agents for Cryptocaryeae (Song et al., 2023). Despite this potential explanation for disjunct distributions, multiple hypotheses can account for the spread of ancestral Lauraceae lineages. For example, the distribution of Cinnamomum group lineages may be explained by an initial dispersal event between Laurasia and Africa, followed by transoceanic long-distance dispersal between Africa and America (Givnish and Renner, 2004). Notably, these lineages may have first dispersed from Africa to Asia during the Eocene–Oligocene via ''Lemurian steppingstones" (Schatz, 1996; Yuan et al., 2005). Alternatively, some lineages may have been dispersed to high-latitude boreotropical floras (Davis et al., 2002), then to Asia and Australia, in the Early Miocene, as well as from North America to South America in the late Early Oligocene (Huang et al., 2016).
Thus far, biogeographic patterns have been explored for relatively few genera or groups, mainly because of the lack of deeply-sampled taxonomic and phylogenic treatments, especially for large, widely-distributed genera and groups. As a result, much more study is needed to resolve current and past biogeographic scenarios for the family.
4.3. PhylogeographyPhylogeography endeavors to understand the processes that underlie the spatial arrangements of genetic variation within and among closely related species (Avise, 2009). Phylogeographic studies of Lauraceae species are predominantly from Perseeae and Laureae in East Asia, except Eusideroxylon zwageri Teijsm. & Binn. in Indonesia (Nurtjahjaningsih et al., 2017) and Laurus azorica (Seub.) Franco in Macaronesia and L. nobilis in the Mediterranean region (Rodríguez-Sánchez et al., 2009).
In East Asia, paleo-biome reconstructions indicate that the distribution of temperate deciduous broad-leaved forests (DBLF) and evergreen broad-leaved forests (EBLF) shifted south to macrorefugia around 25–30 °N and < 24 °N, respectively, during the last glacial maximum (Harrison et al., 2001). Phylogeographic studies have also shown that some DBLF and EBLF remained in northern microrefugia during the last glacial maximum (Qiu et al., 2011). Notably, Lindera obtusiloba (Ye et al., 2017) and L. glauca (Siebold & Zucc.) Blume (Zhu et al., 2020), species with distributions in both DBLF and EBLF apparently survived in northern microrefugia. Sharp north-south genetic discontinuity that dates much older (during the late Pliocene) than the last glacial maximum indicates long-term in situ survival in both regions. In the northern DBLF region, L. obtusiloba survived in multiple refugia, while L. glauca survived in southern costal refugia in the Japanese Archipelago. In the southern EBLF region, L. glauca appears to be demographically stable, as research has found no significant decline in genetic diversity at higher latitudes, in contrast to what the southern microrefugia model would predict (Waters et al., 2013). Although genetic diversity has declined in L. obtusiloba, highly structured lineages reflect limited gene flow between populations.
In EBLF, Lauraceae species are prone to conform to the southern macrorefugia model. In subtropical EBLF, two Machilus species were found to survive in southern macrorefugia: M. pauhoi Kaneh. and M. thunbergii Siebold & Zucc. (Fan et al., 2016; Zhu et al., 2017). Studies have indicated that genetic diversity in Lindera aggregata is negatively correlated with latitude (Ye and Li, 2021). Furthermore, studies have also found distinct subtropical-tropical divergence and northward expansion in both L. aggregata (Ye et al., 2019) and M. thunbergii (Fan et al., 2022). Northern populations of L. aggregata are composed of two distinct clusters, which appear to have colonized the region after the last glacial maximum. In contrast, expansions of the sole northern cluster of M. thunbergii predate the last glacial maximum.
In warm-temperate EBLF, chloroplast-based phylogeographical analysis of Neolitsea sericea (Blume) Koidz. revealed an ancestral status for populations in the southwestern part of the Japanese Archipelago, with later migrations to the Korean Peninsula and Taiwan Island (Lee et al., 2013). Nuclear microsatellite (Zhai et al., 2012) and restriction site-associated DNA sequencing (Cao et al., 2018) suggest that northward migration of N. sericea may have occurred during the LGM via the exposed continental shelf.
Thus, in East Asia Lauraceae species show different phylogeographic histories at different scales. To resolve complex and taxon-specific phylogeographic patterns in Lauraceae, future investigations should include more species from a range of other subclades and high-resolution data.
5. Organelle and nuclear genomes of Lauraceae 5.1. Organelle genomes of LauraceaePlastomes provide robust and significantly supported relationships among deep lineages of Lauraceae (Song et al., 2017a, 2020, 2023; Liu et al., 2021; Yang et al., 2023). Over 830 assembled Lauraceae plastomes have been deposited in GenBank, yet fewer than 10% of Lauraceae species have at least one complete plastome sequence in public databases (NCBI, July 2024). Among these sequenced Lauraceae species, those from Asia with complete plastome constitute the vast majority (90.3%).
Most of these sequenced Lauraceae plastomes display the typical quadripartite structure of angiosperms, including one large single-copy (LSC) region, one small single-copy (SSC) region, and a pair of inverted repeat (IR) regions. However, plastomes from Cassytha, a genus of parasitic vines, have lost one copy of the IR (Song et al., 2017a, 2020; Yang et al., 2023). Plastid genome size ranges from a minimum of 114,215 bp in Cassytha filiformis to a maximum of 158,671 bp in Beilschmiedia brenesii (Li et al., 2020; Yu et al., 2023), showing a 1.39-fold variation in the sizes of published Lauraceae plastomes.
Comparative genomics analysis indicates that the main reasons for plastome size differences include gene duplication, gene loss, and pseudogenes lost (Song et al., 2017a, 2020; Xiao and Ge, 2022; Yang et al., 2023; Wu et al., 2017; Yu et al., 2023; Cao et al., 2024). Comparative genomics analysis in Lauraceae have also identified differences in plastid gene number and locations. Lauraceae plastomes contain 107 (Cassytha filiformis) to 132 (Caryodaphnopsis henryi Airy Shaw) genes, including 73 to 86 protein-coding genes (PCGs), 30 to 37 transfer RNA (tRNA) genes, and 4 to 8 ribosome RNA (rRNA) genes, excluding the pseudogene ѱycf15 (Song et al., 2017a, 2020).
Besides changes in gene copy number, a significant degree of synteny was found within Lauraceae plastomes, indicating a relatively conserved genome structure. However, dozens of micro-inversions, forming stable single-stranded hairpin structures, were identified among Lauraceae plastomes (Song et al., 2015, 2016, 2017b; Tian et al., 2019; Zhu et al., 2023; Cao et al., 2024). All these micro-inversion events present challenges for multiple sequence alignment of Lauraceae plastomes, producing significantly different phylogenetic tree reconstructions as has been observed in waterlily plastomes (Roestel et al., 2024).
The second challenge for phylogenetic tree reconstructions at the species level or within some genera arises from the surprisingly low genetic divergence within most Lauraceae plastomes. Values of mean nucleotide variability range from 0.04% to 0.15% (Song et al., 2015, 2017b, 2018; Tian et al., 2019; Trofimov et al., 2022; Cao et al., 2023, 2024; Zhu et al., 2023).
Despite the overall low genetic divergence, hundreds of mutation sites, including indels and substitutions, exist in the sequence matrix of Lauraceae plastomes, providing enough data to resolve deep-level relationships. For instance, in contrast to morphological studies (Doyle and Endress, 2000; Renner and Chanderbali, 2000), plastid phylogenomic analyses confirm that Lauraceae are monophyletic and sister to Monimiaceae and Hernandiaceae (Song et al., 2020; Li et al., 2021). Studies of Lauraceae plastomes have consistently reconstructed monophyletic groups, with analyses ranging from eight to 189 plastomes revealing major lineages, viz. Hypodaphnideae, Cryptocaryeae, Cassytheae, Neocinnamomeae, Caryodaphnopsideae, Mezilaureae, Perseeae, Laureae and Cinnamomeae (Song et al., 2016, 2017a, 2020; Liu et al., 2021; Yang et al., 2023).
Additional plastomes have been used to resolve the relationships among some species and groups within different genera, including Alseodaphne (Song et al., 2018), Beilschmiedia (Li et al., 2020; Song et al., 2023; Zhu et al., 2024), Caryodaphnopsis (Cao et al., 2024), Cassytha (Yu et al., 2023; Liu et al., 2024), Cinnamomum (Xiao and Ge, 2022), Cryptocarya (Song et al., 2023), Lindera (Zhao et al., 2018; Tian et al., 2019, 2024; Jo et al., 2019; Xiao et al., 2020), Litsea (Liu et al., 2022c; Tian et al., 2024), Machilus (Xiao et al., 2022), Neocinnamomum (Cao et al., 2023), and Ocotea (Trofimov et al., 2022).
Mitogenomes have also provided important data sources for phylogenetic studies in plants and have been sequenced and assembled successfully in many angiosperm families (Xue et al., 2022). However, due to variability of mitogenomes and the difficulty of sequence assembly, circular mitogenomes of Lauraceae have only recently successfully been assembled using next-generation sequencing data (Bi et al., 2024; Song et al., 2024; Yang et al., 2024). Regardless, phylogenomics using mitogenome sequences has the potential to address additional evolutionary questions, as Song et al. (2024) and Zhu et al. (2025) found significant incongruence among the mitochondrial, chloroplast, and nuclear phylogenies, especially for species within Caryodaphnopsideae and Neocinnamomeae.
5.2. Nuclear genomes of LauraceaeGenomics in Lauraceae began quite late. Initially research focused on using Lauraceae chloroplast genomes to resolve phylogenetic relationships and aid in species identification. Chaw et al. (2019) published the first Lauraceae nuclear genome of Camphora micrantha (Hayata) Y. Yang, Bing Liu & Zhi Yang (as Cinnamomum kanehirae Hayata), a rare and endangered species from Taiwan, China. Next, the entire genome of the commercially important Persea americana was sequenced (Rendón-Anaya et al., 2019; Nath et al., 2022), followed by sequences for eight other Lauraceae species: Litsea cubeba (Chen et al., 2020a), Phoebe bournei (Hemsl.) Yen C. Yang (Chen et al., 2020b; Han et al., 2022), Camphora officinarum (as Cinnamomum camphora (L.) J. Presl) (Jiang et al., 2022; Shen et al., 2022; Sun et al., 2022; Wang et al., 2022), Cinnamomum burmannii (Nees & T.Nees) Blume (Li et al., 2022), Lindera glauca (Xiong et al., 2022), Litsea coreana H.Lév (Zhang et al., 2022), Sextonia rubra (Mez) van der Werff (Schmitt et al., 2024), and Cinnamomum chago B.S. Sun & H.L. Zhao (Tao et al., 2024). The genome sizes of the ten species sequenced thus far range from 0.7G to 2.09G, of which the largest is Lindera glauca. However, our preliminary finding indicates that the hemiparasitic Cassytha filiformis may have an even larger genome, estimated at more than 7G (unpublished data).
The genomes of magnoliids, including Lauraceae, offer valuable information about the evolutionary history of flowering plants, although various phylogenetic methodologies and genomic data from different taxa have resulted in incongruent magnoliid phylogenies (Chaw et al., 2019; Chen et al., 2020a, 2020b). Consequently, relationships between monocots, eudicots, and magnoliids remain a subject of debate. The incongruence is possibly biological rather than methodological, with past incomplete lineage sorting leading to the presence of genomic blocks with diverse ancestries in these three major angiosperm lineages (Chaw et al., 2019; Rendon-Anaya et al., 2019; Chen et al., 2020a, 2020b).
Research indicates that Lauraceae may have undergone two whole-genome duplication (WGD) events (Chaw et al., 2019; Rendón-Anaya et al., 2019; Nath et al., 2022; Han et al., 2022; Jiang et al., 2022; Shen et al., 2022; Sun et al., 2022; Xiong et al., 2022; Wang et al., 2022; Zhang et al., 2022). The older WGD event occurred immediately before the divergence of Laurales and Magnoliales. This ancient WGD likely then facilitated genomic expansion and diversification early in the evolution of Lauraceae, setting the stage for subsequent speciation. The more recent WGD event is shared by all lineages of Lauraceae investigated to date. This WGD is believed to have coincided with the establishment of basal lineages during the Late Cretaceous (Chanderbali et al., 2001) and an increase in biodiversity of the family. This second WGD event may also have played a role in driving the evolution of specific metabolic pathways, particularly those involved in the production of aromatic compounds and volatile substances (Xiong et al., 2022).
The confirmation and dating of WGD events typically rely on Ks value analysis—the rate of synonymous substitutions—comparing the Ks value distribution of paralogous gene pairs (Chaw et al., 2019; Shen et al., 2022; Wang et al., 2022; Zhang et al., 2022). This methodology assumes a constant molecular clock rate, that is, a stable rate of synonymous substitutions over time, permitting the calculation of temporal distance based on the accumulation of synonymous substitutions. However, the actual rates of molecular evolution can be influenced by various factors, introducing some degree of uncertainty regarding the precise timing of WGD events. The timing of WGD events in the evolution of Lauraceae remains uncertain (Shen et al., 2022; Wang et al., 2022) and the frequency and impact of WGD require further study.
Lauraceae are rich in polysaccharides, flavonoids, polyphenols, and essential oils. Genomic analysis has revealed key genes involved in the synthesis of terpenoids, flavonoids, aromatic compounds, and D-borneol, helping us understand the metabolic diversity, evolutionary history, and adaptive strategies of this plant family. The terpene synthase (TPS) gene family is involved in the terpenoid pathway, which produces many physiologically important chemicals. These genes encode enzymes that create monoterpenoids, sesquiterpenoids, and diterpenoids, which are essential oil defense, pollinator attractiveness, and environmental stress adaptation elements (Chen et al., 2020a, 2020b; Han et al., 2022). Chalcone synthase (CHS) genes are associated with Lauraceae flavonoids. CHS is the first committed enzyme in the flavonoid biosynthesis pathway to produce flavonoids, which tint plants, provide UV protection, and repel herbivores (Zhang et al., 2022).
Aromatic chemicals in Lauraceae are generated via complex routes that cross the methylerythritol 4-phosphate (MEP) and mevalonate (MVA) pathways. Several of these pathways and their genes, including TPS genes, have been identified and analyzed, revealing the evolutionary origins of the aromatic profiles of Lauraceae species (Xiong et al., 2022). However, the complete pathways and genetic control of secondary metabolite biosynthesis have not been fully elucidated because of the complexity of metabolic pathways and the role of environmental factors in metabolite production. Moreover, the genetic mechanisms underlying the chemical diversity among Lauraceae species are not fully understood because of the difficulties in identifying and characterizing genes responsible for producing different chemotypes.
Addressing unanswered questions in Lauraceae genomics requires a comprehensive strategy. To accomplish this, it is necessary to make use of advanced long-read sequencing technology to achieve accurate genome assembly and annotation, to increase the number of species samples available for comparative genomics research, and to use CRISPR/Cas9 technology. To examine environmental adaptation, it is necessary to perform gene function validation, integrate environmental genomics and ecological niche models, provide novel bioinformatics tools for the processing of enormous amounts of data, and improve international and inter-institutional collaboration. The implementation of these approaches will not only strengthen our understanding of the genetic variability and evolutionary changes in Lauraceae, but will also strengthen our efforts to protect and utilize these plants appropriately.
6. Conclusions and prospectsIn conclusion, the field of Lauraceae systematics and biogeography has witnessed remarkable progress over the past two decades, propelled by significant advancements in phylogenetic and phylogenomic techniques. These technological innovations have revolutionized our understanding of the family's evolutionary history and relationships. Major achievements in this period include the comprehensive classification of the family into nine distinct tribes and the strategic rearrangement of several complex generic groups, providing a more accurate representation of their evolutionary relationships. Research in the Neotropics has focused mainly on extensive field work, the acquisition of new collections, revisions of genera and the description of new species. In contrast, the main focus of research in Asia has been plastid and nuclear genomes, historical biogeography and phylogeography. We recommend that future research in the Neotropics focus more on genomic studies, phylogeography, and historical biogeography, whereas future research in Asia needs to include more botanical exploration, generic revision, and studies of generic delimitations.
Despite substantial strides, numerous challenges persist in fully resolving systematic relationships within Lauraceae. Many groups remain unresolved, necessitating further investigation and potential reclassification. These unresolved areas present exciting opportunities for future research and highlight the complexity of this diverse plant family.
Recent biogeographic studies have provided invaluable insights into the family's pan-tropical distribution pattern. These investigations have challenged previous assumptions about the family's dispersal history, suggesting that recent long-distance dispersal events have played a more significant role than previously thought. This finding has important implications for understanding the family's current distribution and its potential responses to future environmental changes.
As we look to the future of Lauraceae research, a multifaceted approach is crucial for continued progress. The integration of cutting-edge genomic studies with ongoing technical advancements in sequencing and data analysis will provide increasingly detailed and accurate phylogenetic information. Additionally, intensified field work efforts are essential to discover new species, document biodiversity, and collect comprehensive samples from a wide range of geographic locations.
The importance of integrated morphological and molecular analyses cannot be overstated. While molecular data have revolutionized our understanding of evolutionary relationships, morphological studies remain crucial for species identification, description, and understanding adaptive traits. Combining these approaches will provide a more holistic view of Lauraceae evolution and diversity.
A critical aspect of future research lies in ensuring the collection of comprehensive samples from carefully labeled trees. This practice will provide a solid foundation for future studies, allowing for verification and replication of results. It will also facilitate the creation of robust reference collections, which are invaluable for taxonomic work and comparative studies.
Furthermore, as climate change and habitat loss continue to threaten biodiversity worldwide, research on Lauraceae takes on added significance. Understanding the family's biogeography, adaptations, and evolutionary history can provide crucial insights for conservation efforts and predictions of how these important plants may respond to future environmental changes.
In addition, exploring the ecological roles of Lauraceae species in their respective ecosystems will be vital. Many Lauraceae species are keystone species in tropical and subtropical forests, providing food and habitat for numerous animal species. Investigating these ecological interactions can provide a broader context for the family's evolutionary success and importance in global biodiversity. Ultimately, continued research in Lauraceae systematics and biogeography will not only enhance our understanding of this diverse and ecologically significant plant family but also contribute to broader questions about plant evolution, biogeography, and ecology. As we unravel the complexities of Lauraceae, we gain valuable insights into the processes that shape plant diversity across the globe, furthering our comprehension of life's evolutionary journey.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (31970222; 31770569; 31500454; 31500165; 32260060; 32270217; 32260056; 31970223; 32400180); the Science and Technology Basic Resources Investigation Program of China (2017FY100100; 2017FY100102); Biodiversity Conservation Program of Chinese Academy of Sciences (ZSSD-013); National Key Research and Development Program of China (2022YFC2601200; 2023YFF0805800); Yunnan Fundamental Research Projects (202201AS070055; 202301AU070224); the 14th Five-Year Plan of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (XTBG-1450101); Australian Research Council grant (DP130104314); RSNZ Marsden grant (11-UOO-043); Xingdian Talent Support Program (XDRC-QNRC-2022-0323); Shandong Provincial Natural Science Foundation (ZR2022QC214).
CRediT authorship contribution statement
Lang Li: Writing – review & editing, Writing – original draft, Visualization, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization. Bing Liu: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Funding acquisition. Yu Song: Writing – review & editing, Writing – original draft, Formal analysis, Funding acquisition. Hong-Hu Meng: Writing – review & editing, Writing – original draft. Xiu-Qin Ci: Writing – review & editing, Writing – original draft, Visualization, Formal analysis. John G. Conran: Writing – review & editing, Writing – original draft, Funding acquisition. Rogier P.J. de Kok: Writing – review & editing, Writing – original draft. Pedro Luís Rodrigues de Moraes: Writing – review & editing, Writing – original draft, Formal analysis. Jun-Wei Ye: Writing – review & editing, Writing – original draft, Funding acquisition. Yun-Hong Tan: Writing – review & editing, Writing – original draft. Zhi-Fang Liu: Writing – review & editing, Writing – original draft, Funding acquisition. Marlien van der Merwe: Writing – review & editing. Henk van der Werff: Writing – review & editing, Conceptualization. Yong Yang: Writing – review & editing, Funding acquisition, Conceptualization. Jens G. Rohwer: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Jie Li: Writing – review & editing, Writing – original draft, Supervision, Resources, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2025.04.001.
Annotation for "An updated phylogenetic classification of Lauraceae".
Annotation: The genus Porostema comprises three combinations: P. bijuga (Rottb.) Forsyth f., P. guianensis (Aubl.) Forsyth f., and P. sanguinea (Rol.) Forsyth f., none of which has been formally designated as the type species. Notably, P. guianensis is the type species of Ocotea (O. guianensis Aubl.), while P. sanguinea represents the type of Nectandra (N. sanguinea Rol.). Therefore, Porostema is a superfluous name for both Nectandra and Ocotea. To resolve the taxonomic ambiguity surrounding the synonymy attribution of Porostema, we hereby designate P. guianensis as the type species of this genus.
Ocotea Aubl., Hist. Pl. Guiane, 780. 1775. TYPE: Ocotea guianensis Aubl., Hist. Pl. Guiane, 781. 1775.
≡ Porostema Schreb., Gen. Pl., ed. 8[a], 2: 517. 1791. TYPE (here designated): Porostema guianensis (Aubl.) Forsyth f., Bot. Nomencl. 418. 1794.
Allen, C.K., 1938. Studies in the Lauraceae, I. Chinese and Indo-Chinese species of Litsea, Neolitsea, and Actinodaphne. Ann. Mo. Bot. Gard., 25: 361-434. DOI:10.2307/2394482 |
Allen, C.K., 1939. Studies in the Lauraceae Ⅱ. Some critical and new species of Cinnamomum and Neocinnamomum. J. Arnold Arbor., 20: 44-63. DOI:10.5962/p.324598 |
Allen, C.K., 1941. Studies in the Lauraceae, Ⅲ. some critical and new species of Asiatic Lindera, with occasional notes on Litsea. J. Arnold Arbor., 22: 1-31. DOI:10.5962/p.30188 |
Allen, C.K., 1942a. Studies in the Lauraceae, Ⅳ, preliminary study of the Papuasian species collected by the archbold expeditions. J. Arnold Arbor., 23: 112-155. DOI:10.5962/bhl.part.18681 |
Allen, C.K., 1942b. Studies in the Lauraceae, V. some eastern Asiatic species of Beilschmiedia and related genera. J. Arnold Arbor., 23: 444-463. DOI:10.5962/bhl.part.18684 |
Alves, F.M., Souza, V.C., 2013. Phylogenetic analysis of the neotropical genus Mezilaurus and reestablishment of Clinostemon (Lauraceae). Taxon, 62: 281-290. DOI:10.12705/622.5 |
Ara, H., Mia, M.U.K., Khan, B., 2007. An annotated checklist of Lauraceae. Bangladesh. J. Plant Taxon., 14: 147-162. |
Arifiani, D., 2001. Taxonomic revision of Endiandra (Lauraceae) in Borneo. Blumea, 46: 99-124. |
Atkinson, B.A., Stockey, R.A., Rothwell, G.W., et al., 2015. Lauraceous flowers from the Eocene of Vancouver Island: Tinaflora beardiae gen. et sp. nov. (Lauraceae). Int. J. Plant Sci., 176: 567-585. DOI:10.1086/681586 |
Avise, J., 2009. Phylogeography: retrospect and prospect. J. Biogeogr., 36: 3-15. DOI:10.1111/j.1365-2699.2008.02032.x |
Backer, C.A., Bakhuizen, van den Brink, R.C., 1963. Flora of Java, vol. 1. Noordhoff, Groningen.
|
Baitello, J.B., Lorea-Hernández, F.H., Moraes, P.L.R. de, et al., 2003. Lauraceae. In: Wanderley, M.G.L., Shepherd, G.J., Melhem, T.S., et al. (Eds.), Flora Fanerogâmica do Estado de São Paulo. Instituto de Botanica, vol. 3. RiMa, São Paulo, pp. 149-224.
|
Bannister, J.M., Lee, D.E., Conran, J.G., 2012. Lauraceae from rainforest surrounding an early Miocene maar lake, Otago, southern New Zealand. Rev. Palaeobot. Palynol., 178: 13-34. DOI:10.1016/j.revpalbo.2012.03.015 |
Barbosa, T.D.M., Baitello, J.B., Moraes, P.L.R. de, 2012. A família Lauraceae no município de Santa Teresa, Espírito Santo. Bol. Mus. Biol. Mello Leitão, Nova Sér., 30: 5-178. |
Bentham, G., 1880. Laurineae. In: Bentham, G., Hooker, J.D. (Eds.), Genera Plantarum, vol. 3. L. Reeve, London, pp. 146-168.
|
Beurel, S., Bachelier, J.B., Munzinger, J., et al., 2024. First flower inclusion and fossil evidence of Cryptocarya (Laurales, Lauraceae) from Miocene amber of Zhangpu (China). Foss. Rec., 27: 1-11. DOI:10.3897/fr.27.109621 |
Bi, C.W., Sun, N., Han, F.C., et al., 2024. The first mitogenome of Lauraceae (Cinnamomum chekiangense). Plant Divers., 46: 144-148. DOI:10.1016/j.pld.2023.11.001 |
Bhuinya, T., Singh, P., Mukherjee, S.K., 2009. Distribution of the genus Litsea Lam. (Lauraceae) in India with special reference to rare and endemic species. Phytotaxonomy, 9: 116-121. |
Bhuinya, T., Singh, P., Mukherjee, S.K., 2010. An account of the species of Litsea Lam. (Lauraceae) endemic to India. Bangladesh J. Plant Taxon., 17: 183-191. |
Blume, C.L., 1825-1826. Bijdragen tot de Flora van Nederlandsch Indië (Batavia).
|
Blume, C.L., 1836. Eenige waarnemingen omtrent den Culilawan-boom van Rumphius, in het 11de deel, pp 65–69 van zijn Herbarium Amboinense. Rumphia, 1: 46-65. |
Blume, C.L., 1849-1851. Museum Botanicum Lugduno-Batavum, vol. 1 (Leiden).
|
Burger, W.C., 1988. A new genus of Lauraceae from Costa Rica, with comments on problems of generic and specific delimitation within the family. Brittonia, 40: 275-282. DOI:10.2307/2807472 |
Cammerloher, H., 1925. Die Cinnamomum-Arten von Niederländisch-Ostindien. Bull. Jard. Bot. Buitenzorg, ser. ⅲ, 7: 446-497. |
Cantrill, D.J., Wanntorp, L., Drinnan, A.N., 2011. Mesofossil flora from the late Cretaceous of New Zealand. Cretac. Res., 32: 164-173. DOI:10.1016/j.cretres.2010.11.006 |
Cao, Y.N., Wang, I.J., Chen, L.Y., et al., 2018. Inferring spatial patterns and drivers of population divergence of Neolitsea sericea (Lauraceae), based on molecular phylogeography and landscape genomics. Mol. Phylogenet. Evol., 126: 162-172. DOI:10.1109/cc.2018.8387995 |
Cao, Z.Y., Qu, Y.Y., Song, Y., et al., 2024. Comparative genomics and phylogenetic analysis of chloroplast genomes of Asian Caryodaphnopsis taxa (Lauraceae). Gene, 907: 148259. DOI:10.1016/j.gene.2024.148259 |
Cao, Z.Y., Yang, L.Y., Xin, Y.X., et al., 2023. Comparative and phylogenetic analysis of complete chloroplast genomes from seven Neocinnamomum taxa (Lauraceae). Front. Plant Sci., 14: 1205051. DOI:10.3389/fpls.2023.1205051 |
Carpenter, R.J., Jordan, G.J., Hill, R.S., 2007. A toothed Lauraceae leaf from the early Eocene of Tasmania, Australia. Int. J. Plant Sci., 168: 1191-1198. DOI:10.1086/520721 |
Carpenter, R.J., Truswell, E.M., Harris, W.K., 2010. Lauraceae fossils from a volcanic Palaeocene oceanic island, Ninetyeast Ridge, Indian Ocean: ancient long-distance dispersal?. J. Biogeogr., 37: 1202-1213. DOI:10.1111/j.1365-2699.2010.02279.x |
Carter, S.N., 2017. Molecular Systematics of the New Caledonian Cryptocaryeae (Lauraceae). University of Waikato, Hamilton, New Zealand.
|
Cevallos-Ferriz, S.R.S., Catharina, A.S., Kneller, B., 2021. Cretaceous Lauraceae wood from El Rosario, Baja California, Mexico. Rev. Palaeobot. Palynol., 292: 104478. DOI:10.1016/j.revpalbo.2021.104478 |
Chakrabarty, T., Kumar, A., Krishna, G., 2022. A revision of the genus Dehaasia (Lauraceae) in the Indo-Burmese region. Plant Sci. Today, 9: 61-67. DOI:10.14719/pst.1718 |
Chakrabarty, T., Kumar, A., Krishna, G., 2023. A revision of the genus Phoebe (Lauraceae) in the Indo-Burmese region. Phytotaxa, 606: 26-42. |
Chakrabarty, T., Lakra, G.S., Diwakar, P.G., 2010. The family Lauraceae in Andaman and Nicobar islands. In: Ramakrishna, Raghunathan C., Sivaperuman, C. (Eds.), Recent Trends in Biodiversity of Andaman and Nicobar Islands. Zoological Survey of India, Kolkata, pp. 179-193.
|
Chambers, K.L., Poinar, G.O., Chanderbali, A.S., 2012. Treptostemon (Lauraceae), a new genus of fossil flower from mid-Tertiary Dominican amber. J. Bot. Res. Inst. Tex., 6: 551-556. |
Chambers, K.L., Poinar Jr., G.O., Brown, A.E., 2011. A fossil flower of Persea (Lauraceae) in Tertiary Dominican amber. J. Bot. Res. Inst. Tex., 5: 457-462. |
Chanderbali, A.S., 2004. Endlicheria (Lauraceae). Flora Neotropica Monograph 91. New York Botanical Garden Press: pp. 1-141.
|
Chanderbali, A.S., van der Werff, H., Renner, S.S., 2001. Phylogeny and historical biogeography of Lauraceae: evidence from the chloroplast and nuclear genomes. Ann. Mo. Bot. Gard., 88: 104-134. DOI:10.2307/2666133 |
Chandler, M.E.J., 1964. A summary and survey of findings in the light of recent botanical observations. In: The Lower Tertiary Floras of Southern England, Vol. 4. British Museum (Natural History), London, UK, p. 151
|
Chase, M.W., Soltis, D.E., Olmstead, R.G., et al., 1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Ann. Mo. Bot. Gard., 80: 528-548+550-580. DOI:10.2307/2399846 |
Chaw, S.M., Liu, Y.C., Wu, Y.W., et al., 2019. Stout camphor tree genome fills gaps in understanding of flowering plant genome evolution. Nat. Plants, 5: 63-73. DOI:10.1038/s41477-018-0337-0 |
Chen, S.B., Ferry, Slik, J.W., Gao, J., et al., 2015. Latitudinal diversity gradients in bryophytes and woody plants: roles of temperature and water availability. J. Syst. Evol., 53: 535-545. DOI:10.1111/jse.12158 |
Chen, S.P., Sun, W.H., Xiong, Y.F., et al., 2020b. The Phoebe genome sheds light on the evolution of magnoliids. Hortic. Res., 7: 146. DOI:10.1038/s41438-020-00368-z |
Chen, Y.C., Li, Z., Zhao, Y.X., et al., 2020a. The Litsea genome and the evolution of the laurel family. Nat. Commun., 11: 1675. DOI:10.1038/s41467-020-15493-5 |
Christophel, D.C., Rowett, A.I., 1996. Leaf and cuticle atlas of Australian leafy Lauraceae. In: Flora of Australia Supplementary Series, vol. 6, pp. 1-217.
|
Christophersen, E., 1935. Flowering Plants of Samoa, vol. 128. Bernice P. Bishop Museum Bulletin, Hawaii.
|
Co, L.L., 2024. Co's Digital Flora of the Philippines - Lauraceae (philippineplants.org. (Accessed June 2024).
|
Coiffard, C., El, Atfy, H., Renaudie, J., et al., 2023. The emergence of the tropical rainforest biome in the Cretaceous. Biogeosciences, 20: 1145-1154. DOI:10.5194/bg-20-1145-2023 |
Collinson, M.E., Fowler, K., Boulter, M.C., 1981. Floristic changes indicate a cooling climate in the Eocene of southern England. Nature, 291: 315-317. DOI:10.1038/291315a0 |
Conran, J.G., Bannister, J.M., Reichgelt, T., et al., 2016. Epiphyllous fungi and leaf physiognomy indicate an ever-wet humid mesothermal (subtropical) climate in the late Eocene of southern New Zealand. Palaeogeogr. Palaeoclimatol. Palaeoecol., 452: 1-10. DOI:10.1016/j.palaeo.2016.03.032 |
Das, A., 1937. Phoebe of Assam and some new species. Assam Forest Rec. Bot., 2: 1-12. |
Davis, C.C., Bell, C.D., Fritsch, P.W., et al., 2002. Phylogeny of Acridocarpus-Brachylophon (Malpighiaceae): implications for tertiary tropical floras and Afroasian biogeography. Evolution, 56: 2395-2405. |
de Kok, R.P.J., 2015. A revision of Cryptocarya R. Br. (Lauraceae) of Thailand and Indo-China. Gard. Bull. Singapore, 67: 309-349. DOI:10.3850/S2382581215000277 |
de Kok, R.P.J., 2016a. Notes on the monotypic genus Hexapora (Lauraceae), endemic to peninsular Malaysia. Gard. Bull. Singapore, 68: 201-208. DOI:10.3850/S2382581216000156 |
de Kok, R.P.J., 2016b. A revision of Beilschmiedia (Lauraceae) of Peninsular Malaysia. Blumea, 61: 147-164. DOI:10.3767/000651916X693004 |
de Kok, R.P.J., 2016c. A revision of Cryptocarya R. Br. (Lauraceae) of Peninsular Malaysia. Kew Bull., 71: 1-26. DOI:10.18352/tseg.884 |
de Kok, R.P.J., 2019. A revision of Cinnamomum Schaeffer (Lauraceae) of Peninsular Malaysia. Gard. Bull. Singapore, 71: 89-139. DOI:10.26492/gbs71(1).2019-07 |
de Kok, R.P.J., 2021a. A revision of Beilschmiedia (Lauraceae) for Thailand and Indochina. Thai For. Bull. (Bot.), 49: 1-26. DOI:10.20531/tfb.2021.49.1.01 |
de Kok, R.P.J., 2021b. A revision of Litsea (Lauraceae) in Peninsular Malaysia and Singapore. Gard. Bull. Singapore, 73: 81-178. DOI:10.26492/gbs73(1).2021-07 |
de Kok, R.P.J., 2025a. Lauraceae. In: Flora of Laos, Cambodia and Vietnam. Muséum National d'Histoire Naturelle, Paris (in press).
|
de Kok, R.P.J., 2025b. Lauraceae. In: Kiew, R., Chung, R.C.K., Saw, L.G., et al. (Eds.), Flora of Peninsular Malaysia, Series Ⅱ: Seed Plants. Forest Research Institute Malaysia (FRIM), Kuala Lumpur (in press).
|
de Kok, R.P.J., Middleton, D., 2025. Lauraceae (accepted). In: Santisuk, T., Chayamarit, C., Balslev, H. (Eds.), Flora of Thailand. Forest Herbarium. Royal Forest Department, Bangkok.
|
de Kok, R.P.J., Sengun, S., 2020. A revision of Cinnadenia Kosterm. (Lauraceae). Adansonia, Sér., 3(42): 105-112. DOI:10.5252/adansonia2020v42a4 |
de Kok, R.P.J., Thomas, D.C., 2025. Lauraceae. In: Middleton, D. (Ed.), Flora of Singapore. Singapore Botanic Gardens, Singapore (in press).
|
de Kok, R.P.J., Utteridge, T.M.A., 2021. Lauraceae. In: Utteridge, T.M.A., Jennings, L.V.S. (Eds.), Trees of New Guinea. Kew Publishing, Kew, pp. 81-89.
|
Denk, T., Grímsson, F., Kvaček, Z., 2005. The Miocene floras of Iceland and their significance for late Cainozoic North Atlantic biogeography. Bot. J. Linn. Soc., 149: 369-417. DOI:10.1111/j.1095-8339.2005.00441.x |
Doyle, J.J., 1992. Gene trees and species trees: molecular systematics as one-character taxonomy. Syst. Bot., 17: 144-163. DOI:10.2307/2419070 |
Doyle, J.A., Endress, P.K., 2000. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. Int. J. Plant Sci., 161: S121-S153. DOI:10.1086/317578 |
Drinnan, A.N., Crane, P.R., Friis, E.M., et al., 1990. Lauraceous flowers from the Potomac group (mid-Cretaceous) of eastern North America. Bot. Gaz., 151: 370-384. DOI:10.1086/337838 |
Dupéron-Laudoueneix, M., Dupéron, J., 2005. Bois fossiles de Lauraceae: nouvelle découverte au Cameroun, inventaire et discussion. Ann. Paleontol., 91: 127-151. DOI:10.1016/j.annpal.2005.03.002 |
Eklund, H., 2000. Lauraceous flowers from the Late Cretaceous of North Carolina, U.S.A. Bot. J. Linn. Soc., 132: 397-428. DOI:10.1111/j.1095-8339.2000.tb01220.x |
Eklund, H., Kvaček, J., 1998. Lauraceous inflorescences and flowers from the Cenomanian of Bohemia (Czech Republic, Central Europe). Int. J. Plant Sci., 159: 668-686. DOI:10.1086/297585 |
Endemia, 2001. Endemia. nc - Faune & Flore de Nouvelle-Calédonie. onwards. Retrieved 2 Oct 2024 from. http://www.endemia.nc/.
|
Fan, D.M., Hu, W., Li, B., et al., 2016. Idiosyncratic responses of evergreen broad-leaved forest constituents in China to the late Quaternary climate changes. Sci. Rep., 6: 31044. DOI:10.1038/srep31044 |
Fan, D.M., Lei, S.Q., Liang, H., et al., 2022. More opportunities more species: Pleistocene differentiation and northward expansion of an evergreen broad-leaved tree species Machilus thunbergii (Lauraceae) in Southeast China. BMC Plant Biol., 22: 35. DOI:10.1186/s12870-021-03420-9 |
Fijridiyanto, I.A., Murakami, N., 2009. Phylogeny of Litsea and related genera (Laureae-Lauraceae) based on analysis of rpb2 gene sequences. J. Plant Res., 122: 283-298. DOI:10.1007/s10265-009-0218-8 |
Fijridiyanto, I.A., Smets, E., Arifiani, D., 2020. Taxonomic revision of Dehaasia (Lauraceae) in Sumatra. Blumea, 65: 167-175. DOI:10.3767/blumea.2020.65.02.08 |
Franco, M.J., Brea, M., Passeggi, E., et al., 2015. The first record of Lauraceae fossil woods from the Cretaceous Puerto Yeruá Formation of eastern Argentina and palaeobiogeographic implications. Cretac. Res., 56: 388-398. DOI:10.1016/j.cretres.2015.05.014 |
Friis, E.M., Crane, P.R., Pedersen, K.R.
, 2011. Early Flowers and Angiosperm Evolution. Cambridge University Press: Cambridge, UK.
|
Gamble, J.S., 1910a. New Lauraceae from the Malayan region I. Bull. Misc. Inform. Kew, 1910: 142-153. DOI:10.2307/4113176 |
Gamble, J.S., 1910b. New Lauraceae from the Malayan region Ⅱ. Bull. Misc. Inform. Kew, 1910: 218-228. DOI:10.2307/4111848 |
Gamble, J.S., 1910c. New Lauraceae from the Malayan region Ⅲ. Bull. Misc. Inform. Kew, 1910: 312-321. DOI:10.2307/4111876 |
Gamble, J.S., 1910d. New Lauraceae from the Malayan region Ⅳ. Bull. Misc. Inform. Kew, 1910: 357-368. DOI:10.2307/4113248 |
Gangopadhyay, M., Chakrabarty, T., 2005. The genus Cryptocarya R. Br. (Lauraceae) in the Indian subcontinent. J. Econ. Taxon. Bot., 29: 274-293. |
Garrouste, R., Munzinger, J., Leslie, A., et al., 2021. New fossil discoveries illustrate the diversity of past terrestrial ecosystems in New Caledonia. Sci. Rep., 11: 18388. DOI:10.1038/s41598-021-97938-5 |
Geethakumary, M.P., Deepu, S., Pandurangan, A.G., 2021. Synopsis of the genus Cinnamomum Schaeffer (Lauraceae) in India. Plant Sci. Today, 8: 199-209. DOI:10.14719/pst.2021.8.1.1028 |
Givnish, T.J., Renner, S.S., 2004. Tropical intercontinental disjunctions: Gondwana breakup, immigration from the boreotropics, and transoceanic dispersal. Int. J. Plant Sci., 165: S1-S6. DOI:10.1086/424022 |
González, J., Poveda, L.J., 2007. Lauraceae. In: Hammel, B.R., Grayum, M.H., Herrera, C., et al. (Eds.), Manual de Plantas de Costa Rica, Vol. Ⅵ. Dicotiledóneas (Haloragaceae-Phytolaccaceae), Monographs in Systematic Botany from the Missouri Botanical Garden 111. Missouri Botanical Garden Press, St. Louis, pp. 90-172.
|
Gottwald, H., 1992. Hölzer aus marinen Sanden des Oberen Eozän von Helmstedt (Niedersachsen). Palaeontograph. Abteilung B, 225: 27-103. |
Han, X., Zhang, J., Han, S., et al., 2022. The chromosome-scale genome of Phoebe bournei reveals contrasting fates of terpene synthase (TPS)-a and TPS-b subfamilies. Plant Commun., 3: 100410. DOI:10.1016/j.xplc.2022.100410 |
Harrison, S.P., Yu, G., Takahara, H., et al., 2001. Palaeovegetation (Communications arising): diversity of temperate plants in East Asia. Nature, 413: 129-130. DOI:10.1038/35093166 |
Heintz, H., 2007. Untersuchungen zur Morphologie und Ontogenie der Infloreszenzen und Blüten einiger Lauraceae. Unpublished Ph. D. thesis. Universität Hamburg, Hamburg, Germany.
|
Helmstetter, A.J., Ezedin, Z., de Lírio, E.J., et al., 2025. Toward a phylogenomic classification of magnoliids. Am. J. Bot., 112: e16451. DOI:10.1002/ajb2.16451 |
Heo, K., van der Werff, H., Tobe, H., 1998. Embryology and relationships of Lauraceae (Laurales). J. Linn. Soc. Bot., 126: 295-322. DOI:10.1111/j.1095-8339.1998.tb01383.x |
Herendeen, P.S., Crepet, W.L., Nixon, K.C., 1994. Fossil flowers and pollen of Lauraceae from the Upper Cretaceous of New Jersey. Plant Syst. Evol., 189: 29-40. DOI:10.1007/BF00937576 |
Hill, R.S. (Ed.), 2017. History of the Australian Vegetation: Cretaceous to Recent.
University of Adelaide Press, Adelaide, SA.
|
Hô, P.H., 1991. Lauraceae. An Illustrated Flora of Vietnam, vol. 2. Mekong Printing,
Montreal, pp. 619-1249
|
Hô, P.H., 1999. Lauraceae. An Illustrated Flora of Vietnam, vol. 1. Youth Publishing House, Ho Chi Minh City, pp. 343-402.
|
Hooker, J.D., 1890. Laurineae. In: Hooker, J.D. (Ed.), The Flora of British India, vol. 5. L. Reeve, London, pp. 116-189.
|
Huang, H., Li, J., 2018. Flower fossils of Lauraceae in the geological time and its phylogenetic evolutionary significance. Guihaia, 38: 210-219. DOI:10.11931/guihaia.gxzw201709008 |
Huang, J.F., Li, L., van der Werff, H., et al., 2016. Origins and evolution of cinnamon and camphor: A phylogenetic and historical biogeographical analysis of the Cinnamomum group (Lauraceae). Mol. Phylogenet. Evol., 96: 33-44. DOI:10.1016/j.ympev.2015.12.007 |
Hyland, B.P.M., 1989. A revision of Lauraceae in Australia (excluding Cassytha). Aust. Syst. Bot., 2: 135-367. DOI:10.1071/SB9890135 |
Irawan, B., 2004. Ironwood (Eusideroxylon zwageri Teijsm. & Binn.) and its Varieties in Jambi, Indonesia. Cuvillier Verlag, Göttingen.
|
Jiang, R., Chen, X., Liao, X., et al., 2022. A chromosome-level genome of the Camphor Tree and the underlying genetic and climatic factors for its Top-Geoherbalism. Front. Plant Sci., 13: 1-17. DOI:10.1155/2022/5668226 |
Jo, S.J., Kim, Y.K., Cheon, S.H., et al., 2019. Characterization of 20 complete plastomes from the tribe Laureae (Lauraceae) and distribution of small inversions. PLoS One, 14: e0224622. DOI:10.1371/journal.pone.0224622 |
Johnson, M.G., Pokorny, L., Dodsworth, S., et al., 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Syst. Biol., 68: 594-606. DOI:10.1093/sysbio/syy086 |
Julia, S., 2005. A synopsis of the genus Actinodaphne Nees (Lauraceae) in Sabah and Sarawak, Malaysia. Gard. Bull. Singapore, 57: 69-100. |
Julia, S., Soepadmo, E., Yahud, W., 2009. Problems in the generic delimitation between Alseodaphne, Dehaasia and Nothaphoebe (Lauraceae) in Borneo. Blumea, 54: 192-197. DOI:10.3767/000651909X476148 |
Kennedy, E.M., 2003. Late Cretaceous and Paleocene terrestrial climates of New Zealand: leaf fossil evidence from South Island assemblages. New Zeal. J. Geol. Geophys., 46: 295-306. DOI:10.1080/00288306.2003.9515010 |
Klucking, E.P., 1987. Leaf venation patterns. In: Lauraceae, Vol. 2, pp. 1-216. J. Cramer, Berlin-Stuttgart.
|
Kochummen, K.M., 1989. Lauraceae. In: Ng, F.S.P. (Ed.), Tree Flora of Malaya, vol. 4. Longman, Malaysia, pp. 98-178.
|
Kokubugata, G., Nakamura, K., Forster, P.I., et al., 2012. Cassytha pubescens and C. glabella (Lauraceae) are not disjunctly distributed between Australia and the Ryukyu Archipelago of Japan – evidence from morphological and molecular data. Aust. Syst. Bot., 25: 364-373. DOI:10.1071/SB10040 |
Kopp, L.E., 1966. A taxonomic revision of the genus Persea in the Western Hemisphere (Perseae-Lauraceae). Mem. N. Y. Bot. Gard., 14: 1-120. |
Kostermans, A.J.G.H., 1937. Revision of the Lauraceae Ⅱ: the genera Endlicheria, Cryptocarya (American species) and Licaria. Recueil Trav. Bot. Néerl., 34: 500-609. |
Kostermans, A.J.G.H., 1938a. Revision of the Lauraceae Ⅲ. The genera Aiouea, Systemonodaphne, Urbanodendron, Mezilaurus; additions and corrections to Licaria and Cryptocarya. Recueil Trav. Bot. Néerl., 35: 56-129. |
Kostermans, A.J.G.H., 1938b. Revision of the Lauraceae V. A monograph of the genera: Anaueria, Beilschmiedia (American species) and Aniba. Recueil Trav. Bot. Néerl., 35: 834-931. |
Kostermans, A.J.G.H., 1957. Lauraceae. Pengumuman Balai Besar Penjelidikan Kehutanan Indonesia, 57: 1-64. |
Kostermans, A.J.G.H., 1961. The New World species of Cinnamomum Trew (Lauraceae). Reinwardtia, 6: 17-24. |
Kostermans, A.J.G.H., 1973a. A synopsis of Alseodaphne Nees (Lauraceae). Candollea, 28: 93-136. |
Kostermans, A.J.G.H., 1973b. A synopsis of the genus Dehaasia Bl. (Lauraceae). Bot. Jahrb. Syst., 93: 424-480. |
Kostermans, A.J.G.H., 1974a. A monograph of the genus Neocinnamomum Liou Ho. Reinwardtia, 9: 85-96. |
Kostermans, A.J.G.H., 1974b. A monograph of Caryodaphnopsis A. Shaw. Reinwardtia, 9: 123-137. |
Kostermans, A.J.G.H., 1974c. Lauracées. In: Aubréville, A., Leroy, J.F. (Eds.), Flore de la Nouvelle-Calédonie et Dépendances, vol. 5, pp. 1-123.
|
ostermans, A.J.G.H., 1978a. Lauraceae. In: Nasir, E., Ali, S.I. (Eds.), Flora of West Pakistan, vol. 118, pp. 1-13.
|
Kostermans, A.J.G.H., 1978b. Potoxylon, a new Bornean genus of Lauraceae. Malay. Nat. J., 32: 143-147. |
Kostermans, A.J.G.H., 1985. The South Indian species of Cinnamomum Schaeffer (Lauraceae). Bull. Bot. Surv. India, 25: 90-133. |
Kostermans, A.J.G.H., 1986. A monograph of the genus Cinnamomum Schaeffer (Lauraceae) Part Ⅰ. Ginkgoana, 6: 1-171. |
Kostermans, A.J.G.H., 1995. Lauraceae. In: Dassanayake, M.D. (Ed.), A Revised Handbook to the Flora of Ceylon, vol. 9. Balkema, Rotterdam, pp. 105-172.
|
Kostermans, A.J.G.H., 1998. The Burmese Cinnamomum (Lauraceae). Reinwardtia, 11: 195-214. |
Kostermans, A.J.G.H., unpublished. Species of West Malesia (Malay Peninsula, Sumatra, Java, Borneo, and the Lesser Sunda Islands). (Unpublished article in the Kostermans archive in the Naturalis Botany Library).
|
Kremer, A., Hipp, A.L., 2020. Oaks: An evolutionary success story. New Phytol., 226: 987-1011. DOI:10.1111/nph.16274 |
Kress, W.J., DeFilipps, R.A., Farr, E., et al., 2003. A checklist of the trees, shrubs, herbs, and climbers of Myanmar. Contr. U.S. Natl. Herb. 45, 1-590. Department of Botany, Smithsonian Institution.
|
Kubitzki, K., Renner, S.S., 1982. Lauraceae I (Aniba and Aiouea). Flora Neotrop. Monogr. 31, 1-124. New York Botanical Garden Press.
|
Kubitzki, K., Richter, H.G., 1987. Williamodendron Kubitzki & Richter, a new genus of neotropical Lauraceae. Bot. Jahrb. Syst., 109: 49-58. |
Kurz, H.W., 2000. Revision der Gattung Licaria (Lauraceae). Mitt. Inst. Allg. Bot. Hamburg, 28/29: 89-221. |
Kvaček, J., Coiffard, C., Gandolfo, M., et al., 2020. When and why nature gained angiosperms. In: Martinetto, E., Tschopp, E., Gastaldo, R.A. (Eds.), Nature through Time: Virtual Field Trips through the Nature of the Past. Springer International Publishing, Cham, pp. 129-158.
|
Kvaček, J., Svobodová, M., Čepičková, J., et al., 2024. Cenomanian terrestrial paleoenvironments from the Bohemian Cretaceous Basin in Central Europe and their implications for angiosperm paleoecology. Palaeogeogr. Palaeoclimatol. Palaeoecol., 650: 112348. DOI:10.1016/j.palaeo.2024.112348 |
Le Cussan, J., Hyland, B.P.M., Weber, J.Z., 2007. Lauraceae. In: Wilson, A.J.G. (Ed.), Flora of Australia, Winteraceae to Platanaceae, vol. 2. Australian Biological Resources Study: Canberra, ACT, pp. 106-223.
|
Lecomte, H., 1914. Lauraceae. In: Flore Générale de l'Indo-Chine 5. Masson & Cie., Paris, pp. 107-158.
|
Lee, J.H., Lee, D.H., Choi, B.H., 2013. Phylogeography and genetic diversity of East Asian Neolitsea sericea (Lauraceae) based on variations in chloroplast DNA sequences. J. Plant Res., 126: 193-202. DOI:10.1007/s10265-012-0519-1 |
Li, F., Huang, S., Mei, Y., et al., 2022. Genome assembly provided new insights into the Cinnamomum burmannii evolution and D-borneol biosynthesis differences between chemotypes. Ind. Crops Prod., 186: 115181. DOI:10.1016/j.indcrop.2022.115181 |
Li, H.W., 1985. Parallel evolution in Litsea and Lindera of Lauraceae. Acta Bot. Yunnan., 7: 129-135. |
Li, H.W., Li, J., Huang, P.H., et al., 2008b. Lauraceae. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China, vol. 7. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, pp. 102-254.
|
Li, H.W., Liu, B., Davis, C.C., et al., 2020. Plastome phylogenomics, systematics, and divergence time estimation of the Beilschmiedia group (Lauraceae). Mol. Phylogenet. Evol., 151: 106901. DOI:10.1016/j.ympev.2020.106901 |
Li, H.W., Pai, P.Y., Lee, S.K., et al., 1982. Lauraceae. In: Li, H.W. (Ed.), Flora Reipublicae Popularis Sinicae, vol. 31. Science Press, Beijing, pp. 1-463.
|
Li, H.T., Yi, T.S., Gao, L.M., et al., 2021. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants, 5: 461-470. DOI:10.5194/angeo-39-461-2021 |
Li, J., 2001. Systematic Relationships in Litsea Complex (Lauraceae). Unpublished Ph. D. thesis. University of Adelaide, Adelaide, Australia.
|
Li, J., Christophel, D.C., 2000. Systematic relationships within the Litsea complex (Lauraceae): A cladistic analysis based on morphological and leaf cuticle data. Aust. Syst. Bot., 13: 1-13. DOI:10.1071/SB98015 |
Li, J., Christophel, D.C., Conran, J.G., et al., 2004. Phylogenetic relationships within the Litsea complex (Lauraceae) inferred from sequences of the chloroplast gene matK and nuclear ribosomal DNA ITS regions. Plant Syst. Evol., 246: 19-34. DOI:10.1007/s00606-003-0113-z |
Li, J., Conran, J.G., Christophel, D.C., et al., 2008c. Phylogenetic relationships of the Litsea complex and core Laureae (Lauraceae) using ITS and ETS sequences and morphology. Ann. Mo. Bot. Gard., 95: 580-599. DOI:10.3417/2006125.9504 |
Li, J., Xia, N.H., Li, X.W., 2008a. Sinopora, a new genus of Lauraceae from South China. Novon, 18: 199-201. DOI:10.3417/2006126 |
Li, L., Li, J., Conran, J.G., et al., 2007. Phylogeny of Neolitsea (Lauraceae) inferred from Bayesian analysis of nrDNA ITS and ETS sequences. Plant Syst. Evol., 269: 203-221. DOI:10.1007/s00606-007-0580-8 |
Li, L., Li, J., Rohwer, J.G., et al., 2011. Molecular phylogenetic analysis of the Persea group (Lauraceae) and its biogeographic implications on the evolution of tropical and subtropical Amphi-Pacific disjunctions. Am. J. Bot., 98: 1520-1536. DOI:10.3732/ajb.1100006 |
Li, L., Madriñán, S., Li, J., 2016. Phylogeny and biogeography of Caryodaphnopsis (Lauraceae) inferred from low-copy nuclear gene and its sequences. Taxon, 65: 433-443. DOI:10.12705/653.1 |
Li, Q.S., Yang, L.Y., Yu, Q.F., et al., 2023. Nuclear DNA-based phylogenetic analysis of Neocinnamomum species. Plant Genet. Resour., 21: 323-330. DOI:10.1017/s1479262123000771 |
Li, X.W., Li, J., 1991. Notes on the taxonomy and distribution of the genus Caryodaphnopsis of Lauraceae and to discuss the characteristics of its area-type. Acta Bot. Yunnan., 13: 1-17. |
Li, Z.M., Li, J., Li, H.W., 2006. Polyphyly of the genus Actinodaphne (Lauraceae) inferred from the analyses of nrDNA ITS and ETS sequences. Acta Phytotaxon. Sin., 44: 272-285. DOI:10.1360/aps040150 |
Liao, J.C., 1996. Lauraceae in Flora of Taiwan, second ed., vol. 2, pp. 433-451.
|
Little, S.A., Stockey, R.A., Penner, B., 2009. Anatomy and development of fruits of Lauraceae from the middle Eocene Princeton Chert. Am. J. Bot., 96: 637-651. DOI:10.3732/ajb.0800318 |
Liu, C., Chen, H.H., Cai, J., et al., 2022b. Characteristics of the complete plastid genome sequences of the monotypic genus Dodecadenia (Family: Lauraceae) and its phylogenomic implications. Forests, 13: 1240. DOI:10.3390/f13081240 |
Liu, C., Chen, H.H., Tang, L.Z., et al., 2022a. Plastid genome evolution of a monophyletic group in the subtribe Lauriineae (Laureae, Lauraceae). Plant Divers, 44: 377-388. DOI:10.1016/j.pld.2021.11.009 |
Liu, H., 1932. Contribution à l'Étude syst ematique et phytogéographique des Lauracées de Chine et d'Indochine. Jouve, Paris.
|
Liu, Y.C., Ou, C.H., 1969. Revision of the Taiwan Species of Cinnamomum. Q. J. Chin. For., 2(1–8): Ⅰ-Ⅲ. |
Liu, Z.F., Ci, X.Q., Li, L., et al., 2017. DNA barcoding evaluation and implications for phylogenetic relationships in Lauraceae from China. PLoS One, 12: e0175788. DOI:10.1371/journal.pone.0175788 |
Liu, Z.F., Ma, H., Zhang, X.Y., et al., 2022c. Do taxon-specific DNA barcodes improve species discrimination relative to universal barcodes in Lauraceae?. Bot. J. Linn. Soc., 199: 741-753. DOI:10.1093/botlinnean/boab089 |
Liu, Z.F., Ma, H., Ci, X.Q., et al., 2021. Can plastid genome sequencing be used for species identification in Lauraceae?. Bot. J. Linn. Soc., 197: 1-14. DOI:10.1093/botlinnean/boab018 |
Liu, Z.F., Zhang, S.F., Twyford, A.D., et al., 2024. Dense infraspecific sampling reveals cryptic differentiation in the enigmatic hemiparasitic love vine Cassytha filiformis (Lauraceae). J. Syst. Evol., 62: 1238-1254. DOI:10.1111/jse.13069 |
Long, D.G., 1984. Lauraceae. In: Grierson, A.J.C., Long, D.G. (Eds.), Flora of Bhutan, vol. 1, pp. 250-281. Edinburgh.
|
Lorea-Hernández, F.G., 1995. Mocinnodaphne: un genero nuevo de la familia Lauraceae en la flora de Mexico. Acta Bot. Mex., 32: 25-32. DOI:10.21829/abm32.1995.743 |
Lorea-Hernández, F.G., 1996. A Systematic Revision of the Neotropical Species of Cinnamomum Schaeffer (Lauraceae). Unpublished Ph. D. thesis. University of Missouri, St. Louis, U.S.A.
|
Lorea-Hernandez, F.G., 2002. La Familia Lauraceae en el Sur de Mexico: Diversidad, Distribucion y Estado de Conservacion. Bol. Soc. Bot. Mex., 71: 59-70. DOI:10.17129/botsci.1663 |
Mabberley, D.J., Nieto, Feliner, G.
, 2017. Mabberley's Plant-Book. 4. Cambridge, United Kingdom: Cambridge University Press: p. 1102.
|
Maccracken, S.A., Miller, I.M., Johnson, K.R., et al., 2022. Insect herbivory on Catula gettyi gen. et sp. nov. (Lauraceae) from the Kaiparowits Formation (Late Cretaceous, Utah, USA). PLoS One, 17: e0261397. DOI:10.1371/journal.pone.0261397 |
Macphail, M.K., 1980. Fossil and modern Beilschmiedia (Lauraceae) pollen in New Zealand. N. Z. J. Bot., 18: 453-457. DOI:10.1080/0028825X.1980.10425165 |
Madriñán, S., 2004. Rhodostemonodaphne (Lauraceae). Flora Neotrop. Monogr. 92, 1-102. New York Botanical Garden Press.
|
Manchester, S.R., 2014. Revisions to Roland Brown's North American Paleocene flora. Acta Mus. Natl. Pragae Ser. B Hist. Nat., 70: 153-210. |
Mase, K., Tagane, S., Chhang, P., et al., 2020. A taxonomic study of Machilus (Lauraceae) in Cambodia based on DNA barcodes and morphological observations. Acta Phytotaxon. Geobot., 71: 79-101. |
Meng, H.H., Jacques, F.M.B., Su, T., et al., 2014. New biogeographic insight into Bauhinia s.l. (Leguminosae): integration from fossil records and molecular analyses. BMC Evol. Biol., 14: 181. DOI:10.1186/s12862-014-0181-4 |
Meng, H.H., Su, T., Huang, Y.J., et al., 2015. Late Miocene Palaeocarya (Engelhardieae: Juglandaceae) from Southwest China and its biogeographic implications. J. Syst. Evol., 53: 499-511. DOI:10.1111/jse.12145 |
Merrill, E.D., 1917. New Philippine Lauraceae. Philipp. J. Sci., 12: 125-141. |
Miller, K.G., Fairbanks, R.G., Mountain, G.S., 1987. Tertiary oxygen isotope synthesis, sea level history, and continental margin erosion. Paleoceanography, 2: 1-19. DOI:10.1029/PA002i001p00001 |
Miquel, F.A.W., 1858. Flora Indiae Batavae, vol. 1. C.G. van der Post, Amsterdam, pp. 865-1116 (1), 6.
|
Miquel, F.A.W., 1861. Flora Indiae Batavae. Eerste Bijvoegsel 3. C.G. van der Post, Amsterdam, pp. 337-656.
|
Miquel, F.A.W., 1864. Cinnamomi Generis Revisio. Ann. Mus. Bot. Lugduno-Batavi 1. Van der Post, Amsterdam: 257-270. |
Mo, Y.Q., Li, L., Li, J.W., et al., 2017. Alseodaphnopsis: A new genus of Lauraceae based on molecular and morphological evidence. PLoS One, 12: e0186545. DOI:10.1371/journal.pone.0186545 |
Moraes, P.L.R. de, 2007. Taxonomy of Cryptocarya species of Brazil. Abc Taxa, 3: 1-191. DOI:10.11606/issn.2596-2477.i15p1-5 |
Moraes, P.L.R. de, 2018. Flora das cangas da Serra dos Carajás, Pará, Brasil: Lauraceae. Rodriguesia, 69: 81-117. DOI:10.1590/2175-7860201869109 |
Moraes, P.L.R. de, Oliveira, J.M.B., 2007. Flora dos estados de Goiás e Tocantins (Coleçã Rizzo): Lauraceae Juss, vol. 33. PRPPG/UFG, Goiania, p. 154.
|
Moraes, P.L.R. de, Vergne, M.C., 2019a. A synopsis of Lauraceae (excluding Ocotea) from the Reserva Natural Vale, Linhares, Espírito Santo, Brazil. Feddes Repert., 129: 247-303. DOI:10.1590/0100-3984.2018.0084 |
Moraes, P.L.R. de, Vergne, M.C., 2019b. A synopsis of Ocotea (Lauraceae) from the Reserva Natural Vale, Linhares, Espírito Santo, Brazil. Feddes Repert., 130: 117-217. DOI:10.22481/el.v17i3.5943 |
Moreau, J.-D., Gomez, B., Daviero-Gomez, V., et al., 2016. Inflorescences of Mauldinia sp. (Lauraceae) and associated fruits from the Cenomanian of Languedoc Roussillon, France. Cretac. Res., 59: 18-29. DOI:10.1016/j.cretres.2015.10.018 |
Morley, R.J., 2003. Interplate dispersal paths for megathermal angiosperms. Perspect. Plant Ecol. Evol. Syst., 6: 5-20. DOI:10.1078/1433-8319-00039 |
Munzinger, J., Gemmill, C.E.C., 2025. Molecular insights into species delimitations of Cryptocarya, Endiandra, and Beilschmiedia (Lauraceae) in New Caledonia and neighbouring islands. Phytotaxa, 681: 233-264. DOI:10.11646/phytotaxa.681.3.1 |
Munzinger, J., McPherson, G., Bruy, D., 2024. Novitates neocaledonicae XV: two new species of Endiandra R. Br. (Lauraceae) from New Caledonia. Adansonia, 46: 19-30. |
Munzinger, J., McPherson, G., Meyer, S., et al., 2022. Phylogenetic study of the New Caledonian endemic genus Adenodaphne (Lauraceae) confirms its synonymy with Litsea. Bot. Lett., 170: 479-487. |
Nath, O., Fletcher, S.J., Hayward, A., et al., 2022. A haplotype resolved chromosomal level avocado genome allows analysis of novel avocado genes. Hortic. Res., 9: uhac157. DOI:10.1093/hr/uhac157 |
Nees von Esenbeck, C.G.D., Nees von Esenbeck, T.F.L., 1823. De Cinnamomo Disputatio (Bonn).
|
Nees von Esenbeck, C.G.D., 1831. Laurinae Indiae Orientalis. In: Wallich, N. (Ed.),
Plantae Asiaticae Rariores 2. Treuttel & Würtz, London, Paris, Strasburg, pp. 58-76.
|
Nees von Esenbeck, C.G.D., 1836. Systema Laurinarum. Sumtibus Veitii et Sociorum, Berlin ⅷ + 720.
|
Ng, F.S.P., 2005a. Taxonomic notes on Bornean Cryptocarya R. Br. (Lauraceae). Gard. Bull. Singapore, 57: 63-68. |
Ng, F.S.P., 2005b. Taxonomic notes on Bornean Litsea, Lindera, Neolitsea and Iteadaphne (Lauraceae). Gard. Bull. Singapore, 57: 217-246. |
Ngernsaengsaruay, C., Middleton, D.J., Chayamarit, K., 2011. A revision of the genus Litsea Lam. (Lauraceae) in Thailand. Thai Forest. Bull. (Arch. Am. Art), 39: 40-119. |
Nguyễn K. Ð., 2017. Lauraceae. Lauraceae. Flora of Vietnam, vol. 20. Publishing House for Science and Technology, Hanoi, pp. 1-700.
|
Nie, Z.L., Wen, J., Sun, H., 2007. Phylogeny and biogeography of Sassafras (Lauraceae) disjunct between eastern Asia and eastern North America. Plant Syst. Evol., 267: 171-203. |
Nishida, S., 1999. Revision of Beilschmiedia (Lauraceae) in the Neotropics. Ann. Mo. Bot. Gard., 86: 657-701. DOI:10.2307/2666150 |
Nishida, S., 2008. Taxonomic revision of Beilschmiedia (Lauraceae) in Borneo. Blumea, 53: 345-383. DOI:10.3767/000651908X608007e.2012.08.024 |
Nishida, S., de Kok, R., Yang, Y., 2016. Cuticular features of Cryptocarya (Lauraceae) from Peninsular Malaysia, Thailand and Indo-China and its taxonomic implications. Phytotaxa, 244: 26-44. DOI:10.11646/phytotaxa.244.1.2 |
Nishida, S., van der Werff, H., 2007. Are cuticular characters useful in solving generic relationships of problematic species of Lauraceae?. Taxon, 56: 1229-1237. DOI:10.2307/25065914 |
Nishida, S., van der Werff, H., 2011. An evaluation of classification by cuticular characters of the Lauraceae: a comparison to molecular phylogeny. Ann. Mo. Bot. Gard., 98: 348-357. DOI:10.3417/2010054 |
Nishida, S., van der Werff, H., 2014. Do cuticle characters support the recognition of Alseodaphe, Nothaphoebe and Dehaasia as distinct genera?. Reinwardtia, 14: 53-65. DOI:10.14203/reinwardtia.v14i1.395 |
Nurtjahjaningsih, I.L.G., Sukartiningsih, Kurokochi, H., et al., 2017. Genetic structure of the tropical tree Eusideroxylon zwageri in Indonesia revealed by chloroplast DNA phylogeography. Forests, 8: 229. DOI:10.3390/f8070229 |
Penagos Zuluaga, J.C., Queenborough, S.A., Comita, L.S., 2020. Flowering sex ratios and costs of reproduction in gynodioecious Ocotea oblonga (Lauraceae). Biol. J. Linn. Soc., 131: 344-355. DOI:10.1093/biolinnean/blaa117 |
Penagos Zuluaga, J.C., van der Werff, H., Park, B., et al., 2021. Resolved phylogenetic relationships in the Ocotea complex (Supraocotea) facilitate phylogenetic classification and studies of character evolution. Am. J. Bot., 108: 664-679. DOI:10.1002/ajb2.1632 |
Pendry, C.A., 2011. Lauraceae. In: Watson, M.E., Ikeda, H., Rajbhandari, K.R., et al. (Eds.), Flora of Nepal, vol. 3, pp. 21-48. Edinburgh.
|
Pole, M., 1993. Early Miocene flora of the Manuherikia Group, New Zealand. 6. Lauraceae. J. Roy. Soc. N. Z., 23: 303-312. DOI:10.1080/03036758.1993.10721228 |
Pole, M., 2007. Lauraceae macrofossils and dispersed cuticle from the Miocene of southern New Zealand. Palaeontol. Electron., 10(3A): 1-38. |
Pole, M., 2012. Plant macrofossils. In: Gordon, D.P. (Ed.), New Zealand Inventory of Biodiversity, Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi, Vol. 3. Canterbury University Press, Christchurch, NZ, pp. 460-475.
|
Pole, M., Douglas, B., 1999. Plant macrofossils of the Upper Cretaceous Kaitangata coalfield, New Zealand. Aust. Syst. Bot., 12: 331-364. DOI:10.1071/SB98003 |
Pole, M., Vajda, V., 2009. A new terrestrial Cretaceous-Paleogene site in New Zealand – turnover in macroflora confirmed by palynology. Cretac. Res., 30: 917-938. DOI:10.1016/j.cretres.2009.02.007 |
Poole, I., Richter, H.G., Francis, J.E., 2000. Evidence for Gondwana origins for Sassafras (Lauraceae)? Later Cretaceous fossil wood from Antarctica. IAWA J., 21: 463-475. DOI:10.1163/22941932-90000262 |
Pujana, R.R., Jud, N.A., Wilf, P., et al., 2024. Lauraceous fossil woods from the early Eocene of Laguna del Hunco, Argentine Patagonia. Alcheringa early online. DOI:10.1080/03115518.2024.2426030 |
Qin, S.Y., Zuo, Z.Y., Guo, C., et al., 2023. Phylogenomic insights into the origin and evolutionary history of evergreen broadleaved forests in East Asia under Cenozoic climate change. Mol. Ecol., 32: 2850-2868. DOI:10.1111/mec.16904 |
Qiu, Y.X., Fu, C.X., Comes, H.P., 2011. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world's most diverse temperate flora. Mol. Phylogenet. Evol., 59: 225-244. DOI:10.1016/j.ympev.2011.01.012 |
Quinet, A., Andreata, R.H.P., 2002. Reserva Ecológica de Macaé de Cima, Município de Nova Friburgo, Rio de Janeiro, Brasil. Rodriguesia, 53: 59-121. DOI:10.1590/2175-78602002538204 |
Raine, J.I., Mildenhall, D.C., Kennedy, E.M., 2011. New Zealand Fossil Spores and Pollen: an Illustrated Catalogue, fourth ed. (GNS Science Miscellaneous Series No. 4). Retrieved 10 Aug 2024 from. http://pal.gns.cri.nz/catalog.
|
Raj, B., van der Werff, H., 1988. A contribution to the pollen morphology of neotropical Lauraceae. Ann. Mo. Bot. Gard., 75: 130-167. DOI:10.2307/2399470 |
Reid, E.M., Chandler, M.E.J., 1933. The London Clay Flora. British Museum (Natural History), London, UK.
|
Rendón-Anaya, M., Ibarra-Laclette, E., Méndez-Bravo, A., et al., 2019. The avocado genome informs deep angiosperm phylogeny, highlights introgressive hybridization, and reveals pathogen-influenced gene space adaptation. Proc. Natl. Acad. Sci. U.S.A., 116: 17081-17089. DOI:10.1073/pnas.1822129116 |
Richter, H.G., 1981a. Anatomie des sekundären Xylems und der Rinde der Lauraceae. Sonderb. Naturw. Vereins Hamburg, 5: 1-148. |
Richter, H.G., 1981b. Wood and bark anatomy of Lauraceae Ⅰ. Aniba Aublet. IAWA Bull. n.s., 2: 79-87. DOI:10.1163/22941932-90000819 |
Richter, H.G., 1985. Wood and bark anatomy of Lauraceae Ⅱ. Licarial Aublet. IAWA Bull. n.s., 6: 187-199. DOI:10.1177/0002716285482001019 |
Richter, H.G., 1990. Wood and bark anatomy of Lauraceae Ⅲ. Aspidostemon Rohwer & Richter. IAWA Bull. n.s., 11: 47-56. DOI:10.1163/22941932-90001143 |
Richter, H.G., van Wyk, A.E., 1990. Wood and bark anatomy of Lauraceae Ⅳ. Dahlgrenodendron J.J.M. van der Merwe & van Wyk. IAWA Bull., 11: 173-182. DOI:10.1163/22941932-90000513 |
Rieseberg, L.H., Soltis, D.E., 1991. Phylogenetic consequences of cytoplasmic gene flow in plants. Evol. Trends Plants, 5: 65-84. |
Rivas-González, I., Rousselle, M., Li, F., et al., 2023. Pervasive incomplete lineage sorting illuminates speciation and selection in primates. Science, 380: eabn4409. DOI:10.1126/science.abn4409 |
Renner, S.S., Chanderbali, A.S., 2000. What is the relationship among Hernandiaceae, Lauraceae, and Monimiaceae, and why is this question so difficult to answer?. Int. J. Plant Sci., 161: S109-S119. DOI:10.1086/317574 |
Robi, A.J., 2014. A Taxonomic Revision of the Family Lauraceae. Department of Botany, Kannur University, Kannur, India.
|
Rodríguez-Sánchez, F., Guzmán, B., Valido, A., et al., 2009. Late Neogene history of the laurel tree (Laurus L., Lauraceae) based on phylogeographical analyses of Mediterranean and Macaronesian populations. J. Biogeogr., 36: 1270-1281. DOI:10.1111/j.1365-2699.2009.02091.x |
Roestel, J.A., Wiersema, J.H., Jansen, R.K., et al., 2024. On the importance of sequence alignment inspections in plastid phylogenomics – an example from revisiting the relationships of the water-lilies. Cladistics, 40: 469-495. DOI:10.1111/cla.12584 |
Rohde, R., Rudolph, B., Ruthe, K., et al., 2017. Neither Phoebe nor Cinnamomum – the tetrasporangiate species of Aiouea (Lauraceae). Taxon, 66: 1085-1111. DOI:10.12705/665.6 |
Rohwer, J.G., 1986. Prodromus einer Monographie der Gattung Ocotea Aubl. (Lauraceae), sensu lato. Mitt. Inst. Allg. Bot. Hamburg, 20: 3-278. |
Rohwer, J.G., 1988. The genera Dicypellium, Phyllostemonodaphne, Systemonodaphne and Urbanodendron (Lauraceae). Bot. Jahrb. Syst., 110: 157-171. |
Rohwer, J.G., 1993a. Lauraceae. In: Kubizki, K., Rohwer, J.G., Bittrich, V. (Eds.), The Families and Genera of Vascular Plants, vol. 2. Springer-Verlag, Berlin, Germany, pp. 366-391.
|
Rohwer, J.G., 1993b. Lauraceae: Nectandra. Flora Neotropica, Monograph No. 60. The New York Botanical Garden, New York, U.S.A.
|
Rohwer, J.G., 2000. Toward a phylogenetic classification of the Lauraceae: Evidence from matK sequences. Syst. Bot., 25: 60-70. DOI:10.2307/2666673 |
Rohwer, J.G., 2014. Lauraceae (Ocotea con Sabrina A. Schmidt). In: Greuter, W., Rankin Rodríguez, R. (Eds.), Flora de la República de Cuba, vol. 19. Koeltz Scientific Books, Königstein, Germany, 2.
|
Rohwer, J.G., 2018. A contribution to the pollen morphology of the Cryptocarya group (Lauraceae). Grana, 57: 178-213. DOI:10.1080/00173134.2017.1365374 |
Rohwer, J.G., Li, J., Rudolph, B., et al., 2009. Is Persea (Lauraceae) monophyletic? Evidence from nuclear ribosomal ITS sequences. Taxon, 58: 1153-1167. DOI:10.1002/tax.584009 |
Rohwer, J.G., Moraes, P.L.R. de, Rudolph, B., et al., 2014. A phylogenetic analysis of the Cryptocarya group (Lauraceae), and relationships of Dahlgrenodendron, Sinopora, Triadodaphne, and Yasunia. Phytotaxa, 158: 111-132. DOI:10.11646/phytotaxa.158.2.1 |
Rohwer, J.G., Richter, H.G., van der Werff, H., 1991. Two new genera of neotropical Lauraceae and critical remarks on the generic delimitation. Ann. Mo. Bot. Gard., 78: 388-400. DOI:10.2307/2399568 |
Rohwer, J.G., Rudolph, B., 2005. Jumping genera: The phylogenetic positions of Cassytha, Hypodaphnis, and Neocinnamomum (Lauraceae) based on different analyses of trnK intron sequences. Ann. Mo. Bot. Gard., 92: 153-178. |
Ruge, V., 2000. Morphologische Studien an Blüten und Infloreszenzen einiger Lauraceae. Unpublished Diploma thesis. Johannes Gutenberg-Universität Mainz.
|
Schatz, G., 1996. Malagasy/Indo-Australo-Malesian phytogeographic connections. In: Lourenco, W.R. (Ed.), Biogeography of Madagascar. Orstom, Paris, pp. 73-83.
|
Schmitt, S., Heuret, P., Troispoux, V., et al., 2024. Low-frequency somatic mutations are heritable in tropical trees Dicorynia guianensis and Sextonia rubra. Proc. Natl. Acad. Sci. U.S.A., 121: e2313312121. DOI:10.1073/pnas.2313312121 |
Scotese, C.R., 2014a. Atlas of Neogene Paleogeographic Maps (Mollweide Projection), Maps 1-7, Volume 1, the Cenozoic, PALEOMAP Atlas for ArcGIS. PALEOMAP Project, Evanston, IL.
|
Scotese, C.R., 2014b. Atlas of Paleogene Paleogeographic Maps (Mollweide Projection), Maps 8-15, Volume 1. The Cenozoic, PALEOMAP Atlas for ArcGIS. PALEOMAP Project, Evanston, IL.
|
Shen, T., Qi, H., Luan, X., et al., 2022. The chromosome-level genome sequence of the camphor tree provides insights into Lauraceae evolution and terpene biosynthesis. Plant Biotechnol. J., 20: 244-246. DOI:10.1111/pbi.13749 |
Shi, G.L., Xie, Z.M., Li, H.M., 2014. High diversity of Lauraceae from the Oligocene of Ningming, South China. Palaeoworld, 23: 336-356. DOI:10.1016/j.palwor.2014.08.001 |
Smith, A.C., 1981. Lauraceae and Cassythaceae in Flora Vitiensis Nova 2. Pacific Tropical Botanical Gardens, Hawaii, pp. 113-142.
|
Snow, D.W., 1981. Tropical frugivorous birds and their food plants: a world survey. ATBC Journal, 13: 1-14. DOI:10.2307/2387865 |
Soh, W.K., 2011. Taxonomic revision of Cinnamomum (Lauraceae) in Borneo. Blumea, 56: 241-264. DOI:10.3767/000651911X615168 |
Soltis, D.E., Kuzoff, R.K., 1995. Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). Evolution, 49: 727-742. DOI:10.2307/2410326 |
Soltis, P.S., Soltis, D.E., 2020. Plant genomes: Markers of evolutionary history and drivers of evolutionary change. Plants People Planet, 3: 74-82. |
Song, Y., Dong, W.P., Liu, B., et al., 2015. Comparative analysis of complete chloroplast genome sequences of two tropical trees Machilus yunnanensis and Machilus balansae in the family Lauraceae. Front. Plant Sci., 6: 662. DOI:10.1007/s11596-015-1486-2 |
Song, Y., Xia, S.W., Tan, Y.H., et al., 2023. Phylogeny and biogeography of the Cryptocaryeae (Lauraceae). Taxon, 72: 1244-1261. DOI:10.1002/tax.13084 |
Song, Y., Yao, X., Liu, B., et al., 2018. Complete plastid genome sequences of three tropical Alseodaphne trees in the family Lauraceae. Holzforschung, 72: 337-345. DOI:10.1515/hf-2017-0065 |
Song, Y., Yao, X., Tan, Y.H., et al., 2016. Complete chloroplast genome sequence of the avocado: gene organization, comparative analysis, and phylogenetic relationships with other Lauraceae. Can. J. For. Res., 46: 1293-1301. DOI:10.1139/cjfr-2016-0199 |
Song, Y., Yao, X., Tan, Y.H., et al., 2017b. Comparative analysis of complete chloroplast genome sequences of two subtropical trees, Phoebe sheareri and Phoebe omeiensis (Lauraceae). Tree Genet. Genome, 13: 120. DOI:10.1007/s11295-017-1196-y |
Song, Y., Yu, Q.F., Zhang, D., et al., 2024. New insights into the phylogenetic relationships within the Lauraceae from mitogenomes. BMC Biology, 22: 241. DOI:10.1186/s12915-024-02040-7 |
Song, Y., Yu, W.B., Tan, Y.H., et al., 2017a. Evolutionary comparisons of the chloroplast genome in Lauraceae and insights into loss events in the Magnoliids. Genome Biol. Evol., 9: 2354-2364. DOI:10.1093/gbe/evx180 |
Song, Y., Yu, W.B., Tan, Y.H., et al., 2020. Plastid phylogenomics improve phylogenetic resolution in the Lauraceae. J. Syst. Evol., 58: 423-439. DOI:10.1111/jse.12536 |
Stapf, O., 1909. Laurineae. In: Thiselton-Dyer, W.T. (Ed.), Flora of Tropical Africa, vol. 6. Lovell Reeve & Co., London, United Kingdom, pp. 171-188, 1.
|
Sun, W.H., Xiang, S., Zhang, Q.G., et al., 2022. The camphor tree genome enhances the understanding of magnoliid evolution. J. Genet. Genomics, 49: 249-253. DOI:10.1016/j.jgg.2021.11.001 |
Takahashi, M., Herendeen, P.S., Xiao, X., et al., 2014. Lauraceous fossil flowers from the Kamikitaba Assemblage (Coniacian, Late Cretaceous) of northeastern Japan (Lauraceae). Syst. Bot., 39: 715-724. DOI:10.1600/036364414X681464 |
Tanaros, M., Vajrodaya, S., Chayamarit, K., 2010. Taxonomic study of the genus Actinodaphne Nees (Lauraceae) in Thailand. Thai J. Bot., 2: 7-23. |
Tao, L., Guo, S., Xiong, Z., et al., 2024. Chromosome-level genome assembly of the threatened resource plant Cinnamomum chago. Sci. Data, 11: 1-9. DOI:10.1038/s41597-023-02657-3 |
Taylor, D.W., 1988. Eocene floral evidence of Lauraceae: corroboration of the North American megafossil record. Am. J. Bot., 75: 948-957. DOI:10.1002/j.1537-2197.1988.tb08799.x |
Taylor, T.N., Taylor, E.L., Krings, M., 2009. Paleobotany. In: The Biology and Evolution of Fossil Plants, second ed. Academic Press, Burlington, MA.
|
Teschner, H., 1923. Die Lauraceae Nordost-Neu-Guineas. Bot. Jahrb. Syst., 58: 380-440. |
Tetsana, N., 2005. Taxonomic Revision of Genus Beilschmiedia Nees (Lauraceae) in Thailand. Unpublished Master of Science thesis. Kasetsart University, Bangkok, Thailand.
|
Tian, X., Ye, J., Song, Y., 2019. Plastome sequences help to improve the systematic position of trinerved Lindera species in the family Lauraceae. PeerJ, 7: e7662. DOI:10.7717/peerj.7662 |
Tian, X.Y., Guo, J., Song, Y., et al., 2024. Intraspecific differentiation of Lindera obtusiloba as revealed by comparative plastomic and evolutionary analyses. Ecol. Evol., 14: e11119. DOI:10.1002/ece3.11119 |
Tosolini, A.-M.P., Cantrill, D.J., Francis, J.E., 2021. Paleocene high-latitude leaf flora of Antarctica Part 1: entire-margined angiosperms. Rev. Palaeobot. Palynol., 285: 104317. DOI:10.1016/j.revpalbo.2020.104317 |
Trofimov, D., Cadar, D., Schmidt-Chanasit, J., et al., 2022. A comparative analysis of complete chloroplast genomes of seven Ocotea species (Lauraceae) confirms low sequence divergence within the Ocotea complex. Sci. Rep., 12: 1120. DOI:10.1038/s41598-021-04635-4 |
Trofimov, D., Moraes, P.L.R. de, Rohwer, J.G., 2019. Towards a phylogenetic classification of the Ocotea complex (Lauraceae): Classification principles and reinstatement of Mespilodaphne. Bot. J. Linn. Soc., 190: 25-50. DOI:10.1093/botlinnean/boz010 |
Trofimov, D., Rohwer, J.G., 2018. Epidermal features allowing identification of evolutionary lineages in the Ocotea complex (Lauraceae). Perspect. Plant Ecol. Evol. Syst., 31: 17-35. DOI:10.1016/j.ppees.2017.12.003 |
Trofimov, D., Rohwer, J.G., 2020. Towards a phylogenetic classification of the Ocotea complex (Lauraceae): an analysis with emphasis on the Old World taxa and description of the new genus Kuloa. Bot. J. Linn. Soc., 192: 510-535. DOI:10.1093/botlinnean/boz088 |
Trofimov, D., Rudolph, B., Rohwer, J.G., 2016. Phylogenetic study of the genus Nectandra (Lauraceae), and reinstatement of Damburneya. Taxon, 65: 980-996. DOI:10.12705/655.3 |
Tsui, H.P., 1987. A study on the system of Lindera. Acta Phytotaxon. Sin., 25: 161-171. |
Upchurch Jr., G.R., Dilcher, D.L., 1990. Cenomanian angiosperm leaf megafossils, Dakota Formation, Rose Creek locality, Jefferson County, southeastern Nebraska. US Geol. Surv. Bull., 1915: 1-55. |
Vadala, A.J., Greenwood, D.R., 2001. Australian Paleogene vegetation and environments: evidence for palaeo-Gondwanic elements in the fossil records of Lauraceae and Proteaceae. In: Metcalfe, I., Smith, J.M.B., Davidson, I. (Eds.), Faunal and Floral Migrations and Evolution in SE Asia-Australasia. Swets & Zeitlinger Publishers, Lisse, Netherlands, pp. 196-201.
|
van der Merwe, A.M., Crayn, D.M., Ford, A.J., et al., 2016. Evolution of Australian Cryptocarya (Lauraceae) based on nuclear and plastid phylogenetic trees: evidence of recent landscape-level disjunctions. Aust. Syst. Bot., 29: 157-166. DOI:10.1071/SB16023 |
van der Merwe, J.M., van, Wyk, A.E., Kok, P.D.F., 1988. Dahlgrenodendron, a remarkable new genus of Lauraceae from Natal and Pondoland. S. Afr. J. Bot., 54: 80-88. DOI:10.1016/S0254-6299(16)31366-7 |
van der Merwe, J.M., van, Wyk, A.E., Kok, P.D.F., 1990. Pollen types in the Lauraceae. Grana, 29: 185-196. DOI:10.1080/00173139009427751 |
van der Werff, H., 1987. A revision of Mezilaurus (Lauraceae). Ann. Mo. Bot. Gard., 74: 153-182. DOI:10.2307/2399274 |
van der Werff, H., 1988. Eight new species and one new combination of neotropical Lauraceae. Ann. Mo. Bot. Gard., 75: 402-419. DOI:10.2307/2399431 |
van der Werff, H., Endress, P.K., 1991. Gamanthera (Lauraceae), a new genus from Costa Rica. Ann. Mo. Bot. Gard., 78: 401-408. DOI:10.2307/2399569 |
van der Werff, H., 1993. A revision of the genus Pleurothyrium (Lauraceae). Ann. Mo. Bot. Gard., 80: 39-118. DOI:10.2307/2399821 |
van der Werff, H., 1995. Lauraceae. In: Stannard, B.L. (Ed.), Flora of the Pico das Almas. Royal Botanic Gardens, Kew, pp. 363-368.
|
van der Werff, H., 1996. Ocotea ikonyokpe, a new species of Lauraceae from Cameroon. Novon, 6: 460-462. DOI:10.2307/3392056 |
van der Werff, H., 1997. Sextonia, a new genus of Lauraceae from South America. Novon, 7: 436-439. DOI:10.2307/3391778 |
van der Werff, H., 2001a. An annotated key to the genera of Lauraceae in the flora Malesiana region. Blumea, 46: 125-140. |
van der Werff, H., 2001b. Lauraceae. In: Stevens, W.D., Ulloa, C.U., Pool, A., et al. (Eds.), Flora de Nicaragua. Angiospermas (Fabaceae-Oxalidaceae). Monographs in Systematic Botany from the Missouri Botanical Garden, vol. 85. Missouri Botanical Garden Press, St. Louis, pp. 1190-1206, 2.
|
van der Werff, H., 2002. Lauraceae. In: Mori, S.A., Cremers, G., Gracie, C.A., et al. (Eds.), Guide to the Vascular Plants of Central French Guiana. Part 2. Dicotyledons. Memoirs of the New York Botanical Garden, vol. 76. New York Botanical Garden Press, New York, pp. 370-384, 2.
|
van der Werff, H., 2003. A synopsis of the genus Beilschmiedia (Lauraceae) in Madagascar. Adansonia, Sér., 3(25): 77-92. |
van der Werff, H., 2006. A revision of the Malagasy endemic genus Aspidostemon Rohwer & Richter (Lauraceae). Adansonia, Sér, 3(28): 7-44. |
van der Werff, H., 2010. Lauraceae. In: Vásquez Martínez, R., Gonz ales, R.R., van der Werff, H. (Eds.), Flora del Río Cenepa, Amazonas, Perú, Vol. 2. Angiospermae (Gentianaceaee-Zingiberaceae). Monographs in Systematic Botany from the Missouri Botanical Garden 114. Missouri Botanical Garden Press, St. Louis, pp. 832-859.
|
van der Werff, H., 2013a. Nomenclatural notes on Cryptocarya R. Br. (Lauraceae) from Madagascar. Candollea, 6: 303-306. |
van der Werff, H., 2013b. A revision of the genus Ocotea Aubl. (Lauraceae) in Madagascar and the Comoro Islands. Adansonia, Sér., 3(35): 235-279. |
van der Werff, H., 2017. The genera of Lauraceae in Madagascar with nomenclatural novelties in Cryptocarya. Candollea, 72: 323-328. DOI:10.15553/c2017v722a8 |
van der Werff, H., 2019. Alseodaphnopsis (Lauraceae) revisited. Blumea, 64: 186-189. DOI:10.3767/blumea.2019.64.02.10 |
van der Werff, H., 2022. Andea, a new genus of neotropical Lauraceae. Ann. Mo. Bot. Gard., 107: 422-431. DOI:10.3417/2022748 |
van der Werff, H., 2025. Lauraceae. In: Ulloa, C.U., Hernández, H.M., Barrie, F.R., et al. (Eds.), Flora Mesoamericana: Cycadaceae a Cactaceae, vol. 2. Missouri Botanical Garden Press, St. Louis (in press).
|
van der Werff, H., Nishida, S., 2010. Yasunia (Lauraceae), a new genus with two species from Ecuador and Peru. Novon, 20: 493-502. DOI:10.3417/2010030 |
van der Werff, H., Richter, H.G., 1985. Caryodaphnopsis Airy-Shaw (Lauraceae), a genus new to the neotropics. Syst. Bot., 10: 166-173. DOI:10.2307/2418342 |
van der Werff, H., Richter, H.G., 1996. Toward an improved classification of Lauraceae. Ann. Mo. Bot. Gard., 83: 409-418. DOI:10.2307/2399870 |
van der Werff, H., Rohwer, J.G., 1999. Lauraceae. In: Steyermark, J.A., Berry, P.E., Yatskievych, K., et al. (Eds.), Flora of the Venezuelan Guayana, vol. 5. Missouri Botanical Garden Press, St. Louis, pp. 700-750.
|
van der Werff, H., Lorea-Hernandez, F., 1997. Flora del Bajio y de regiones adyacentes. Familia Lauraceae. Fasciculo, 56: 1-58. |
van der Werff, H., Zanotti, C.A., Ospina, J.C., 2015. Lauraceae. In: Zuloaga, F.O., Belgrano, M.J., Anton, A.M. (Eds.), Flora de la República Argentina, vol. 15. Instituto de Botanica Darwinion, San Isidro, pp. 41-57.
|
Vicentini, A., van der Werff, H., Nicolau, S.A., 1999. Lauraceae. In: Ribeiro, J.E.L.S., Hopkins, M.J.G., Vicentini, A., et al. (Eds.), Flora da Reserva Ducke. Guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazània Central. Manaus: INPA-DFID, Manaus, pp. 150-179.
|
von Balthazar, M., Pedersen, K.R., Crane, P.R., et al., 2007. Potomacanthus lobatus gen. et sp. nov., a new flower of probable Lauraceae from the Early Cretaceous (early to middle Albian) of eastern North America. Am. J. Bot., 94: 2041-2053. DOI:10.3732/ajb.94.12.2041 |
Wagner, W.L., Herbst, D.R., Sohmer, S.H., 1990. Manual for the Flowering Plants of Hawai'i. University of Hawaii Press (Bishop Museum Special Publications 83).
|
Wang, X.D., Xu, C.Y., Zheng, Y.J., et al., 2022. Chromosome-level genome assembly and resequencing of camphor tree (Cinnamomum camphora) provides insight into phylogeny and diversification of terpenoid and triglyceride biosynthesis of Cinnamomum. Hortic. Res., 9: uhac216. DOI:10.1093/hr/uhac216 |
Wang, Z.H., Li, J., Conran, J.G., et al., 2010. Phylogeny of the southeast Asian endemic genus Neocinnamomum H. Liu (Lauraceae). Plant Syst. Evol., 290: 173-184. DOI:10.1007/s00606-010-0359-1 |
Wang, Z., Sun, B., Sun, F., et al., 2017. Identification of two new species of Meliolinites associated with Lauraceae leaves from the middle Miocene of Fujian, China. Mycologia, 109: 676-689. |
Wang, Z., Sun, F., Wang, J., et al., 2019. New fossil leaves and fruits of Lauraceae from the Middle Miocene of Fujian, southeastern China differentiated using a cluster analysis. Hist. Biol., 31: 581-599. DOI:10.1080/08912963.2017.1379517 |
Waters, J.M., Fraser, C.I., Hewitt, G.M., 2013. Founder takes all: density-dependent processes structure biodiversity. Trends Ecol. Evol., 28: 78-85. DOI:10.1016/j.tree.2012.08.024 |
Weakley, A.S., Kees, J.C., Sorrie, B.A., et al., 2023. Studies in the vascular flora of the southeastern United States. IX. J. Bot. Res. Inst. Tex., 17: 191-257. DOI:10.17348/jbrit.v17.i1.1293 |
Weber, J.Z., 1981. A taxonomic revision of Cassytha (Lauraceae) in Australia. J. Adel. Bot. Gard., 3: 187-262. |
Weber, J.Z., 2007. Cassytha. In: Wilson, A.G. (Ed.), Flora of Australia 2, pp. 117-136 (ABRS/CSIRO Publishing: Melbourne).
|
Weber, L.C., Forster, P.I., 2021. Endiandra wongawallanensis L. Weber (Lauraceae), a new species from south-east Queensland allied to. E. floydii B. Hyland. Austrobaileya, 11: 155-169. DOI:10.5962/p.366381 |
Wieringa, J.J., Simons, E.L.A.N., 2024. Cassytha (Lauraceae) in Africa and the description of a new species from Gabon. Blumea, 69: 201-210. |
Wilkinson, D.M., 1997. Plant colonization: are wind dispersed seeds. really dispersed by birds at larger spatial and temporal scales?. J. Biogeogr., 24: 61-65. DOI:10.1111/j.1365-2699.1997.tb00050.x |
Wolfe, J.A., 1975. Some aspects of plant geography of the northern hemisphere during the late Cretaceous and Tertiary. Ann. Mo. Bot. Gard., 62: 264-279. DOI:10.2307/2395198 |
Wolfe, J.A., 1997. Relations of environmental change to angiosperm evolution during the late Cretaceous and Tertiary. In: Iwatsuki, K., Raven, P.H. (Eds.), Evolution and Diversification of Land Plants. Springer-Verlag, Tokyo, Japan, pp. 269-290.
|
Wu, C.S., Wang, T.J., Wu, C.W., et al., 2017. Plastome evolution in the sole hemiparasitic genus laurel dodder (Cassytha) and insights into the plastid phylogenomics of Lauraceae. Genome Biol. Evol., 9: 2604-2614. DOI:10.1093/gbe/evx177 |
Wu, J.Y., Sun, B.N., Xie, S.P., et al., 2008. Two Neogene Machilus (Lauraceae) fossil leaves from Tengchong, Yunnan Province and its paleoenvironmental significance. Geol. J. China Univ., 14: 90-98. |
Xiao, T.W., Ge, X.J., 2022. Plastome structure, phylogenomics, and divergence times of tribe Cinnamomeae (Lauraceae). BMC Genomics, 23: 642. DOI:10.1186/s12864-022-08855-4 |
Xiao, T.W., Xu, Y., Jin, L., et al., 2020. Conflicting phylogenetic signals in plastomes of the tribe Laureae (Lauraceae). PeerJ, 8: e10155. DOI:10.7717/peerj.10155 |
Xiao, T.W., Yan, H.F., Ge, X.J., 2022. Plastid phylogenomics of tribe Perseeae (Lauraceae) yields insights into the evolution of East Asian subtropical evergreen broad-leaved forests. BMC Plant Biol., 22: 32. DOI:10.1186/s12870-021-03413-8 |
Xiong, B., Zhang, L., Xie, L., et al., 2022. Genome of Lindera glauca provides insights into the evolution of biosynthesis genes for aromatic compounds. iScience, 25: 104761. DOI:10.1016/j.isci.2022.104761 |
Xu, L.L., Yu, R.M., Lin, X.R., et al., 2021. Different rates of pollen and seed gene flow cause branch-length and geographic cytonuclear discordance within Asian butternuts. New Phytol., 232: 388-403. DOI:10.1111/nph.17564 |
Xue, J.Y., Dong, S.S., Wang, M.Q., et al., 2022. Mitochondrial genes from 18 angiosperms fill sampling gaps for phylogenomic inferences of the early diversification of flowering plants. J. Syst. Evol., 60: 773-788. DOI:10.1111/jse.12708 |
Yang, S.T., Huang, J.P., Qu, Y.Y., et al., 2024. Phylogenetic incongruence in an Asiatic species complex of the genus Caryodaphnopsis (Lauraceae). BMC Plant Biol., 24: 616. DOI:10.1145/3643832.3661845 |
Yang, Y., Zhang, L., Liu, B., et al., 2012. Leaf cuticular anatomy and taxonomy of Syndiclis (Lauraceae) and its allies. Syst. Bot., 37: 861-878. DOI:10.1600/036364412X656518 |
Yang, Z., Ferguson, D.K., Yang, Y., 2023. New insights into the plastome evolution of Lauraceae using herbariomics. BMC Plant Biol., 23: 387. DOI:10.1007/978-3-031-33380-4_30 |
Yang, Z., Ferguson, D.K., Yang, Y., 2025. Differential plastome diversification of the Lauraceae between tropical regions of Asia and America. Bot. J. Linn. Soc. (Submitted).
|
Yang, Z., Liu, B., Yang, Y., Ferguson, D.K., 2022. Phylogeny and taxonomy of Cinnamomum (Lauraceae). Ecol. Evol., 12: e9378. DOI:10.1002/ece3.9378 |
Ye, J.W., Bai, W.N., Bao, L., et al., 2017. Sharp genetic discontinuity in the arid-sensitive species Lindera obtusiloba (Lauraceae): solid evidence supporting the Tertiary floral subdivision in East Asia. J. Biogeogr., 44: 2082-2095. DOI:10.1111/jbi.13020 |
Ye, J.W., Li, D.Z., Hampe, A., 2019. Differential Quaternary dynamics of evergreen broadleaved forests in subtropical China revealed by phylogeography of Lindera aggregata (Lauraceae). J. Biogeogr., 46: 1112-1123. DOI:10.1111/jbi.13547 |
Ye, J.W., Li, D.Z., 2021. Distinct late Pleistocene subtropical-tropical divergence revealed by fifteen low-copy nuclear genes in a dominant species in South-East China. Sci. Rep., 11: 4147. DOI:10.1038/s41598-021-83473-w |
Yu, Q.F., Tan, Y.H., Yu, W.B., et al., 2023. Comparative analyses of eight complete plastid genomes of two hemiparasitic Cassytha vines in the family Lauraceae. Front. Genet., 14: 1192170. DOI:10.3389/fgene.2023.1192170 |
Yuan, Y.M., Wohlhauser, S., Möller, M., et al., 2005. Phylogeny and biogeography of Exacum (Gentianaceae): a disjunctive distribution in the Indian Ocean Basin resulted from long distance dispersal and extensive radiation. Syst. Biol., 54: 21-34. DOI:10.1080/10635150590905867 |
Zeng, G., Liu, B., Rohwer, J.G., et al., 2021. Leaf epidermal micromorphology defining the clades in Cinnamomum (Lauraceae). PhytoKeys, 182: 125-140. DOI:10.3390/polym14010125 |
Zeng, G., Liu, B., van der Werff, H., et al., 2014. Origin and Evolution of the unusual leaf epidermis of Caryodaphnopsis (Lauraceae). Perspect. Plant Ecol. Evol. Syst., 16: 296-309. DOI:10.1016/j.ppees.2014.07.003 |
Zhai, S.N., Comes, H.P., Nakamura, K., et al., 2012. Late Pleistocene lineage divergence among populations of Neolitsea sericea (Lauraceae) across a deep sea-barrier in the Ryukyu Islands. J. Biogeogr., 39: 1347-1360. DOI:10.1111/j.1365-2699.2012.02685.x |
Zhang, B., Yao, X., Chen, H.F., Lu, L., 2022. High-quality chromosome-level genome assembly of Litsea coreana L. provides insights into magnoliids evolution and flavonoid biosynthesis. Genomics, 114: 110394. DOI:10.1016/j.ygeno.2022.110394 |
Zhang, Q., Sun, T.T., Omollo, W.O., et al., 2024a. An integrative taxonomy of Asian Caryodaphnopsis (Lauraceae) based on morphology and phylogenomics. Taxon, 73: 949-970. DOI:10.1002/tax.13223 |
Zhang, R., Huang, L.L., Li, S.F., et al., 2024b. Fossil woods of Cryptocarya (Lauraceae) from the middle Miocene of Southwest China. Rev. Palaeobot. Palynol., 324: 105096. DOI:10.1016/j.revpalbo.2024.105096 |
Zhao, M.L., Song, Y., Ni, J., et al., 2018. Comparative chloroplast genomics and phylogenetics of nine Lindera species (Lauraceae). Sci. Rep., 8: 8844. DOI:10.1038/s41598-018-27090-0 |
Zhu, Q., Liao, B.Y., Li, P., et al., 2017. Phylogeographic pattern suggests a general northeastward dispersal in the distribution of Machilus pauhoi in South China. PLoS One, 12: e0184456. DOI:10.1371/journal.pone.0184456 |
Zhu, S.S., Comes, H.P., Tamaki, I., et al., 2020. Patterns of genotype variation and demographic history in Lindera glauca (Lauraceae), an apomict-containing dioecious forest tree. J. Biogeogr., 47: 2002-2016. DOI:10.1111/jbi.13874 |
Zhu, W., Tan, Y.H., Zhou, X.X., et al., 2023. The complete plastid genome sequences of the Belian (Eusideroxylon zwageri): comparative analysis and phylogenetic relationships with other magnoliids. Forests, 14: 2443. DOI:10.3390/f14122443 |
Zhu, W., Zhang, D., Xu, W., et al., 2025. Comparative genomics and phylogenetic analysis of mitochondrial genomes of Neocinnamomum. BMC Plant Biol., 25: 289. DOI:10.1007/s44211-024-00697-2 |
Zhu, W., Zhang, H.R., Li, Q.S., et al., 2024. Complete plastid genome sequences of three tropical African Beilschmiediineae trees (Lauraceae: Crytocaryeae). Forests, 15: 832. DOI:10.3390/f15050832 |
Zuntini, A.R., Carruthers, T., Maurin, O., et al., 2024. Phylogenomics and the rise of the angiosperms. Nature, 629: 843-850. DOI:10.1038/s41586-024-07324-0 |



