2. Southern Research Station, USDA Forest Service, 2500 Shreveport Highway, Pineville, LA 71360, USA
For converting wood into liquid materials under mild conditions using an organic solvent and an acid catalyst, research has focused on wood waste as a raw material for manufacturing bio-products. Several studies were conducted to formulate liquefied wood resins for bonding plywood (Hassan et al. 2009; Hse et al. 2009), composite panels (Pan et al. 2009), and phenolic-based foam (Huang et al. 2011). Although pilot-scale evaluation of liquefied wood resins as novalac moldings and polyurethane foam showed encouraging results in Japan (Yao 2003), full scale commercial production has yet to be realized. It is necessary to develop an efficient, cost effective, and environmentally friendly liquefaction technique for the commercialization of the wood liquefaction process.
It is generally recognized that the current high cost for wood liquefaction is largely due to the following factors: first, the conventional liquefaction technique has the inherent disadvantages of long heating time and low energy efficiency; second, in order to achieve a satisfactory extent of liquefaction, large amounts of phenol are needed. As a result, a large amount of un-reacted phenol remains after the liquefaction reaction and must be removed from the liquefied mixtures. This treatment raises the cost and complexity of the liquefaction process. Finally, the liquefaction process relies on expensive equipment because of the large amount of strong sulfuric acid used as a catalyst. Sulfuric acid is extremely corrosive and difficult to handle for safety and environmental reasons. Therefore, numerous studies have focused on improvement of these cost factors. For instance, weaker phosphoric acid (Lin et al. 1994) and oxalic acid (Alma et al. 1995) were substituted for sulfuric acid in wood liquefaction with phenol; novalac resin was successfully fabricated using the phenolated liquefied wood mixture containing large amounts of free phenol to react in situ with formaldehyde (Li et al. 2012); and faster liquefaction was accomplished by using ethylene carbonate in the presence of an acid catalyst (Yamada and Ono 1999) and supercritical phenol without an acid catalyst (Lee and Ohkita 2003).
Microwave heating is an alternative to conventional heating methods. Microwave irradiation has been increasingly used in many areas of biomass processing because of its higher heating efficiency, increase in reaction rate, and reduction of reaction time (Gabhane et al. 2011). Microwave-assisted pretreatment enhances enzymatic hydrolysis of rice straw (Gong et al. 2010) and switchgrass (Keshwani and Cheng 2009). Microwave pyrolysis has been employed to pyrolyze large biomass rapidly and consequently has attracted increasing attention (Lei et al. 2009; Zhao et al. 2010). Microwave radiation was used as the sole heating source to achieve complete liquefaction of hardwoods in 7 min at temperatures above 190 ℃ with glycols and organic acid (Kržan and Žagar 2009). We examined microwave-assisted liquefaction of sweetgum wood in phenol with sulfuric and phosphoric acid catalysts. We developed a novel process to achieve rapid and efficient transformation of lignocellulosic material into a bio-polymer precursor. In comparing our results to those from conventional oil bath heating, we used FTIR to characterize the liquefied wood residue and liquefied product.
Materials and methods MaterialsSawdust of sweetgum (Liquidambar styraciflua) was ground to pass a 0.15 mm sieve (100 mesh), and air dried to a moisture content of 3 % before use. Liquid industrial grade phenol (90 % concentration) was used as the liquefaction reagent, and tetrahydrofuran (THF) high-performance liquid chromatography (HPLC) grade reagent was also used. All the other chemicals were analytical reagents.
Microwave liquefactionWood meal (100 g), phenol (150–250 g), polyethylene glycol 400 (10 %, based on phenol), sulfuric acid catalyst (1.5 %, based on phenol), and phosphoric acid catalyst (1.5 %, based on phenol) were mixed thoroughly in a mixer. Approximately 15 g mixture was then weighed into a 100 ml Teflon reaction vessel containing a magnetic stirring bar. The vessel caps were perfectly tightened to prevent release of the resulting gas. The liquefactions were carried out in a Milestone Ethos EX microwave oven equipped with Terminal 640 Easy Control Software. Each experiment was duplicated. In this study, the temperature was increased from room temperature to 150 ℃ at a heating rate of 32.5 ℃ min-1 and then was held constant for 2.5–15 min.
Oil bath liquefactionFor comparative purposes, wood liquefaction was also performed in an oil bath using the same wood meal mixture as in the microwave liquefaction. Approximately 15 g of the wood meal mixture was added to a 100-mL three-neck glass reactor equipped with a condenser and stirring system. The reaction flask was then immersed in an oil bath preheated to 150 ℃. The liquefaction time was 30–90 min. Each experiment was repeated in duplicate.
Measurement of residue contentAbout 5 g of the liquefied product was diluted with an acetone/water binary solvent (acetone/water = 7/3) and filtered with Whatman fast flow filter paper in vacuo. The residue was dried to a constant weight at 105 ℃ in an oven, and then the residue content was calculated.
FTIR spectroscopyFTIR analysis of the liquefied wood residues, liquefied wood products and black solid residues was performed by a Nicolet Nexus 670 spectrometer equipped with a Thermo Nicolet Golden Gate MKII Single Reflection ATR accessory. A small amount of sample was placed directly on the diamond crystal. Data collection was performed at a 4-cm-1 spectral resolution and 32 scans were taken per sample. FTIR spectra were normalized with respect to the most intense band. The second derivative spectrum was obtained by using a 13-point smooth.
Results and discussion Residue contentTable 1 shows the change in residue content under various conditions of microwave heating in comparison with residue content after oil bath liquefaction. As expected, the residue content decreased as the phenol to wood (P/W) ratio increased. At a microwave heating rate of 32.5 ℃, the temperature raised rapidly to 150 ℃ at 4.6 min. The liquefied residue content declined rapidly to 10–17 % within 2.5 min of reaction time at 150 ℃ under microwave heating, indicating a high efficiency of microwave-assisted wood liquefaction. The P/W ratio interacted with the liquefaction reaction times to affect the liquefied residues. At high P/W ratios (i.e., 2/1 and 2.5/1) the liquefied residues decreased with increasing liquefaction time. At low P/W ratios (i.e., 1.5/1), liquefied residues decreased when the liquefaction time increased to 15 min., and further increasing liquefaction time yielded greater quantities of liquefied residue. These results indicate that a lower P/W ratio tends to result in re-condensation of LW to insoluble products due to an insufficient amount of phenol. Re-condensation of LW was reported by Lin et al. (2001) and Zhang et al. (2006).
|
|
Table 1 Effects of P/W ratio on residue content by reaction time |
In general, the residue contents from microwave liquefaction for 2.5–15 min were substantially lower than those from oil bath liquefaction for 30–90 min (Table 1). The residue content reached 17–27 % for oil bath liquefaction at 30 min. It slowly decreased to 4–26 % when the liquefaction time increased to 120 min. Especially, at low P/W ratios (i.e., 1.5/1), the residue content remained greater than 20 % at 120 min. The necessary reaction time for microwave heating to achieve a residue content similar to oil bath heating was at least one sixth, one eighteenth, and one twenty-fourth than that by oil bath heating for P/W ratios of 2.5/1, 2/1, and 1.5/1, respectively. This indicates that microwave radiation achieved more rapid and efficient thermochemical conversion of wood than conventional oil bath heating. Furthermore, the clear advantage of microwave over oil bath liquefaction was most significant as the P/W ratio decreased. Although wood has a weak microwave absorption ability, water and phosphoric acid are excellent absorbing agents for microwave energy, especially water (Wang et al. 2008). In addition, sulfuric acid, phenol, and polyethylene glycol 400 all have permanent dipole moments, and are responsive to microwave fields. As a result, rapid heating throughout the entire reactor system is achieved as soon as the microwave radiates, resulting in rapid completion of wood liquefaction. The oil bath wood liquefaction system is a heterogeneous solid– liquid reaction with poor heat and mass transformation in which inefficient stirring in the initial stage leads to gradual and tedious liquefaction of the wood.
FTIR analysis of the liquefied wood residueFTIR spectra of sound wood and liquefied wood residue from microwave and oil bath liquefaction are shown in Fig. 1. For sharper differentiation of the bands, the second derivative of the spectra is shown (Fig. 1). It is clear that there were significant changes in IR spectra between sound wood and liquefied wood residue. Band assignments discussed below are given according to Faix (1991) and Schwanninger et al. (2004).
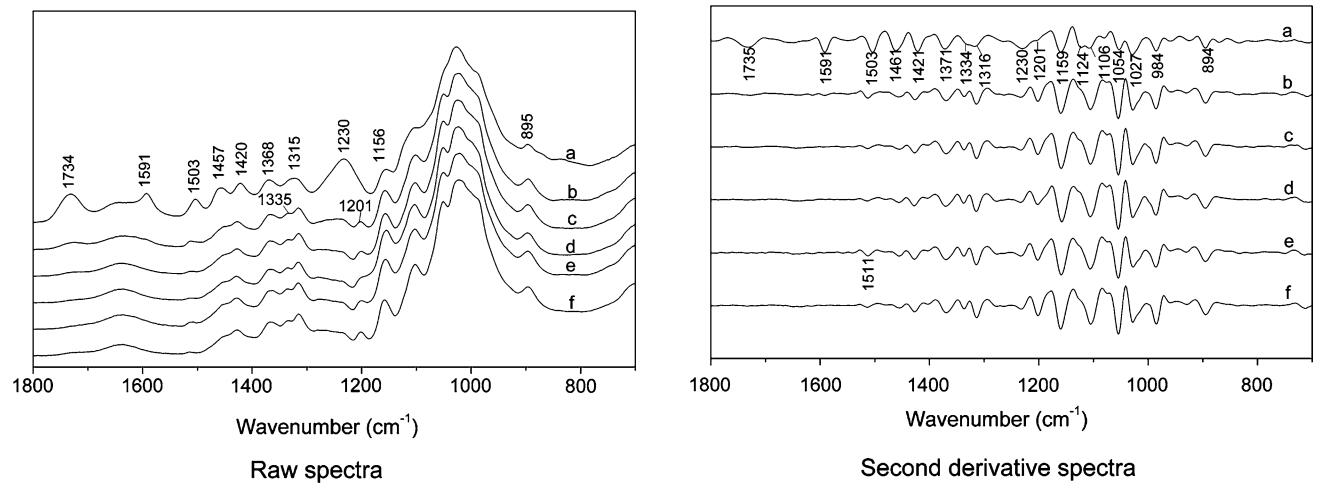
|
Fig. 1 FTIR spectra of sound wood (a), liquefied wood residue at P/W ratio of 1.5/1 by microwave for 2.5 min (b), 5 min (c), 10 min (d), 15 min (e) and at P/W ratio of 2/1 by oil bath for 90 min (f) |
The absorbance at 1735 cm-1, assigned to unconjugated C=O stretch in hemicellulose, significantly decreased after 2.5 min of microwave liquefaction, and was almost absent after 10 min of microwave liquefaction, indicating that hemicellulose was sensitive to the microwave liquefaction process.
The bands at 1, 591 (syringyl (S) > guaiacyl (G)), 1, 503 (G > S) and 1457 cm-1, resulting from the aromatic skeletal vibrations in lignin, rapidly decreased during the initial stage, indicating the relative ease of lignin liquefaction. The band at 1591 cm-1 almost disappeared after 5 min of liquefaction, whereas the band at 1503 cm-1 retained a very weak absorbance after 10 min of liquefaction. The behaviors of these two bands indicate that the syringyl units were more sensitive than the guaiacyl units to microwave liquefaction. The band at 1124 cm-1 (typical for S units), almost missing in the residue after 5 min of liquefaction, supports the above conclusion. According to the reaction pathway of the β–O–4 lignin model compound suggested by Lin et al. (2001), the β-aryl ether of the syringyl unit was cleaved much faster than was that of the guaiacyl unit under acid conditions. The slight increase of absorbance at 1503 cm-1 indicates the occurrence of a recondensation reaction after 10 min of liquefaction, resulting from the uncondensed C-5 position at the guaiacyl unit.
The cellulose bands at 1157 cm-1 (C–O–C asymmetric vibration), 1106 cm-1 (glucose ring asymmetric valence vibration), and 895 cm-1 (anomere C-groups) gradually became predominant with increased microwave time, showing that cellulose was most difficult to liquefy. An independent band at 1202 cm-1 (OH in-plane bending in cellulose Ⅰ and cellulose Ⅱ) appearrd, which is not observed in the spectrum of sound wood. The presence of the 1202 cm-1 band is attributed to a decrease in the intensity of the 1231 cm-1 band (Syringyl ring breathing and C–O stretching in lignin and xylan), which further confirms that hemicellulose and lignin were liquefied faster than cellulose during phenol liquefaction.
The doublet at 1335 and 1316 cm-1 is assigned to the OH in plane bending and CH2 wagging and is related to the contents in crystallized cellulose Ⅰ and amorphous cellulose (Colom and Carrillo 2002). In Fig. 1, a shoulder at 1335 cm-1, which is not observed in the spectrum of sound wood, developed in the band at 1316 cm-1 after 2.5 min of microwave radiation, resulting from the decrease in the intensity of the 1316 cm-1 band. An increase in the ratio of 1335/1316 cm-1 suggests a decrease in wood crystallinity, meaning that the cellulose crystalline region was destroyed after only 2.5 min of microwave liquefaction at 150 ℃. Cellulose crystallinity is also measured based on the bandheight ratio 1421/895 cm-1 (A1421/A895) (Oh et al. 2005). In order to amplify small differences in the IR spectra, the band height was determined based on the second derivative IR spectra. A1421/A895, which changed from 1.813 to 0.636 after 2.5 min of microwave liquefaction, indicating that the crystalline cellulose was significantly degraded and in agreement with the above finding. A1421/A895 had a slight increase from 0.636 to 0.725 for liquefaction periods up to 15 min, indicating that the amorphous region was more influenced by microwave liquefaction than the crystalline region.
The spectrum of the residue from oil bath heating was similar to that of the residue from the microwave heating. Therefore, there was no significant difference in the chemical components of residues between these two methods.
FTIR analysis of black solid residuesA small quantity of black solid material was produced at P/W ratio of 1.5/1, and there were no black solids at P/W ratios of 2/1 and 2.5/1. Therefore, we used the FTIR spectra of the black material to study the effects of P/W ratio on wood liquefaction.
Figure 2 shows that the IR spectra of black solids obtained at a P/W ratio of 1.5/1 for different liquefaction times were similar, but significantly different from the IR spectrum of wood powder. This meant that the overwhelming majority of wood chemical components were decomposed and carbonized according to a similar pathway during microwave liquefaction. The presence of absorption peaks, attributed to hemicellulose (1694 cm-1, C=O stretch), cellulose (1341 cm-1, O–H in plane deformation; 1167 cm-1, C–O– C asymmetric vibration), and lignin (1594, 1503 and 1453 cm-1, aromatic skeletal vibrations) suggest, according to Schwanninger et al. (2004), that a few wood residual fragments still existed in the black solid residue. It is possible that carbonization of some wood powder happened to a certain extent before complete liquefaction, or even liquefaction did not happen at all.
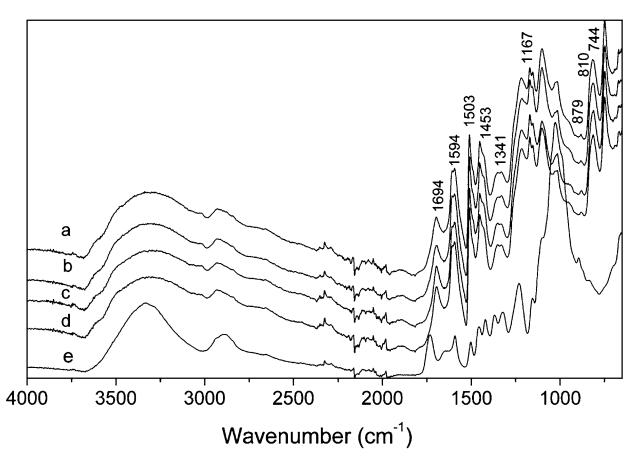
|
Fig. 2 FTIR spectra of black solids at P/W ratio of 1.5/1 by microwave for 2.5 min (a), 5 min (b), 10 min (c), 15 min (d) and sound wood (e) |
The indication of a substitution pattern of the phenol can be obtained due to out-of-plane vibration of aromatic C–H in the region from 900 to 650 cm-1 (Zhang et al. 2006). The bands at 744 cm-1 (adjacent four hydrogen atoms on the benzene ring), 810 cm-1 (adjacent two hydrogen atoms) and 879 cm-1 (lone hydrogen atom on the benzene ring), suggest the presence of p-substitution, o-substitution, and di-and/or tri-substitution of phenol, respectively. Absorbance at 700 cm-1 was not found, indicating un-reacted phenol was absent in the black solid. The above results show that the wood chemical components were subjected to decomposition, phenolation, and carbonation during wood liquefaction at a P/W ratio of 1.5/1, and the side effect of carbonization was due to the lack of phenol and localized superheating.
We noticed that black solid was produced only at a P/W ratio of 1.5/1, and it disappeared as the P/W ratio increased from 1.5/1 to 2/1 and 2.5/1. This further demonstrates that the occurrence of carbonization resulted from a shortage of phenol. Thus, an increase in the P/W ratio can supress carbonization.
FTIR analysis of liquefied woodThe IR spectra of LW were measured to define their chemical modifications and structural changes resulting from the liquefaction process. There were clear differences between sound wood and LW both in the absorbance and shapes of the bands, and in their locations (Fig. 3).
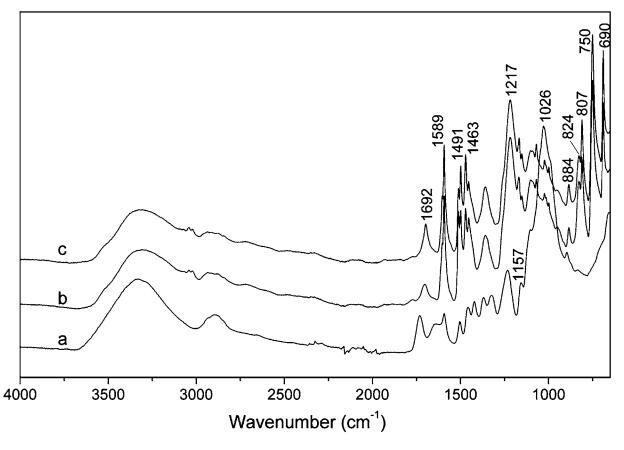
|
Fig. 3 FTIR spectra of sound wood (a), LW from P/W ratio of 2/1 by oil bath for 90 min (b) and by microwave for 10 min (c) |
The characteristic unconjugated C=O stretch in hemicellulose shifts from 1735 to 1692 cm-1, and a series of bands from 1157 to 884 cm-1, mainly assigned to C–O absorption from all components of wood, decreased significantly as a result of liquefaction. The bands from 1589 to 1463 cm-1, assigned to aromatic skeletal vibrations, showed a notable increase, and the number of bands increased from three in sound wood to five in LW. These results indicate that the structure of the three main wood components was degraded to a significant extent, and unreacted phenol might have been retained in LW.
One of the main differences between sound wood and LW is the appearance of an out of plane vibration of aromatic C-H from 900 to 650 cm-1, where the information about the substitution patterns of the phenol can be obtained (Zhang et al. 2006). The strong bands at 750 and 690 cm-1 are assigned to five adjacent hydrogen atoms on the benzene ring, confirming that a great deal of un-reacted phenol was still present in LW. The strong band at 750 cm-1 is assigned to four adjacent hydrogen atoms on the benzene ring, indicating the existence of o-substitution of phenol. The band at 824 cm-1, assigned to two adjacent hydrogen atoms on the benzene ring, suggests the presence of p-substitution of phenol. The bands at 884 cm-1, assigned to a lone hydrogen atom on the benzene ring, indicates phenol with di-and/or tri-substitution, and it is certain that phenol with di-substitution was present in LW because of the strong band at 807 cm-1. The substitution patterns of phenol suggest that wood liquefaction with phenol is a complicated process including various reactions between phenol and the wood components.
As expected, the IR spectra of microwaved LW were almost identical to those of LW from oil bath, indicating the similarity of their chemical components and of their substitution patterns between liquefied wood components and phenol.
ConclusionMicrowave heating had a dramatic effect on the rate of wood liquefaction in phenol. Microwave liquefaction had more obvious advantage over oil bath liquefaction when low P/W ratios were used. Carbonization of liquefied wood was detected when using microwave heating at low a P/W ratio, and resulted from the insufficient amount of phenol and localized superheating. The results from the IR evaluation showed that chemical components and substitution patterns of bonded phenol from microwave heating were similar to those from the oil bath. Microwave heating is an effective and promising alternative method for traditional heating in wood phenol liquefaction.
AcknowledgmentsThe authors gratefully acknowledge the Southern Research Station, USDA Forest Service, USA for kindly providing the experimental facilities for this study. The financial support of "948 Project" of State Forestry Administration (2012-4-28) is gratefully acknowledged.
Alma MH, Yoshioka M, Yao YG, Shiraishi N (1995) Preparation of oxalic acid-catalyzed resinified phenolated wood and its characterization. J Jpn Wood Res Soc 41: 1122-1131. |
Colom X, Carrillo F (2002) Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur Polym J 38: 2225-2230. DOI:10.1016/S0014-3057(02)00132-5 |
Faix O (1991) Classification of lignin from different botanical origins by FT-IR spectroscopy. Holzforschung 45(Suppl): 21-27. |
Gabhane J, Prince William SPM, Vaidya AN, Mahapatra K, Chakrabarti T (2011) Influence of heating source on the efficacy of lignocellulosic pretreatment-a cellulosic ethanol perspective. Biomass Bioenergy 35: 96-102. DOI:10.1016/j.biombioe.2010.08.026 |
Gong GF, Liu DY, Huang YD (2010) Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw. Biosyst Eng 107: 67-73. DOI:10.1016/j.biosystemseng.2010.05.012 |
Hassan EB, Kim M, Wan H (2009) Phenol-formaldehyde-type resins made from phenol-liquefied wood for the bonding of particleboard. J Appl Polymer Sci 112: 1436-1443. DOI:10.1002/app.v112:3 |
Hse CY, Feng F, Pan H (2009) Bond quality of phenol-based adhesives containing liquefied creosote-treated wood. In: Hse CY, Jiang ZH, Kuo ML (eds) Advanced biomass science and technology for bio-based products: proceedings. Chinese Academy of Forestry, Beijing, pp 87-95
|
Huang YB, Zheng ZF, Feng H, Pan H (2011) Phenolic foam from liquefied products of walnut shell in phenol. Adv Mater Res 236-238: 241-246. DOI:10.4028/www.scientific.net/AMR.236-238 |
Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100: 1515-1523. DOI:10.1016/j.biortech.2008.09.035 |
Kržan A, Žagar E (2009) Microwave driven wood liquefaction with glycols. Bioresour Technol 100: 3143-3146. DOI:10.1016/j.biortech.2009.01.057 |
Lee SH, Ohkita T (2003) Rapid wood liquefaction by supercritical phenol. Wood Sci Technol 37: 29-38. DOI:10.1007/s00226-003-0167-7 |
Lei HW, Ren SJ, Julson J (2009) The effects of reaction temperature and time and particle size of corn stover on microwave pyrolysis. Energy Fuels 23: 3254-3261. DOI:10.1021/ef9000264 |
Li GY, Hse CY, Qin TF (2012) Preparation and characterization of novolak phenol formaldehyde resin from liquefied brown-rotted wood. J Appl Polymer Sci 125: 3142-3147. DOI:10.1002/app.v125.4 |
Lin LZ, Yoshioka M, Yao YG, Shiraishi N (1994) Liquefaction of wood in the presence of phenol using phosphoric acid as a catalyst and the flow properties of the liquefied wood. J Appl Polymer Sci 52: 1629-1636. DOI:10.1002/app.1994.070521111 |
Lin LZ, Nakagame S, Yao YG, Yoshioka M, Shiraishi N (2001) Liquefaction mechanism of β-O-4 lignin model compound in the presence of phenol under acid catalysts part 2. React Behav Pathways. Holzforschung 55: 625-630. |
Oh SY, Yoo D, Shin Y, Kim HC, Kim HY, Chung YS, Park WH, Youke JH (2005) Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr Res 340: 2376-2391. DOI:10.1016/j.carres.2005.08.007 |
Pan H, Shupe T, Hse CY (2009) Characterization of novolac type liquefied wood/phenol/formaldehyde (LWPF) resin. Eur J Wood Prod 67: 427-437. |
Schwanninger M, Rodrigues JC, Pereira H, Hinterstoisser B (2004) Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc 36: 23-40. DOI:10.1016/j.vibspec.2004.02.003 |
Wang XH, Chen HP, Luo K, Shao JA, Yang HP (2008) The influence of microwave drying on biomass pyrolysis. Energy Fuels 22: 67-74. DOI:10.1021/ef700300m |
Yamada T, Ono H (1999) Rapid liquefaction of lignocellulosic waste by using ethylene carbonate. Bioresour Technol 70: 61-67. DOI:10.1016/S0960-8524(99)00008-5 |
Yao YG (2003) Potential utilization of bio-based waste. In: Kyoto Shinbun, No. 43609
|
Zhang YC, Ikeda A, Hori N, Takemura A, Ono H, Yamada T (2006) Characterization of liquefied product from cellulose with phenol in the presence of sulfuric acid. Bioresour Technol 97: 313-321. DOI:10.1016/j.biortech.2005.02.019 |
Zhao XQ, Song ZL, Liu HZ, Li ZQ, Li LZ, Ma CY (2010) Microwave pyrolysis of corn stalk bale: a promising method for direct utilization of large-sized biomass and syngas production. J Anal Appl Pyrolysis 89: 87-94. DOI:10.1016/j.jaap.2010.06.001 |
 2015, Vol. 26
2015, Vol. 26
