| 纳米管状埃洛石的应用矿物学研究进展 |
2. 中国科学院大学,北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
埃洛石是一种具层状结构的1 : 1型铝硅酸盐矿物,是高岭石的水合多型矿物[1]。其分子式为Al2Si2O5(OH)4·nH2O,其中n=0时为7 Å埃洛石,n=2时为10 Å埃洛石。10 Å埃洛石在室温或略微加热时易不可逆地失去层间水转变为7 Å埃洛石。本文在不需区分水合状态时,将二者统称为埃洛石。晶体结构由一层硅氧四面体片和一层铝氧八面体片构成,由于二者间的尺寸不匹配,结构单元层中产生结构应力,而水分子层的存在使相邻结构单元层无法通过氢键维持平衡,因此,结构单元层采取相邻四面体反向旋转和/或卷曲的方式消除结构应力(图 1)[2]。由于结晶条件和地质产出环境的差异,埃洛石具有不同的蚀变程度和水合状态,可呈管状、球状、片状等多种形貌,但以管状形貌最为常见。这种以纳米管状形态产出的埃洛石(以下简称为HNT)的形态和尺寸各异(如长管、短管、部分展开管和套管)[3];其管内径通常为10~100 nm,管外径为30~190 nm,管长为0.02~30 μm[4]。
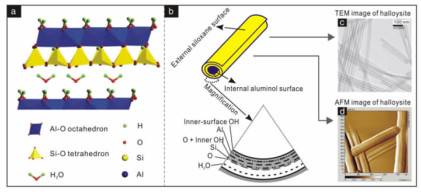 |
| 图 1 埃洛石的结构和形貌图:(a)10 Å埃洛石的晶体结构示意图;(b)埃洛石纳米管结构示意图;(c,d)埃洛石的透射电子显微镜(TEM)和原子力显微镜(AFM)图[1] Fig.1 Structure and morphology of halloysite: (a) Crystalline structure of halloysite-10 Å; (b) Nanotubular structure of halloysite; (c-d) TEM and AFM images of halloysite[1] |
与目前受关注度和研究程度均更高的碳纳米管相比,HNT在某些方面具有优势。HNT在地球上储量丰富,中国、法国、美国、土耳其、比利时和新西兰等都有HNT的大量产出,动辄以千吨计,易满足工业量产需要,而碳纳米管多以克为量级。作为天然产出的纳米材料,HNT的开采耗能少,环境污染小。HNT的价格约4美元/kg,远低于碳纳米管的价格(500美元/kg)[5-6]。HNT生物相容性好,而碳纳米管具有毒性。HNT的内、外表面具有不同的化学成分,内表面和管端分布有铝羟基,外表面主要为硅氧烷(由于空位等缺陷的出现也含有一定数量的硅羟基);内、外表面均可改性,且可进行选择性改性[7-8],进而实现更多可能的功能化[9]。而较之另一种天然产出的纳米管——伊毛缟石纳米管[10],HNT的内腔孔径大得多,可容纳大尺寸分子(如酶)[11]。另外,伊毛缟石在地球的储量较HNT少得多,目前尚未发现具有工业开采价值的矿体。
由于具有中空的纳米管状结构和独特的表面荷电性(外表面带负电而内腔表面带正电)[12],HNT已广泛应用于医学与生物医学(如用于药物负载与缓释、组织支架和癌细胞分离)、纳米复合材料(如用作填料、涂层、载体、模板和纳米反应容器)、环境污染治理(如用于污染物的吸附固定和催化降解)、陶瓷工业(用作原料)、新型能源(如用作储氢材料)和食品(用作防腐剂载体)等领域[13-14]。下面将对HNT在上述领域的研究进展进行综述。
1 医学与生物医学已有大量研究关注到HNT在医学与生物医学领域的应用[15]。HNT作为一种天然产出的纳米管状矿物,具有廉价易得、生物相容性好和环境友好等特点,已广泛应用于药物或生物活性分子的负载与缓释、组织支架和癌细胞分离等领域。
1.1 生物相容性与细胞毒性HNT的主要化学成分为氧化铝和氧化硅,含少量氧化铁、氧化钙、氧化钾等杂质,基本不含或仅含极其微量的有害元素(如铅、汞、铬和镉)。因此,HNT常被当作一种绿色环保的硅酸盐材料[16]。大量的生物体外实验证明HNT具有良好的生物相容性[17-19]。Kamalieva等[20]研究了不同浓度的HNT对模型癌细胞的毒性,发现低浓度的HNT对细胞的毒害性小,虽影响细胞的生物化学作用,但未改变细胞形态,也并未进入细胞核。生物聚合物(纤维素、壳聚糖)/HNT复合材料在浓度不超过0.5 mg/mL时,对细胞培养是安全的[21]。对秀丽隐杆线虫,HNT的安全浓度为1 mg/mL[22];而对于尾草履虫,HNT的安全浓度更高,达10 mg/mL[23]。另外,Bellani等[24]发现负载钯纳米颗粒的HNT对萝卜未表现生态毒性。
近年来,研究者们也开展了一些HNT生物相容性和细胞毒性相关的生物体内实验。Wang等[25]研究了纯化HNT经口服后对小鼠肝脏的毒性,发现,低剂量(5 mg/kg BW)的HNT未表现出肝脏毒性,并可刺激小鼠生长;而中高剂量(50和300 mg/kg BW)的HNT则会引发肝脏氧化应激反应,并抑制小鼠生长。另外,高剂量会导致Al在肝脏中积累,进而引起肝脏功能障碍和病理变化[26]。他们还研究了口服纯化HNT对小鼠肺的毒性,低剂量(5 mg/kg BW)的HNT未表现肺部毒性;而高剂量(50 mg/kg BW)的HNT可从胃肠道中被吸收而沉积在肺部,引起肺纤维化[27]。综上,一定浓度范围内的HNT对细胞、微生物和动植物都是安全的。
1.2 药物负载与缓释较丰富的表面基团、较大长径比和中空管状结构使HNT成为一种用于缓释控释的理想载体[28-29]。一方面,药物分子在HNT表面发生不断吸附与解吸,使得药物从HNT中释放缓慢;另一方面,HNT管腔内、外液体交换速率较低,导致负载于管腔内的药物释放速率下降[30]。目前,利用HNT作为药物(如硝苯地平、地塞米松、呋喃苯胺酸、布洛芬和异烟肼)的负载与缓释已成为医学与生物医学领域的研究热点[30-33]。Tan等[13]发现布洛芬以纳米晶或无定形态负载于HNT内腔和外表面,有机硅烷改性可提升布洛芬的负载量,并通过增强对布洛芬的亲和力实现其可控释放(图 2)[34]。此外,3-氨基丙基三乙氧基硅烷(APTES)改性HNT作为环丙沙星的载体,可防止其在口服后与体内的铁络合而失效[35]。Liu等[36]以负载了己酮可可碱的有机改性HNT和可压性淀粉为原料制备了水凝胶,该水凝胶在存在H2O2时发生分解,从而实现对己酮可可碱的智能释放。
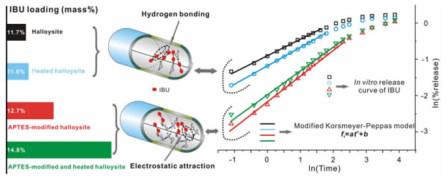 |
| 图 2 3-氨基丙基三乙氧基硅烷(APTES)改性前后的HNT用于布洛芬的负载和释放[34] Fig.2 Schematic representation of HNT before and after APTES modification for ibuprofen loading and release[34] |
除药物外,HNT还可用于负载生物活性分子(如酶、核酸),以克服其低效、易分解和具毒副作用等缺点。当前,RNA干扰技术已成为癌症的潜在治疗方法,它可特定地抑制某一目标基因的表达,实现治疗癌症的目的。Liu等[37]将HNT负载小干扰RNA(siRIPK4)以治疗膀胱癌(图 3),增大了小干扰RNA的血清稳定性,延长了其在血液中的循环时间,促进了小干扰RNA在细胞内的吸收和肿瘤处的集聚,显著抑制了膀胱癌基因(RIPK4)的表达,且无副作用。
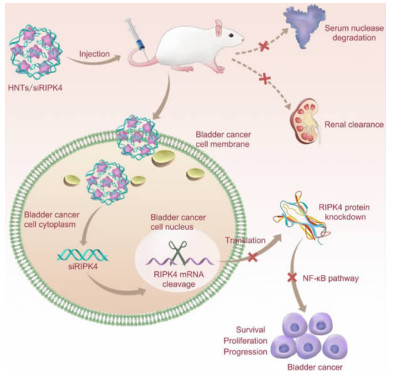 |
| 图 3 HNT/siRIPK4复合物的体内传输、RIPK4抑制和膀胱癌治疗过程示意图[37] Fig.3 Schematic diagram of in vivo delivery of HNT/siRIPK4 complexes for RIPK4 silencing and bladder cancer therapy[37] |
1.3 组织支架
HNT还可用于制备组织支架[38]。Nitya等[39]将HNT添加到聚己酸内酯(PCL)中,制备PCL/HNT支架,该支架较纯PCL支架具有更高机械强度,且在模拟体液中表现出更高的蛋白质吸附能力和更强的矿化作用。Liu等[40]制备了壳聚糖/HNT复合支架,该支架较纯壳聚糖支架具有更高的抗压强度、压缩模量和热稳定性,且表现出好的细胞相容性。随后,他们以类似方法制备了藻朊酸盐/ HNT复合物支架[41]。Wei等[42]将负载庆大霉素的HNT加入到聚甲基丙烯酸甲酯骨接合剂中,降低了固化温度,提升了抗拉强度及其与牛股骨的黏附力。
1.4 其它应用我国最早的药学专著《神农本草经》即有HNT入药的记载,其主要功效为涩肠、止血、收湿、生肌等[16, 43]。鉴于其独特的一维纳米管状结构和良好的生物相容性,HNT还被用作牙齿填料、骨内植入物、超声造影剂,或用于癌细胞分离[27, 44-45]。He等[46]发现,HNT可从癌症病人血液中高效捕获肿瘤细胞,这些被捕获的细胞表现更加舒展的形貌。HNT也可用于诱导细胞定向,Liu等[47]通过蒸发诱导自组装法制备了同心环状HNT阵列,用于诱导成肌细胞沿垂直于环的方向生长。此外,HNT/量子点复合材料可被活细胞内化,并表现强而稳定的荧光和显著的纳米管光散射,或可用于细胞检测[48]。
1.5 评述HNT用作药物载体,不仅可有效保护药物分子在到达靶组织前不被破坏,还可实现药物的缓慢释放。此外,还可用聚合物阻塞管端进一步延长释放时间[49]或通过制备复合材料实现智能释放[36]。因此,HNT用作药物载体及各类药用辅料已成为国内外药剂学研究热点之一[50]。细胞靶向治疗是目前精准医学发展的前沿,应继续发掘天然HNT在该领域的潜在应用。然而,需要注意的是,HNT及其复合材料在人体内的相容性和细胞毒性还无定论,需要深入开展相关工作。而且,HNT难以在生物体内降解,也在一定程度上限制了HNT在医药中的应用[51]。
2 纳米复合材料由于具有结构和表面性质特殊、储量丰富和廉价易得等优点,HNT作为天然纳米材料被广泛用于制备聚合物/HNT复合材料,包括结构复合材料和功能复合材料。前者主要利用HNT的高强度、高模量制备具有优异机械性能的复合材料,而后者利用HNT的独特结构制备具有高阻燃、高防腐和超疏水特性的复合材料[52]。聚合物/HNT复合材料具有易于加工、成本较低、热和机械性能好、环境友好和生产效率高等特点,在民用或工业产品领域具有广阔的应用前景[16, 53]。在上述复合材料中,HNT可以不同形式(如填料、涂层、载体等)赋存,并赋予复合材料多样的功能。
2.1 填料近年来,HNT被用作有发展潜力的纳米填料来提升聚合物的性能。由于具有纳米管状结构和极性表面,HNT可不经表面改性而较好地分散在极性聚合物基体(如环氧树脂、聚酰胺等)中[52, 54]。加之其弹性模量高(可达130 GPa)[55]具有一定韧性,HNT被认为是一种潜在的增强增韧高分子材料的优质纳米填料[56-57]。在复合材料中,HNT会形成一种“骨架”,提升复合材料的强度和内部结合力,同时骨架中可负载活性组分来赋予提供聚合物更多功能[58]。
目前,HNT作为填料应用最广泛的材料是橡胶和塑料。天然橡胶、丁苯橡胶、乙丙橡胶和羧基丁苯橡胶等均可用于制备橡胶/HNT复合材料[16, 59-60]。HNT在橡胶中分散较为均匀,表现出显著的补强效应,还可负载抗氧化剂等功能试剂提高橡胶的热稳定性、抗老化性和耐磨损性。如何毅等[61]将外表面负载TiO2的HNT用作环氧树脂复合涂层的填料,提升了其耐磨性和耐腐蚀性。HNT也可作为填料用于制备热塑性塑料(如尼龙6、聚丙烯、聚乙烯等)[62]和热固性塑料[63]。塑料/HNT复合材料具有显著提升的机械强度、热性能和阻燃性能。美国Natural Nano公司已经将尼龙/HNT复合材料和聚丙烯/HNT复合材料的商业化产品推向市场。与其它复合材料相比,这些材料具有轻质高强、加工性能好、热性能和韧性高和价格低廉等优势[53]。而且,这些产品的性能可以根据客户需求进行定制[16]。
2.2 涂层HNT可作为涂层或涂层添加剂来制备功能复合材料[64-65]。如,HNT涂覆在聚氨酯泡沫上可降低其可燃性并捕获燃烧产生的有害气体[66],涂覆在醋酸纤维素表面可提升其防污能力[67],涂覆在头发上可用于染色或医疗[68]。此外,可实现HNT在涂层中的定向排列,来扩展其应用领域。Wu等[69]在不同条纹宽度的3D打印聚乳酸阵列表面负载了一层聚多巴胺@HNT膜,用于诱导细胞定向生长。该膜可有效地提升聚乳酸阵列的表面粗糙度和亲水性,有利于细胞的黏附和增值。聚丙烯腈多孔膜涂覆一高有序排列的HNT层,表现出高盐渗透作用下优异的染料排斥性,可用于染料提纯或浓缩[70]。
2.3 载体纳米复合材料制备过程中,HNT也常被用作金属纳米颗粒、酶等催化剂的载体,并提升其催化性能。一方面,独特的管状结构有利于提高催化剂的分散性和表面可利用性(与污染物接触的面积);另一方面,管状内腔可实现催化剂的局部浓缩和限域而表现出协同效应[71]。HNT负载金纳米颗粒用于选择性地氧化苯甲醇[72],负载Cu-Ni合金纳米颗粒来催化氧化汽车尾气并防止催化剂发生结块[73],负载淀粉酶可提高其催化活性和稳定性[74]。除用于催化外,还可赋予复合材料更多功能。如,HNT负载Ag-ZnO或CeO2-ZnO所制备得到的纳米复合材料表现出增强的抗菌性能[75-76],负载抗腐蚀剂用于金属防腐蚀[64, 77-79],负载抗微生物剂制备具防污性质的新型油漆复合物,通过抗微生物剂的缓慢而持续的释放来防止贝壳和其它海洋微生物附着在船体上。
2.4 模板HNT形貌独特,长径比大,表面多孔,可作为模板来制备多孔炭和聚合物纳米管等。Liu等[80]以HNT为模板,以蔗糖为碳源合成了具有较宽孔径分布(尤其在介孔范围)的多孔炭,用作双电层电容器的电极材料。Li等[81]用原子转移自由基聚合法,在HNT的内腔和外壁上沉积一层聚合物,经炭化和去模板,制得碳纳米管。类似地,Zhang等[82]制得聚苯胺纳米管。Wang等[83]以HNT为模板,以糠醇为碳源,合成了含中孔的纳米炭片,其具有高的比表面积和中孔率。由于去模板过程中酸与硅酸盐反应释放大量热和SiF4气体,纳米炭片没有复制模板的管状结构。通过降低酸浓度来减缓反应进程,周述慧等[84]以天然HNT为模板,以蔗糖为碳源合成了具有“壳-核”结构的一维管状中孔炭,其比表面积高达1 000 m2/g。程志林等[85]以HNT的中空结构为模板,以聚乙烯醇为碳源,通过一步法制备了碳纳米管和碳纳米棒混合纳米碳材料,其中碳纳米棒的比例随聚乙烯醇量增加而增加。此外,通过在荷负电的HNT表面组装阳离子型双亲分子,经硅烷化和焙烧,可制备以HNT为中心沿其管轴方向排列的介孔型沸石MCM-41[86]。
HNT的一维纳米介孔内腔可充当模板实现可控尺寸的金、银等纳米颗粒或纳米棒的快速高产合成(图 4),管内腔的纳米颗粒可通过电子束进行操纵,这可能用于纳米颗粒生长过程的基础研究[87]。HNT内腔被用作模板来合成Ru/ HNT“核-壳”管状催化剂用于催化芳香烃加氢反应[88],也被用来合成CdS/HNT“核-壳”管状催化剂用于可见光催化产氢[89]。
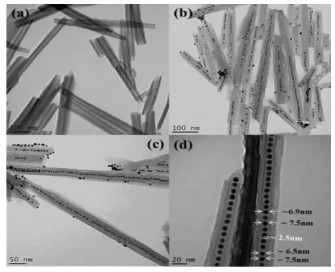 |
| 图 4 HNT为模板合成金纳米颗粒的TEM图:(a)HNT;(b-d)负载金纳米颗粒的HNT[87] Fig.4 TEM images of gold nanoparticles synthesized using HNT as template: (a) HNT; (b-d) HNT loaded with gold nanoparticles[87] |
2.5 纳米反应器
HNT内腔可用于封装酶,以提供更长储存时间、更高耐受温度和更多功能,而管端开口可允许小分子进入管内以实现生物催化。Shchukin等[11]将脲酶负载于HNT内腔来催化尿素的水解,在管内腔成功合成了亚稳态的球霰石,而在HNT外表面和溶液中均未发现CaCO3相(图 5)。该方法或可用于制备两种性质差异极大的物质构成的复杂无机“核-壳”纳米材料。
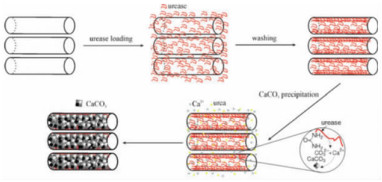 |
| 图 5 HNT内腔中脲酶催化CaCO3合成[11] Fig.5 Schematic illustration of the urease-catalyzed synthesis of CaCO3 inside HNT[11] |
2.6 评述
纳米颗粒在基体中的分散性和纳米颗粒与基体的界面相容性是决定聚合物纳米复合材料性能的两个主要因素[90]。受尺寸效应、表面电子效应以及表面羟基所成氢键等影响,HNT在基质中易发生团聚,常规的共混方式难以实现HNT在聚合物基体中的良好分散。即使在HNT与极性聚合物混合时,也不可避免地出现粒径为几微米的团聚体[57]。为提高HNT在基体中的分散性,改善其与基体的相容性,往往需要通过物理或化学方法对HNT进行适当的表面改性[91]。由于内表面分布大量铝羟基,外表面也有一定数量的硅羟基,HNT易于改性。在纳米复合材料中,应用最多的是对HNT外表面和边缘进行改性。常见的改性方法包括偶联剂改性、插层改性、表面包覆改性、自由基改性和表面活性剂改性等[1, 92-93]。
3 环境污染治理由于具有储量丰富、廉价易得、环境相容性好、比表面积大、表面基团丰富和吸附效率高等特点,以及独特介孔型管状内腔和带不同电荷的内、外表面,HNT已被广泛用于环境污染修复领域,不仅可以直接用于污染物的吸附固定,还可用于多种有机污染物的催化降解。
3.1 吸附固定HNT对染料(如甲基蓝、中性红和龙胆紫)[6, 14, 94]、重金属离子(如铬酸根离子、铅离子)[95-97]、放射性元素(如铀)[98]、铵根离子[99]和有机污染物(如抗生素、挥发性有机物)[100]等均具有好的吸附性能,且可在较短时间内达到吸附平衡,是一种潜在的廉价吸附剂[94]。而且,可通过物理或化学方法改性进一步提升HNT的吸附性能[101]。如,酸处理HNT的比表面积和孔容大大增加[102],对染料、挥发性有机物等表现出更好的吸附性能[103-104]。纳米铁氧化物改性HNT可通过静电引力、离子交换和路易斯酸碱作用等多种机制吸附磷酸根离子,增大了吸附量的同时提升了对磷酸根的亲和力[105]。此外,将HNT制备成功能膜用于水净化,是目前一个值得关注的应用方向[106]。
3.2 催化降解HNT及其改性产物可直接用于污染物的催化降解。如,HNT可催化月桂酸发生甲基酯化[107],而酸处理HNT可催化聚苯乙烯降解为苯乙烯和二乙烯基苯[108]。也有不少关于HNT及其改性产物作为催化剂载体用于降解污染物的报道[109]。如,HNT可负载金属卟啉催化有机质的氧化[110],负载Ru用于氨的分解[111]等。此外,HNT可用于制备和稳定油水乳剂,所得乳剂表现界面催化反应性,可用于处理石油泄漏[112]。HNT具有高长径比的管状形貌,可增大颗粒从油-水界面分离的能量,有利于提高乳剂的稳定性[113]。Yu等[114]将两亲性聚类肽改性的HNT用作石油泄漏修复领域的乳剂稳定剂,可以有效减小界面张力,提升HNT在油-水界面的热动力学倾向性,还可以增大乳剂的黏性以防止油滴聚集。此外,较之妨碍油降解细菌生长的传统表面活性剂,嫁接聚类肽的HNT无细胞毒性,可促进油降解细菌的生长。Zhou等[115]通过一步法制备了超亲水且在水中超疏油(Superhydrophilic-underwater superoleophobic)的HNT@聚偏氟乙烯膜,可用于从废水中快速而有效地分离乳剂油和染料,且表现出好的防污性能和优异的再循环能力。
3.3 评述最近,笔者课题组发现HNT与某些盐类化合物共煅烧可显著促进其结构无序化,使结构铝在较低温度下即可发生“活化”。如,将HNT与稀土氧化物通过共煅烧复合,使结构铝高效活化,所得复合物对磷酸根离子的吸附容量达到了同类研究报道的最高值[116]。未来可开展更多关于HNT这一独特性质在污染物吸附去除领域的研究。另外,有机改性HNT制备和稳定乳剂用于石油泄漏修复具有光明的应用前景,需深入开展更多研究工作。然而,需要注意的是,虽然无机或有机改性可大大提升埃洛石在环境污染修复领域的应用性能,但考虑到其用量大等特点,需综合考虑污染物去除效率与经济成本等诸多因素。
4 其它领域陶瓷工业是HNT的一个传统应用领域,也是目前最主要的应用领域[117-118]。每年陶瓷工业消耗成千上万吨HNT[119]。HNT作为天然纳米管,具有纤维增强功能,与填充剂、流平剂混合后具有骨架支撑结构,可显著增强配体强度,是制备超薄精细陶瓷的理想原料。HNT也可作为矿物原料制备莫来石-锆石复合物[120]和沸石分子筛[121]。HNT在能源领域也有应用。由于具备高比表面积、中空管状结构和大层间域,HNT具有作为储氢材料的潜力。而且,HNT易于改性,或可通过负载过渡金属进一步提升其吸氢储氢能力[122]。Liang等[123]将超疏水性聚二甲硅氧烷改性的HNT用于负载相变材料,明显提升了相变材料的热稳定性,或可用于太阳能储存系统。HNT还可用于制备无溶剂纳米流体,该流体在室温下表现流动性,且可通过HNT在不同温度下的热处理调控其流变性[124]。Xu等[125]以HNT为原料制备了大尺度定向膜,膜中纳米管间的均一空隙可用于离子传导。HNT可用作动物饲料添加剂减轻玉米烯酮对动物的毒性[126-127],也可用于制备抗菌性食品包装材料[128],在食品或饲料领域具有应用潜力。
5 结论与展望埃洛石是地球上产出的仅有的几种纳米管状矿物之一,是一种储量丰富、廉价易得的天然介孔型纳米材料。近年来,随着纳米科学与技术的发展,埃洛石相关应用研究发展迅速。由于具有低细胞毒性、高生物相容性、环境友好以及介孔型内腔孔,埃洛石可用于药物或生物活性分子的可控释放、医疗植入物、癌细胞分离、组织工程支架、化妆品、纳米反应容器和纳米模板、污染物的吸附固定和催化降解等,在医学与生物医学、纳米复合材料、环境污染治理等诸多领域具有广阔的应用前景,是我国具有资源优势的重要非金属矿产资源。
当前,埃洛石作为一维纳米材料的科学意义和经济价值越来越受到学术界乃至产业界的重视。尤其是,埃洛石作为药物和生物活性分子载体的研究是目前的热点。虽然已有大量研究证明埃洛石具有良好的生物相容性和低的细胞毒性,但仍需进一步开展埃洛石及其衍生品在人体内的相容性和细胞毒性的相关实验研究。另一方面,不同产地乃至同一产地不同矿区的管状埃洛石的微形貌(如管内/外径、管长、表面缺陷)存在较大差异[1, 3, 91],这些差异势必影响埃洛石的应用性能。另外,埃洛石的内、外表面均可通过物理或化学方法进行选择性改性来提升其应用性能。因此,需深入开展埃洛石“结构-性质-应用”研究,进一步拓展埃洛石的应用领域,提升其应用性能,从而实现埃洛石这一天然纳米管状矿物的高科技、高附加值开发利用。
| [1] |
Yuan P, Tan DY, Annabi-Bergaya F. Properties and applications of halloysite nanotubes:Recent research advances and future prospects[J]. Applied Clay Science, 2015, 112: 75-93. |
| [2] |
袁鹏, 杜培鑫, 周军明, 等. 铝硅酸盐纳米矿物的地质意义和资源价值再认识[J]. 岩石学报, 2019, 35: 164-176. DOI:10.18654/1000-0569/2019.01.13 |
| [3] |
谭道永, 曲天晨, 董发勤, 等. 管状埃洛石的微结构对其负载活性的制约[J]. 矿物学报, 2018, 38(4): 437-442. |
| [4] |
Yuan P, Southon PD, Liu ZW, et al. Functionalization of halloysite clay nanotubes by grafting with gamma-aminopropyltriethoxysilane[J]. Journal of Physical Chemistry C, 2008, 112(40): 15742-15751. DOI:10.1021/jp805657t |
| [5] |
Lvov YM, Shchukin DG, Möhwald H, et al. Halloysite clay nanotubes for controlled release of protective agents[J]. ACS Nano, 2008, 2(5): 814-820. DOI:10.1021/nn800259q |
| [6] |
Liu RC, Zhang B, Mei DD, et al. Adsorption of methyl violet from aqueous solution by halloysite nanotubes[J]. Desalination, 2011, 268(1-3): 111-116. DOI:10.1016/j.desal.2010.10.006 |
| [7] |
Tan DY, Yuan P, Liu D, et al. Surface modifications of halloysite[M]//Yuan P, Thill A, Annabi-Bergaya F. Nanosized tubular clay minerals. Amsterdam: Elsevier, 2016: 167-201.
|
| [8] |
袁鹏. 纳米结构矿物的特殊结构和表-界面反应性[J]. 地球科学, 2018, 43(5): 1384-1407. |
| [9] |
Ma W, Wu H, Higaki Y, et al. Halloysite nanotubes:Green nanomaterial for functional organic-inorganic nanohybrids[J]. The Chemical Record, 2018, 18: 1-15. DOI:10.1002/tcr.201880101 |
| [10] |
Du P, Yuan P, Thill A, et al. Insights into the formation mechanism of imogolite from a full-range observation of its sol-gel growth[J]. Applied Clay Science, 2017, 150: 115-124. DOI:10.1016/j.clay.2017.09.021 |
| [11] |
Shchukin DG, Sukhorukov GB, Price RR, et al. Halloysite nanotubes as biomimetic nanoreactors[J]. Small, 2005, 1(5): 510-513. DOI:10.1002/smll.200400120 |
| [12] |
Yuan P, Southon PD, Liu ZW, et al. Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release[J]. Nanotechnology, 2012, 23(37): 375705. DOI:10.1088/0957-4484/23/37/375705 |
| [13] |
Tan DY, Yuan P, Annabi-Bergaya F, et al. Natural halloysite nanotubes as mesoporous carriers for the loading of ibuprofen[J]. Microporous and Mesoporous Materials, 2013, 179: 89-98. DOI:10.1016/j.micromeso.2013.05.007 |
| [14] |
Luo P, Zhao YF, Zhang B, et al. Study on the adsorption of neutral red from aqueous solution onto halloysite nanotubes[J]. Water Research, 2010, 44(5): 1489-1497. DOI:10.1016/j.watres.2009.10.042 |
| [15] |
Lvov YM, DeVilliers MM, Fakhrullin RF. The application of halloysite tubule nanoclay in drug delivery[J]. Expert Opinion on Drug Delivery, 2016, 13(7): 977-986. DOI:10.1517/17425247.2016.1169271 |
| [16] |
刘明贤.具有新型界面结构的聚合物-埃洛石纳米复合材料[D].广州: 华南理工大学, 2010. http://cdmd.cnki.com.cn/Article/CDMD-10561-2010229227.htm
|
| [17] |
Vergaro V, Abdullayev E, Lvov YM, et al. Cytocompatibility and uptake of halloysite clay nanotubes[J]. Biomacromolecules, 2010, 11(3): 820-826. DOI:10.1021/bm9014446 |
| [18] |
Lai XY, Agarwal M, Lvov YM, et al. Proteomic profiling of halloysite clay nanotube exposure in intestinal cell co-culture[J]. Journal of Applied Toxicology, 2013, 33(11): 1316-1329. |
| [19] |
Ahmed FR, Shoaib MH, Azhar M, et al. In-vitro assessment of cytotoxicity of halloysite nanotubes against HepG2, HCT116 and human peripheral blood lymphocytes[J]. Colloids and Surfaces B-Biointerfaces, 2015, 135: 50-55. DOI:10.1016/j.colsurfb.2015.07.021 |
| [20] |
Kamalieva RF, Ishmukhametov IR, Batasheva SN, et al. Uptake of halloysite clay nanotubes by human cells:Colourimetric viability tests and microscopy study[J]. Nano-Structures & Nano-Objects, 2018, 15: 54-60. |
| [21] |
Cavallaro G, Lazzara G, Konnova S, et al. Composite films of natural clay nanotubes with cellulose and chitosan[J]. Green Materials, 2014, 2(4): 232-242. DOI:10.1680/gmat.14.00014 |
| [22] |
Fakhrullina GI, Akhatova FS, Lvov YM, et al. Toxicity of halloysite clay nanotubes in vivo:A Caenorhabditis elegans study[J]. Environmental Science-Nano, 2015, 2(1): 54-59. DOI:10.1039/C4EN00135D |
| [23] |
Kryuchkova M, Danilushkina A, Lvov Y, et al. Evaluation of toxicity of nanoclays and graphene oxide in vivo:A Paramecium caudatum study[J]. Environmental Science-Nano, 2016, 3(2): 442-452. DOI:10.1039/C5EN00201J |
| [24] |
Bellani L, Giorgetti L, Riela S, et al. Ecotoxicity of halloysite nanotube-supported palladium nanoparticles in Raphanus sativus L[J]. Environmental Toxicology and Chemistry, 2016, 35(10): 2503-2510. DOI:10.1002/etc.3412 |
| [25] |
Wang X, Gong J, Gui Z, et al. Halloysite nanotubes-induced Al accumulation and oxidative damage in liver of mice after 30-day repeated oral administration[J]. Environmental Toxicology, 2018, 33: 623-630. DOI:10.1002/tox.22543 |
| [26] |
王雪, 徐小龙, 龚家春, 等. 埃洛石纳米管的口服毒性研究(英文)[J]. 中国科学技术大学学报, 2017, 47(12): 988-995. DOI:10.3969/j.issn.0253-2778.2017.12.003 |
| [27] |
Wang X, Gong J, Rong R, et al. Halloysite nanotubes-induced Al accumulation and fibrotic response in lung of mice after 30-day repeated oral administration[J]. Journal of Agricultural and Food Chemistry, 2018, 66(11): 2925-2933. DOI:10.1021/acs.jafc.7b04615 |
| [28] |
Leporatti S. Halloysite clay nanotubes as nano-bazookas for drug delivery[J]. Polymer International, 2017, 66(8): 1111-1118. DOI:10.1002/pi.5347 |
| [29] |
Hanif M, Jabbar F, Sharif S, et al. Halloysite nanotubes as a new drug-delivery system:A review[J]. Clay Minerals, 2016, 51(3): 469-477. DOI:10.1180/claymin.2016.051.3.03 |
| [30] |
Price RR, Gaber BP, Lvov Y. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral[J]. Journal of Microencapsulation, 2001, 18(6): 713-722. DOI:10.1080/02652040010019532 |
| [31] |
Veerabadran NG, Price RR, Lvov YM. Clay nanotubes for encapsulation and sustained release of drugs[J]. Nano, 2007, 2(2): 115-120. DOI:10.1142/S1793292007000441 |
| [32] |
Ward CJ, Song S, Davis EW. Controlled release of tetracycline-HCl from halloysite-polymer composite films[J]. Journal of nanoscience and nanotechnology, 2010, 10(10): 6641-6649. DOI:10.1166/jnn.2010.2647 |
| [33] |
Carazo E, Borregosánchez A, Garcíavillén F, et al. Assessment of halloysite nanotubes as vehicles of isoniazid[J]. Colloids & Surfaces B Biointerfaces, 2017, 160: 337-344. |
| [34] |
Tan DY, Yuan P, Annabi-Bergaya F, et al. Loading and in vitro release of ibuprofen in tubular halloysite[J]. Applied Clay Science, 2014, 96: 50-55. DOI:10.1016/j.clay.2014.01.018 |
| [35] |
Rawtani D, Pandey G, Tharmavaram M, et al. Development of a novel 'nanocarrier' system based on halloysite nanotubes to overcome the complexation of ciprofloxacin with iron:An in vitro approach[J]. Applied Clay Science, 2017, 150: 293-302. DOI:10.1016/j.clay.2017.10.002 |
| [36] |
Liu F, Bai L, Zhang H, et al. Smart H2O2-responsive drug delivery system made by halloysite nanotubes and carbohydrate polymers[J]. ACS Applied Materials & Interfaces, 2017, 9(37): 31626. |
| [37] |
Liu J, Zhang Y, Zeng Q, et al. Delivery of RIPK4 small interfering RNA for bladder cancer therapy using natural halloysite nanotubes[J]. Science Advances, 2019, 5(9): eaaw6499. DOI:10.1126/sciadv.aaw6499 |
| [38] |
Ana CS, Caroline F, Francisco V, et al. Halloysite clay nanotubes for life sciences applications:From drug encapsulation to bioscaffold[J]. Advances in Colloid and Interface Science, 2018, 257: 58-70. DOI:10.1016/j.cis.2018.05.007 |
| [39] |
Nitya G, Nair GT, Mony U, et al. In vitro evaluation of electrospun PCL/nanoclay composite scaffold for bone tissue engineering[J]. Journal of Materials Science-Materials in Medicine, 2012, 23(7): 1749-1761. DOI:10.1007/s10856-012-4647-x |
| [40] |
Liu MX, Wu CC, Jiao YP, et al. Chitosan-halloysite nanotubes nanocomposite scaffolds for tissue engineering[J]. Journal of Materials Chemistry B, 2013, 1(15): 2078-2089. DOI:10.1039/c3tb20084a |
| [41] |
Liu MX, Dai LB, Shi HZ, et al. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering[J]. Materials Science & Engineering C-Materials for Biological Applications, 2015, 49: 700-712. |
| [42] |
Wei WB, Abdullayev E, Hollister A, et al. Clay nanotube/poly(methyl methacrylate) bone cement composites with sustained antibiotic release[J]. Macromolecular Materials and Engineering, 2012, 297(7): 645-653. DOI:10.1002/mame.201100309 |
| [43] |
Alavi M, Totonchi A, Okhovat MA, et al. The effect of a new impregnated gauze containing bentonite and halloysite minerals on blood coagulation and wound healing[J]. Blood Coagulation & Fibrinolysis, 2014, 25(8): 856-859. |
| [44] |
Hughes AD, King MR. Use of naturally occurring halloysite nanotubes for enhanced capture of flowing cells[J]. Langmuir, 2010, 26(14): 12155-12164. DOI:10.1021/la101179y |
| [45] |
Hughes AD, Mattison J, Powderly JD, et al. Rapid isolation of viable circulating tumor cells from patient blood samples[J]. Jove-Journal of Visualized Experiments, 2012(64): 4248. |
| [46] |
He R, Liu MX, Shen Y, et al. Large-area assembly of halloysite nanotubes for enhancing the capture of tumor cells[J]. Journal of Materials Chemistry B, 2017, 5(9): 1712-1723. DOI:10.1039/C6TB02538B |
| [47] |
Liu M, Huo Z, Liu T, et al. Self-assembling halloysite nanotubes into concentric ring patterns in a sphere-on-flat geometry[J]. Langmuir, 2017, 33: 3088-3098. DOI:10.1021/acs.langmuir.6b04460 |
| [48] |
Stavitskaya AV, Novikov AA, Kotelev MS, et al. Fluorescence and cytotoxicity of cadmium sulfide quantum dots stabilized on clay nanotubes[J]. Nanomaterials, 2018, 8: 806391. |
| [49] |
Abdullayev E, Lvov Y. Clay nanotubes for corrosion inhibitor encapsulation:Release control with end stoppers[J]. Journal of Materials Chemistry, 2010, 20(32): 6681-6687. DOI:10.1039/c0jm00810a |
| [50] |
杜阳, 刘颖, 冯年平. 埃洛石:缓释药物的新型载体[J]. 药学进展, 2012, 36(7): 315-320. DOI:10.3969/j.issn.1001-5094.2012.07.004 |
| [51] |
Donaldson L. Halloysite clay nanotubes hold promise[J]. Materials Today, 2016, 19(1): 5-6. |
| [52] |
Du ML, Guo BC, Jia DM. Newly emerging applications of halloysite nanotubes:A review[J]. Polymer International, 2010, 59(5): 574-582. |
| [53] |
张俊珩.埃洛石纳米管的表面改性及其对环氧树脂复合材料结构与性能的影响[D]广州: 华南理工大学, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10561-1012452857.htm
|
| [54] |
伍巍, 吴鹏君, 何丁, 等. 埃洛石纳米管在高分子纳米复合材料中的应用进展[J]. 化工进展, 2011, 30(12): 2647-2651, 2657. |
| [55] |
Lu D, Chen HB, Wu JS, et al. Direct measurements of the young's modulus of a single halloysite nanotube using a transmission electron microscope with a bending stage[J]. Journal of Nanoscience and Nanotechnology, 2011, 11(9): 7789-7793. DOI:10.1166/jnn.2011.4720 |
| [56] |
Abdullayev E, Lvov Y. Halloysite clay nanotubes as a ceramic "skeleton" for functional biopolymer composites with sustained drug release[J]. Journal of Materials Chemistry B, 2013, 1(23): 2894-2903. DOI:10.1039/c3tb20059k |
| [57] |
Lvov Y, Abdullayev E. Functional polymer-clay nanotube composites with sustained release of chemical agents[J]. Progress in Polymer Science, 2013, 38(10-11): 1690-1719. DOI:10.1016/j.progpolymsci.2013.05.009 |
| [58] |
Lvov Y, Wang W, Zhang L, et al. Halloysite clay nanotubes for loading and sustained release of functional compounds[J]. Advanced Materials, 2015, 28(6): 1227-1250. |
| [59] |
Ismail H, Pasbakhsh P, Fauzi MNA, et al. Morphological, thermal and tensile properties of halloysite nanotubes filled ethylene propylene diene monomer (EPDM) nanocomposites[J]. Polymer Testing, 2008, 27(7): 841-850. DOI:10.1016/j.polymertesting.2008.06.007 |
| [60] |
Du ML, Guo BC, Lei YD, et al. Carboxylated butadiene-styrene rubber/halloysite nanotube nanocomposites:Interfacial interaction and performance[J]. Polymer, 2008, 49(22): 4871-4876. DOI:10.1016/j.polymer.2008.08.042 |
| [61] |
何毅, 丁亿鑫, 章杰, 等. TiO_2负载埃洛石纳米管杂化材料的制备及其在环氧复合涂层中的应用[J]. 涂料工业, 2015, 45(5): 1-6. DOI:10.3969/j.issn.0253-4312.2015.05.001 |
| [62] |
Ning N-Y, Yin Q-J, Luo F, et al. Crystallization behavior and mechanical properties of polypropylene/halloysite composites[J]. Polymer, 2007, 48(25): 7374-7384. DOI:10.1016/j.polymer.2007.10.005 |
| [63] |
Deng SQ, Zhang JN, Ye L, et al. Toughening epoxies with halloysite nanotubes[J]. Polymer, 2008, 49(23): 5119-5127. DOI:10.1016/j.polymer.2008.09.027 |
| [64] |
Abdullayev E, Price R, Shchukin D, et al. Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole[J]. ACS Applied Materials & Interfaces, 2009, 1(7): 1437-1443. |
| [65] |
Liu MX, Zhang Y, Zhou CR. Nanocomposites of halloysite and polylactide[J]. Applied Clay Science, 2013, 75-76: 52-59. DOI:10.1016/j.clay.2013.02.019 |
| [66] |
Smith RJ, Holder KM, Ruiz S, et al. Environmentally benign halloysite nanotube multilayer assembly significantly reduces polyurethane flammability[J]. Advanced Functional Materials, 2017, 28(27): 1703289. |
| [67] |
Liu YC, Tu WW, Chen MY, et al. A mussel-induced method to fabricate reduced graphene oxide/halloysite nanotubes membranes for multifunctional applications in water purification and oil/water separation[J]. Chemical Engineering Journal, 2018, 336: 263-277. DOI:10.1016/j.cej.2017.12.043 |
| [68] |
Lazzara G, Cavallaro G, Panchal A, et al. An assembly of organic-inorganic composites using halloysite clay nanotubes[J]. Current Opinion in Colloid & Interface Science, 2018, 35: 42-50. |
| [69] |
Wu F, Zheng JQ, Li ZX, et al. Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation[J]. Chemical Engineering Journal, 2019, 359: 672-683. DOI:10.1016/j.cej.2018.11.145 |
| [70] |
Qin LJ, Zhao YF, Liu JD, et al. Oriented clay nanotube membrane assembled on microporous polymeric substrates[J]. ACS Applied Materials & Interfaces, 2016, 8(50): 34914-34923. |
| [71] |
Massaro M, Colletti CG, Lazzara G, et al. Halloysite nanotubes as support for metal-based catalysts[J]. Journal of Materials Chemistry A, 2017, 5(26): 13276-13293. DOI:10.1039/C7TA02996A |
| [72] |
Philip A, Lihavainen J, Keinänen M, et al. Gold nanoparticle-decorated halloysite nanotubes-Selective catalysts for benzyl alcohol oxidation[J]. Applied Clay Science, 2017, 143: 80-88. DOI:10.1016/j.clay.2017.03.015 |
| [73] |
Sanchez-Ballester NM, Ramesh GV, Tanabe T, et al. Activated interiors of clay nanotubes for agglomeration-tolerant automotive exhaust remediation[J]. Journal of Materials Chemistry A, 2015, 3(12): 6614-6619. DOI:10.1039/C4TA06966H |
| [74] |
Pandey G, Munguambe DM, Tharmavaram M, et al. Halloysite nanotubes-An efficient 'nano-support' for the immobilization of α-amylase[J]. Applied Clay Science, 2017, 136: 184-191. DOI:10.1016/j.clay.2016.11.034 |
| [75] |
Shu Z, Zhang Y, Yang Q, et al. Halloysite nanotubes supported Ag and ZnO nanoparticles with synergistically enhanced antibacterial activity[J]. Nanoscale Research Letters, 2017, 12(1): 135. DOI:10.1186/s11671-017-1859-5 |
| [76] |
Shu Z, Zhang Y, Ouyang J, et al. Characterization and synergetic antibacterial properties of ZnO and CeO2 supported by halloysite[J]. Applied Surface Science, 2017, 420: 833-838. DOI:10.1016/j.apsusc.2017.05.219 |
| [77] |
Zahidah KA, Kakooei S, Ismail MC, et al. Halloysite nanotubes as nanocontainer for smart coating application:A review[J]. Progress in Organic Coatings, 2017, 111: 175-185. DOI:10.1016/j.porgcoat.2017.05.018 |
| [78] |
王绮, 李澄, 郑顺丽, 等. 缓蚀剂在埃洛石上的担载与释放规律研究[J]. 材料导报, 2016, 30(8): 61-64. |
| [79] |
Shchukin DG, Lamaka SV, Yasakau KA, et al. Active anticorrosion coatings with halloysite nanocontainers[J]. The Journal of Physical Chemistry C, 2008, 112(4): 958-964. DOI:10.1021/jp076188r |
| [80] |
Liu GY, Kang FY, Li BH, et al. Characterization of the porous carbon prepared by using halloysite as template and its application to EDLC[J]. Journal of Physics and Chemistry of Solids, 2006, 67(5-6): 1186-1189. DOI:10.1016/j.jpcs.2006.01.044 |
| [81] |
Li CP, Liu JG, Qu XZ, et al. Polymer-modified halloysite composite nanotubes[J]. Journal of Applied Polymer Science, 2008, 110(6): 3638-3646. DOI:10.1002/app.28879 |
| [82] |
Zhang L, Liu P. Facile fabrication of uniform polyaniline nanotubes with tubular aluminosilicates as templates[J]. Nanoscale Research Letters, 2008, 3(8): 299-302. DOI:10.1007/s11671-008-9155-z |
| [83] |
Wang AP, Kang FY, Huang ZH, et al. Synthesis of mesoporous carbon nanosheets using tubular halloysite and furfuryl alcohol by a template-like method[J]. Microporous and Mesoporous Materials, 2008, 108(1-3): 318-324. DOI:10.1016/j.micromeso.2007.04.021 |
| [84] |
周述慧, 传秀云. 埃洛石为模板合成中孔炭[J]. 无机材料学报, 2014, 29(6): 584-588. |
| [85] |
程志林, 曹宝冲, 刘赞. 埃洛石纳米管模板法一步法制备一维碳纳米管/碳纳米棒混合纳米碳材料[J]. 无机化学学报, 2018, 34(10): 1808-1816. DOI:10.11862/CJIC.2018.228 |
| [86] |
Glotov A, Levshakov N, Stavitskaya A, et al. Templated self-assembly of ordered mesoporous silica on clay nanotubes[J]. Chemical Communications, 2019, 55(38): 5507-5510. DOI:10.1039/C9CC01935A |
| [87] |
Rostamzadeh T, Khan MSI, Riche K, et al. Rapid and controlled in situ growth of noble netal nanostructures within halloysite clay nanotubes[J]. Langmuir, 2017, 33(45): 13051-13059. DOI:10.1021/acs.langmuir.7b02402 |
| [88] |
Vinokurov V, Glotov A, Chudakov Y, et al. Core/shell ruthenium-halloysite nanocatalysts for hydrogenation of phenol[J]. Industrial & Engineering Chemistry Research, 2017, 56(47): 14043-14052. |
| [89] |
Vinokurov VA, Stavitskaya AV, Ivanov EV, et al. Halloysite nanoclay based CdS formulations with high catalytic activity in hydrogen evolution reaction under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(12): 11316-11323. |
| [90] |
孙攀, 刘国明, 吕冬, 等. 埃洛石纳米管增强聚合物复合材料研究进展[J]. 中国科学:技术科学, 2015, 45(6): 602-616. |
| [91] |
Yuan P, Thill A, Bergaya F. Nanosized Tubular Clay Minerals[M]. Amsterdam: Elsevier, 2016.
|
| [92] |
秦嘉旭, 赵斌伟, 林雅逢, 等. 埃洛石纳米管的改性及应用研究[J]. 河南化工, 2011, 28(11): 27-30. DOI:10.3969/j.issn.1003-3467.2011.11.007 |
| [93] |
Zhang HL, Cheng C, Song HZ, et al. A facile one-step grafting of polyphosphonium onto halloysite nanotubes initiated by Ce(Ⅳ)[J]. Chemical Communications, 2019, 55(8): 1040-1043. DOI:10.1039/C8CC08667B |
| [94] |
Zhao M, Liu P. Adsorption behavior of methylene blue on halloysite nanotubes[J]. Microporous and Mesoporous Materials, 2008, 112(1): 419-424. |
| [95] |
Krawczyk-Coda M. Halloysite nanotubes as a solid sorbent in ultrasound-assisted dispersive micro solid-phase extraction for the determination of bismuth in water samples using high-resolution continuum source graphite-furnace atomic absorption spectrometry[J]. Spectrochimica Acta Part B-Atomic Spectroscopy, 2017, 129: 21-27. DOI:10.1016/j.sab.2017.01.003 |
| [96] |
陈廷方, 易发成, 冯启明, 等. 北川埃洛石黏土对Sr、Co、Cs的吸附性能研究[J]. 中国矿业, 2011, 20(3): 74-77. DOI:10.3969/j.issn.1004-4051.2011.03.021 |
| [97] |
Maziarz P, Matusik J. The effect of acid activation and calcination of halloysite on the efficiency and selectivity of Pb(Ⅱ), Cd(Ⅱ), Zn(Ⅱ) and As(Ⅴ) uptake[J]. Clay Minerals, 2016, 51(3): 385-394. DOI:10.1180/claymin.2016.051.3.06 |
| [98] |
Kilislioglu A, Bilgin B. Adsorption of uranium on halloysite[J]. Radiochimica Acta, 2002, 90(3): 155-160. |
| [99] |
Zheng YA, Wang AQ. Enhanced adsorption of ammonium using hydrogel composites based on chitosan and halloysite[J]. Journal of Macromolecular Science Part A-Pure and Applied Chemistry, 2010, 47(1): 33-38. |
| [100] |
Deng LL, Yuan P, Liu D, et al. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors[J]. Applied Clay Science, 2017, 143: 184-191. DOI:10.1016/j.clay.2017.03.035 |
| [101] |
Zhou TZ, Li CP, Jin HL, et al. Effective adsorption/reduction of Cr(Ⅵ) oxyanion by halloysite@polyaniline hybrid nanotubes[J]. ACS Applied Materials & Interfaces, 2017, 9(7): 6030-6043. |
| [102] |
Abdullayev E, Joshi A, Wei WB, et al. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide[J]. ACS Nano, 2012, 6(8): 7216-7226. DOI:10.1021/nn302328x |
| [103] |
Shu Z, Chen Y, Zhou J, et al. Preparation of halloysite-derived mesoporous silica nanotube with enlarged specific surface area for enhanced dye adsorption[J]. Applied Clay Science, 2016, 132: 114-121. |
| [104] |
Deng L, Yuan P, Liu D, et al. Effects of calcination and acid treatment on improving benzene adsorption performance of halloysite[J]. Applied Clay Science, 2019, 181: 105240. DOI:10.1016/j.clay.2019.105240 |
| [105] |
Almasri DA, Saleh NB, Atieh MA, et al. Adsorption of phosphate on iron oxide doped halloysite nanotubes[J]. Scientific Reports, 2019, 9: 3232. DOI:10.1038/s41598-019-39035-2 |
| [106] |
Yu L, Wang H, Zhang Y, et al. Recent advances in halloysite nanotube derived composites for water treatment[J]. Environmental Science-Nano, 2016, 3(1): 28-44. DOI:10.1039/C5EN00149H |
| [107] |
Zatta L, Gardolinski JEFD, Wypych F. Raw halloysite as reusable heterogeneous catalyst for esterification of lauric acid[J]. Applied Clay Science, 2011, 51(1-2): 165-169. DOI:10.1016/j.clay.2010.10.020 |
| [108] |
Tae JW, Jang BS, Kim JR, et al. Catalytic degradation of polystyrene using acid-treated halloysite clays[J]. Solid State Ionics, 2004, 172(1-4): 129-133. DOI:10.1016/j.ssi.2004.05.013 |
| [109] |
Wang R, Jiang G, Ding Y, et al. Photocatalytic activity of heterostructures based on TiO2 and halloysite nanotubes[J]. ACS Appl Mater Interfaces, 2011, 3(10): 4154-4158. DOI:10.1021/am201020q |
| [110] |
Machado GS, Castro KADF, Wypych F, et al. Immobilization of metalloporphyrins into nanotubes of natural halloysite toward selective catalysts for oxidation reactions[J]. Journal of Molecular Catalysis A-Chemical, 2008, 283(1-2): 99-107. DOI:10.1016/j.molcata.2007.12.009 |
| [111] |
Wang L, Chen JL, Ge L, et al. Halloysite-nanotube-supported Ru nanoparticles for ammonia catalytic decomposition to produce COx-free hydrogen[J]. Energy & Fuels, 2011, 25(8): 3408-3416. |
| [112] |
von Klitzing R, Stehl D, Pogrzeba T, et al. Halloysites stabilized emulsions for hydroformylation of long chain olefins[J]. Advanced Materials Interfaces, 2017, 4: 1600435. DOI:10.1002/admi.201600435 |
| [113] |
Owoseni O, Zhang YH, Su Y, et al. Tuning the wettability of halloysite clay nanotubes by surface carbonization for optimal emulsion stabilization[J]. Langmuir, 2015, 31(51): 13700-13707. DOI:10.1021/acs.langmuir.5b03878 |
| [114] |
Yu T, Swientoniewski LT, Omarova M, et al. Investigation of amphiphilic polypeptoid-functionalized halloysite nanotubes as emulsion stabilizer for oil spill remediation[J]. ACS Applied Materials & Interfaces, 2019, 11(31): 27944-27953. |
| [115] |
Zhou L, He Y, Shi H, et al. One-pot route to synthesize HNTs@PVDF membrane for rapid and effective separation of emulsion-oil and dyes from waste water[J]. Journal of Hazardous Materials, 2019, 380: 120865. DOI:10.1016/j.jhazmat.2019.120865 |
| [116] |
Wei YF, Yuan P, Liu D, et al. Activation of natural halloysite nanotubes by introducing lanthanum oxycarbonate nanoparticles via co-calcination for outstanding phosphate removal[J]. Chemical Communications, 2019, 55(14): 2110-2113. DOI:10.1039/C8CC10314C |
| [117] |
Wilson I. Kaolin and halloysite deposits of China[J]. Clay Minerals, 2004, 39(1): 1-15. DOI:10.1180/0009855043910116 |
| [118] |
Wilson MJ. Clay mineralogical and related characteristics of geophagic materials[J]. Journal of Chemical Ecology, 2003, 29(7): 1525-1547. DOI:10.1023/A:1024262411676 |
| [119] |
Joussein E, Petit S, Churchman J, et al. Halloysite clay minerals - A review[J]. Clay Minerals, 2005, 40(4): 383-426. DOI:10.1180/0009855054040180 |
| [120] |
Raghdi A, Heraiz M, Sahnoune F, et al. Mullite-zirconia composites prepared from halloysite reaction sintered with boehmite and zirconia[J]. Applied Clay Science, 2017, 146: 70-80. DOI:10.1016/j.clay.2017.05.037 |
| [121] |
赵亚婔.埃洛石纳米管及其改性产品在废水处理中的应用研究[D]郑州: 郑州大学, 2010. http://cdmd.cnki.com.cn/Article/CDMD-10459-1011017727.htm
|
| [122] |
Ramadass K, Sathish CI, Johns A, et al. Characterization and hydrogen storage performance of halloysite nanotubes[J]. Journal of nanoscience and nanotechnology, 2019, 19(12): 7892-7898. DOI:10.1166/jnn.2019.16751 |
| [123] |
Liang W, Wu Y, Sun H, et al. Halloysite clay nanotubes based phase change material composites with excellent thermal stability for energy saving and storage[J]. RSC Advances, 2016, 6(24): 19669-19675. DOI:10.1039/C5RA27964J |
| [124] |
Du P, Liu D, Yuan P, et al. Controlling the macroscopic liquid-like behaviour of halloysite-based solvent-free nanofluids via a facile core pretreatment[J]. Applied Clay Science, 2018, 156: 126-133. DOI:10.1016/j.clay.2018.01.037 |
| [125] |
Xu P, Zhou Y, Cheng H. Large-scale orientated self-assembled halloysite nanotubes membrane with nanofluidic ion transport properties[J]. Applied Clay Science, 2019, 180: 105184. DOI:10.1016/j.clay.2019.105184 |
| [126] |
Gao R, Meng Q, Li J, et al. Modified halloysite nanotubes reduce the toxic effects of zearalenone in gestating sows on growth and muscle development of their offsprings[J]. Journal of Animal Science and Biotechnology, 2016, 7: 14. DOI:10.1186/s40104-016-0071-2 |
| [127] |
Yin ST, Meng QW, Zhang BR, et al. Alleviation of zearalenone toxicity by modified halloysite nanotubes in the immune response of swine[J]. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure & Risk Assessment, 2015, 32(1): 87-99. |
| [128] |
Tas C, Hendessi S, Baysal M, et al. Halloysite nanotubes/polyethylene nanocomposites for active food packaging materials with ethylene scavenging and gas barrier properties[J]. Food and Bioprocess Technology, 2017, 10(4): 789-798. DOI:10.1007/s11947-017-1860-0 |
 2019
2019



