双水相系统(ATPS)是一种由两相组合在一起高于临界值时形成的互不相溶的水相系统,其组成可以是结构不同的聚合物[1],一种聚合物和一种盐[2],离子液体和一种盐[3],或小分子有机溶剂和一种盐[4]。与传统的分离萃取方法相比,双水相具有反应时间短、耗能低等优点,并且现已广泛地应用于分离萃取DNA[5]、抗体[6]、蛋白质[7]等,具有良好的研究潜力。
Gutowski等人提出了一种新的双水相体系-离子液体-盐[8]。对于离子液体的定义是由特定的阳离子和阴离子组成的具有不易挥发、不易燃等优点的低温熔盐。近年来,在蛋白质、抗生素、氨基酸等生物分离分析中得到了广泛地研究与应用[9~11]。目前离子液体双水相体系主要集中于咪唑类离子液[12, 13],针对于吡啶类离子液体双水相体系的报道较少。研究表明,吡啶类离子液体在既能形成双水相体系的同时也具有良好的萃取分离性能[14~16]。此外,离子液体存在价格昂贵的缺点,寻找便宜、高效的离子液体是目前研究关注的焦点与热点。氯盐离子液体具有独特的性质且价格低廉,常用作改性离子液体的基质,氯化N-丁基吡啶双水相体系的研究尚未见报到。同时,磷酸氢二钾作为双水相体系中常用的盐,具有良好的分离性能,易于形成双水相体系。离子液体-盐双水相体系组成不同,对于温度的响应规律也不同。
本文将离子液体氯化N-丁基吡啶和磷酸氢二钾相结合,形成了三元双水相体系,并利用浊点法在308.15、318.15、328.15 K三个温度下测定双节线数据和液液相平衡数据,采用经验方程进行拟合,并研究温度对该体系相平衡的影响,得到的结果有助于对离子液体的应用进行工业化推广。
2 实验部分 2.1 主要仪器与试剂分析天平(塞多利斯科学仪器(北京)有限公司);101-1A型超级恒温水浴锅(南京多助科技发展有限公司),氯化N-丁基吡啶[Epy]Cl (上海成捷化学有限公司)纯度≥99.9%,磷酸氢二钾[K2HPO4·3H2O](国药集团化学试剂有限公司),纯度≥99.0%。所有试剂使用时均无需进行进一步纯化。实验用水为二次蒸馏水。
2.2 实验方法双节线曲线利用浊点滴定法测定[17, 18]。把已知质量分数的离子液体[Epy]Cl溶液放在比色管中,向里滴加一定质量分数的盐溶液[K2HPO4·3H2O]直到混合溶液出现浊点。然后滴加一定量的水直到浊点刚好澄清,算出此时各个组分的质量百分含量。然后继续滴加盐溶液直到下一个浊点出现。反复操作。实验过程中质量用分析天平测量,比色管分别在温度为308.15、318.15、328.15 K的恒温水浴锅内操作。
双水相系线的测定:同样在温度为308.15、318.15、328.15 K的恒温水浴锅内进行,一定量的离子液体[Epy]Cl,盐[K2HPO4·3H2O]和水加入到离心管中,然后以2000 r×min-1的速度离心操作10 min,再将离心管放置在水浴中恒温静置24 h,保证双水相完全形成。上下相中离子液体是通过液相色谱测定阳离子得到,盐的浓度通过原子吸收测定钾离子含量得到,水通过质量守恒定律相减得到[19, 20]。
系线的校对:达到相平衡后的双水相体系,准确分离上下相,并分别精确称量上下相的质量。根据双水相体系的总组成,利用杠杆原理,通过软件MATLAB可快速计算出离子液体双水相体系液液相平衡(ILATPS)上相和下相中的离子液体[Epy]Cl和盐[K2HPO4·3H2O]的含量[21~23]。
系线长度TLL,系线斜率S利用如下公式而得:
| $ TLL = {\left[{{{\left( {w_1^t-w_1^b} \right)}^2} + {{\left( {w_2^t-w_2^b} \right)}^2}} \right]^{0.5}} $ | (1) |
| $ S = \left( {w_1^t-w_1^b} \right)/\left( {w_2^t-w_2^b} \right) $ | (2) |
其中w1t,w1b,w2t,w2b分别为离子液体[Epy]Cl上相,下相,盐[K2HPO4·3H2O]上相,下相的质量百分数。
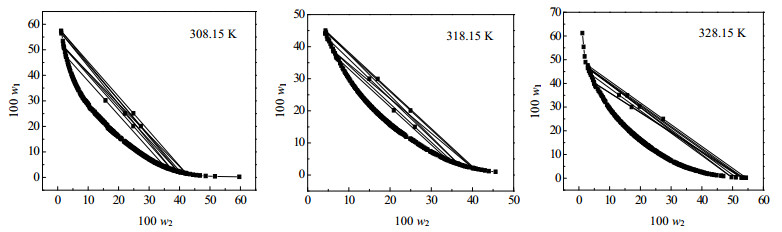
3 结果与讨论 3.1 双节线数据的关联对三元系统氯化N-丁基吡啶([Epy]Cl)+磷酸氢二钾(K2HPO4·3H2O)+水(H2O)双水相体系在T=308.15、318.15、328.15 K三个温度下的双节线和系线数据绘制图见图 1,并采用Merchuk方程[24]对双节线数据进行关联,方程如下所示:
| $ {w_1} = a\exp \left( {bw_2^{0.5}-cw_2^3} \right) $ | (3) |
|
图 1 [Epy]Cl + K2HPO4·3H2O + H2O体系在温度为308.15K、318.15K和328.15K下的双节线数据和系线数据 Fig.1 Binodal curves and tie lines of [Epy]Cl + K2HPO4·3H2O + H2O ATPSs at 308.15, 318.15 and 328.15 K |
式中w1、w2分别代表离子液体[Epy]Cl和盐K2HPO4·3H2O的质量分数,方程(3)拟合得到的a,b,c,R2和相对标准偏差sd在表 1中给出。
| 表 1 Epy]Cl-K2HPO4·3H2O-H2O三元体系关于方程(3)的参数结果及标准偏差 Table 1 Parameters of Eq.(3) and sd data for the [Epy]Cl-K2HPO4·3H2O-H2O systems |
为了提高精确度,采用另外两个非线性方程对双节线进行拟合:
| $ {w_1} = \exp \left( {a + b{w_2}^{0.5} + c{w_2} + d{w_2}^2} \right) $ | (4) |
| $ {w_1} = {a_1}\exp \left( {-\frac{{{w_2}}}{{{b_1}}}} \right) + {a_2}\exp \left( {-\frac{{{w_2}}}{{{b_2}}}} \right) + c $ | (5) |
同样,由方程(4)拟合得到的参数a,b,c,d,R2,sd和方程(5)拟合得到的参数a1,b1,a2,b2,c,R2,sd分别在表 2和表 3中给出从表 1,表 2和表 3可以看出,三个经验方程对所研究体系均具有良好的拟合效果。通过比较三个方程的线性相关系数R2和相对标准偏差(sd)可以看出方程(3)的拟合效果最好。表明Merchuk方程在离子液体双水相体系的拟合过程中具有更好的适用性[25]。
| 表 2 [Epy]Cl-K2HPO4·3H2O-H2O三元体系关于方程(4)的参数结果及标准偏差 Table 2 Parameters of Eq.(4) and sd data for the [Epy]Cl-K2HPO4·3H2O-H2O systems |
| 表 3 [Epy]Cl-K2HPO4·3H2O-H2O三元体系关于方程(5)的参数结果及标准偏差 Table 3 Parameters of Eq.(5) and sd data for the [Epy]Cl-K2HPO4·3H2O-H2O systems |
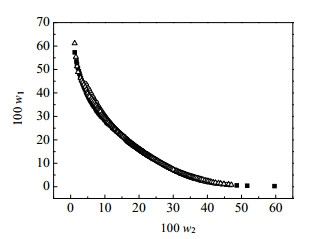
三元系统氯化N-丁基吡啶([Epy]Cl)+磷酸氢二钾(K2HPO4·3H2O)+水(H2O)双水相体系在T=308.15、318.15、328.15 K三个温度下的温度对比图如图 2所示。由图中可以看出三条双节线轨迹相互重叠,表明了温度对该体系分相能力没有影响。这与[C4mim][Br]+K2HPO4+H2O体系随温度基本没有变化相同[26],但与氨基酸咪唑盐离子液体双水相随温度下降成相能力增强的规律不同[27],也不同于[C4mim][BF4] + Na3C6H5O7/(NH4)3C6H5O7+ H2O体系和经典的聚合物双水相体系随温度的变化规律[28]。
|
图 2 温度对[Epy]Cl-K2HPO4·3H2O-H2O双水相体系的影响 Fig.2 Effect of temperature on binodal curves for the [Epy]Cl-K2HPO4·3H2O-H2O aqueous two-phase system ■ 308.15 K ○ 318.15 K △ 328.15 K |
氯化N-丁基吡啶([Epy]Cl)+磷酸氢二钾(K2HPO4·3H2O)+水(H2O)双水相体系在T=308.15、318.15、328.15 K三个温度下的液液相平衡数据、系线的长度及斜率如表 4所示。
| 表 4 [Epy]Cl-K2HPO4·3H2O-H2O三元体系在三个温度下的液液相平衡数据、系线长度及斜率 Table 4 Liquid-liquid equilibrium data, tie-line TLL and Sfor the [Epy]Cl-K2HPO4·3H2O-H2O systems at different temperatures |
采用Setschenow-type方程(6)[29]和另外一个由两个参数组成的方程(7)进行关联:
| $ \ln \left( {\frac{{c_1^t}}{{c_1^b}}} \right) = {k_{IL}}\left( {c_1^b-c_1^t} \right) + {k_s}\left( {c_2^b-c_2^t} \right) $ | (6) |
| $ \ln \left( {\frac{{w_2^t}}{{w_2^b}}} \right) = \beta + k\left( {w_1^b-w_1^t} \right) $ | (7) |
其中c1,c2,kIL,ks分别代表离子液体摩尔浓度、盐摩尔浓度、离子液体活度系数和盐析常数。上标t,b分别代表离子液体相和盐相。所得相关系数(R2)和标准偏差(sd)如表 5所示。w1、w2分别代表离子液体[Epy]Cl和盐K2HPO4·3H2O的质量分数,β,k分别代表盐析常数和活度系数。所得的相关系数(R2)和标准偏差(sd)列于表 6。
| 表 5 [Epy]Cl-K2HPO4·3H2O-H2O三元体系关于方程(6)的参数结果及标准偏差 Table 5 Parameters of Eq (6) and sd data for the [Epy]Cl-K2HPO4·3H2O-H2O systems |
| 表 6 [Epy]Cl-K2HPO4·3H2O-H2O三元体系关于方程(7)的参数结果及标准偏差 Table 6 Parameters of Eq.(7) and sd data for the [Epy]Cl-K2HPO4·3H2O-H2O systems |
从表中可以看出,利用方程(6)、(7)对所研究的离子液体双水相体系进行关联,结果令人满意。方程(7)的拟合精度更高一些。
4 结论离子液体氯化N-丁基吡啶([Epy]Cl)可以和磷酸氢二钾(K2HPO4·3H2O)在水溶液中形成双水相体系。采用常见的经验公式对得到的液液相平衡数据进行拟合,Merchuk方程对于双节线的拟合精度更高。相图表明该体系上下相组成服从杠杆原理。由于不同离子液体对温度的敏感程度不同,对于本体系的离子液体,温度对体系的分相能力影响不明显。从相图得到的规律为萃取分离提供分相基础,同时加入盐有助于离子液体从水溶液中析出,可作为离子液体回收再利用的理论依据。
| [1] | Shibusawa Y, Takeuchi N, Sugawara K , et al. Aqueous-aqueous two-phase systems composed of low molecular weight of polyethylene glycols and dextrans for counter-current chromatographic purification of proteins[J]. Journal of Chromatography, B , 2006, 844 (2) : 217-222. DOI:10.1016/j.jchromb.2006.07.028. |
| [2] | Li L, Liu F, Kong X , et al. Investigation of a liquid-liquid extraction system based on non-ionic surfactant-salt-H2O and mechanism of drug extraction[J]. Analytica Chimica Acta , 2002, 452 (2) : 321-328. DOI:10.1016/S0003-2670(01)01471-4. |
| [3] | Ferguson J, Baxter A, Young P , et al. Detection of chloramphenicol and chloramphenicol glucuronide residues in poultry muscle, honey, prawn and milk using a surface plasmon resonance biosensor and Qflex kit chloramphenicol[J]. Analytica Chimica Acta , 2005, 529 (1-2) : 109-113. DOI:10.1016/j.aca.2004.11.042. |
| [4] | Liu H L, He C Y, Wen D W , et al. Extraction of testosterone and epitestosterone in human urine using 2-propanol-salt-H2O system[J]. Analytica Chimica Acta , 2006, 557 (1-2) : 329-336. DOI:10.1016/j.aca.2005.10.020. |
| [5] | Johansson H O, Matos T B, Lus J S , et al. Plasmid DNA partitioning and separation using poly (ethyleneglycol)/poly (acrylate)/salt aqueous two-phase systems[J]. Journal of Chromatography, A , 2012, 1233 (7) : 30-35. |
| [6] | Wu Q, Lin D Q, Zhang Q L , et al. Evaluation of poly (ethylene glycol)/hydroxypropyl starch aqueous two-phase system for the separation of monoclonal antibodies from cell culture supernatant[J]. Journal of Separation Science , 2014, 37 (4) : 447-453. DOI:10.1002/jssc.v37.4. |
| [7] | Ibarra-Herrera C C, Aguilar O, Rito-Palomares M . Application of an aqueous two-phase systems strategy for the potential recovery of a recombinant protein from alfalfa (Medicago sativa)[J]. Separation and Purification Technology , 2011, 77 (1-2) : 94-98. |
| [8] | Gutowski K E, Broker G A, Willauer H D , et al. Controlling the aqueous miscibility of ionic liquids:aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations[J]. Journal of the American Chemical Society , 2003, 125 (22) : 6632-6633. DOI:10.1021/ja0351802. |
| [9] | Pei Y, Wang J, Wu K , et al. Ionic liquid-based aqueous two-phase extraction of selected proteins[J]. Separation and Purification Technology , 2009, 64 (3) : 288-295. DOI:10.1016/j.seppur.2008.10.010. |
| [10] | Li C X, Han J, Wang Y , et al. Extraction and mechanism investigation of trace roxithromycin in real water samples by use of ionic liquid salt aqueous two-phase system[J]. Analytica Chimica Acta , 2009, 653 (2) : 178-183. DOI:10.1016/j.aca.2009.09.011. |
| [11] | Ventura S P M, Neves C M S S, Freire M G , et al. Evaluation of anion influence on the formation and extraction capacity of ionic-liquid-based aqueous biphasic systems[J]. Journal of Physical Chemistry B , 2009, 113 (27) : 9304-9310. DOI:10.1021/jp903286d. |
| [12] | ZHANG Chen(张宸), CHEN Qiao-li(陈巧丽), WU Ke-jun(吴可君) , et al. Study of liquid-liquid equilibrium data for H2O-(NH3)2SO4-[Bmim][PF6] -diacetylmonoxime system([Bmim][PF6] -丁酮肟-水-硫酸铵体系液液平衡的研究)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2015, 29 (6) : 1313-1319. |
| [13] | CHEN Ting(陈婷), MA Ai-qing(马爱青), WANG Fei(王菲) , et al. Aqueous two-phase system (ATPS) containing ionic liquids[Cnmim]Br and SDS(SDS/[Cnmim]Br/H2O体系的双水相性质)[J]. Journal of East China University of Science and Technology (Natural Science Edition)(华东理工大学学报(自然科学版)) , 2012, 38 (2) : 137-141. |
| [14] | NA Ji(那吉), YANG Qing-hai(杨青海), DONG Xue-chang(董学畅) , et al. Extraction and separation of Rutin in ionic liquid aqueous two-phase system(离子液体[BPy]BF4双水相萃取芦丁的研究)[J]. Yunnan Chemical Technology(云南化工) , 2008, 35 (3) : 36-41. |
| [15] | ZHAO Di-hai(赵弟海), ZENG Yan-bo(曾延波), LI Lei(李蕾) , et al. Determination of chloramphenicol in eggs using an aqueous two phase systems of pyridine ionic liquid and salt(一种用于鸡蛋中氯霉素残留测定的吡啶类离子液体双水相体系)[J]. Chinese Journal of Analytical Chemistry(分析化学) , 2009, 37 (3) : 445-448. |
| [16] | Lu X J, Luo Q X, Xu Z Y , et al. Extraction and mechanistic investigation of trace dibutyl phthalate using an ionic liquid aqueous two-phase system[J]. New Journal of Chemistry , 2015, 39 (8) : 6223-6230. DOI:10.1039/C5NJ00232J. |
| [17] | Lu Y, Hao T F, Zhou Y , et al. Aqueous two-phase systems of polyoxyethylene lauryl ether and potassium gluconate/potassium oxalate/potassium citrate at different temperature-experimental results and modeling of (liquid+liquid) equilibrium data[J]. The Journal of Chemical Thermodynamics , 2014, 71 (4) : 137-147. |
| [18] | Wang Y, Han J, Liu J , et al. Liquid-liquid equilibrium phase behavior of imidazolium-based ionic liquid aqueous two-phase systems composed of 1-alkyl-3-methyl imidazolium tetrafluoroborate and different electrolytes ZnSO4, MgSO4 and Li2SO4 at 298.15 K:Experimental and correlation[J]. Thermochimica Acta , 2013, 557 (10) : 68-76. |
| [19] | LI Yu-liang(李宇亮), ZHAO Xue-wei(赵学伟), YANG Li-heng(杨丽衡) , et al. Measurement and correlation of liquid-liquid equilibrium data for aqueous two--phase system of[Epy]BF4-(NH4)3C6H5O7-H2O([Epy]BF4-(NH4)3C6H5O7-H2O双水相体系液液相平衡测定及其关联)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2014, 28 (2) : 212-217. |
| [20] | Li C X, Han J, Wang Y , et al. Phase behavior for the aqueous two-phase systems containing the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and kosmotropic salts[J]. Journal of Chemical and Engineering Data , 2010, 55 (3) : 1087-1092. DOI:10.1021/je900533h. |
| [21] | Dimitrijevic A, Trticpetrovic T, Vranes S , et al. Liquid-liquid equilibria in aqueous 1-alkyl-3-methylimidazolium and 1-butyl-3-ethylimidazolium-based ionic liquid[J]. Journal of Chemical and Engineering Data , 2016, 61 (1) : 549-555. DOI:10.1021/acs.jced.5b00697. |
| [22] | Han J, Wang Y, Li Y F , et al. Equilibrium phase behavior of aqueous two-phase systems containing 1-Alkyl-3-methylimidazolium tetrafluoroborate and ammonium tartrate at different temperatures:experimental determination and correlation[J]. Journal of Chemical and Engineering Data , 2011, 56 (9) : 3679-3687. DOI:10.1021/je2006055. |
| [23] | LU Yang(逯洋), TAN Zhen-jiang(谭振江), YAN Yong-sheng(闫永胜) . The design and implementation of the data processing system for liquid-liquid equilibrium experiments of ATPS based on VB and MATLAB(基于VB和MATLAB的双水相体系液-液相平衡数据处理系统的设计与实现)[J]. Computer and Applied Chemistry(计算机与应用化学) , 2012, 29 (10) : 1195-1198. |
| [24] | Merchuk J C, Andrews B A, Asenjo J A . Aqueous two-phase systems for protein separation:studies on phase inversion[J]. Journal of Chromatography, B , 1998, 711 (1-2) : 285-293. DOI:10.1016/S0378-4347(97)00594-X. |
| [25] | Wu B, Zhang Y M, Wang H P . Aqueous biphasic systems of hydrophilic ionic liquids+sucrose for separation[J]. Journal of Chemical and Engineering Data , 2008, 53 (4) : 983-985. DOI:10.1021/je700729p. |
| [26] | Pei Y C, Wang J J, Liu L , et al. Liquid-liquid equilibria of aqueous biphasic systems containing selected imidazolium ionic liquids and salts[J]. Journal of Chemical and Engineering Data , 2007, 52 (5) : 2026-2031. DOI:10.1021/je700315u. |
| [27] | Wu C Z, Wang J J, Li Z Y , et al. Relative hydrophobicity between the phases and partition of cytochrome-c in glycine ionic liquids aqueous two-phase systems[J]. Journal of Chromatography, A , 2013, 1305 (1) : 1-6. |
| [28] | Han J, Pan R, Xie X Q , et al. Liquid-liquid equilibria of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate+sodium and ammonium citrate aqueous two-phase systems at (298.15, 308.15 and 323.15) K[J]. Journal of Chemical and Engineering Data , 2010, 55 (9) : 3749-3754. DOI:10.1021/je1002797. |
| [29] | Hey M J, Jackson D P, Yan H . The salting-out effect and phase separation in aqueous solutions of electrolytes and poly (ethylene glycol)[J]. Polymer , 2005, 46 (8) : 2567-2572. DOI:10.1016/j.polymer.2005.02.019. |




