骨质疏松症(osteoporosis,OP)是以骨强度下降,导致骨折风险增加为特征的全身性骨骼疾病。随着人口老龄化,骨质疏松症已成为影响人群健康的严重问题,导致患者生活质量下降,也给社会经济带来沉重负担[1-2]。2006年流行病学调查显示我国OP患者近7 000万,骨量减少已超2亿[3]。OP的严重后果是轻微创伤即引发骨折,其危害巨大,是老年患者致残和致死的主要原因之一[4]。OP是受多因素影响的复杂疾病,遗传、环境、内分泌等因素共同导致骨转换异常、骨量减低和骨强度下降[1]。骨转换是成骨细胞介导的骨形成和破骨细胞介导的骨吸收保持动态平衡并不断更新的过程[5-6]。OP的重要病理生理机制是骨转换失衡,骨吸收超过骨形成,导致骨密度(bone mineral density,BMD)减低,骨强度下降,骨折风险增加[2]。
成骨细胞来源于间充质干细胞,产生Ⅰ型胶原、骨钙素等多种骨基质蛋白,参与骨骼合成代谢及微损伤修复。成骨细胞的分化、成熟及活性主要受Wnt信号通路的调控,通过Wnt蛋白与由卷曲受体和低密度脂蛋白受体相关蛋白(low density lipoprotein receptor-related protein,LRP)家族LRP5和6组成的受体复合物结合激活Wnt通路,稳定细胞内β-连环蛋白,后者进入细胞核,调节多种基因的转录,增加间充质干细胞向成骨细胞分化,并促进成骨细胞的成熟和存活[7-8](图 1)。硬骨抑素(sclerostin,SOST)和Dickkopf-1(DKK1)是Wnt通路的天然拮抗剂,能与Wnt共受体LRP5/6结合,阻止β-连环蛋白进入细胞核,抑制Wnt通路的活化,减少成骨细胞的分化与成熟[9-10]。此外,甲状旁腺素(parathyroid hormone,PTH)可降低SOST的表达,促进成骨细胞分化,促进骨形成[11]。
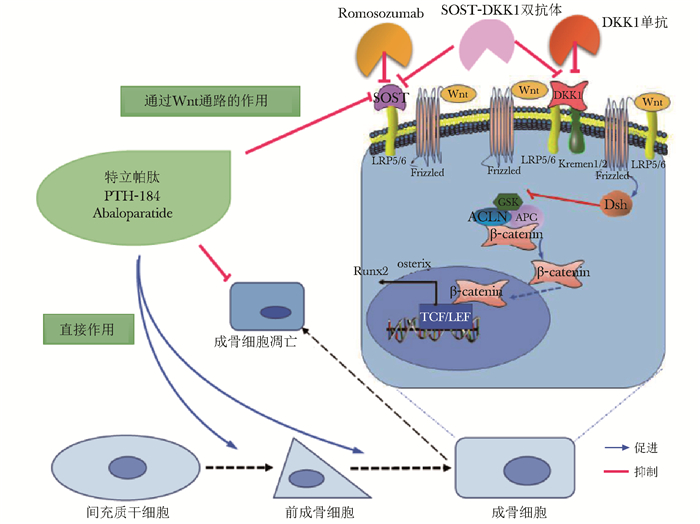
|
| 图 1 骨形成促进剂的作用机制 Figure 1 Mechanism of osteoanabolic agents PTH抑制SOST产生,促进成骨细胞分化,抑制成骨细胞凋亡; Wnt通路:在成骨细胞表面,Wnt蛋白结合由LRP5/6及卷曲蛋白(Frizzled)组成的复合物后,促进散乱蛋白(DSH)的激活,后者抑制糖原合成酶激酶3β(GSK),解离由GSK、轴素蛋白(AXIN)和结直肠腺瘤性息肉基因(APC)组成的多蛋白降解复合物,使其不能降解β-连环蛋白(β-catenin); β-连环蛋白转移到细胞核内,与转录因子(主要是T细胞因子/淋巴增强因子,TCF/LEF)结合,激活与成骨细胞分化和骨形成相关的多种靶基因的转录(如Runx2和Osterix等); SOST与LRP5/6结合,DKK1与LRP5/6和Kremen1/2结合,均能够抑制Wnt通路的激活; 针对SOST和DKK1的单克隆抗体能够阻断上述作用,激活Wnt通路 |
基于上述成骨细胞分化、成熟及活化的调控机制,近年来,骨形成促进剂研究取得了长足进展,其明显促进骨形成,增加骨密度,降低骨折风险。骨形成促进剂的作用机制如图 1所示。此类药物主要包括PTH类似物、Abaloparatide、Romosozumab、DKK1单克隆抗体、DKK1和SOST双抗体,本文综述上述药物在OP中的作用机制、临床治疗效果和安全性等的研究进展。
PTH及其类似物甲状旁腺素是甲状旁腺分泌的84个氨基酸的多肽,可促进骨钙释放,肾小管及肠道对钙的回吸收,在钙稳态调节中发挥重要作用[12]。PTH对骨转换呈双相调节,持续高水平PTH会明显增加破骨细胞活性、促进骨吸收,导致骨丢失[13]。短期间断PTH则明显增加成骨细胞介导的骨形成,使骨量增加[14]。PTH还抑制SOST的产生,活化Wnt通路,促进成骨细胞分化,抑制成骨细胞凋亡[11, 15](图 1)。
PTH1-34特立帕肽(Teriparatide)是重组人PTH氨基端1-34片段,小剂量、间歇使用可刺激成骨细胞活性,促进骨形成[16-17]。多项研究均提示特立帕肽能够增加骨量、改善骨微结构、提高骨强度,降低椎体和非椎体骨折的风险[18-20]。在纳入1 637例有椎体骨折史的绝经后女性的临床研究中,患者每日皮下注射特立帕肽20 μg治疗21个月,椎体和髋部骨密度分别增加约9%和3%[16]。特立帕肽显著降低骨折发生率,椎体骨折率在安慰剂组为14%,特立帕肽20 μg/d和40 μg/d组分别为5%和4%,较安慰剂组下降65%和69%[16]。研究发现特立帕肽增加椎体和髋部骨密度优于阿仑膦酸钠[18]。特立帕肽治疗24个月,降低绝经后女性椎体骨折风险的作用优于利塞膦酸钠[21]。一项评估特立帕肽对骨质疏松患者髋部和上肢骨折影响的Meta分析纳入23项随机临床试验,包含8 644例患者,其中3 893例接受特立帕肽治疗,平均治疗18个月后,共34例出现髋部骨折,其中特立帕肽组患者髋部骨折风险较对照组降低56%[22]。
特立帕肽对于男性OP也有一定的治疗效果。237名骨质疏松男性随机接受特立帕肽20 μg/d,40 μg/d及安慰剂治疗11个月,特立帕肽20 μg/d和40 μg/d组椎体BMD分别增加5.9%和9%,股骨颈BMD分别增加1.5%和2.9%[23]。特立帕肽对糖皮质激素诱导的OP亦有效。在一项为期36个月的随机对照试验中,泼尼松导致的骨质疏松患者,随机接受特立帕肽和阿仑膦酸钠治疗,两组腰椎骨密度平均增加11%和5.3%,特立帕肽组新发椎体骨折率明显低于阿仑膦酸钠组[24]。还有研究显示特立帕肽有利于骨折愈合,102例非手术治疗的桡骨远端骨折女性分别接受特立帕肽和安慰剂治疗,特立帕肽组骨折愈合时间较安慰剂组显著缩短[25]。
特立帕肽总体安全性良好,常见不良反应包括恶心、肢体疼痛、头痛和眩晕。高钙血症和高尿钙也较常见,多为轻度[16]。值得注意的是,大鼠在接受人类4~28倍剂量的PTH1-34后,骨肉瘤瘤风险明显增加[26],而100多万例接受特立帕肽治疗的患者中,仅报告了3例骨肉瘤,未超过一般人群骨肉瘤的患病率[27],但目前仍建议特立帕肽疗程不超过24个月。
PTH1-84重组人全段甲状旁腺素(PTH1-84)主要用于甲状旁腺功能减退症的治疗,其对OP亦有治疗效果。TOP研究发现,2 532例绝经后骨质疏松女性接受PTH1-84治疗18个月,腰椎BMD增加6.9%,初次椎体骨折发生风险降低68%[28]。PTH1-84治疗36个月,腰椎BMD增加8.5%,全髋和股骨颈BMD增加3.2%和3.4%[29]。PTH1-84治疗能够显著缩短骨质疏松性骨折的愈合时间,并明显减轻患者疼痛[30]。近期研究发现PTH1-84治疗18个月能够增加绝经后骨质疏松患者的骨小梁面积骨密度[31]。
PTH1-84最常见的不良反应为高尿钙、高钙血症和恶心,且发生率高于PTH1-34[32],可能与PTH1-84半衰期较长有关。此外PTH对钙稳态和骨重塑的调节作用不仅涉及氨基端与PTH1R的结合作用,还涉及羧基端结构域的受体。PTH全段和羧基端片段能促进细胞凋亡,这可能减少骨肉瘤发生的风险[33-34],但目前PTH1-84仅在欧洲被批准用于绝经后OP的治疗,建议疗程也不超过24个月。
Abaloparatide甲状旁腺素相关肽(parathyroid hormone-related peptide,PTHrP)在肺、骨、心、脑、皮肤等组织表达,其氨基端36个氨基酸与PTH高度同源,能与PTH受体结合,促进前成骨细胞分化,抑制成骨细胞凋亡(图 1)。Abaloparatide是人工合成的PTHrP1-34类似物,其41%的氨基酸与人PTH1-34同源。Abaloparatide可显著增加皮质骨厚度和骨小梁数量[35-36]。PTH1R有两种构象:RG和R0,与特立帕肽相比,Abaloparatide对RG的选择性更高,作用时间更短,产生的下游信号更多,促骨形成作用更强,对骨吸收影响较小,致高钙血症的风险较低[37]。
Abaloparatide显著提升绝经后骨质疏松患者的骨密度。在Ⅱ期临床研究中,222例绝经后骨质疏松女性每日接受Abaloparatide 20、40、80 μg,特立帕肽20 μg或安慰剂治疗24周,Aabaloparatide呈剂量依赖性明显增加全髋、股骨颈和腰椎BMD。ACTIVE的Ⅲ期临床试验[38],纳入2 463例骨折高风险的绝经后骨质疏松女性,每日皮下注射Abaloparatide 80 μg、特立帕肽20 μg或安慰剂治疗18个月,Abaloparatide分别增加全髋、股骨颈、腰椎骨密度4.3%、4.0%和10.4%,Abaloparatide组、特立帕肽组和安慰剂组新发椎体骨折率分别为0.6%,0.84%,4.2%,Abaloparatide和特立帕肽组骨折率较安慰剂组分别降低了86%和80%。Abaloparatide还降低非椎体骨折及临床骨折的风险[37]。对其中80岁以上患者行亚组分析,Abaloparatide使全髋、股骨颈、腰椎BMD分别增加3.9%、3.6%、12.1%,表明其对超老龄患者亦有效[39]。
Abaloparatide的安全性较好,常见不良反应为恶心、头痛和头晕,其高钙血症发生率较特立帕肽低,疗程亦建议不超过24个月[38]。
基于Wnt通路的药物治疗 Romosozumab硬骨抑素主要由骨细胞产生,能够抑制Wnt和骨形态发生蛋白通路,影响成骨细胞分化和功能,减少骨形成[7](图 1)。SOST还通过核因子κB活化因子受体配体通路,增加破骨细胞作用[40]。Romosozumab是人源化的SOST单克隆抗体,通过抑制SOST发挥促骨形成的作用[41]。近期,Romosozumab刚刚在日本和美国食品药品监督管理局(Food and Drug Administration, FDA)获批用于治疗具有脆性骨折的严重OP患者。
Romosozumab能促进去卵巢食蟹猴的骨形成,增加骨皮质厚度和骨小梁数量,提升骨强度[42]。Romosozumab增加绝经后女性的骨密度,促进骨形成、抑制骨吸收。在Ⅱ期临床试验中,419例绝经后女性随机接受每月或每3月不同剂量的Romosozumab注射、每周服70 mg阿仑膦酸钠、每日注射20 μg特立帕肽或安慰剂,治疗12个月。结果显示,Romosozumab明显升高骨形成指标、降低骨吸收指标,显著增加腰椎、全髋和股骨颈骨密度,其中Romosozumab 210 mg/月组腰椎骨密度增加11.3%,显著优于阿仑膦酸钠、特立帕肽及安慰剂组[43]。亚组分析提示其明显增加皮质厚度和骨小梁数量,改善骨微结构[44]。FRAME Ⅲ期临床试验共纳入7 180例绝经后骨质疏松女性,随机接受每月皮下注射Romosozumab 210 mg或安慰剂,治疗12个月,Romosozumab组新发椎体骨折及临床骨折率均显著低于安慰剂组[45]。ARCH研究中,4 093例骨质疏松性骨折的绝经后妇女随机接受每月Romosozumab 210 mg或每周阿仑膦酸钠70 mg治疗12个月,两组再接受阿仑膦酸钠治疗12个月,共治疗24个月后,Romosozumab组新发椎体骨折率为6.2%,显著低于阿仑膦酸钠组(11.9%),其临床和髋部骨折风险也明显低于阿仑膦酸钠组[46]。
Romosozumab在男性OP中的治疗效果也得到证实。BRIDGE研究纳入245例骨质疏松男性,163例每月接受210 mg Romosozumab,82例接受安慰剂治疗,12个月后,Romosozumab组腰椎、全髋及股骨颈的骨密度分别增加12.1%、2.5%和2.2%,显著优于安慰剂组[47]。
Romosozumab的总体安全性较好,常见不良事件包括注射部位反应和抗Romosozumab的抗体产生。值得关注的是,ARCH研究中Romosozumab组和阿仑膦酸钠组分别有50例(2.5%)和38例(1.9%)患者出现严重心脑血管不良事件[46],包括缺血性心脏病和脑血管疾病。BRIDGE研究中也观察到心血管事件风险的增加[47],而FRAME研究则显示各组心血管事件发生率无差异[45]。
针对DKK1的单克隆抗体DKKl主要由成骨细胞和骨细胞表达,通过结合单通道跨膜受体蛋白Kremen1/Kremen2及LRP5/LRP6共受体,引起LRP5/LRP6内化和降解,降低β-连环蛋白稳定性,抑制成骨细胞分化和活性[1, 48]。DKK1基因失活性突变引起骨量增加[49],其过表达则致骨密度降低[50]。DKK1的单克隆抗体能中和DKK1,激活Wnt信号通路,促进骨形成、增加骨密度(图 1)。予去卵巢骨质疏松小鼠及恒河猴DKKl单抗治疗,显示骨形成增加,BMD升高[51-52]。DKK1单抗还促进啮齿动物骨折愈合,增加骨痂形成和机械强度[53]。
目前DKKl抗体在人类的临床研究主要用于治疗多发性骨髓瘤骨病,它能够逆转骨髓瘤细胞分泌的DKK1对Wnt信号通路的抑制,从而增加骨密度,延缓溶骨性病变的发展[54],其在OP的治疗潜力巨大。近期,有研究在啮齿类和灵长类动物中使用针对DKK1和SOST的双抗体,其促骨形成的效果优于单一特异性抗体[55]。SOST抗体和SOST-DKK1双抗体能够增加去卵巢大鼠牙槽嵴的体积和高度,减少下颌骨骨丢失,后者作用更明显[56]。阻断SOST和DKK1的双抗体可能具有更强的促骨形成作用,值得深入研究。
展望随着OP发病机制研究的深入,抗OP药物治疗取得了快速发展。近十余年新型骨形成促进剂的研究取得长足进展。PTH和PTHrP类似物通过刺激骨形成,有效增加骨密度并减少椎体和非椎体的骨折风险。针对硬骨抑素及DKK1的单克隆抗体、针对SOST-DKK1的双抗体,可强有效地激活Wnt通路,促进骨形成,增加骨密度,表现出抗骨质疏松的巨大潜力,值得进一步研发及应用。
| [1] | Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future[J]. Lancet, 2011, 377: 1276–1287. DOI:10.1016/S0140-6736(10)62349-5 |
| [2] | Compston JE, McClung MR, Leslie WD. Osteoporosis[J]. Lancet, 2019, 393: 364–376. DOI:10.1016/S0140-6736(18)32112-3 |
| [3] | 中华医学会骨质疏松和骨矿盐疾病分会. 原发性OP诊疗指南(2017)[J]. 中华骨质疏松和骨矿盐疾病杂志, 2017, 10: 413–444. DOI:10.3969/j.issn.1674-2591.2017.05.002 |
| [4] | Katsoulis M, Benetou V, Karapetyan T, et al. Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project[J]. J Intern Med, 2017, 281: 300–310. DOI:10.1111/joim.12586 |
| [5] | Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility[J]. N Engl J Med, 2006, 354: 2250–2261. DOI:10.1056/NEJMra053077 |
| [6] | Martin T, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption[J]. Crit Rev Eukar Gene, 2009, 19: 73–88. DOI:10.1615/CritRevEukarGeneExpr.v19.i1.40 |
| [7] | Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments[J]. Nat Med, 2013, 19: 179–192. DOI:10.1038/nm.3074 |
| [8] | Ke HZ, Richards WG, Li X, et al. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases[J]. Endocrine Rev, 2012, 33: 747–783. DOI:10.1210/er.2011-1060 |
| [9] | Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone[J]. Bone, 2017, 96: 29–37. DOI:10.1016/j.bone.2016.10.007 |
| [10] | Butler JS, Queally JM, Devitt BM, et al. Silencing Dkk1 expression rescues dexamethasone-induced suppression of primary human osteoblast differentiation[J]. BMC Musculoskel Dis, 2010, 11: 210. DOI:10.1186/1471-2474-11-210 |
| [11] | Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH[J]. Bone, 2007, 40: 1434–1446. DOI:10.1016/j.bone.2007.03.017 |
| [12] | Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism[J]. Lancet (London, England), 2018, 391: 168–178. DOI:10.1016/S0140-6736(17)31430-7 |
| [13] | Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton[J]. Curr Opin Pharmacol, 2015, 22: 41–50. DOI:10.1016/j.coph.2015.03.005 |
| [14] | Fujihara R, Mashiba T, Yoshitake S, et al. Weekly teriparatide treatment increases vertebral body strength by improving cortical shell architecture in ovariectomi-zed cynomolgus monkeys[J]. Bone, 2019, 121: 80–88. DOI:10.1016/j.bone.2019.01.006 |
| [15] | Keller H, Kneissel M. SOST is a target gene for PTH in bone[J]. Bone, 2005, 37: 148–158. DOI:10.1016/j.bone.2005.03.018 |
| [16] | Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis[J]. New Engl J Med, 2001, 344: 1434–1441. DOI:10.1056/NEJM200105103441904 |
| [17] | Dempster DW, Cosman F, Zhou H, et al. Effects of daily or cyclic teriparatide on bone formation in the iliac crest in women on no prior therapy and in women on alendronate[J]. J Bone Miner Res, 2016, 31: 1518–1526. DOI:10.1002/jbmr.2822 |
| [18] | Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis[J]. N Engl J Med, 2007, 357: 2028–2039. DOI:10.1056/NEJMoa071408 |
| [19] | Graeff C, Chevalier Y, Charlebois M, et al. Improvements in vertebral body strength under teripara-tide treatment assessed in vivo by finite element analysis: results from the EUROFORS study[J]. J Bone Miner Res, 2009, 24: 1672–1680. DOI:10.1359/jbmr.090416 |
| [20] | Keaveny TM, Donley DW, Hoffmann PF, et al. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis[J]. J Bone Mineral Res, 2007, 22: 149–157. |
| [21] | Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial[J]. Lancet, 2018, 391: 230–240. DOI:10.1016/S0140-6736(17)32137-2 |
| [22] | Diez-Perez A, Marin F, Eriksen EF, et al. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and Meta-analysis[J]. Bone, 2019, 120: 1–8. DOI:10.1016/j.bone.2018.09.020 |
| [23] | Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide[human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis[J]. J Bone Miner Res, 2003, 18: 9–17. DOI:10.1359/jbmr.2003.18.1.9 |
| [24] | Saag KG, Zanchetta JR, Devogelaer JP, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial[J]. Arth Rheum, 2009, 60: 3346–3355. DOI:10.1002/art.24879 |
| [25] | Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures[J]. J Bone Mineral Res, 2010, 25: 404–414. DOI:10.1359/jbmr.090731 |
| [26] | Vahle JL, Long GG, Sandusky G, et al. Bone neoplasms in F344 rats given teriparatide[rhPTH(1-34)] are dependent on duration of treatment and dose[J]. Toxicol Pathol, 2004, 32: 426–438. DOI:10.1080/01926230490462138 |
| [27] | Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience[J]. J Bone Mineral Res, 2012, 27: 2419–2428. DOI:10.1002/jbmr.1800 |
| [28] | Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial[J]. Ann Intern Med, 2007, 146: 326–339. DOI:10.7326/0003-4819-146-5-200703060-00005 |
| [29] | Zanchetta JR, Bogado CE, Cisari C, et al. Treatment of postmenopausal women with osteoporosis with PTH(1-84) for 36 months: treatment extension study[J]. Curr Med Res Opin, 2010, 26: 2627–2633. DOI:10.1185/03007995.2010.524121 |
| [30] | Peichl P, Holzer LA, Maier R, et al. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women[J]. J Bone Joint Surg Am Vol, 2011, 93: 1583–1587. DOI:10.2106/JBJS.J.01379 |
| [31] | Cipriani C, Pepe J, Silva BC, et al. Comparative effect of rhPTH(1-84) on bone mineral density and trabecular bone score in hypoparathyroidism and postmenopausal osteoporosis[J]. J Bone Miner Res, 2018, 33: 2132–2139. DOI:10.1002/jbmr.3554 |
| [32] | Kraenzlin ME, Meier C. Parathyroid hormone analo-gues in the treatment of osteoporosis[J]. Nat Rev Endocrinol, 2011, 7: 647–656. DOI:10.1038/nrendo.2011.108 |
| [33] | Murray TM, Rao LG, Divieti P, et al. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligands[J]. Endocrine Rev, 2005, 26: 78–113. DOI:10.1210/er.2003-0024 |
| [34] | Wysolmerski JJ. Parathyroid hormone-related protein: an update[J]. J Clin Endocrinol Metab, 2012, 97: 2947–2956. DOI:10.1210/jc.2012-2142 |
| [35] | Bilezikian JP, Hattersley G, Fitzpatrick LA, et al. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): a 24-week randomized clinical trial[J]. Osteoporos Int, 2018, 29: 323–328. DOI:10.1007/s00198-017-4304-9 |
| [36] | Chew CK, Clarke BL. Abaloparatide: recombinant human PTHrP (1-34) anabolic therapy for osteoporosis[J]. Maturitas, 2017, 97: 53–60. DOI:10.1016/j.maturitas.2016.12.003 |
| [37] | Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial[J]. JAMA, 2016, 316: 722–733. DOI:10.1001/jama.2016.11136 |
| [38] | Leder BZ, O'Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis[J]. J Clin Endocrinol Metab, 2015, 100: 697–706. DOI:10.1210/jc.2014-3718 |
| [39] | McClung MR, Harvey NC, Fitzpatrick LA, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis[J]. Menopause, 2018, 25: 767–771. DOI:10.1097/GME.0000000000001080 |
| [40] | Wijenayaka AR, Kogawa M, Lim HP, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway[J]. PLoS One, 2011, 6: e25900. DOI:10.1371/journal.pone.0025900 |
| [41] | Padhi D, Jang G, Stouch B, et al. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody[J]. J Bone Miner Res, 2011, 26: 19–26. DOI:10.1002/jbmr.173 |
| [42] | Ominsky MS, Boyd SK, Varela A, et al. Romoso-zumab improves bone mass and strength while maintaining bone quality in ovariectomized cynomolgus monkeys[J]. J Bone Miner Res, 2017, 32: 788–801. DOI:10.1002/jbmr.3036 |
| [43] | McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density[J]. N Engl J Med, 2014, 370: 412–420. DOI:10.1056/NEJMoa1305224 |
| [44] | Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater gains in spine and hip strength for romoso-zumab compared with teriparatide in postmenopausal women with low bone mass[J]. J Bone Mineral Res, 2017, 32: 1956–1962. DOI:10.1002/jbmr.3176 |
| [45] | Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis[J]. N Engl J Med, 2016, 375: 1532–1543. DOI:10.1056/NEJMoa1607948 |
| [46] | Saag KG, Petersen J, Brandi ML, et al. Romoso-zumab or alendronate for fracture prevention in women with osteoporosis[J]. N Engl J Med, 2017, 377: 1417–1427. DOI:10.1056/NEJMoa1708322 |
| [47] | Lewiecki EM, Blicharski T, Goemaere S, et al. A phase Ⅲ randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis[J]. J Clin Endocrinol Metab, 2018, 103: 3183–3193. DOI:10.1210/jc.2017-02163 |
| [48] | Rossini M, Gatti D, Adami S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis[J]. Calcified Tissue Int, 2013, 93: 121–132. DOI:10.1007/s00223-013-9749-z |
| [49] | Morvan F, Boulukos K, Clement-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass[J]. J Bone Mineral Res, 2006, 21: 934–945. DOI:10.1359/jbmr.060311 |
| [50] | Guo J, Liu M, Yang D, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation[J]. Cell Metab, 2010, 11: 161–171. DOI:10.1016/j.cmet.2009.12.007 |
| [51] | Li X, Grisanti M, Fan W, et al. Dickkopf-1 regul-ates bone formation in young growing rodents and upon traumatic injury[J]. J Bone Mineral Res, 2011, 26: 2610–2621. DOI:10.1002/jbmr.472 |
| [52] | Glantschnig H, Scott K, Hampton R, et al. A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody[J]. J Pharmacol Exp Therapeut, 2011, 338: 568–578. DOI:10.1124/jpet.111.181404 |
| [53] | Jin H, Wang B, Li J, et al. Anti-DKK1 antibody promotes bone fracture healing through activation of beta-catenin signaling[J]. Bone, 2015, 71: 63–75. DOI:10.1016/j.bone.2014.07.039 |
| [54] | van Andel H, Kocemba KA, Spaargaren M, et al. Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options[J]. Leukemia, 2019, Epub ahead of print. |
| [55] | Florio M, Gunasekaran K, Stolina M, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair[J]. Nat Commun, 2016, 7: 11505. DOI:10.1038/ncomms11505 |
| [56] | Liu M, Kurimoto P, Zhang J, et al. Sclerostin and DKK1 inhibition preserves and augments alveolar bone volume and architecture in rats with alveolar bone loss[J]. J Dent Res, 2018, 97: 1031–1038. DOI:10.1177/0022034518766874 |
| (收稿日期:2019-03-19) |

