多孔材料是一种由贯通或封闭的孔道所构筑的功能性材料,因其具有优良物理化学性能,在航空、航天、化工、建材、冶金、原子能、石化、机械、医药和环保等诸多领域具有广阔的应用和发展前景。为了赋予该类材料特殊性能以满足某一特定应用需求,相关研究者探索并研发了多种制备方法以达到对材料结构、性能的可控。模板法是制备多孔材料最常用的方法之一,该方法操作简单易行、重复率高、预见性好,且对所制备材料结构易于控制。
模板法是以模板为主体构形去控制、影响和修饰材料的形貌,控制尺寸进而决定材料性质的一种合成方法。根据所用模板剂自身的特点和限域能力的不同,模板剂可以分为以共价键维持其特定结构的硬模板(hard template)和以分子间或分子内的弱相互作用维持其特定结构的软模板(soft template)两类[1-2]。常见的硬模板有:二氧化硅颗粒[3-4]、二氧化钛颗粒[5-6]、碳颗粒[7]、碳酸钙颗粒[8-9]等;软模板则有:乳化液滴[10-11]、表面活性剂胶束[12-14]、聚合物泡囊[15]以及气泡模板[16]等。传统模板法合成多孔材料不但生产成本高,且需要后期处理以去除模板,而且还存在孔结构有可能坍塌和一些模板剂难以完全去除的问题。例如,Liu等[3]以SiO2微球为模板剂,通过先制备核-壳结构SiO2@壳聚糖纳米粒子,再氢氟酸去除二氧化硅核得到壳聚糖中空纳米球。Chen等[8]和Gao等[9]以纳米CaCO3为模板,分别制备了具有核-壳结构的CaCO3@SiO2和CaCO3@TiO2复合氧化物,然后酸性去除CaCO3核得到中空SiO2和TiO2纳米球体。Fendler等[12]以含有乙基、甲基丙烯酸酯、丁二炔、异氰基和苯乙烯基团的二烷基表面活性剂形成稳定的囊泡,并以其作模板剂制备中空结构材料,通过后期焙烧去除表面活性剂。气泡模板法则不用考虑后期模板剂的去除问题,操作简单、成本低且不污染环境。
气泡模板法合成多孔材料一般包括产生气泡乳液(即形成气泡模板剂)和悬浮颗粒在气泡表面上的沉积/吸附,进而通过颗粒的进一步生长/聚集形成壳层结构等过程。气泡模板剂形成方法主要有:化学反应法、气体鼓入法、原位电解制气法和超声诱导法。本文按气泡模板剂形成方法系统论述了气泡模板法在多孔材料合成方面的研究进展。
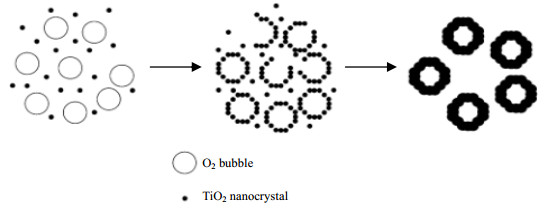
2 气泡模板法合成多孔材料 2.1 化学反应产生气泡模板剂气泡可以通过化学反应产生。双氧水在碱性条件下会缓慢分解产生氧气,O2气泡能提供一个稳定的中心,吸附合成体系中悬浮的材料前驱体而制得空心球结构。Long等[16]在乙醇体系中,以Ti(SO4)2为钛源,以NH4Cl催化双氧水分解形成气泡模板。由于系统不稳定,配位化合物分解产生的TiO2纳米晶体趋于聚集,在界面能量下降的情况下,纳米小晶粒可聚集在气、液界面周围,最后形成TiO2空心球体(图 1)。所得到球体样品的直径在800~1000 nm,平均孔径约为4 nm,比表面积达到268.2 m2·g-1。Li等[17]采用类似的方法批量生产了直径约1 μm的等级结构的TiO2空心球体,测量显示这些球体具有直径约50 nm的孔。Yang等[18]以双氧水分解形成的O2气泡为模板,合成具有均匀直径约500 nm的CoOOH等级结构空心球。
|
图 1 TiO2空心球结构形成机理示意图[16] Fig.1 Schematic diagram of the formation mechanism of TiO2 hollow spheres [16] |
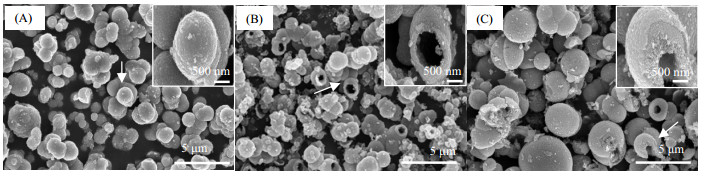
本课题组以Ti(SO4)2为钛源,以NH4Cl催化双氧水分解形成的O2气泡为模板,水/溶剂热法合成了TiO2中空微球(如图 2所示)。通过考察不同的参数发现,随着合成体系中水量的增加形成中空结构的难度加大,这归因于O2在水中的溶解度比有机溶剂中小且稳定性相对较差;有机溶剂的类型对所合成样品的形貌也具有一定影响,其可能是由于气泡在不同溶剂体系中具有不同的界面张力引起;另外还发现提高前驱体浓度会使所得到球体壁厚增加,即使增加双氧水的用量也得到了相同结果,这可能是由于O2在合成体系下气泡溶解已达到饱和状态气泡模板数量不再增加所致。
|
图 2 制备样品的SEM图片 Fig.2 SEM micrographs of prepared samples (A) reaction solvent: methanol 38 mL (B) reaction solvent: ethanol 38 mL (C) 4 times of the amount of reactants (Ti(SO4)2, H2O2 and NH4Cl) were added |
尿素应用性价比高,水溶性好,而且分解产生的NH3和CO2可作为气泡模板剂构筑多孔结构。Sun等[19]采用尿素作为气泡源,通过水热法很容易地合成了具有空心结构钒酸铋。水热条件下,尿素在溶液中水解产生NH3和CO2气泡软模板;同时钒酸铋水热晶化并形成截角八面体,这些八面体在气泡表面聚集,通过Ostwald熟化形成致密的球壳结构。所制备的钒酸铋空心球体直径在4~5 μm,且球壳由大小为0.5~1 μm截角八面体构成。Zhou等[20]采用类似的方法制备了多级结构Sn2Nb2O7空心球,样品具有孔径约为4 nm较为均匀的孔道,比表面积和孔体积分别为58.3 m2·g-1和0.12 cm3·g-1,该样品具有很高的光催化活性。Chen等[21]采用FeCl3·6H2O和尿素为原料,在不添加任何其它添加剂的情况下,在乙二醇(EG)溶液中合成了直径大小为200~500 nm的磁铁矿(Fe3O4)空心球。Kuang等[22]以尿素水解释放的微泡为模板制造具有蓬松表面的NiO空心球,微球的平均直径为2 μm,壳层厚度约为400~700 nm;这种蓬松的表面不仅具有高比表面积,而且还有助于气体扩散。陈小梅等[23]设计了一种气泡模板辅助合成具有空心结构铁酸盐微球的方法,该法以乙二醇为溶剂,尿素为气泡模板反应剂和矿化剂,聚乙二醇为气泡稳定剂,制备了大小为350 nm均匀的CoFe2O4和ZnFe2O4的空心微球。Weng等[24]也通过尿素分解形成的气泡软模板,成功合成直径为(157±26) nm的Fe/Ga基氧化物纳米球。Yan等[25]通过尿素分解产生的气泡模板合成具有多孔半壳的Pd3Pt合金。Wang等[26]以尿素分解产生的CO2气泡为模板,成功制备了具有大表面积的g-C3N4。
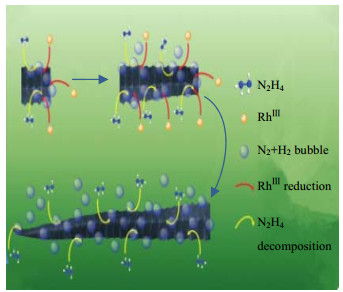
肼化学性质不稳定,高温易分解产生N2、NH3和H2,且具有强还原性。采用肼作还原剂产生N2气泡模板,水热条件下制备ZnSe空心球的工作已有大量报道。Peng等[27]用肼作还原剂在水热条件下,首次合成了直径为3 μm的ZnSe半导体微球。Wang等[28]用类似的方法成功合成了由60~100 nm纳米粒子聚集而成的ZnSe空心球,当体系中含NaOH时,中空球的尺寸为2~3 μm,而不使用NaOH时,球体尺寸为200~400 nm。Jiang等[29]则合成了表面积为77.13 m2·g-1、平均孔径约为20 nm的ZnSe中空纳米球。此外,Liu等[30]和Lin等[31]都成功制备了ZnSe空心球结构。Lin等[31]通过改变pH来控制ZnSe团聚体孔的形态和尺寸,在pH值为10.5和11.5下分别获得珊瑚状和荔枝状团聚体,其孔径可在20~50 nm范围调节。关于以肼为气泡源制备其它空心球结构材料的还有:Wu等[32]合成了VOOH空心球体,Zuo等[33]合成粒径约100 nm均匀的空心结构IF(富勒烯)-MoS2纳米笼;Guo等[34]利用肼还原钴络合物时释放的NH3、N2和H2作为模板合成了480~850 nm大小的Co空心球,通过球体后期自发地自组装形成链状的Co中空链结构,其链长度约10 μm。近期报道,Zhu等[35]在溶液相合成过程中,通过肼分解反应引起海马尾状分支的形成,RhⅢ还原产生的Rh核催化肼分解产生的H2和N2气泡,为分支的生长提供了丰富的微小气泡模板;具体而言,Rh优先沉积在气泡之间的间隙区域,导致形成凹面,如图 3所示。
|
图 3 海马尾状分支的形成机制示意图[35] Fig.3 Schematic diagram of the formation mechanism of hippocampus tail-like branches [35] |
气泡模板法在制备空心金属硫化物球体方面也有较广的应用。比如,Chen等[36]利用硫脲水解的反应,所产生的CO2(优于可溶性较好的NH3)在水热条件下形成气态空穴为气泡模板剂,制备得CuS中空微球,球体和内孔的平均直径分别为6和3 μm。Liu等[37]和Gu等[38]通过硫代乙酰胺分解产生的H2S气泡周围的小颗粒聚集,分别制备了直径为0.5~1 μm的CuS中空球和ZnS中空纳米微球。Zhao等[39]以谷胱甘肽为气泡源产生CO2气泡模板,制备了CuS中空微球,壳厚和直径分别约为45 nm和1.9 μm。Liu等[40]通过Zn(NO3)2·2H2O与Na2S2O3·2H2O作用产生的SO2气泡模板剂,溶剂热法合成了均匀的ZnS中空微球,其直径约2~3 μm。Zhang等[41]和Li等[42]以溶液中KBH4释放的H2气泡为模板,分别合成了具有约20 nm均匀壁厚的ZnS纳米空心球和由直径为100 nm的ZnO纳米棒构成,直径为2~4 μm的中空微球。此外,在合成空心ZnO、TiO2等方面也有大量报道。Zhang等[43]以乙醇为溶剂,以反应中产生的NH3气泡为模板,合成了直径约450 nm和长度约4 μm的中空管状ZnO(图 4)。Yan等[44]以所产生的CO2气泡为模板,制备了直径约10 μm,由多面体颗粒构成的新型复合ZnO中空结构材料。Li等[45]用H2气泡模板法简单制备了具有蜂窝状上层结构的ZnO/g-C3N4复合光催化剂,蜂窝巢的孔径在50~300 nm。由于空心球型TiO2具有特殊的结构性质,在很多领用有广泛的应用,通过气泡模板法制备中空TiO2纳米微球,目前已成为研究热点之一[16-17, 46]。Kim等[46]以溶剂热反应过程中四丁基氢氧化铵(TBAH)分解产生的C4H8气泡为模板,合成了壳厚约为250 nm,平均直径1.5~4 μm可控的TiO2纳米空心微球。Wang等[47]利用甲胺分解产生的NH3气泡为模板,制备了壳厚为60~140 nm,直径为2~3 μm的SnO2中空微球。Li等[48]利用气泡-模板辅助生长机制合成SnO2纳米环。Ding等[49]以反应中产生的CO2气泡为模板,制备了多级孔型Fe3O4/C纳米复合微球,其开孔孔径为68 nm,球壁厚度为1 nm,且均匀嵌入约8.1 nm的Fe3O4纳米晶体以及8.8 nm的纳米孔。Valladares等[50]以相似的方法合成了α-Fe2O3中空微球,且表征结果表明中空球的生长取决于气泡生长。
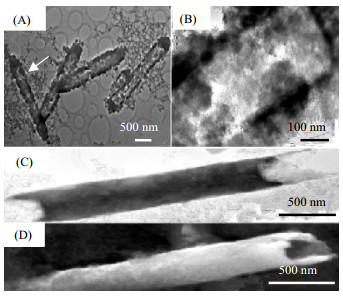
|
图 4 中空管状ZnO的TEM照片(A); 单个ZnO管的放大(B); 具有破坏末端的中空管状ZnO的TEM照片(C)和SEM照片(D)[43] Fig.4 TEM micrographs of prepared tubular ZnO (A); an individual ZnO tube (B); TEM (C) and SEM (D) micrographs of tubular ZnO with destroyed ends [43] |
利用化学反应产生气泡模板构筑多孔结构在其它材料方面也有一定报道。比如,Zhu等[51]利用NH3·H2O和Na2S2O3分解产生气泡模板,合成了CdLa2S4空心球/还原型氧化石墨烯复合材料;其它中空结构材料还有等级聚酰亚胺中空球体[52],La2O3:Yb[53],BaCO3[54],纳米中空g-C3N4[55],介孔CaF2:Yb[56],中孔结构(CuIn)0.2Zn1.6S2(CIZS)中空球[57],Ni0.25Co0.1Mn0.65CO3微球[58],自掺杂多孔碳[59],InVO4空心球[60]和UO2中空纳米球[61]等。Guo等[62]以氯化铵受热分解产生的NH3和HCl气泡为模板,获得了松散的泡沫状结构石墨烯碳氮化物(g-C3N4)。以还原剂(硼氢化钠)的氧化和水解原位形成的氢气泡为模板,可制备双金属AuPt纳米线网络[63],三金属Pt53Ru39Ni8纳米海绵[64],三金属PdNiRu合金纳米链状网络[65]和Pt纳米线网络[66]等。此外,Zhu等[67]以反应产生的NH3为模板,在正辛醇/水混合体系中成功制备了超长且柔性的项链状纳米结构(长度约10 μm,直径约30 nm)的氢氧化镉,且项链状一维纳米线由直径为5 nm初级纳米颗粒构成。
以化学反应产生气泡模板的方法所合成的多孔材料大部分为中空球体,少数为中空管状[43]、网状[62-66]或链状结构[67]等,所构筑的孔道大小一般为2~50 nm的介孔孔道,仅部分样品孔径能达到50~300 nm。化学反应产生气泡模板法,使用原位产生的气泡作为软模板,通常反应条件温和,操作简单方便,通用性强可用于合成各种中空结构材料;并且此方法具有可调控性,可以通过改变反应条件,对内部纳米晶体尺寸、球体直径及孔径大小等进行有效调控,从而合成内部掺入或表面改性的理想实体。但此法产生气泡模板剂的速度与前驱体在气泡模板表面聚集速度匹配性的调控、气泡模板剂尺寸及分布的可调控性等一系列的问题还需进一步探索。
2.2 鼓入气体产生气泡模板剂向合成体系中鼓入气体是形成气泡模板剂的另一种方式。Han等[68-69]将CO2/N2气泡鼓入CaCl2溶液中,合成了粒径为1~5 μm的中空CaCO3球。CO2气泡不仅作构建中空结构CaCO3的模板剂,且水解形成CO32-与母体溶液的Ca2+在气泡表面上形成CaCO3核,通过形成一个强有利的三相气-固-液接触,稳定在气泡表面而形成壳层结构。马保民等[70]也用类似的方法得到纳米中空CaCO3微球。Rudloff等[71-72]以鼓入的CO2气泡为模板,通过首先形成CaCO3的环状结构,然后环生长成中空半球,该材料由10~15 nm的球霰石纳米晶体初级单元构成。此外,Han等[73]将氨气借助毛细管鼓入到所制备的溶胶中,利用氨的催化将二氧化硅溶胶在气泡表面形成胶凝,从而得到中空凝胶颗粒,且颗粒表面为多孔性微观结构(图 5)。Ma等[74]采用鼓入的N2气泡作为软模板,在惰性气泡表面得到具有介孔结构PdCoP合金纳米粒子网络(ANN),其比表面积可达73.1 m2·g-1。

|
图 5 溶液中的凝胶颗粒[73] Fig.5 Gel particles in solution[73] (A) gel on bubble (B) floating gel particle (C) dried gel particle: optical image (D) SEM micrograph of particle surface |
以向合成体系中鼓入气体产生气泡模板的方法合成多孔材料中多数样品为中空球体且具有介孔结构,仅少数样品具有网孔结构[74]。该方法对气泡模板形成速度、尺寸及分布等具有一定的可控性,但所形成气泡模板剂尺寸与鼓入气体所用管口大小有直接关系,所以,由气体鼓入法所得到气泡模板在未进行特殊处理的情况下较难达到气泡的超微化。该法合成工艺简单,成本低,不需要其它物质辅助产气,不但避免了焙烧除模板,且不需要消耗其它化合物,具有绿色无污染的优点,是简单、方便快捷制备高比表面积多孔催化剂的途径。
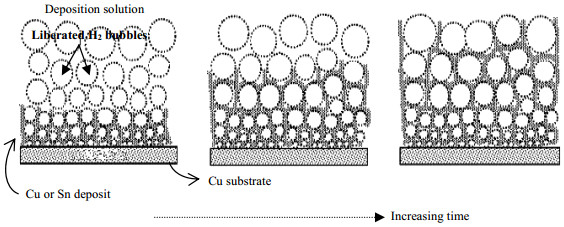
2.3 电解产生气泡模板剂通过电解原位形成气泡动态模板并电沉积制备多孔材料已有诸多报道。这种方法通常以阴极析出的氢气为模板,在高度极化的阴极表面金属离子和氢离子同时被还原从而得到多孔性结构。图 6简单描述了电解电沉积合成多孔金属材料的机理。首先,在阴极材料基体表面电解形成大量的氢气泡,气泡占据的位置不能形成沉积层,被电还原的金属只能在气泡“模板”间的空隙间沉积。另一方面,由于金属沉积速率较快,电极周围的金属离子被快速消耗掉,再加上电解析出的氢气不断向外扩散阻碍了金属离子从母液高浓度区向电极表面附近离子低浓度区域的扩散,因此被还原的金属粒子只能在气泡间的空隙中连续生长,而且随着时间延长气泡与气泡不断合并,待气泡长到一定程度就会最终脱离基体,最终得到具有高孔隙率和高比表面积的多孔自支撑型金属薄膜[75]。
Tsai等[76]利用类似的原位还原技术,以阴极析氢产生的气泡为模板电沉积成功制备了多孔锌膜。孙雅峰等[77]用相同的方法制备了多孔镍、铜薄膜,同时制得铅纳米线三维多孔薄膜。随后,Li等[78]报道了3D多孔铜膜,所得平均孔径为10~40 μm,膜厚度为6~20 μm;而Chung等[79]制得了孔径为20~45 μm的多孔银。杨桂美等[75]在玻碳电极上电沉积形成了3D多孔Pd、Pd/Pt合金和铜膜,该法方便快捷。Zhuo等[80]采用同样的方法得到了具有丰富孔道的树枝状Ni-Sn泡沫,通过改变电解质温度其孔道在10~40 μm调变,而且电解液离子浓度对孔结构也具有明显影响,研究发现从富含金属离子的电解质溶液中获得的树枝状Ni-Sn泡沫外层存在大量孔径为2~5 nm介孔孔道。另外,Zhang等[81]通过电沉积法制备三维网状结构铜泡沫发现,随着沉积时间由5 s延长到20 s时,沉积层外层孔道从32.37 μm增大到了74.22 μm;而且基础溶液中阴离子浓度也对孔径具有一定影响,当向体系中添加的HCl由10 mol·L-1增加到30 mol·L-1时所合成样品平均孔径由77.11 μm增大到105.03 μm;孔壁致密的铜泡沫可以在电流密度反复多次电沉积获得。Kim等[82]在制备得Pt-Cu合金纳米结构薄膜研究中得出了相似结果,当电解液中Cu离子源浓度从0.08 mol·L-1提高到0.25 mol·L-1,所得样品的孔径由(16±3) μm增大到(32±13) μm。采用相似方法制备的多孔结构材料还有Cu泡沫[83],Ni-Cu骨架泡沫[84-85],Pd-Au多孔泡沫薄膜[86],三维Ni/Al涂层[87],树状铋薄膜电极[88],高度多孔的铜和钴基双金属泡沫[89],微米/纳米多孔氧化锰电极[90],三维多孔Ni-Co/Ni泡沫电极[91],三维多结构微/纳米多孔NiAg薄膜[92],Pd和PdNi泡沫[93],多孔Cu纳米膜[94-96],3D大孔石墨烯[97],海绵状骨架镍[98-99],富Zn的Cu-Zn合金[100],针状多孔铟电极[101]以及近期报道的3D多孔Ni@Cu支架[102]等。
此外,有趣的是,Stone等[103]以阴极析出的气泡为模板,制得管状铁氧化物。在该过程中水和H+在阴极表面被还原,产生氢氧根离子和氢气,NH4+被去质子化产生氨气,这些混合气体支撑气-液界面处形成沉淀膜,气泡脱离电极表面时留下延伸管的沉积材料环,且该过程不断地延续下去。随后,Li等[104]同样以阴极析出的氢气泡为“动态模板”,电沉积法制备了多孔CeO2和CeO2纳米片阵列簇。在沉积过程中,当Ce(NO3)3浓度和阴极电流密度都较低时,有利于所产生气泡的合并而形成大气泡,故而电沉积只发生在气泡之间,从而构成多孔结构(如图 7C-1),孔径大小约2 μm;当Ce(NO3)3浓度增加时,CeO2的沉积速率也增加,使所产生气泡受到阻碍而不能快速的合并,故而大多数气泡尺寸较小;因此在电沉积过程中仅有少量大气泡和许多小气泡(如图 7C-2、3),故而形成如图 7C-3所示的许多小气泡位于沉积物之间的气泡阵列,最后构筑成CeO2的纳米片阵列,同时大气泡导致CeO2纳米片阵列间的分离。因此,CeO2纳米片阵列的形成是电沉积过程与气体逸出过程竞争的结果。与上述不同,Comisso等[105]以阳极析出的氧气泡为模板,电沉积法制得了多孔PdO2层材料,孔径为1~10 μm。
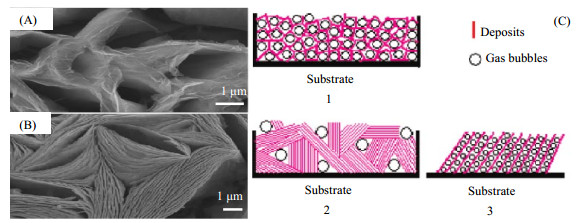
|
图 7 所制备沉积物的SEM图像[104] Fig.7 SEM micrographs of deposits[104] (A) porous CeO2 deposits (B) clusters of CeO2 nanosheet arrays (C-1) formation of porous structure (C-2) nanosheet array clusters (C-3) nanosheet arrays |
以电解产生气泡动态模板的方法所制得的多孔材料大多数具有三维网孔结构,也有部分文献报道合成了管状[103]、纳米片阵列簇结构[104]的样品。采用该法所构建的孔道孔径一般较大,孔径范围较宽一般有几微米到几十微米,甚至上百微米,故而该方法适用制备超大孔型多孔无机材料。通过改变合成体系中离子浓度、电沉积时间、电解体系温度等因素,孔道尺寸在一定范围内可调。通过电解原位形成气泡动态模板并电沉积制备多孔材料,是一种比较新颖的方法,此法合成快速简便,成本低廉,对操作条件无严格要求,但以此法构筑孔道结构的研究中仍缺乏对孔道结构调控较为系统的研究,相信经过广大研究者的不断努力,采用气泡辅助的电化学合成路线可以拓展制备类型孔道结构甚至是多级孔道的构筑。
2.4 超声诱导产生气泡模板剂气泡也可通过超声诱导产生。已有研究报道通过超声处理相应的前驱体溶液,产生的空穴微泡能以常规液-液乳剂相似的方式稳定存在,因此其也可以作为软模板合成中空颗粒[106]。Prouzet等[107]利用超声波对含有表面活性剂和二氧化硅前驱体(TEOS)水溶液进行处理产生空化气泡,其在溶液中被捕获作为模板剂,从而制得具有介孔壳的亚微米中空二氧化硅球。Rana等[108]采用超声介导的超分子模板技术制备了介孔二氧化硅囊泡。表面活性剂和硅酸盐物种在空化气泡的空气-水界面附近协同组装形成泡状形态模板,以此所制得的囊泡直径为50~500 nm,壳层厚度为25~35 nm,最大孔径为2.4 nm。Zheng等[109]以阴离子表面活性剂十二烷基硫酸钠(SDS)泡囊为模板,采用超声波辐射方法,成功制备了100~200 nm大小的CdSe空心球,球壁由平均为3~4 nm纳米晶CdSe颗粒组成,研究结果表明超声辐射是得到CdSe空心球结构的先决条件。随后,Zhu等[110]通过超声诱导初始前驱体在空化微泡气/液界面反应,制备得CdSe空心球体。Shchukin等[111]通过自组装以空气微泡为模板制备了聚电解质微胶囊;该过程首先聚(烯丙基胺盐酸盐) (PAH)被吸收在气泡的表面上,然后吸附带相反电荷的聚(苯乙烯磺酸盐) (PSS),重复此过程即可得到聚电解质微胶囊(图 8)。Deng等[112]利用超声波辅助一锅法超声化学大规模地制备了分层中空CuO亚微球,样品的平均孔径为3.64 nm。已报到采用相似方法,制备的多孔性中空材料还有PbS空心纳米球[113]、无定形磷酸钙中空纳米球[114]、中空CeO2微球[115]等。
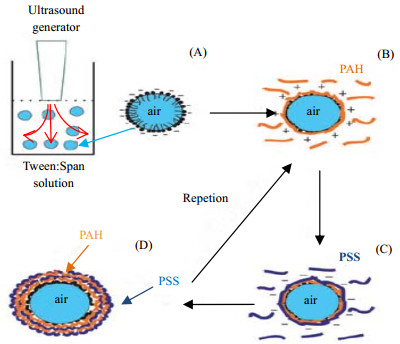
|
图 8 在“空气”芯上形成聚电解质胶囊的示意图[111] Fig.8 Schematic diagram of the formation of polyelectrolyte capsules on "air" core [111] (A)Tween:Span mixture was used for air microbubble formation (B~D)Stabilized by electrostatic assembly of PAH/PSS multilayers |
利用超声诱导产生气泡模板法所构筑的孔道多为介孔结构,样品通常为中空型球体。但该方法需要表面活性剂辅助形成稳定存在的微泡,即所制得样品中有可能含有表面活性剂,故而在应用中有可能影响材料性能,需要考虑后期如何将表面活性剂从所制备的材料中去除。
3 结语气泡模板法因操作条件温和、简便、低成本,较绿色环保,故而气泡模板法在多孔材料合成方面的研究报道越来越多,展现出该方法在多孔材料合成领域的广阔应用前景。目前,气泡模板法合成多孔材料仍处于起步阶段,故而对气泡模板法构筑多孔结构的认识还不够深入,仍然存在一些亟待解决的问题:(1)前驱体粒子性质对气泡模板剂稳定性的影响规律;(2)气泡模板剂形成的速度与前驱体在气泡模板表面聚集速度匹配性的调控;(3)溶剂性质对气泡稳定性及构筑多孔结构的影响;(4)气泡模板剂尺寸及分布的可调控性等一系列的问题。随着科研工作者对该方法的不断探讨,上述问题必将被逐渐认识并解决,气泡模板法在多孔材料合成领域也将更具有广阔的应用前景。
| [1] | CAI Bin(蔡彬), HU Wei(胡炜), DU Bao-ji(杜宝吉), et al. Research progress of template-methods and its application in nano-materials(模板法及其在纳米材料制备领域的应用研究进展)[J]. Materials Review(材料导报), 2010, 24(15): 107-112. |
| [2] | DING Wei(丁巍), WANG Ding-cong(王鼎聪), ZHAO De-zhi(赵德智), et al. Development and application of templates in nanomaterials syntheses(模板剂在合成纳米材料中的应用与发展)[J]. Chemistry(化学通报), 2016, 79(6): 490-495. |
| [3] | Liu Y L, Wu Y H, Tsai W B, et al. Core-shell silica@chitosan nanoparticles and hollow chitosan nanospheres using silica nanoparticles as templates:preparation and ultrasound bubble application[J]. Carbohydrate Polymers, 2011, 84(2): 770-774. DOI:10.1016/j.carbpol.2010.03.033. |
| [4] | Zhang Z W, Wang P, Wang F, et al. Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template[J]. Chinese Journal of Chemical Engineering, 2018, 26(5): 1229-1234. DOI:10.1016/j.cjche.2017.12.016. |
| [5] | Chen G C, Kuo C Y, Lu S Y. A general process for preparation of core-shell particles of complete and smooth shells[J]. Journal of the American Ceramic Society, 2005, 88(2): 277-283. DOI:10.1111/jace.2005.88.issue-2. |
| [6] | Zhao W, Liu N, Wang H, et al. Sacrificial template synthesis of core-shell SrTiO3/TiO2, heterostructured microspheres photocatalyst[J]. Ceramics International, 2017, 43(6): 4807-4813. DOI:10.1016/j.ceramint.2016.12.009. |
| [7] | Zhu K, Sun J, Zhang H, et al. Carbon as a hard template for nano material catalysts[J]. Journal of Natural Gas Chemistry, 2012, 21(3): 215-232. DOI:10.1016/S1003-9953(11)60357-5. |
| [8] | Chen J F, Ding H M, Wang J X, et al. Preparation and characterization of porous hollow silica nanoparticles for drug delivery application[J]. Biomaterials, 2004, 25(4): 723-727. DOI:10.1016/S0142-9612(03)00566-0. |
| [9] | Gao L, Luo L, Chen J. Synthesis of hollow titania using nanosized calcium carbonate as a template[J]. Chemistry Letters, 2005, 34(2): 138-139. DOI:10.1246/cl.2005.138. |
| [10] | Mcdonald C J, Bouck K J, Chaput A B, et al. Emulsion polymerization of voided particles by encapsulation of a nonsolvent[J]. Macromolecules, 2000, 33(5): 1593-1605. DOI:10.1021/ma991284e. |
| [11] | Han J, Song G, Guo R. A facile solution route for polymeric hollow spheres with controllable size[J]. Advanced Materials, 2006, 18(23): 3140-3144. DOI:10.1002/(ISSN)1521-4095. |
| [12] | Fendler J H. Polymerized surfactant vesicles:novel membrane mimetic systems[J]. Science, 1984, 223(4639): 888-894. DOI:10.1126/science.223.4639.888. |
| [13] | Bunton C A, Nome F, Quina F H, et al. Ion binding and reactivity at charged aqueous interfaces[J]. Accounts of Chemical Research, 1991, 24(12): 357-364. DOI:10.1021/ar00012a001. |
| [14] | Li Y, Li X, Li Y, et al. Controlled self-assembly behavior of an amphiphilic bisporphyrin-bipyridinium-palladium complex:from multibilayer vesicles to hollow capsules[J]. Angewandte Chemie International Edition, 2006, 45(22): 3639-3643. DOI:10.1002/(ISSN)1521-3773. |
| [15] | Rana R, Murthy V, Yu J, et al. Nanoparticle self-assembly of hierarchically ordered microcapsule structures[J]. Advanced Materials, 2005, 17(9): 1145-1150. DOI:10.1002/(ISSN)1521-4095. |
| [16] | Long L, Zhang H, Ye M, et al. Ammonia cation-assisted bubble template for synthesizing hollow TiO2 nanospheres and their application in lithium ion storage[J]. RSE Advances, 2015, 5(16): 12224-12229. DOI:10.1039/C4RA14476G. |
| [17] | Li X X, Xiong Y J, Li Z Q, et al. Large-scale fabrication of TiO2 hierarchical hollow spheres[J]. Inorganic Chemistry, 2006, 45(9): 3493-3495. DOI:10.1021/ic0602502. |
| [18] | Yang J, Sasaki T. Synthesis of CoOOH hierarchically hollow spheres by nanorod self-assembly through bubble templating[J]. Chemistry of Materials, 2008, 20(5): 2049-2056. DOI:10.1021/cm702868u. |
| [19] | Sun J, Chen G, Wu J, et al. Bismuth vanadate hollow spheres:bubble template synthesis and enhanced photocatalytic properties for photodegradation[J]. Applied Catalysis B:Environmental, 2013, 132-133: 304-314. DOI:10.1016/j.apcatb.2012.12.002. |
| [20] | Zhou C, Zhao Y, Bian T, et al. Bubble template synthesis of Sn2Nb2O7 hollow spheres for enhanced visible-light-driven photocatalytic hydrogen production[J]. Chemical Communications, 2013, 49(84): 9872-9874. DOI:10.1039/c3cc45683h. |
| [21] | Chen X, Zhang Z, Li X, et al. Hollow magnetite spheres:synthesis, characterization, and magnetic properties[J]. Chemical Physics Letters, 2006, 422(1-3): 294-298. DOI:10.1016/j.cplett.2006.02.082. |
| [22] | Kuang C, Zeng W, Ye H, et al. A novel approach for fabricating NiO hollow spheres for gas sensors[J]. Physica E:Low-Dimensional Systems and Nanostructures, 2018, 97: 314-316. DOI:10.1016/j.physe.2017.12.006. |
| [23] | CHEN Xiao-mei(陈小梅), GUAN Xiang-feng(关翔锋), LI Li-ping(李莉萍), et al. Bubble template synthesis of MFe2O4 (M=Co, Zn) hollow microspheres and their application in li-ion batteries(MFe2O4 (M=Co, Zn)中空微球的气泡模板法合成及在锂离子电池中的应用)[J]. Chemical Journal of Chinese Universities(高等学校化学学报), 2011, 32(3): 624-629. |
| [24] | Weng C H, Huang C C, Yeh C S, et al. Synthesis of hollow, magnetic Fe/Ga-based oxide nanospheres using a bubble templating method in a microfluidic system[J]. Microfluidics & Nanofluidics, 2009, 7(6): 841-848. |
| [25] | Yan X X, Hu X J, Fu G T, et al. Facile synthesis of porous Pd3Pt half-shells with rich "active sites" as efficient catalysts for formic acid oxidation[J]. Small, 2018, 14(13): e 1703940. DOI:10.1002/smll.v14.13. |
| [26] | Wang X L, Yang H G. Facile fabrication of high-yield graphitic carbon nitride with a large surface area using bifunctional urea for enhanced photocatalytic performance[J]. Applied Catalysis B:Environmental, 2017, 205: 624-630. DOI:10.1016/j.apcatb.2017.01.013. |
| [27] | Peng Q, Dong Y J, Li Y D. ZnSe semiconductor hollow microspheres[J]. Angewandte Chemie International Edition, 2003, 42(26): 3027-3030. DOI:10.1002/anie.200250695. |
| [28] | Wang H, Du F. Hydrothermal synthesis of ZnSe hollow micropheres[J]. Crystal Research & Technology, 2006, 41(4): 323-327. |
| [29] | Jiang C, Zhang W, Zou G, et al. Synthesis and characterization of ZnSe hollow nanospheres via a hydrothermal route[J]. Nanotechnology, 2005, 16(4): 551-554. DOI:10.1088/0957-4484/16/4/036. |
| [30] | Liu X, Ma J, Peng P, et al. Ionic liquid-assisted complex-solvothermal synthesis of ZnSe hollow microspheres[J]. Materials Science & Engineering B, 2008, 150(2): 89-94. |
| [31] | Lin H Y, Wei J D, Ou C C, et al. Hydrothermal synthesis and characterization of mesoporous zinc selenide agglomerates by nitrogen bubble templates[J]. Journal of Alloys & Compounds, 2011, 509(25): 7009-7015. |
| [32] | Wu C. Z, Xie Y, Lei L Y, et al. Synthesis of new-phased VOOH hollow "dandelions" and their application in lithium-ion batteries[J]. Advanced Materials, 2006, 18(13): 1727-1732. DOI:10.1002/(ISSN)1521-4095. |
| [33] | Zuo X, Chang K, Zhao J, et al. Bubble-template-assisted synthesis of hollow fullerene-like MoS2nanocages as a lithium ion battery anode material[J]. Journal of Materials Chemistry A, 2016, 4(1): 51-58. DOI:10.1039/C5TA06869J. |
| [34] | Guo L, Liang F, Wen X, et al. Uniform magnetic chains of hollow cobalt mesospheres from one-pot synthesis and their assembly in solution[J]. Advanced Functional Materials, 2007, 17(3): 425-430. DOI:10.1002/(ISSN)1616-3028. |
| [35] | Zhu J Y, Li F M, Yao L, et al. In situ bubble template-assisted synthesis of phosphonate-functionalized Rh nanodendrites and their catalytic application[J]. Crystengcomm, 2017, 19(21): 2946-2952. DOI:10.1039/C7CE00606C. |
| [36] | Chen X, Wang Z, Wang X, et al. Synthesis of novel copper sulfide hollow spheres generated from copper (Ⅱ)-thiourea complex[J]. Journal of Crystal Growth, 2004, 263(1-4): 570-574. DOI:10.1016/j.jcrysgro.2003.12.004. |
| [37] | Liu J, Xue D. Solvothermal synthesis of CuS semiconductor hollow spheres based on a bubble template route[J]. Journal of Crystal Growth, 2009, 311(3): 500-503. DOI:10.1016/j.jcrysgro.2008.09.025. |
| [38] | Gu F, Li C Z, Wang S F, et al. Solution-phase synthesis of spherical zinc sulfide nanostructures[J]. Langmuir, 2006, 22(3): 1329-1332. DOI:10.1021/la052539m. |
| [39] | Zhao L, Tao F, Quan Z, et al. Bubble template synthesis of copper sulfide hollow spheres and their applications in lithium ion battery[J]. Materials Letters, 2012, 68: 28-31. DOI:10.1016/j.matlet.2011.09.108. |
| [40] | Liu X, Cui J, Zhang L, et al. A solvothermal route to semiconductor ZnS micrometer hollow spheres with strong photoluminescence properties[J]. Materials Letters, 2006, 60(20): 2465-2469. DOI:10.1016/j.matlet.2006.01.019. |
| [41] | Zhang H, Zhang S, Pan S, et al. A simple solution route to ZnS nanotubes and hollow nanospheres and their optical properties[J]. Nanotechnology, 2004, 15(8): 945-948. DOI:10.1088/0957-4484/15/8/012. |
| [42] | Li L, Yang H, Qi G, et al. Synthesis and photoluminescence of hollow microspheres constructed with ZnO nanorods by H2 bubble templates[J]. Chemical Physics Letters, 2008, 455(1-3): 93-97. DOI:10.1016/j.cplett.2008.02.071. |
| [43] | Zhang J, Sun L, Liao C, et al. A simple route towards tubular ZnO[J]. Chemical Communications, 2002(3): 262-263. DOI:10.1039/b108863g. |
| [44] | Yan C, Xue D. Polyhedral construction of hollow ZnO microspheres by CO2 bubble templates[J]. Journal of Alloys & Compounds, 2007, 431(1-2): 241-245. |
| [45] | Li L, Sun S Q, Wang Y X, et al. Facile synthesis of ZnO/g-C3N4 composites with honeycomb-like structure by H2 bubble templates and their enhanced visible light photocatalytic performance[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2018, 355: 16-24. DOI:10.1016/j.jphotochem.2017.12.016. |
| [46] | Kim Y J, Chai S Y, Lee W I. Control of TiO2 structures from robust hollow microspheres to highly dispersible nanoparticles in a tetrabutylammonium hydroxide solution[J]. Langmuir, 2007, 23(19): 9567-9571. DOI:10.1021/la700797v. |
| [47] | Wang H, Liang J, Fan H, et al. Synthesis and gas sensitivities of SnO2, nanorods and hollow microspheres[J]. Journal of Solid State Chemistry, 2008, 181(1): 122-129. DOI:10.1016/j.jssc.2007.11.010. |
| [48] | Li S H, Chu Z, Meng F F, et al. Highly sensitive gas sensor based on SnO2 nanorings for detection of isopropanol[J]. Journal of Alloys and Compounds, 2016, 688: 712-717. DOI:10.1016/j.jallcom.2016.07.248. |
| [49] | Ding C, Zeng Y, Cao L, et al. Hierarchically porous Fe3O4/C nanocomposite microspheres via a CO2 bubble-templated hydrothermal approach as high-rate and high-capacity anode materials for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(16): 5898-5908. DOI:10.1039/C5TA10726A. |
| [50] | Valladares L D L S, Félix L L, Suarez S M E, et al. Preparation and crystallization of hollow α-Fe2O3, microspheres following the gas-bubble template method[J]. Materials Chemistry & Physics, 2016, 169: 21-27. |
| [51] | Zhu L, Oh W C. Bubble template synthesis of CdLa2S4 hollow spheres/reduced graphene oxide nanocomposites as efficient and sustainable visible-light driven photocatalysts[J]. RSE Advances, 2015, 5(110): 90321-90334. DOI:10.1039/C5RA18014G. |
| [52] | Yan Y, Lu C, Xiang L, et al. Preparation of hierarchical polyimide hollow spheres via a gas bubble templated transimidization induced crystallization process[J]. Polymer Bulletin, 2012, 69(6): 675-684. DOI:10.1007/s00289-012-0754-6. |
| [53] | Lv R, Yang G, Dai Y, et al. Self-produced bubble-template synthesis of La2O3:Yb/Er@Au hollow spheres with markedly enhanced luminescence and release properties[J]. Crystengcomm, 2014, 16(41): 9612-9621. DOI:10.1039/C4CE01063A. |
| [54] | Yu S H, C lfen H, Xu A W, et al. Complex spherical BaCO3 superstructures self-assembled by a facile mineralization process under control of simple polyelectrolytes[J]. Crystal Growth & Design, 2004, 4(1): 33-37. |
| [55] | SHI Lei (石磊). Synthesis and property of carbon nitride based photocatalytic materials (氮化碳基光催化材料的制备及性能) [D]. Haerbin (哈尔滨): Harbin Institute of Technology (哈尔滨工业大学), 2016. |
| [56] | ZHANG Xiao-zeng (张小增). Preparation and properties of rare earth fluoride upconversion nanoluminescent materials (稀土氟化物上转换纳米发光材料的制备及性能研究) [D]. Ganzhou (赣州): Jiangxi University of Science and Technology (江西理工大学), 2016. |
| [57] | Chen Y, Qin Z, Guo X, et al. One-step hydrothermal synthesis of (CuIn)0.2Zn1.6S2 hollow sub-microspheres for efficient visible-light-driven photocatalytic hydrogen generation[J]. International Journal of Hydrogen Energy, 2016, 41(3): 1524-1534. DOI:10.1016/j.ijhydene.2015.11.087. |
| [58] | Chen Z, Yan X, Ming X, et al. Building honeycomb-like hollow microsphere architecture in a bubble template reaction for high-performance lithium-rich layered oxide cathode materials[J]. ACS Applied Materials & Interfaces, 2017, 9(36): 30617-30625. |
| [59] | Cao C, Wei L, Su M, et al. Template-free and one-pot synthesis of N-doped hollow carbon tube@hierarchically porous carbon supporting homogeneous AgNPs for robust oxygen reduction catalyst[J]. Carbon, 2017, 112: 27-36. DOI:10.1016/j.carbon.2016.10.083. |
| [60] | You Z, Su Y, Yu Y, et al. Preparation of g-C3N4, nanorod/InVO4, hollow sphere composite with enhanced visible-light photocatalytic activities[J]. Applied Catalysis B:Environmental, 2017, 213: 127-135. DOI:10.1016/j.apcatb.2017.05.015. |
| [61] | Wang Y M, Chen Q D, Shen X H, et al. One-step synthesis of hollow UO2 nanospheres via radiolytic reduction of ammonium uranyl tricarbonate[J]. Chinese Chemical Letters, 2017, 28(2): 197-200. DOI:10.1016/j.cclet.2016.06.035. |
| [62] | Guo Q, Zhang Y, Zhang H S, et al. 3D foam strutted graphene carbon nitride with highly stable optoelectronic properties[J]. Advanced Functional Materials, 2017, 27(42): e1703711. DOI:10.1002/adfm.v27.42. |
| [63] | Liu L, Chen L X, Wang A J, et al. Hydrogen bubbles template-directed synthesis of self-supported AuPt nanowire networks for improved ethanol oxidation and oxygen reduction reactions[J]. International Journal of Hydrogen Energy, 2016, 41(21): 8871-8880. DOI:10.1016/j.ijhydene.2016.03.208. |
| [64] | Shi Y C, Yuan T, Feng J J, et al. Rapid fabrication of support-free trimetallic Pt53Ru39Ni8 nanosponges with enhanced electrocatalytic activity for hydrogen evolution and hydrazine oxidation reactions[J]. Journal of Colloid & Interface Science, 2017, 505: 14-22. |
| [65] | Yuan T, Wang A, Fang K, et al. Hydrogen evolution-assisted one-pot aqueous synthesis of hierarchical trimetallic PdNiRu nanochains for hydrazine oxidation reaction[J]. Journal of Energy Chemistry, 2017, 26(6): 1231-1237. DOI:10.1016/j.jechem.2017.08.003. |
| [66] | Chen S S, Shi Y C, Wang A J, et al. Free-standing Pt nanowire networks with clean surfaces:highly sensitive electrochemical detection of nitrite[J]. Journal of Electroanalytical Chemistry, 2017, 791: 131-137. DOI:10.1016/j.jelechem.2017.03.016. |
| [67] | Zhu J, Qian X. Necklace-like nanostructures of cadmium hydroxide:controlled synthesis with bubble-template and its separation property on dye[J]. Solid State Sciences, 2008, 10(11): 1577-1583. DOI:10.1016/j.solidstatesciences.2008.02.010. |
| [68] | Hadiko G, Han Y S, Fuji M, et al. Synthesis of hollow calcium carbonate particles by the bubble templating method[J]. Materials Letters, 2005, 59(19-20): 2519-2522. DOI:10.1016/j.matlet.2005.03.036. |
| [69] | Han Y, Fuji M, Shchukin D, et al. A new model for the synthesis of hollow particles via the bubble templating method[J]. Crystal Growth & Design, 2009, 9(8): 3771-3775. |
| [70] | MA Bao-min (马保民). Synthesize nano-CaCO3 particles with bubble biomimetic templates (泡沫仿生模板法合成纳米碳酸钙) [D]. Jinan (济南): Shandong University (山东大学), 2008. |
| [71] | Rudloff J, Colfen H. Superstructures of temporarily stabilized nanocrystalline CaCO3 particles:morphological control via water surface tension variation[J]. Langmuir, 2004, 20(3): 991-996. DOI:10.1021/la0358217. |
| [72] | Rudloff J, Antonietti M, C lfen H, et al. Double-hydrophilic block copolymers with monophosphate ester moieties as crystal growth modifiers of CaCO3[J]. Macromolecular Chemistry & Physics, 2002, 203(4): 627-635. |
| [73] | Han Y S, Tarutani Y, Fuji M, et al. Synthesis of hollow silica particle by combination of bubble templating method and sol-gel transformation[J]. Advanced Materials Research, 2006, 11-12: 673-676. DOI:10.4028/www.scientific.net/AMR.11-12. |
| [74] | Ma Y, Wang R, Wang H, et al. Room-temperature synthesis with inert bubble templates to produce "clean" PdCoP alloy nanoparticle networks for enhanced hydrazine electro-oxidation[J]. RSC Advances, 2015, 5(13): 9837-9842. DOI:10.1039/C4RA14423F. |
| [75] | YANG Gui-mei (杨桂美). The application of hydrogen bubble template electrodepositon porous metal materials (氢气泡模板沉积多孔金属材料及其应用研究) [D]. Hefei (合肥): University of Science and Technology of China (中国科学技术大学), 2011. |
| [76] | Tsai W L, Hsu P C, Hwu Y, et al. Electrochemistry:building on bubbles in metal electrodeposition[J]. Nature, 2002, 417(6885): 139-139. DOI:10.1038/417139a. |
| [77] | SUN Ya-feng (孙雅峰). Electrodeposition of porous metal films on a template of hydrogen bubbles (氢气泡模板法电沉积制备多孔金属薄膜) [D]. Jinhua (金华): Zhejiang Normal University (浙江师范大学), 2006. |
| [78] | Li Y, Jia W Z, Song Y Y, et al. Superhydrophobicity of 3D porous copper films prepared using the hydrogen bubble dynamic template[J]. Chemistry of Materials, 2007, 19(23): 5758-5764. DOI:10.1021/cm071738j. |
| [79] | Cherevko S, Xing X, Chung C H. Electrodeposition of three-dimensional porous silver foams[J]. Electrochemistry Communications, 2010, 12(3): 467-470. DOI:10.1016/j.elecom.2010.01.021. |
| [80] | Zhuo K, Jeong M G, Shin M S, et al. Morphological variation of highly porous Ni-Sn foams fabricated by electro-deposition in hydrogen-bubble templates and their performance as pseudo-capacitors[J]. Applied Surface Science, 2014, 322: 15-20. DOI:10.1016/j.apsusc.2014.10.007. |
| [81] | Zhang W, Ding C, Wang A, et al. 3-D network pore structures in copper foams by electrodeposition and hydrogen bubble templating mechanism[J]. Journal of the Electrochemical Society, 2015, 162(8): D365-D370. DOI:10.1149/2.0591508jes. |
| [82] | Kim Y, Lee H, Lim T, et al. Non-conventional Pt-Cu alloy/carbon paper electrochemical catalyst formed by electrodeposition using hydrogen bubble as template[J]. Journal of Power Sources, 2017, 364: 16-22. DOI:10.1016/j.jpowsour.2017.08.016. |
| [83] | Lee H, Kim S K, Sang H A. Electrochemical preparation of Ag/Cu and Au/Cu foams for electrochemical conversion of CO2 to CO[J]. Journal of Industrial and Engineering Chemistry, 2017, 54: 218-225. DOI:10.1016/j.jiec.2017.05.036. |
| [84] | Mirzaee M, Dehghanian C, Bokati K S. One-step electrodeposition of reduced graphene oxide on three-dimensional porous nano nickel-copper foam electrode and its use in supercapacitor[J]. Journal of Electroanalytical Chemistry, 2018, 813: 152-162. DOI:10.1016/j.jelechem.2018.02.032. |
| [85] | Mirzaee M, Dehghanian C, Bokati K S. ERGO grown on Ni-Cu foam frameworks by constant potential method as high performance electrodes for supercapacitors[J]. Applied Surface Science, 2018, 436: 1050-1060. DOI:10.1016/j.apsusc.2017.12.145. |
| [86] | Liu J, Wang J, Kong F, et al. Facile preparation of three-dimensional porous Pd-Au films and their electrocatalytic activity for methanol oxidation[J]. Catalysis Communications, 2016, 73: 22-26. DOI:10.1016/j.catcom.2015.09.033. |
| [87] | Guo X, Li X, Wei Z, et al. Rapid fabrication and characterization of superhydrophobic tri-dimensional Ni/Al coatings[J]. Applied Surface Science, 2016, 387: 8-15. DOI:10.1016/j.apsusc.2016.06.068. |
| [88] | Zhou H, Hou H, Dai L, et al. Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb (Ⅱ) and Cd (Ⅱ)[J]. Chinese Journal of Chemical Engineering, 2016, 24(3): 410-414. DOI:10.1016/j.cjche.2015.08.012. |
| [89] | Eugénio S, Demirci U B, Silva T M, et al. Copper-cobalt foams as active and stable catalysts for hydrogen release by hydrolysis of sodium borohydride[J]. International Journal of Hydrogen Energy, 2016, 41(20): 8438-8448. DOI:10.1016/j.ijhydene.2016.03.122. |
| [90] | Nguyen T, Boudard M, Carmezim M J, et al. Hydrogen bubbling-induced micro/nano porous MnO2 films prepared by electrodeposition for pseudocapacitor electrodes[J]. Electrochimica Acta, 2016, 202: 166-174. DOI:10.1016/j.electacta.2016.03.204. |
| [91] | Guo F, Cao D, Du M, et al. Enhancement of direct urea-hydrogen peroxide fuel cell performance by three-dimensional porous nickel-cobalt anode[J]. Journal of Power Sources, 2016, 307: 697-704. DOI:10.1016/j.jpowsour.2016.01.042. |
| [92] | Yu X, Wang M, Wang Z, et al. 3D multi-structural porous NiAg films with nanoarchitecture walls:high catalytic activity and stability for hydrogen evolution reaction[J]. Electrochimica Acta, 2016, 211: 900-910. DOI:10.1016/j.electacta.2016.06.062. |
| [93] | Li R, Mao H, Zhang J, et al. Rapid synthesis of porous Pd and PdNi catalysts using hydrogen bubble dynamic template and their enhanced catalytic performance for methanol electrooxidation[J]. Journal of Power Sources, 2013, 241: 660-667. DOI:10.1016/j.jpowsour.2013.05.032. |
| [94] | Liu R, Ye K, Gao Y, et al. Preparation of three-dimensional porous Cu film supported on Cu foam and its electrocatalytic performance for hydrazine electrooxidation in alkaline medium[J]. Materials Science and Engineering B, 2016, 210: 51-56. DOI:10.1016/j.mseb.2016.05.005. |
| [95] | Singh H, Dheeraj P B, Singh Y P, et al. Electrodeposition of porous copper as a substrate for electrocatalytic material[J]. Journal of Electroanalytical Chemistry, 2017, 785: 1-7. DOI:10.1016/j.jelechem.2016.12.013. |
| [96] | Majidi M R, Ghaderi S. Hydrogen bubble dynamic template fabrication of nanoporous Cu film supported by graphene nanaosheets:a highly sensitive sensor for detection of nitrite[J]. Talanta, 2017, 175: 21-29. DOI:10.1016/j.talanta.2017.07.020. |
| [97] | Yang X, Liu A, Zhao Y, et al. Three-dimensional macroporous polypyrrole-derived graphene electrode prepared by the hydrogen bubble dynamic template for supercapacitors and metal-free catalysts[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23731-23740. |
| [98] | Kim S W, Kim I H, Kim S I, et al. Nickel hydroxide supercapacitor with a theoretical capacitance and high rate capability based on hollow dendritic 3D-nickel current collectors[J]. Chemistry An Asian Journal, 2017, 12(12): 1291-1296. DOI:10.1002/asia.v12.12. |
| [99] | Ashassi-Sorkhabi H, Badakhshan P L. Electrochemical synthesis of three-dimensional porous networks of nickel with different micro-nano structures for the fabrication of Ni/MnOx, nanocomposites with enhanced supercapacitive performance[J]. Applied Surface Science, 2017, 419: 165-176. DOI:10.1016/j.apsusc.2017.04.254. |
| [100] | Varzi A, Mattarozzi L, Cattarin S, et al. 3D porous Cu-Zn alloys as alternative anode materials for Li-Ion batteries with superior low T performance[J]. Advanced Energy Materials, 2018, 8(1): e1870001. DOI:10.1002/aenm.v8.1. |
| [101] | Xia Z, Freeman M, Zhang D, et al. Highly selective electrochemical conversion of CO2 to HCOOH on dendritic indium foams[J]. Chemelectrochem, 2018, 5(2): 253-259. DOI:10.1002/celc.201700935. |
| [102] | Xu Y, Menon A S, Harks P P, et al. Honeycomb-like porous 3D nickel electrodeposition for stable Li and Na metal anodes[J]. Energy Storage Materials, 2018, 12: 69-78. DOI:10.1016/j.ensm.2017.11.011. |
| [103] | Stone D A, Cummins H Z. Tubular precipitation and redox gradients on a bubbling template[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(32): 11537-11541. DOI:10.1073/pnas.0404544101. |
| [104] | Li G R, Qu D L, Yu X L, et al. Microstructural evolution of CeO2 from porous structures to clusters of nanosheet arrays assisted by gas bubbles via electrodeposition[J]. Langmuir, 2008, 24(8): 4254-4259. DOI:10.1021/la7037526. |
| [105] | Comisso N, Cattarin S, Guerriero P, et al. Electrochemical behaviour of porous PbO2, layers prepared by oxygen bubble templated anodic deposition[J]. Electrochimica Acta, 2016, 200: 259-267. DOI:10.1016/j.electacta.2016.03.184. |
| [106] | Shchukin D G, M hwald H. Sonochemical nanosynthesis at the engineered interface of a cavitation microbubble[J]. Physical Chemistry Chemical Physics, 2006, 8(30): 3496-3506. DOI:10.1039/B606104D. |
| [107] | Prouzet E, Cot F, Boissière C, et al. Nanometric hollow spheres made of MSU-X-type mesoporous silica[J]. Journal of Materials Chemistry, 2002, 12(5): 1553-1556. DOI:10.1039/b111236h. |
| [108] | Rana R K, Mastai Y, Gedanken A. Acoustic cavitation leading to the morphosynthesis of mesoporous silica vesicles[J]. Advanced Materials, 2002, 14(19): 1414-1418. DOI:10.1002/1521-4095(20021002)14:19<1414::AID-ADMA1414>3.0.CO;2-F. |
| [109] | Zheng X, Xie Y, Zhu L, et al. Formation of vesicle-templated CdSe hollow spheres in an ultrasound-induced anionic surfactant solution[J]. Ultrasonics Sonochemistry, 2002, 9(6): 311-316. DOI:10.1016/S1350-4177(02)00086-X. |
| [110] | Zhu J J, Xu S, Wang H, et al. Sonochemical synthesis of CdSe hollow spherical assemblies via an in-situ template route[J]. Advanced Materials, 2003, 15(2): 156-159. DOI:10.1002/(ISSN)1521-4095. |
| [111] | Shchukin D G, K hler K, M hwald H, et al. Gas-filled polyelectrolyte capsules[J]. Angewandte Chemie International Edition, 2005, 44(21): 3310-3314. DOI:10.1002/(ISSN)1521-3773. |
| [112] | Deng C, Hu H, Ge X, et al. One-pot sonochemical fabrication of hierarchical hollow CuO submicrospheres[J]. Ultrasonics Sonochemistry, 2011, 18(5): 932-937. DOI:10.1016/j.ultsonch.2011.01.007. |
| [113] | Wang S F, Gu F, Lü M K. Sonochemical synthesis of hollow PbS nanospheres[J]. Langmuir, 2006, 22(1): 398-401. DOI:10.1021/la0518647. |
| [114] | Cai Y, Pan H, Xu X, et al. Ultrasonic controlled morphology transformation of hollow calcium phosphate nanospheres:a smart and biocompatible drug release system[J]. Chemistry of Materials, 2007, 19(13): 3081-3083. DOI:10.1021/cm070298t. |
| [115] | Zhang L, Sun Y, Jiu H, et al. Bubble template synthesis of hollow CeO2 microspheres through a solvothermal approach[J]. Iet Micro & Nano Letters, 2011, 6(1): 22-25. |