2. 浙江大学 化学工程与生物工程学院, 浙江 杭州 310058;
3. 浙江师范大学 地理与环境科学学院, 浙江 金华 321004
2. College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310058, China;
3. College of Geography and Environmental Sciences, Zhejiang Normal University, Jinhua 321004, China
挥发性有机化合物(volatile organic compounds, VOCs)的工业源主要包括石油炼制、煤炭加工、油类储运和销售过程、涂料和农药生产等,生活源包括建筑装饰装修、餐饮服务等[1],VOCs排放能引发雾霾、光化学烟雾等一系列大气复合污染环境问题[2~3]。2021年11月,中共中央、国务院印发《关于深入打好污染防治攻坚战的意见》,要求安全高效推进挥发性有机物综合治理,目标到2025年,挥发性有机物排放总量比2020年下降10%[4]。因此,VOCs有效控制是当前大气污染防治的重要内容。
以2019年为例,我国工业源VOCs排放量达1 330.49万吨,生活源VOCs排放量为489.56万吨[5]。采用吸附、热处理、低温等离子体等方法直接处置会导致巨大的化学能源浪费[6~7],并存在成本高、二次污染、效率不高等缺点[8]。微生物燃料电池(microbial fuel cell,MFC)通过电极与微生物间的相互作用,将微生物降解有机物产生的电子提取出来,从而将化学能转化为电能[9-12]。MFC处理VOCs不仅具有传统生物法成本低、二次污染少、适应性广的优势[13],而且不需要额外燃料、电能的输入[6],还能够将2.2%~37%的碳固定在生物质内[14],具有显著的低碳优势。因此,采用MFC净化VOCs是碳达峰碳中和背景下VOCs废气处理技术发展更新的绿色低碳方案。
MFC系统去除气态VOCs分为3个过程:1)VOCs的气-液传质过程;2)VOCs由液相扩散进入生物膜;3)生物膜中VOCs的微生物降解。VOCs生物净化效率会受到气-液传质、生物膜活性等多方面因素影响,疏水性VOCs主要受气-液传质限制,亲水性VOCs主要受微生物活性限制,而对于中等亲水VOCs,气-液传质和微生物活性共同决定了去除率[13, 15-16]。实现传质过程及降解反应过程的强化,对于加快MFC在VOCs废气净化中的应用具有重要科学意义和应用价值。本研究围绕MFC净化VOCs废气技术,重点阐述以传质-反应过程强化为目标的反应器构型创新、高效电极设计、电子传递提升等研究进展,探讨该技术研发所面临的瓶颈及未来研究方向。
2 MFC降解气态VOCs的基本原理 2.1 MFC中的电子传递MFC中气态VOCs净化的基本原理如图 1所示[17-21],主要涉及了气-液传质、液-生物膜传质、生物降解3个基本步骤,与传统的生物膜法主要区别在于MFC中存在电极这一额外的电子受体。由于生物膜附着在电极上,微生物和电极之间产生快速的原位电子转移,促进了VOCs的降解和同步产电。微生物和电极基底之间的电子转移是MFC中最为复杂及关键的过程,也是加速降解反应的突破口之一。该过程可分为直接电子传递(direct electron transfer,DET)和间接电子传递(mediated electron transfer,MET)2种。DET通过胞体与电极以及细胞间的物理接触(细胞色素、纳米导线等)完成电子传输,而MET则是利用氧化还原活性的电子介体实现电极与微生物之间的电子传递[22-24]。总体而言,DET过程相较于MET过程电子传递路径更短、电子传递损失更低,能够实现更有效的电子传递[24-26]。也有研究证实,当DET、MET 2种电子转移方式结合时,产电细菌Shewanella oneidensis MR-1的产电性能明显增强,这为MFC中电子传递增强、反应加速提供了思路[27-28]。
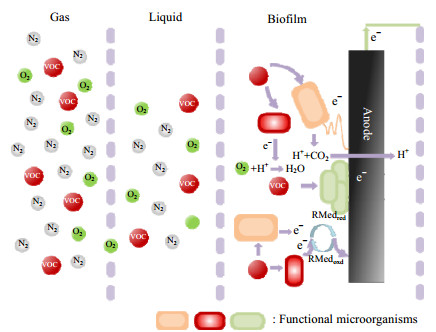
|
图 1 MFC净化VOCs的传质-反应过程及界面电子传递机制(参考文献[21]) Fig.1 Mass transfer, reaction, and interfacial electron transfer processes of VOCs degradation in MFC (reproduced from reference[21]) |
MFC中的功能微生物可以分为产电细菌和VOCs降解细菌,产电细菌主要存在于阳极表面或周围,而VOCs降解菌在电极生物膜外侧及培养基中占主导地位[20, 29],2类功能菌在MFC阳极中共生协作,实现VOCs生物降解同步产电。例如,在以苯为底物的MFC中,尽管在阳极室中苯降解菌Peptococcaceae的含量 < 1%,但苯的降解依旧进行[29],表明更多微生物可以以苯的中间代谢产物作为代谢底物。另外,在MFC接种一种苯酚特异降解菌,也依旧能够产电,但产电功率密度比常见产电菌低一个数量级[30],表明具有高电化学活性的产电菌对于提高MFC的库仑效率有着重要作用。目前,研究人员已筛选出超过30种的产电菌株,如表 1所示,分布于Proteobacteria(变形菌)、Firmicutes(厚壁菌)、Acidobacteria(酸性细菌)、Actinobacteria(放线菌)、Eumycota(真菌)和Chlorophyta(绿藻)等门。
|
|
表 1 已筛选出的MFC中的产电细菌 Table 1 Exoelectrogens isolated from MFC systems |
为了实现不同VOCs底物的能量转化,更多的产电菌被挖掘出来,尤其是那些在极端环境中可以生存和繁衍的微生物对于难降解VOCs的净化及能量回收具有重要意义。例如,Bacillus cereus WL027对质量浓度为0.2 mg·L−1的Cr2+、Co2+、Pb2+和Cu2+等重金属离子有较好的耐受性,同时能在高盐(30 g·L−1 NaCl)条件下降解苯胺,以菌株WL027构建的MFC降解苯胺体系最大电压达到179 mV,库仑效率为64.25%,在能量转化效率方面较普通产电菌有明显优势[67]。Monzon等[68]在处理石油行业产生的100 g·L−1 NaCl高盐废水(含有芳香烃)的MFC阳极中接种Marinobacter hydrocarbonoclasticus与H. praevalens,达到的最大稳定电压为(0.155±0.23) V,对石油的去除效率达到68%,库仑效率达到10%。此外,Adelaja等[69]在MFC反应器中接种厌氧消化污泥,实现对混合石油烃(菲和苯)的去除。在NaCl质量分数达1.5% 的高盐度条件下,仍可以保持良好的电化学和降解性能;同时在40 ℃时,降解速率和电流密度与30 ℃相比均提高了1倍,证实了MFC在炎热气候地区(如非洲)用于地下水中石油污染问题处理的潜在应用价值。
探索电子传递途径对于选择有效措施以增强电子转移并因此提高气态VOCs的去除率至关重要,专用的VOCs降解菌和产电菌可能会竞争电子[70],高效协作的功能菌群对VOCs降解速率、产电能力也起着决定性作用。目前,MFC处理VOCs的研究主要集中在去除效率、产电能力。以甲苯为例,如表 2所示,关于电子转移机制和功能微生物的种间关系的探索有限,相关深入研究的开展有助于获得更好的降解及产电协同性能。
|
|
表 2 甲苯为底物的MFC在不同操作条件下的功能菌群 Table 2 Main functional bacteria in toluene-driven MFC under different operating conditions |
在MFC体系中,传质和微生物降解两个过程的速率共同决定气态VOCs去除效率,两者的加速是MFC中VOCs净化及产电过程强化的基本思路。例如,在传质方面,改进反应器或电极构型可以获得更大的传质面积,在生物降解方面,通过电极的修饰、添加氧化还原介体、外加电位驯化等方式可以提升微生物活性或者反应中电子传递速率。在此基础上,将MFC与其他工艺耦合,开发新型的VOCs净化耦合体系也是一种可行的方法。
3.1 MFC反应器构型设计双室MFC是最为常见的MFC构型,如图 2(a)所示,厌氧条件下的阳极室生物膜中的微生物会分解底物并释放出质子和电子,质子经质子交换膜(proton exchange membrane, PEM)传递至阴极,电子经外电路传递至阴极形成回路,氧化剂(通常为O2)在阴极获得电子与质子结合生成H2O,从而达到去除各种底物的目的[76-78]。
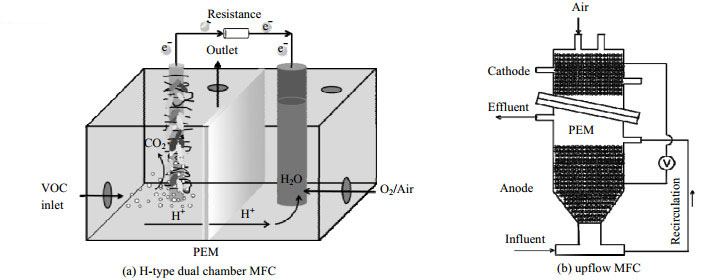
|
图 2 H型双室MFC及升流式MFC结构示意图(参考文献[76, 79]) Fig.2 Schematic diagram of H-type dual chamber MFC and upflow MFC (reproduced from references[76, 79]) |
影响双室MFC性能的结构因素主要包括进出气口位置、电极材料及阴阳极间隔、MFC的内阻和外阻、PEM材料类型等。进出气口相对位置决定了气液接触时间及传质推动力,从而影响VOCs传质效果。较小的外电阻可以降低电子的传递阻力提升电流密度,但会降低输出电压,不利于输出功率的提高;当生物电极内阻与外阻接近时,MFC输出功率密度可达到最高水平。一般将DuPont Nafion 117作为PEM隔开两室;常用阳极材料为碳纸、碳刷等碳质材料,成本低、导电性较好[80-84]。然而双室MFC中一半的反应器体积未直接用以VOCs净化,导致VOCs降解率及输出功率密度偏低。因此,双室MFC的构型改进应提高气-液传质速率、微生物附着量、有效阳极体积为目标[17, 20, 29]。研究人员依据升流式厌氧污泥床反应器(upflow anaerobic sludge bed,UASB)原理,将双室MFC改进为升流式MFC[29, 79],如图 2(b),阴阳极均为石墨纤维,将阴极堆叠在阳极室的上部,通过类似于填料塔同向吸收的操作显著增大气-液接触面积,有利于VOCs的气液传质,并且用于泵送VOCs的能耗较普通双室MFC中的鼓泡方式低。实际上,能够去除VOCs的微生物不仅仅局限于厌氧微生物,好氧微生物的降解动力学反而更占优势[76, 83, 85]。在好氧条件下,仍然能构建MFC系统实现VOCs降解同步产电,即单室MFC系统。单室MFC中阴、阳极在同一室工作,避免了阴极室无效反应器体积,同时O2的存在能够促进难降解VOCs分子的断键、开环过程,有助于VOCs净化负荷的提高。普遍存在的负面影响是O2会与电极形成电子竞争,从而降低MFC产电功率及库仑效率。但也有研究证实,以苯系物为底物的MFC好氧条件下最大功率密度达到(1.71±0.5) W·m−3,相比厌氧条件下提升了4.75倍[86]。因此,单室好氧MFC的恰当选用,能够达到难降解VOCs净化效果及产电性能的协同提升。
3.2 相际传质强化 3.2.1 气体扩散电极气体扩散电极通过使气态VOCs或O2与电极进行接触,直接吸附并扩散进入生物膜或者电极基底发生氧化还原反应。气体电极辅助MFC结构如图 3(a)所示[87],将疏水性气体扩散层(gas diffusion layer,GDL)和聚四氟乙烯层(polytetrafluoroethylene,PTFE)喷射到阳极的气体侧上,VOCs直接传递到电极另一侧生物膜,阴极上直接发生O2还原反应,在反应器外表面生成水。由于省去了液相吸收的传质过程,同时为气相和生物膜提供很大的界面面积,从而大大提高了传质效率,显著降低气液吸收、液固吸附对VOCs降解的速率限制。例如,在气体扩散阳极辅助MFC中,MFC最大功率密度提高了约8倍(从(0.72±0.02)到(6.19±0.45) mW·m−2),甲苯去除效率提高了1倍(从(43.1±2.7)% 到(91.2±2.4)%)[18],甲苯净化负荷((274.5±14.4) g·m−3·h−1)高于或接近目前报道的生物过滤池(255 g·m−3·h−1)[88]和生物滴滤池(275 g·m−3·h−1)的最高去除负荷[89]。
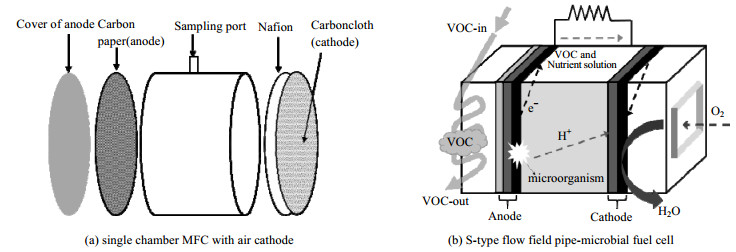
|
图 3 气体电极辅助MFC结构示意图(参考文献[17, 87]) Fig.3 Schematic diagram of gas electrode assisted MFC systems (reproduced from references[17, 87]) |
在气体扩散电极中,可以改进气体分布器结构,进一步增加气-液接触面积,进而提高传质速率。Wu等[17]研制了一种S型流场管-微生物燃料电池(S-type flowfield pipe-microbial fuel cell,SFP-MFC),如图 3(b),SFP-MFC以聚乙烯醇和膜电极组件为气体扩散膜和PEM,在聚乙烯醇层和碳布之间掺杂导电炭黑构建复合阳极,S型流动增加了VOCs实际接触时间,有效提高了吸附传质效果,在空床停留时间为14.35 s、进气负荷为63~3 700 g·m−3·h−1的条件下,对目标污染物乙酸乙酯的去除能提高90%,空床停留时间比生物滴滤器缩短了一半以上。需要注意的是,尽管气体分布器结构的改进可以增强气-液传质过程,但当前仍然存在分布器制作复杂、成本高的缺点。
3.2.2 三维电极填料塔具备较高的比表面积,能够提供丰富的吸收及微生物附着位点,将MFC与之耦合,以导电填料为基底制备具备三维结构的生物膜电极,从而构建新型的生物膜填料塔-微生物燃料电池系统(biopacking tower-microbial fuel cell,BPT-MFC)。如图 4(a)所示,Wu等[19-20]采用与石墨棒结合的导电焦炭为三维阳极,焦炭为气-液传质和生物膜附着提供了高表面积,同时用聚乙烯醇和膜电极组件构建空气阴极高效传递质子,乙酸乙酯去除负荷由14.41提升到29.58 g·m−3·h−1,最大功率密度达到49.1 mW·m−2。该课题组也设计了一种中空空气阴极辅助生物膜填料塔-微生物燃料电池系统[90],如图 4(b),将空气阴极置于BPT-MFC中心,增大PEM对H2O的附着和保留程度,提高阴极接收质子和O2的能力,加速其表面的还原反应。经过参数优化后,乙酸乙酯去除容量高达66.3 g·m−3·h−1,最大功率密度为146.8 mW·m−2,较此前BPT-MFC分别提升至2.24及2.99倍。以上研究结果证实了基于三维生物电极构建BPT-MFC实现VOCs快速降解及高效产电的可行性,将其与空气阴极耦合,可以较好地提升传质和电子传递效率,具有较好的参考价值,但是由于微生物的大量生长,其库仑效率水平不高,同时对于疏水性VOCs的吸收效果离实际应用仍有较大差距,有待采用双相吸收等方法进一步提升。
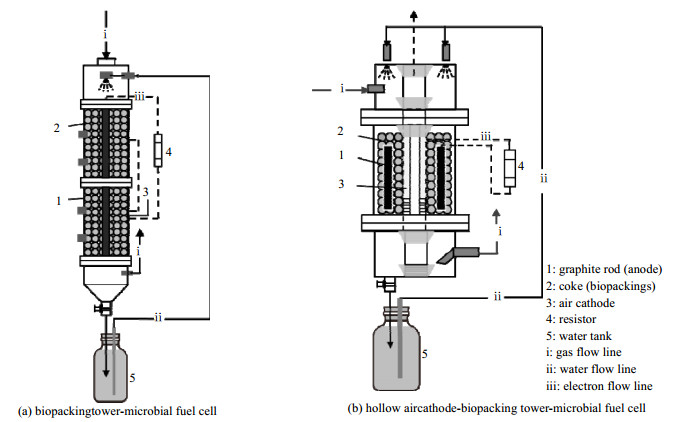
|
图 4 三维电极在VOCs净化MFC中的应用(参考文献[20, 90]) Fig.4 MFC systems with three-dimensional electrodes for VOCs treatment (reproduced from references[20, 90]) |
采用MFC净化苯[91]、甲苯[75]、苯酚[81]、1, 2-二氯乙烷[80]、氯酚[92]、2, 4-二氯酚[70]、2, 4, 6-三氯苯酚[92]等不同种类VOCs,并协同产电的可行性已经得到证实。MFC的运行能够通过电极和微生物之间的电子传递作用刺激微生物更快地降解有机物并导出电子,在闭路条件下,MFC的VOCs去除率往往高于开路条件。然而,含有苯环、卤素基团等结构的VOCs可生化性差,导致降解速率不高,同时产电功率较乙酸钠、乳酸等易降解有机物低一个数量级以上,库仑效率都低于12%。例如,常规易降解有机物驱动的MFC输出功率密度最大文献数据是6 630 mW·m−2[93],而甲苯驱动的MFC最大输出功率密度在100 mW·m−2以下[75, 76, 94]。难降解VOCs有限的降解速率限制了MFC库仑效率,其不完全矿化和发酵等导致了呼吸链中电子的未完全释放,MFC能量输出难达到理想水平[75, 81, 83, 91]。电能输出不高严重阻碍了MFC的接受度和应用,MFC中VOCs降解反应的强化和电子的外运,成为MFC技术应用于VOCs废气净化所面临的重要挑战。
电极修饰是改善微生物细胞与电极之间的电子传递路径的有效手段。聚吡咯、聚苯胺导电聚合物是因导电性强而被称为“合成金属”,具备较好的热稳定性和环境稳定性[95],是一类常用的电极修饰材料。例如,将聚吡咯改性电极应用到MFC中,结果发现在乙酸钠基质中其最大功率密度比未改性的不锈钢电极增加了29倍[96]。碳纳米管、石墨烯等新型碳材料具有较好的导电性、化学稳定性,其小尺寸效应能很好地促进电子转移[62, 97],在文献中取得的强化结果都在2倍以上。银、金或铁等构建的金属纳米颗粒能够直接进入细胞膜间质,直接替代微生物细胞膜上蛋白发挥快速电子转移功能[98-99],从而从细胞尺度上提升MFC产电性能。目前,MFC最大的输出功率密度为6 630 mW·m−2,就是通过将还原氧化石墨烯及银纳米颗粒复合修饰电极应用于希瓦氏菌Shewanella降解乳酸获得[93],表明不同尺度修饰材料的耦合,能够从细胞活性、胞外电子传递等多方面实现MFC降解和协同产电能力的提升。
在电解液中添加氧化还原介体可为微生物构筑人工的间接电子传递通道。电极修饰多数是构建微生物和电极基底间的低阻力电子传递通道,而在阳极室中加入低浓度的铁氰化钾、绿脓菌素和中性红等作为电子介体[29, 75, 94],可以在已有的电子传递基础上,额外增加人工的电子传递通道,以增强间接电子传递的方式提高MFC内的电子传递速率,提高其产电性能和库仑效率[30, 75, 81, 83, 94]。例如,Lin等[94]研究发现,当向阳极添加适量的中性红和铁氰化钾时产电量分别增加51%及39.3%,但甲苯降解时间反而比无介质的MFC长1.56~2.15倍;Wu等[75]补充绿脓菌素使甲苯为底物的MFC最大功率密度从4.69增加到21.76 mW·m−2,同时库仑效率从0.83% 增加到11.62%。这些结果表明,外源氧化还原介体可能存在抑制微生物活性的负面影响,导致完全降解VOCs的时间延长,内源性氧化还原介体对微生物活性不会产生显著不利影响,且有助于库仑效率的提高。
除了上述方法,依据降低难降解VOCs对微生物活性抑制、加速“VOCs氧化→细胞膜→胞外电子传递→电极基底”路径上电子传递的原理,还有不同的强化手段被研究者提出并得到验证。例如,Lu等[100]为了克服高浓度甲硫醚对微生物的毒性并增强产电能力,添加乙酸钠作为共底物,通过共代谢作用使MFC甲硫醚降解活性提高52.5%,最大输出电压提高16.5倍。You等[101]采用不同的外加电势驯化MFC生物阳极,使得生物电极内阻降低24%,输出功率密度最高提升了29%。外加电势并未对电极表面微生物菌群结构有明显影响,而是改变了细胞膜表面蛋白,调控了生物膜活性。微生物对阳极电位的生理适应是MFC性能变化的主要原因[102],然而依旧不能排除阳极电位长期驯化造成微生物遗传变化对MFC性能的影响[103]。You等[104]还通过在以邻二甲苯为底物的MFC中添加Shewanella oneidensis MR-1,利用希瓦氏菌高效直接的电子传递能力,构建其与邻二甲苯降解菌协作共生菌群,诱导生成大量的细菌纳米导线,邻二甲苯去除效率提升1.7倍,最大功率密度提升77%。
综上,MFC中反应过程的强化,其基本思路在于微生物活性及“微生物-电极”间电子传递速率的提升。往往通过电极修饰改善微生物细胞与电极之间的直接电子传递路径,提升微生物负载量及活性,可以加速微生物体内反应的进行,提升MFC性能;而当电活性菌具有间接电子传递能力时,添加额外的氧化还原介体,为微生物构筑人工的间接电子传递通道,能有效提升产电性能和库仑效率;此外,电压驯化、菌群结构等调控方法也可以改善微生物的降解活性以及产电能力。
3.4 生物光电燃料电池MFC中VOCs净化包含了传质及反应2类过程,传质-反应协同强化是拓宽MFC适用范围、加快其应用的有效途径。例如,生物光电燃料电池技术就兼具了气体扩散电极传质强化及光催化反应强化作用两方面优势,是一种新型MFC。Chen等[105]在阳极室中利用聚偏氟乙烯/碳纤维布扩散膜组件加速VOCs传质进入液相,并结合过硫酸盐和活性炭纤维基的光电空气阴极构建了生物光电燃料电池。在阴极室中,活化过氧硫酸盐在UV光解、光电化学催化的作用下,产生自由基直接氧化甲苯;在生物阳极室中进行VOCs吸附/扩散和生物降解,并为阴极光化学反应过程提供电能。该集成系统在紫外光作用下,对317 mg·kg−1甲苯具有95%的去除效率,并具有稳定的电压输出(0.4 V)。Dai等[106]将纳米金刚石修饰的ZnO光电阴极与生物阳极相结合,该复合体系对甲苯的去除率为60.65%,高于单独光催化作用的37.16% 和单独MFC作用的17.81%。光照射下产生的峰值功率密度为120 mW·m−2,最大电流密度为1.07 A·m−2,分别为黑暗条件下的1.57倍和1.37倍。Wang等[107]开发了一种高效、经济的生物光电催化反应器,该反应器将生物阳极与TiO2光电催化空气阴极相结合,乙酸乙酯降解动力学较单独生物阳极和单独光催化反应器之和仍然提升了两倍。进一步地,采用聚偏氟乙烯膜替代普通质子交换膜作为空气阴极膜组件将该生物光电MFC输出功率密度由59.6提升至92.8 mW·cm−2。值得一提的是,这一功率密度远高于目前报道的常规MFC最高值(0.663 mW·cm−2)[93]。
4 结语及展望MFC促进VOCs降解并同步产电具备充分的可行性,在减污降碳的背景下,该技术用于VOCs废气处理符合国家战略要求。然而MFC输出功率、库仑效率尚未达到可应用的水平,特别是底物为含苯环、卤素取代基、硫等疏水性、难降解VOCs时,功率密度目前基本未超过100 mW·m−2 [93],这一现状严重制约了该技术的进一步发展及实际应用。传质、反应过程的协同强化仍然是MFC净化VOCs废气面临的关键技术壁垒。
传质强化方面,需要解决气-液传质、液-固(生物膜)传质两个步骤的速率限制。气液传质强化需要聚焦于气液接触面积、传质推动力的改善,例如填料塔等高效吸收反应器构型与MFC耦合相较于普通的鼓泡方式可以显著提升传质面积,两相分配生物反应器与MFC的耦合,可以采用非水相提高液相平衡浓度从而增强传质推动力,开发吸附性能良好的电极基底可以加速VOCs由液相进入生物膜。另外,气体扩散电极能够直接缩短VOCs由气相进入微生物的传质路径,并且能够避免MFC阴极室的无效反应器体积,具有较好的参考价值。
反应强化方面,在细胞尺度上,可筛选耐受性好、降解活性高的特异性微生物,并采用诱导合成或人工装配的方法提升微生物细胞膜上的导电结构丰度,从而加速代谢产生的电子向胞外导出。在生物膜尺度上,调控降解菌及产电菌之间的协作关系,平衡VOCs能量外输、微生物消耗2个方面的比例,既维持良好的生物膜活性,又尽可能提高产电功率密度。生物电极尺度上,最为关键的是改善“微生物-电极”界面电子传递,电极修饰方法的选择中,不仅需要考虑材料的生物相容性、导电性,还需要考虑修饰结构与生物膜的构效关系,避免修饰结构仅仅提升生物膜底部的电子传递,构建贯穿生物膜的导电结构能够提升整个生物膜的产电水平。反应器尺度上,可以在设计时优化2个电极之间的相对位置,MFC运行时添加盐类降低内阻、添加外加电子介体促进电子转移也是有效的优化方法。
目前的大多数研究仍仅关注VOCs去除的性能,除了传质-反应过程强化之外,未来应该在探索电子转移途径、反应动力学、功能菌种间关系调控和MFC构型创新设计等方面开展进一步的工作,以实现MFC中VOCs废气快速净化协同高效能量回收,填补基础研究与实际应用间的差距。另外,采用MFC净化VOCs的研究目前主要集中在单一污染物上,针对实际应用中复杂组分废气的净化,是MFC技术实际应用需要解决的一个关键问题。依旧可以围绕传质-反应强化的基本原理,抓住其中的关键限速步骤,实现MFC中复杂VOCs废气净化的过程强化,有以下几个方面值得探究:1)关键污染物的气液传质强化;2)多功能混合菌群的驯化;3)关键难降解VOCs去除的强化;4)不同VOCs组分净化过程中的相互作用。
| [1] |
中华人民共和国生态环境部. 挥发性有机物(VOCs)污染防治技术政策[EB/OL]. (2013-05-24) [2022-01-20]. http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/wrfzjszc/201306/t20130603_253125.shtml. Ministry of Ecology and Environment of the People's Republic of China. Volatile Organic Compounds (VOCs) Pollution PreventionTechnology Policy [EB/OL]. (2013-05-24) [2022-01-20]. http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/wrfzjszc/201306/t20130603_253125.shtml. |
| [2] |
FUZZI S, BALTENSPERGER U, CARSLAW K, et al. Particulate matter, air quality and climate: Lessons learned and future needs[J]. Atmospheric Chemistry and Physics, 2015, 15(14): 8217-8299. DOI:10.5194/acp-15-8217-2015 |
| [3] |
PöSCHL U, SHIRAIWA M. Multiphase chemistry at the atmosphere–biosphere interface influencing climate and public health inthe anthropocene[J]. Chemical Reviews, 2015, 115(10): 4440-4475. DOI:10.1021/cr500487s |
| [4] |
中共中央, 国务院. 中共中央国务院关于深入打好污染防治攻坚战的意见[EB/OL]. (2021-11-08) [2022-01-20]. https://www.mee.gov.cn/zcwj/zyygwj/202111/t20211108_959456.shtml. Communist Party of China Central Committee, State Council of the People's Republic of China. Opinions of the central committee of the communist party of China and the state council on deepening the battle of pollution prevention and control [EB/OL]. (2021-11-08) [2022-01-20]. https://www.mee.gov.cn/zcwj/zyygwj/202111/t20211108_959456.shtml. |
| [5] |
钟美芳, 田俊泰, 叶代启. "十四五"我国VOCs排放总量控制方案研究与建议[J]. 环境影响评价, 2021, 43(2): 1-9. ZHONG M F, TIAN J T, YE D Q. China's total VOCs control program research and suggestionsduring the 14th five-year period[J]. Environmental Impact Assessment, 2021, 43(2): 1-9. |
| [6] |
LI M Z, HUANG Z H, KANG F Y. Progress of volatile organic compounds control technology[J]. Chemical Industry and Engineering, 2015, 32(3): 2-9. |
| [7] |
BAILóN L, NIKOLAUSZ M, KäSTNER M, et al. Removal of dichloromethane from waste gases in one-and two-liquid-phase stirred tank bioreactors and biotrickling filters[J]. Water Research, 2009, 43(1): 11-20. DOI:10.1016/j.watres.2008.09.031 |
| [8] |
MUDLIAR S, GIRI B, PADOLEY K, et al. Bioreactors for treatment of VOCs and odours–A review[J]. Journal of Environmental Management, 2010, 91(5): 1039-1054. DOI:10.1016/j.jenvman.2010.01.006 |
| [9] |
VENKATA MOHAN S, VELVIZHI G, ANNIE MODESTRA J, et al. Microbial fuel cell: Critical factors regulating bio-catalyzed electrochemical process and recent advancements[J]. Renewable and Sustainable Energy Reviews, 2014, 40: 779-797. DOI:10.1016/j.rser.2014.07.109 |
| [10] |
ORTIZ-MARTíNEZ V M, SALAR-GARCíA M J, DE LOS RíOS A P, et al. Developments in microbial fuel cell modeling[J]. Chemical Engineering Journal, 2015, 271: 50-60. DOI:10.1016/j.cej.2015.02.076 |
| [11] |
EBRAHIMI A, NAJAFPOUR G D, YOUSEFI KEBRIA D. Performance of microbial desalination cell for salt removal and energy generation using different catholyte solutions[J]. Desalination, 2018, 432: 1-9. DOI:10.1016/j.desal.2018.01.002 |
| [12] |
SANTORO C, ARBIZZANI C, ERABLE B, et al. Microbial fuel cells: From fundamentals to applications. A review[J]. Journal of Power Sources, 2017, 356: 225-244. DOI:10.1016/j.jpowsour.2017.03.109 |
| [13] |
MUNOZ R, DAUGULIS A J, HERNANDEZ M, et al. Recent advances in two-phase partitioning bioreactors for the treatment of volatile organic compounds[J]. Biotechnology Advances, 2012, 30(6): 1707-1720. DOI:10.1016/j.biotechadv.2012.08.009 |
| [14] |
BORDOLOI A, GOSTOMSKI P A. Fate of degraded pollutants in waste gas biofiltration: An overview of carbon end-points[J]. Biotechnology Advances, 2019, 37(4): 579-588. DOI:10.1016/j.biotechadv.2018.09.002 |
| [15] |
DWIVEDI P, GAUR V, SHARMA A, et al. Comparative study of removal of volatile organic compounds by cryogenic condensation and adsorption by activated carbon fiber[J]. Separation and Purification Technology, 2004, 39(1/2): 23-37. |
| [16] |
HERNANDEZ M, QUIJANO G, MUNOZ R, et al. Modeling of VOC mass transfer in two-liquid phase stirred tank, biotrickling filter and airlift reactors[J]. Chemical Engineering Journal, 2011, 172(2/3): 961-969. |
| [17] |
WU C H, TSAI Y Y, LIN C W. Modifying membrane anode in a microbial fuel cell to improve removal of gaseous ethyl acetate without reducing generation of electricity[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 62: 169-176. DOI:10.1016/j.jtice.2016.02.001 |
| [18] |
LI J, LI M, ZHANG J, et al. A microbial fuel cell capable of converting gaseous toluene to electricity[J]. Biochemical Engineering Journal, 2013, 75: 39-46. DOI:10.1016/j.bej.2013.03.015 |
| [19] |
WU C H, LIN C W. Electricity generation and kinetic aspects of a biotrickling filter-microbial fuel cell for the biofiltration of ethyl acetate vapor from waste gas[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 68: 332-337. DOI:10.1016/j.jtice.2016.09.023 |
| [20] |
WU C H, SHIH J C, LIN C W. Continuous production of power using microbial fuel cells with integrated biotrickling filter for ethyl acetate-contaminated air stream treatment[J]. International Journal of Hydrogen Energy, 2016, 41(47): 21945-21954. DOI:10.1016/j.ijhydene.2016.08.186 |
| [21] |
ZHANG S H, YOU J P, KENNES C, et al. Current advances of VOCs degradation by bioelectrochemical systems: A review[J]. Chemical Engineering Journal, 2018, 334: 2625-2637. DOI:10.1016/j.cej.2017.11.014 |
| [22] |
SHI L, ROSSO K, CLARKE T, et al. Molecular underpinnings of Fe(Ⅲ) oxide reduction by Shewanella oneidensis MR-1[J]. Frontiers in Microbiology, 2012, 3: 50. |
| [23] |
YU Y Y, GUO C X, YONG Y C, et al. Nitrogen doped carbon nanoparticles enhanced extracellular electron transfer for high-performance microbial fuel cells anode[J]. Chemosphere, 2015, 140: 26-33. DOI:10.1016/j.chemosphere.2014.09.070 |
| [24] |
PATIL S A, HäGERHäLL C, GORTON L. Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems[J]. Bioanalytical Reviews, 2012, 4(2): 159-192. |
| [25] |
RABAEY K, BOON N, HöFTE M, et al. Microbial phenazine production enhances electron transfer in biofuel cells[J]. Environmental Science and Technology, 2005, 39(9): 3401-3408. DOI:10.1021/es048563o |
| [26] |
KRACKE F, VASSILEV I, KRöMER J O. Microbial electron transport and energy conservation – The foundation for optimizing bioelectrochemical systems[J]. Frontiers in Microbiology, 2015, 6: 575. |
| [27] |
SCHRöDER U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency[J]. Physical Chemistry Chemical Physics, 2007, 9(21): 2619-2629. DOI:10.1039/B703627M |
| [28] |
PATIL S A, HASAN K, LEECH D, et al. Improved microbial electrocatalysis with osmium polymer modified electrodes[J]. Chemical Communications, 2012, 48(82): 10183-10185. DOI:10.1039/c2cc34903e |
| [29] |
RAKOCZY J, FEISTHAUER S, WASMUND K, et al. Benzene and sulfide removal from groundwater treated in a microbial fuel cell[J]. Biotechnology Bioengineering, 2013, 110(12): 3104-3113. DOI:10.1002/bit.24979 |
| [30] |
FRIMAN H, SCHECHTER A, IOFFE Y, et al. Current production in a microbial fuel cell using a pure culture of Cupriavidus basilensis growing in acetate or phenol as a carbon source[J]. Microbial Biotechnology, 2013, 6(4): 425-434. DOI:10.1111/1751-7915.12026 |
| [31] |
XING D F, ZUO Y, CHENG S A, et al. Electricity generation by Rhodopseudomonas palustris DX-1[J]. Environmental Science and Technology, 2008, 42(11): 4146-4151. DOI:10.1021/es800312v |
| [32] |
ZUO Y, XING D F, REGAN J M, et al. Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell[J]. Applied and Environmental Microbiology, 2008, 74(10): 3130-3137. DOI:10.1128/AEM.02732-07 |
| [33] |
BOROLE A P, O'NEILL H, TSOURIS C, et al. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum[J]. Biotechnology Letters, 2008, 30(8): 1367-1372. DOI:10.1007/s10529-008-9700-y |
| [34] |
RABAEY K, VAN DE SOMPEL K, MAIGNIEN L, et al. Microbial fuel cells for sulfide removal[J]. Environmental Science and Technology, 2006, 40(17): 5218-5224. DOI:10.1021/es060382u |
| [35] |
CHAUDHURI S K, LOVLEY D R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells[J]. Nature Biotechnology, 2003, 21(10): 1229-1232. DOI:10.1038/nbt867 |
| [36] |
RINGEISEN B R, HENDERSON E, WU P K, et al. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10[J]. Environmental Science and Technology, 2006, 40(8): 2629-2634. DOI:10.1021/es052254w |
| [37] |
KIM H J, PARK H S, HYUN M S, et al. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens[J]. Enzyme and Microbial Technology, 2002, 30(2): 145-152. DOI:10.1016/S0141-0229(01)00478-1 |
| [38] |
COX H, SEXTON T, SHAREEFDEEN Z M, et al. Thermophilic biotrickling filtration of ethanol vapors[J]. Environmental Science & Technology, 2001, 35(12): 2612-2619. |
| [39] |
LIU J, QIAO Y, LU Z S, et al. Enhance electron transfer and performance of microbial fuel cells by perforating the cell membrane[J]. Electrochemistry Communications, 2012, 15(1): 50-53. DOI:10.1016/j.elecom.2011.11.018 |
| [40] |
SHAHI A, RAI B N, SINGH R S. Analysis of metabolites and carbon balance in the biofilteration of cumene using loofa sponge as biofilter media[J]. Applied Biochemistry and Biotechnology, 2016, 180: 338-348. DOI:10.1007/s12010-016-2102-z |
| [41] |
PARK D H, ZEIKUS J G. Improved fuel cell and electrode designs for producing electricity from microbial degradation[J]. Biotechnology and Bioengineering, 2003, 81(3): 348-355. DOI:10.1002/bit.10501 |
| [42] |
ZHANG L X, ZHOU S G, ZHUANG L, et al. Microbial fuel cell based on Klebsiella pneumoniae biofilm[J]. Electrochemistry Communications, 2008, 10(10): 1641-1643. DOI:10.1016/j.elecom.2008.08.030 |
| [43] |
NIMJE V R, CHEN C Y, CHEN C C, et al. Microbial fuel cell of Enterobacter cloacae: Effect of anodic pH microenvironment on current, power density, internal resistance and electrochemical losses[J]. International Journal of Hydrogen Energy, 2011, 36(17): 11093-11101. DOI:10.1016/j.ijhydene.2011.05.159 |
| [44] |
LUO J M, YANG J, HE H H, et al. A new electrochemically active bacterium phylogenetically related to Tolumonas osonensis and power performance in MFCs[J]. Bioresource Technology, 2013, 139: 141-148. DOI:10.1016/j.biortech.2013.04.031 |
| [45] |
CHOI Y-J, KIM N-J, KIM S-H, et al. Dynamic behaviors of redox mediators within the hydrophobic layers as an important factor for effective microbial fuel cell operation[J]. Korean Chemical Society, 2003, 24(4): 437-440. DOI:10.5012/bkcs.2003.24.4.437 |
| [46] |
XING D F, CHENG S A, LOGAN B E, et al. Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction[J]. Applied Microbiology and Biotechnology, 2010, 85(5): 1575-1587. DOI:10.1007/s00253-009-2240-0 |
| [47] |
RABAEY K, BOON N, SICILIANO S D, et al. Biofuel cells select for microbial consortia that self-mediate electron transfer[J]. Applied and Environmental Microbiology, 2004, 70(9): 5373-5382. DOI:10.1128/AEM.70.9.5373-5382.2004 |
| [48] |
VEGA C A, FERNáNDEZ I. Mediating effect of ferric chelate compounds in microbial fuel cells with Lactobacillus plantarum, Streptococcus lactis, and Erwinia dissolvens[J]. Bioelectrochemistry and Bioenergetics, 1987, 17(2): 217-222. DOI:10.1016/0302-4598(87)80026-0 |
| [49] |
XU S T, LIU H. New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell[J]. Journal of Applied Microbiology, 2011, 111(5): 1108-1115. DOI:10.1111/j.1365-2672.2011.05129.x |
| [50] |
ISHII S, WATANABE K, YABUKI S, et al. Characterization of electrode reducing rates of Geobacter sulfurreducens and an enriched electricity-generating mixed consortium in a microbial fuel cell[J]. Applied and Environmental Microbiology, 2008, 74: 7348-7355. DOI:10.1128/AEM.01639-08 |
| [51] |
MIN B, CHENG S, LOGAN B E. Electricity generation using membrane and salt bridge microbial fuel cells[J]. Water Research, 2005, 39(9): 1675-1686. DOI:10.1016/j.watres.2005.02.002 |
| [52] |
HOLMES D E, NICOLL J S, BOND D R, et al. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell[J]. Applied and Environmental Microbiology, 2004, 70(10): 6023-6030. DOI:10.1128/AEM.70.10.6023-6030.2004 |
| [53] |
HOLMES D E, BOND D R, LOVLEY D R. Electron transfer by Desulfobulbus propionicus to Fe(Ⅲ) and graphite electrodes[J]. Applied and Environmental Microbiology, 2004, 70(2): 1234-1237. DOI:10.1128/AEM.70.2.1234-1237.2004 |
| [54] |
KANG C S, EAKTASANG N, KWON D-Y, et al. Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell[J]. Bioresource Technology, 2014, 165: 27-30. DOI:10.1016/j.biortech.2014.03.148 |
| [55] |
FEDOROVICH V, KNIGHTON M C, PAGALING E, et al. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell[J]. Applied and Environmental Microbiology, 2009, 75(23): 7326. DOI:10.1128/AEM.01345-09 |
| [56] |
PARK H S, KIM B H, KIM H S, et al. A novel electrochemically active and Fe(Ⅲ)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell[J]. Anaerobe, 2001, 7(6): 297-306. DOI:10.1006/anae.2001.0399 |
| [57] |
NIESSEN J, SCHRöDER U, SCHOLZ F. Exploiting complex carbohydrates for microbial electricity generation – A bacterial fuel cell operating on starch[J]. Electrochemistry Communications, 2004, 6(9): 955-958. DOI:10.1016/j.elecom.2004.07.010 |
| [58] |
MARSHALL C W. Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica[J]. Energy & Environmental Science, 2009, 2(6): 699-705. |
| [59] |
NIMJE V R, CHEN C Y, CHEN C C, et al. Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell[J]. Journal of Power Sources, 2009, 190(2): 258-263. DOI:10.1016/j.jpowsour.2009.01.019 |
| [60] |
LUO J M, LI M, ZHOU M H, et al. Characterization of a novel strain phylogenetically related to Kocuria rhizophila and its chemical modification to improve performance of microbial fuel cells[J]. Biosensors and Bioelectronics, 2015, 69: 113-120. DOI:10.1016/j.bios.2015.02.025 |
| [61] |
LIU M, YUAN Y, ZHANG L X, et al. Bioelectricity generation by a Gram-positive Corynebacterium sp. strain MFC03 under alkaline condition in microbial fuel cells[J]. Bioresource Technology, 2010, 101(6): 1807-1811. DOI:10.1016/j.biortech.2009.10.003 |
| [62] |
ROSAS-LAVERDE N M, PRUNA A, BUSQUETS-MATAIX D. Improving electrochemical properties of polypyrrole coatings by graphene oxide and carbon nanotubes[J]. Nanomaterials, 2020, 10(3): 507. DOI:10.3390/nano10030507 |
| [63] |
PRASAD D, ARUN S, MURUGESAN M, et al. Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cell[J]. Biosensors and Bioelectronics, 2007, 22(11): 2604-2610. DOI:10.1016/j.bios.2006.10.028 |
| [64] |
SIU C, CHIAO M. A microfabricated PDMS microbial fuel cell[J]. Journal of Microelectromechanical Systems, 2008, 17(6): 1329-1341. DOI:10.1109/JMEMS.2008.2006816 |
| [65] |
HASLETT N D, RAWSON F J, BARRIëRE F, et al. Characterisation of yeast microbial fuel cell with the yeast Arxula adeninivorans as the biocatalyst[J]. Biosensors and Bioelectronics, 2011, 26(9): 3742-3747. DOI:10.1016/j.bios.2011.02.011 |
| [66] |
HOU Q J, NIE C L, PEI H Y, et al. The effect of algae species on the bioelectricity and biodiesel generation through open-air cathode microbial fuel cell with kitchen waste anaerobically digested effluent as substrate[J]. Bioresource Technology, 2016, 218: 902-908. DOI:10.1016/j.biortech.2016.07.035 |
| [67] |
王丽丽. 可降解苯酚的高效产电菌株的分离筛选及生物学特性研究[D]. 哈尔滨: 哈尔滨理工大学, 2016. WANG L L. Isolation, screening and biological characteristics reasearch of highly-effective electricigens for phenol degradation [D]. Harbin: Harbin University of Science and Technology, 2016. |
| [68] |
MONZON O, YANG Y, KIM J, et al. Microbial fuel cell fed by Barnett Shale produced water: power production by hypersaline autochthonous bacteria and coupling to a desalination unit[J]. Biochemical Engineering Journal, 2017, 117: 87-91. DOI:10.1016/j.bej.2016.09.013 |
| [69] |
ADELAJA O, KESHAVARZ T, KYAZZE G. The effect of salinity, redox mediators and temperature on anaerobic biodegradation of petroleum hydrocarbons in microbial fuel cells[J]. Journal of Hazardous Materials, 2015, 283: 211-217. DOI:10.1016/j.jhazmat.2014.08.066 |
| [70] |
HASSAN H, JIN B, DONNER E, et al. Microbial community and bioelectrochemical activities in MFC for degrading phenol and producing electricity: Microbial consortia could make differences[J]. Chemical Engineering Journal, 2018, 332: 647-657. DOI:10.1016/j.cej.2017.09.114 |
| [71] |
PEREIRA-MEDRANO A G, KNIGHTON M, FOWLER G J S, et al. Quantitative proteomic analysis of the exoelectrogenic bacterium Arcobacter butzleri ED-1 reveals increased abundance of a flagellin protein under anaerobic growth on an insoluble electrode[J]. Journal of Proteomics, 2013, 78: 197-210. DOI:10.1016/j.jprot.2012.09.039 |
| [72] |
KODAMA Y, SHIMOYAMA T, WATANABE K. Dysgonomonas oryzarvi sp. nov., isolated from a microbial fuel cell[J]. Microbiology Society, 2012, 62(12): 3055-3059. |
| [73] |
LAZAROAIE M. The tolerance of gram-negative bacterial strain to saturated and aromatic hydrocarbons[J]. Romanian Biotechnological Letters, 2007, 12(4): 3329-3338. |
| [74] |
HU J, ZHANG L L, CHEN J M, et al. Performance and microbial analysis of a biotrickling filter inoculated by a specific bacteria consortium for removal of a simulated mixture of pharmaceutical volatile organic compounds[J]. Chemical Engineering Journal, 2016, 304: 757-765. DOI:10.1016/j.cej.2016.06.078 |
| [75] |
WU C H, YEI-POLE I, CHIU Y H, et al. Enhancement of power generation by toluene biodegradation in a microbial fuel cell in the presence of pyocyanin[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(5): 2319-2324. DOI:10.1016/j.jtice.2014.05.019 |
| [76] |
ZHANG S H, YOU J P, AN N, et al. Gaseous toluene powered microbial fuel cell: Performance, microbial community, and electron transfer pathway[J]. Chemical Engineering Journal, 2018, 351: 515-522. DOI:10.1016/j.cej.2018.06.027 |
| [77] |
BAJRACHARYA S, SHARMA M, MOHANAKRISHNA G, et al. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond[J]. Renewable Energy, 2016, 98: 153-170. DOI:10.1016/j.renene.2016.03.002 |
| [78] |
LOGAN B E, HAMELERS B, ROZENDAL R, et al. Microbial fuel cells: Methodology and technology[J]. Environmental Science and Technology, 2006, 40(17): 5181-5192. DOI:10.1021/es0605016 |
| [79] |
HE Z, MINTEER S D, ANGENENT L T. Electricity generation from artificial wastewater using an upflow microbial fuel cell[J]. Environmental Science and Technology, 2005, 39(14): 5262-5267. DOI:10.1021/es0502876 |
| [80] |
PHAM H, BOON N, MARZORATI M, et al. Enhanced removal of 1, 2-dichloroethane by anodophilic microbial consortia[J]. Water Research, 2009, 43(11): 2936-2946. DOI:10.1016/j.watres.2009.04.004 |
| [81] |
LUO H P, LIU G L, ZHANG R D, et al. Phenol degradation in microbial fuel cells[J]. Chemical Engineering Journal, 2009, 147(2): 259-264. |
| [82] |
AULENTA F, REALE P, CATERVI A, et al. Kinetics of trichloroethene dechlorination and methane formation by a mixed anaerobic culture in a bio-electrochemical system[J]. Electrochim Acta, 2008, 53(16): 5300-5305. DOI:10.1016/j.electacta.2008.02.084 |
| [83] |
WU C H, LAI C Y, LIN C W, et al. Generation of power by microbial fuel cell with ferricyanide in biodegradation of benzene[J]. CLEAN-Soil Air Water, 2013, 41(4): 390-395. DOI:10.1002/clen.201200198 |
| [84] |
AULENTA F, TOCCA L, VERDINI R, et al. Dechlorination of trichloroethene in a continuous-flow bioelectrochemical reactor: Effect of cathode potential on rate, selectivity, and electron transfer mechanisms[J]. Environmental Science and Technology, 2011, 45(19): 8444-8451. DOI:10.1021/es202262y |
| [85] |
LIU Q, CHEN Y, WANG J D, et al. Electrochemical oxidation of 1, 4-dichlorobenzene on platinum electrodes in acetonitrile-water solution: Evidence for direct and indirect electrochemical oxidation pathways[J]. International Journal of Electrochemical Science, 2011, 6(7): 2366-2384. |
| [86] |
CHENG H Y, LIANG B, MU Y, et al. Stimulation of oxygen to bioanode for energy recovery from recalcitrant organic matter aniline in microbial fuel cells (MFCs)[J]. Water Research, 2015, 81: 72-83. DOI:10.1016/j.watres.2015.05.012 |
| [87] |
LIU H, LOGAN B E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane[J]. Environmental Science and Technology, 2004, 38(14): 4040-4046. DOI:10.1021/es0499344 |
| [88] |
ZILLI M, PALAZZI E, SENE L, et al. Toluene and styrene removal from air in biofilters[J]. Process Biochemistry, 2001, 37(4): 423-429. DOI:10.1016/S0032-9592(01)00228-X |
| [89] |
LAURENZIS A, HEITS H, WüBKER S-M, et al. Continuous biological waste gas treatment in stirred trickle-bed reactor with discontinuous removal of biomass[J]. Biotechnology Bioengineering, 1998, 57(4): 497-503. DOI:10.1002/(SICI)1097-0290(19980220)57:4<497::AID-BIT14>3.0.CO;2-9 |
| [90] |
LIN C W, TSAO C Y, JIANG S L, et al. Enhanced gaseous ethyl acetate degradation and power generation by a bioelectrochemical system[J]. Chemical Engineering Journal, 2018, 344: 270-276. DOI:10.1016/j.cej.2018.03.093 |
| [91] |
骆海萍, 张翠萍, 宋海红, 等. 降解苯的微生物燃料电池产电性能研究[J]. 中山大学学报(自然科学版), 2010, 49(1): 113-118. LUO H P, ZHANG C P, SONG H H, et al. Electricity production of microbial fuel cell with biodegradation of benzene[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2010, 49(1): 113-118. |
| [92] |
HUANG L P, SHI Y H, WANG N, et al. Anaerobic/aerobic conditions and biostimulation for enhanced chlorophenols degradation in biocathode microbial fuel cells[J]. Biodegradation, 2014, 25(4): 615-632. DOI:10.1007/s10532-014-9686-1 |
| [93] |
CAO B, ZHAO Z, PENG L, et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells[J]. Science, 2021, 373(6561): 1336-1340. DOI:10.1126/science.abf3427 |
| [94] |
LIN C W, WU C H, CHIU Y H, et al. Effects of different mediators on electricity generation and microbial structure of a toluene powered microbial fuel cell[J]. Fuel, 2014, 125: 30-35. DOI:10.1016/j.fuel.2014.02.018 |
| [95] |
ATES M. A review study of (bio) sensor systems based on conducting polymers[J]. Materials Science and Engineering: C, 2013, 33(4): 1853-1859. DOI:10.1016/j.msec.2013.01.035 |
| [96] |
PU K B, MA Q, CAI W F, et al. Polypyrrole modified stainless steel as high performance anode of microbial fuel cell[J]. Biochemical Engineering Journal, 2018, 132: 255-261. DOI:10.1016/j.bej.2018.01.018 |
| [97] |
WU Z, WANG Z, YU F, et al. Variation in chemical, colloidal and electrochemical properties of carbon nanotubes with the degree of carboxylation[J]. Journal of Nanoparticle Research, 2017, 19(1): 16. DOI:10.1007/s11051-016-3697-2 |
| [98] |
MOHAMED H O, SAYED E T, OBAID M, et al. Transition metal nanoparticles doped carbon paper as a cost-effective anode in a microbial fuel cell powered by pure and mixed biocatalyst cultures[J]. International Journal of Hydrogen Energy, 2018, 43(46): 21560-21571. DOI:10.1016/j.ijhydene.2018.09.199 |
| [99] |
熊仕昌, 管玉江, 贾显乐, 等. 银掺杂碳纳米管电极在微生物燃料电池中的应用[J]. 扬州大学学报(自然科学版), 2015, 18(1): 74-78. XIONG S C, GUAN Y J, JIA X L, et al. Application of Ag-CNTs electrode in microbial fuel cell[J]. Journal of Yangzhou University(Natural Science Edition), 2015, 18(1): 74-78. |
| [100] |
LU Y, FENG K, WU C, et al. Co-substrate-assisted dimethyl sulfide degradation and electricity generation in a microbial fuel cell[J]. Energy & Fuels, 2021, 36(1): 514-520. |
| [101] |
YOU J P, CHEN H, XU L L, et al. Anodic-potential-tuned bioanode for efficient gaseous toluene removal in an MFC[J]. Electrochim Acta, 2021, 375: 137992. DOI:10.1016/j.electacta.2021.137992 |
| [102] |
COMMAULT A S, LEAR G, WELD R J. Comment on microbial community composition is unaffected by anode potential[J]. Environmental Science and Technology, 2014, 48(24): 14851-14852. DOI:10.1021/es501982m |
| [103] |
COMMAULT A S, LEAR G, PACKER M A, et al. Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells[J]. Bioresource Technology, 2013, 139: 226-234. DOI:10.1016/j.biortech.2013.04.047 |
| [104] |
YOU J P, DENG Y Y, CHEN H, et al. Enhancement of gaseous o-xylene degradation in a microbial fuel cell by adding Shewanella oneidensis MR-1[J]. Chemosphere, 2020, 252: 126571. DOI:10.1016/j.chemosphere.2020.126571 |
| [105] |
CHEN Q Y, LIU L F. Integrating anodic membrane diffusion/biodegradation with UV photolysis, Adsorptive oxidation by activation of peroxymonosulfate over activated carbon fiber based photo cathode in one reactor system for removing toluene gas[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104143. DOI:10.1016/j.jece.2020.104143 |
| [106] |
DAI Y X, GUO Y J, WANG J, et al. A vertically configured photocatalytic-microbial fuel cell for electricity generation and gaseous toluene degradation[J]. Chemosphere, 2021, 285: 131530. DOI:10.1016/j.chemosphere.2021.131530 |
| [107] |
WANG L H, LIU L F, YANG F L. Efficient gas phase VOC removal and electricity generation in an integrated bio-photo-electro-catalytic reactor with bio-anode and TiO2 photo-electro-catalytic air cathode[J]. Bioresource Technology, 2018, 270: 554-561. DOI:10.1016/j.biortech.2018.09.041 |




