扩展功能
文章信息
- 陈颖聪, 高媛雪, 温慧军
- CHEN Ying-Cong, GAO Yuan-Xue, WEN Hui-Jun
- 青年初诊2型糖尿病患者颈动脉斑块与血小板-淋巴细胞比值及中性粒细胞-淋巴细胞比值的相关分析
- A correlation analysis of carotid plaques with platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in young patients newly diagnosed with type 2 diabetes mellitus
- 国际神经病学神经外科学杂志, 2020, 47(6): 575-580
- Journal of International Neurology and Neurosurgery, 2020, 47(6): 575-580
-
文章历史
收稿日期: 2020-05-26
修回日期: 2020-11-09
2型糖尿病(type 2 diabetes mellitus, T2DM)合并脑梗死的风险是非2型糖尿病患者的2~4倍[1]。血小板诱导的炎症反应在糖尿病患者动脉粥样硬化形成有着重要作用,可导致动脉粥样硬化发生[2]。血小板―淋巴细胞比值(platelet-lymphocyte ratio, PLR)和中性粒细胞―淋巴细胞比值(neutrophil-lymphocyte ratio, NLR)是两种可重复、容易获得并广泛应用的炎性标志物,与动脉硬化程度密切相关[3]。已有研究报道,PLR和NLR作为炎症指标与2型糖尿病视网膜病变、2型糖尿病肾病密切相关[4]。目前,有关PLR、NLR与青年初诊2型糖尿病患者颈动脉粥样硬化斑块之间关系的相关临床报道较少。本研究通过颈部血管B超判断青年初诊2型糖尿病患者颈动脉粥样硬化的存在,使用半定量法[5]判断患者斑块的严重程度,同时检测患者的血常规及相关生化指标,探讨青年初诊2型糖尿病患者颈动脉斑块与PLR和NLR的关系。
1 研究对象与方法 1.1 研究对象通过查阅病历,收集宝鸡市中心医院糖尿病科2014年3月至2019年11月住院的青年初诊2型糖尿病患者268例的临床病历,所有参与本实验者均获得本人或者家属的知情同意并签署知情同意书,并经宝鸡市中心医院医学伦理委员会批准(伦理编号BJ2016024)。
入选标准:①依照《中国2型糖尿病防治指南(2017年修订版)》的标准[6],随机静脉血糖>11.1 mmol/L或空腹血糖>7.0 mmo1/L,口服葡萄糖耐量试验(oral glucose tolerance test, OGTT)2 h血糖>11.1 mmol/L及伴有糖尿病症状;②距入院48 h以内;③初诊2型糖尿病患者;④未采用饮食控制或口服降糖药者;⑤发病年龄18~45岁;⑥入院48 h内完成颈动脉超声检查。
排除标准:①其他类型糖尿病;②合并严重心肝肾功能损伤等基础疾病;③同时患有自身免疫性疾病、甲状腺及甲状旁腺疾病、恶性肿瘤及其他内分泌疾病者;④接受糖皮质激素治疗或激素替代治疗者;⑤既往有糖尿病的患者;⑥临床资料及既往病史不完善者。
1.2 研究方法 1.2.1 分组根据血管内―中膜厚度(intima-media thickness, IMT)的检查结果,将初诊2型糖尿病的青年患者(观察组)分为内中膜增厚组(116例)及斑块组(152例);斑块组根据半定量法[13]评估斑块的严重程度,分为I级斑块组(62例)、II级斑块组(47例)及III级斑块组(43例)。另外选取同期健康体检的颈动脉内膜正常的青年104例作为对照组。收集所有被试者的性别、年龄、颈动脉硬化相关危险因素等资料,比较各组间PLR及NLR。
1.2.2 计算IMT及斑块等级根据文献[7]测定IMT的标准为:①IMT < 1.0 mm为颈动脉超声阴性;②IMT≥1.0 mm为颈动脉超声阳性,其中,1.0 mm < IMT≤1.2 mm为内―中膜增厚,IMT≥1.3 mm为斑块形成。按照半定量法测定[13]评估动脉斑块严重程度:Ⅰ级为单侧斑块≤2.1 mm;Ⅱ级为单侧斑块>2.1 mm或双侧均有斑块且其中至少一侧斑块≤2.1 mm;Ⅲ级为双侧斑块均>2.1 mm。
1.2.3 相关危险因素诊断标准依据中国高血压病[8]、高血脂[9]、高同型半胱氨酸血症[10]、吸烟[11]及饮酒[12]等标准,严格患者纳入及排除标准。
1.3 统计学方法采用SPSS 20.0软件进行统计学分析。计量资料以均数±标准差(x±s)表示,两组间比较采用独立样本t检验,多组间两两比较采用SNK-q检验,非正态分布的计量资料采用非参数检验;计数资料采用例或例(%)表示,比较采用卡方检验。相关性研究采用Pearson相关分析,相关因素分析采用多因素Logistic回归分析,非正态分布的资料采用Spearman相关分析。通过受试者工作特征曲线(receiver operating characteristic, ROC)和曲线下面积(area under the cure, AUC)评价PLR、NLR在青年初诊2型糖尿病合并颈动脉硬化患者诊断中的价值。P < 0.05为差异有统计学意义。
2 结果 2.1 两组间基线资料比较收集观察组和对照组的性别、年龄及颈动脉硬化相关因素等资料进行比较。见表 1。
| 项目 | 观察组(n=268) | 对照组(n=104) | t/χ2值 | P值 |
| 女/男/例 | 176/92 | 65/39 | 0.090 | 0.894 |
| 年龄/岁 | 36.8±7.4 | 35.9±8.1 | 1.876 | 0.119 |
| 高血压例(%) | 94(35.1) | 16(15.4) | 5.473 | 0.035 |
| 吸烟例(%) | 85(31.7) | 21(20.2) | 4.357 | 0.039 |
| 饮酒例(%) | 68(25.4) | 14(13.5) | 2.158 | 0.056 |
| 白细胞/(109/L) | 6.28±1.39 | 5.97±1.26 | 1.536 | 0.621 |
| 血小板/(109/L) | 269.26±45.19 | 234.11±38.46 | 1.156 | 0.053 |
| 中性粒细胞/(109/L) | 5.14±1.07 | 4.15±1.12 | 2.103 | 0.056 |
| 淋巴细胞/(109/L) | 1.16±0.43 | 1.56±0.34 | 1.934 | 0.061 |
| 胆固醇/(mmol/L) | 4.30±1.05 | 4.08±1.14 | 1.413 | 0.097 |
| 三酰甘油/(mmol/L) | 1.76±0.68 | 1.45±0.72 | 0.685 | 0.538 |
| 低密度脂蛋白/(mmol/L) | 3.16±0.67 | 2.13±0.48 | 3.802 | 0.031 |
| 糖化血红蛋白/(mmol/L) | 7.83±1.42 | 4.56±0.75 | 24.37 | 0.000 |
| 同型半胱氨酸/(μmol/L) | 18.21±11.53 | 14.13±9.18 | 1.293 | 0.059 |
| NLR | 3.31±0.79 | 1.79±0.48 | 10.162 | 0.021 |
| PLR | 152.13±51.69 | 72.30±31.59 | 15.286 | 0.009 |
对表 1中差异有统计学意义的因素进行Logistic回归分析,结果显示糖化血红蛋白、高血压病、高血脂、吸烟及PLR数值、NLR数值均可增加颈动脉硬化发病风险(P < 0.05)。见表 2。
| 自变量 | B | SE | P | OR | 95%CI |
| 糖化血红蛋白 | 1.423 | 0.548 | 0.014 | 2.641 | 1.230~5.514 |
| 高血压 | 0.948 | 0.442 | 0.009 | 3.487 | 1.612~7.783 |
| 低密度脂蛋白 | 1.614 | 0.589 | 0.038 | 3.541 | 1.132~4.896 |
| 吸烟 | 1.396 | 0.453 | 0.026 | 2.593 | 1.469~4.487 |
| PLR | 0.062 | 0.008 | 0.023 | 1.021 | 1.003~1.053 |
| NLR | 0.049 | 0.006 | 0.039 | 2.349 | 1.034~5.357 |
斑块组患者PLR及NLR数值高于内中膜增厚组,差异有统计学意义(P < 0.05)。见表 3。
| 组别 | 例数 | PLR | NLR |
| 内中膜增厚组 | 116 | 103.28±35.46 | 2.11±0.57 |
| 斑块组 | 152 | 153.19±32.58 | 3.09±0.62 |
| t值 | 7.189 | 5.387 | |
| P值 | 0.024 | 0.037 |
III级斑块组患者PLR及NLR数值高于II级斑块组和I级斑块组,差异有统计学意义(P < 0.05);II级斑块组患者PLR及NLR数值水平高于I级斑块组,差异有统计学意义(P < 0.05)。见表 4。
| 斑块等级 | 例数 | PLR | NLR |
| I级斑块组 | 62 | 136.34±43.72 | 2.41±0.71 |
| II级斑块组 | 47 | 149.57±39.58① | 2.98±0.83① |
| III级斑块组 | 43 | 168.28±41.30①② | 3.81±0.59①② |
| 注:①与I级斑块组比较,P<0.05;②与II级斑块组比较,P<0.05 | |||
随着斑块等级的增大,患者的PLR及NLR数值也增大,PLR及NLR数值与斑块等级呈正相关。见图 1。
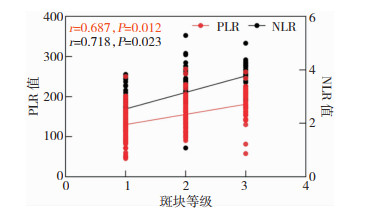
|
| 图 1 PLR及NLR数值与斑块等级的相关性 |
通过ROC和AUC评价PLR、NLR对青年初诊2型糖尿病合并颈动脉硬化患者的诊断作用。PLR的AUC为0.722(95%CI: 0.663~0.790),截断值为111.086,灵敏度和特异性分别为0.789、0.612;NLR的AUC为0.653 (95%CI: 0.586~0.723),截断值为2.240,灵敏度和特异性分别为0.809、0.511。见表 5和图 2。
| 指标 | AUC | 灵敏度 | 特异性 | 截断值 | 95%CI | P值 |
| PLR | 0.722 | 0.789 | 0.612 | 111.086 | 0.663~0.790 | 0.017 |
| NLR | 0.653 | 0.809 | 0.511 | 2.240 | 0.586~0.722 | 0.009 |
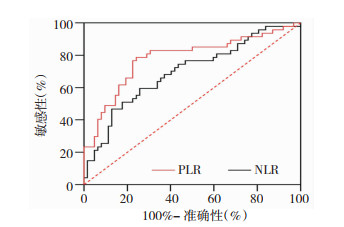
|
| 图 2 PLR和NLR的灵敏度和特异性 |
随着社会发展及生活节奏加快,青年人群T2DM发病率逐渐升高[13]。青年T2DM人群对临床治疗的依从性低,增加了颈动脉硬化的发病风险[14]。研究表明,慢性炎症是T2DM血管病变的重要决定因素[15]。有研究指出,高血压病[16]、高血脂[17]、吸烟[18]及高血糖[19]是T2DM前期患者颈动脉内中膜增高的重要危险因素,研究报道,T2DM的颈动脉斑块发病率为64.3%[20]。本研究结果显示,青年初诊T2DM患者发生颈动脉硬化的危险因素主要是高血压病、糖尿病、高血脂、吸烟,与既往研究结果基本一致。
有研究表明,45岁以下初诊T2DM患者显著增加微血管患病率的风险[21]。T2DM导致的动脉硬化的病理改变是血管损伤及炎症反应,检测炎症反应对分析动脉硬化有重要意义[22]。血液PLR及NLR是2种可重复且容易获得的炎症标志物[23-24],既往研究表明,PLR水平增高会显著增加脑梗死患者斑块脱落的风险[25],NLR是动脉硬化斑块形成及狭窄的重要危险因素,其与血糖调节、胰岛素抵抗、T2DM视网膜病变及肾病相关[26]。
本研究结果显示,青年初诊T2DM患者入院时PLR及NLR数值明显高于对照组。PLR及NLR数值升高的原因可能是T2DM本身是炎症反应,高血糖引起的一系列炎症反应导致血管内皮和血管的异常以及介导平滑肌细胞功能代谢异常[22], 炎症反应在机体蔓延,因此一系列炎症因子释放入血,导致数值明显升高,但具体机制目前仍不清楚,有待进一步研究。多因素Logistic回归分析显示,PLR及NLR数值与初诊青年2型糖尿病患者动脉斑块的发病密切相关,表明PLR及NLR数值作为炎症指标可以对患者颈动脉斑块进行早期筛查及评估,与既往研究结果基本一致。
IMT是动脉硬化的早期阶段,超声多普勒是检测动脉硬化的重要辅助检查工具[27],其可较准确的测量颈动脉IMT,对斑块稳定性作出评价[28]。研究表明,颈动脉IMT是脑血管疾病的独立危险因素[29],体内高血糖是颈动脉粥样硬化斑块(carotid atherosclerotic plaque, CAP)发生的重要原因[30]。本研究应用超声多普勒评估IMT厚度及斑块严重程度,结果显示,III级斑块组患者PLR及NLR数值高于II级斑块组和I级斑块组,II级斑块组患者PLR及NLR数值高于I级斑块组。随着斑块等级的增高,患者PLR及NLR数值也增大。Pearson相关性分析显示,PLR及NLR数值与斑块等级呈正相关。导致此结果的可能原因是糖尿病患者体内慢性炎症导致炎症介质释放增多,引起血小板活化,使血小板升高[31],应激导致淋巴细胞再分布[32],使淋巴细胞减少,其在保护斑块稳定中发挥重要作用[33],血小板可增强淋巴细胞在血管壁的募集[2]。有研究认为高血小板数量和低淋巴细胞计数可能加速狭窄和斑块不稳定性[25]。
本研究结果提示PLR及NLR数值与颈动脉斑块等级有关,表明其数值变化可能反映颈动脉斑块的严重程度。其可作为评估颈动脉严重程度的有效指标,对于指导个体化的院外抗血小板及抗动脉硬化治疗有重要价值。
但是,本研究也有局限性:第一:由于客观条件限制,本研究未对患者进行跟踪随访,不了解动脉硬化斑块与PLR及NLR数值动态演变过程;第二:血常规检测在不同时期变化大,存在检验误差,需要多次检测,计算平均值,才能使结果更可靠;第三:本研究纳入的病例数较少,PLR数值及NLR数值与动脉硬化之间的关系及确切机制尚不明确,仍需大样本的基础研究及临床研究进一步证实。
| [1] |
Duksal T, Tiftikcioglu BI, Bilgin S, et al. Role of inflammation in sensory neuropathy in prediabetes or diabetes[J]. Acta Neurol Scand, 2016, 133(5): 384-390. DOI:10.1111/ane.12474 |
| [2] |
Yildiz A, Yuksel M, Oylumlu M, et al. The utility of the platelet-lymphocyte ratio for predicting no reflow in patients with ST-segment elevation myocardial infarction[J]. Clin Appl Thromb Hemost, 2015, 21(3): 223-228. DOI:10.1177/1076029613519851 |
| [3] |
Corriere T, Di Marca S, Cataudella E, et al. Neutrophil-to-lymphocyte ratio is a strong predictor of atherosclerotic carotid plaques in older adults[J]. Nutr Metab Cardiovasc Dis, 2018, 28(1): 23-27. DOI:10.1016/j.numecd.2017.10.022 |
| [4] |
Wang RT, Zhang JR, Li Y, et al. Neutrophil-lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes[J]. J Diabetes Complications, 2015, 29(2): 245-249. DOI:10.1016/j.jdiacomp.2014.11.006 |
| [5] |
国家卫生计生委脑卒中防治工程委员会. 中国脑卒中血管超声检查指导规范[J]. 中华医学超声杂志(电子版), 2015, 12(8): 599-610. DOI:10.3877/cma.j.issn.1672-6448.2015.08.004 |
| [6] |
中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2017年版)[J]. 中华糖尿病杂志, 2018, 10(1): 4-67. DOI:10.3760/cma.j.issn.1674-5809.2018.01.003 |
| [7] |
Shinozaki N, Ogata N, Ikari Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting[J]. J Stroke Cerebrovasc Dis, 2014, 23(10): 2622-2625. DOI:10.1016/j.jstrokecerebrovasdis.2014.06.007 |
| [8] |
中国高血压防治指南修订委员会. 中国高血压防治指南2010[J]. 中华高血压杂志, 2011, 19(8): 701-743. |
| [9] |
中国成人血脂异常防治指南修订联合委员会. 中国成人血脂异常防治指南(2016年修订版)[J]. 中国循环杂志, 2016, 31(10): 937-953. DOI:10.3969/j.issn.1000-3614.2016.10.001 |
| [10] |
Kim JM, Park KY, Shin DW, et al. Relation of serum homocysteine levels to cerebral artery calcification and atherosclerosis[J]. Atherosclerosis, 2016, 254(3): 200-204. |
| [11] |
Kawle AP, Nayak AR, Lande NH, et al. Comparative evaluation of risk factors, outcome and biomarker levels in young and old acute ischemic stroke patients[J]. Ann Neurosci, 2015, 22(2): 70-77. |
| [12] |
Renna R, Pilato F, Profice P, et al. Risk factor and etiology analysis of ischemic stroke in young adult patients[J]. J Stroke Cerebrovasc Dis, 2014, 23(3): e221-e227. DOI:10.1016/j.jstrokecerebrovasdis.2013.10.008 |
| [13] |
Li Y, Cai YF, Zhao M, et al. Risk factors between intracranial-extracranial atherosclerosis and anterior-posterior circulation stroke in ischaemic stroke[J]. Neurol Res, 2017, 39(1): 30-35. DOI:10.1080/01616412.2016.1250856 |
| [14] |
Hu C, Jia WP. Diabetes in China:epidemiology and genetic risk factors and their clinical utility in personalized medication[J]. Diabetes, 2018, 67(1): 3-11. DOI:10.2337/dbi17-0013 |
| [15] |
Kacířová M, Zmeškalová A, Kořínková L, et al. Inflammation:major denominator of obesity, type 2 diabetes and Alzheimer's disease-like pathology?[J]. Clin Sci (Lond), 2020, 134(5): 547-570. DOI:10.1042/CS20191313 |
| [16] |
Słomka T, Drelich-Zbroja A, Jarząbek M, et al. Intima-media complex thickness and carotid atherosclerotic plaque formation in Lublin's population in the context of selected comorbidities[J]. J Ultrason, 2018, 18(73): 133-139. DOI:10.15557/JoU.2018.0019 |
| [17] |
Bulut A, Avci B. Carotid intima-media thickness values are significantly higher in patients with prediabetes compared to normal glucose metabolism[J]. Medicine (Baltimore), 2019, 98(44): e17805. DOI:10.1097/MD.0000000000017805 |
| [18] |
Trojova I, Kozarova M, Petrasova D, et al. Circulating lipopolysaccharide-binding protein and carotid intima-media thickness in obstructive sleep apnea[J]. Physiol Res, 2018, 67(1): 69-78. |
| [19] |
Manzoni G, Oltolini A, Perra S, et al. Resting whole body energy metabolism in class 3 obesity; from preserved insulin sensitivity to overt type 2 diabetes[J]. Diabetes Metab Syndr Obes, 2020, 13(5): 489-497. |
| [20] |
Seo DH, Kim SH, Song JH, et al. Presence of carotid plaque is associated with rapid renal function decline in patients with type 2 diabetes mellitus and normal renal function[J]. Diabetes Metab J, 2019, 43(6): 840-853. DOI:10.4093/dmj.2018.0186 |
| [21] |
Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas:global estimates for the prevalence of diabetes for 2015 and 2040[J]. Diabetes Res Clin Pract, 2017, 128(7): 40-50. |
| [22] |
马肃, 李涵, 王文宗. 结合珠蛋白作为急性脑梗死患者预后标志物的研究[J]. 国际神经病学神经外科学杂志, 2020, 47(3): 268-271. |
| [23] |
Ozgonul C, Sertoglu E. Accurate use of neutrophil/lymphocyte ratio in patients with age-related macular degeneration[J]. Ocul Immunol Inflamm, 2016, 24(3): 359-360. |
| [24] |
Ozgonul C, Sertoglu E, Ayyildiz O, et al. Novel biomarkers for patients with idiopathic acute anterior uveitis:neutrophil to lymphocyte ratio and platelet to lymphocyte ratio[J]. Int J Ophthalmol, 2017, 10(2): 262-266. |
| [25] |
Yüksel M, Yıldız A, Oylumlu M, et al. The association between platelet/lymphocyte ratio and coronary artery disease severity[J]. Anatol J Cardiol, 2015, 15(8): 640-647. DOI:10.5152/akd.2014.5565 |
| [26] |
Īdil Soylu A, Arıkan Cortcu S, Uzunkaya F, et al. The correlation of the platelet-to-lymphocyte ratio with the severity of stenosis and stroke in patients with carotid arterial disease[J]. Vascular, 2017, 25(3): 299-306. DOI:10.1177/1708538116673770 |
| [27] |
Øygarden H. Carotid intima-media thickness and prediction of cardiovascular disease[J]. J Am Heart Assoc, 2017, 6(1): e005313. |
| [28] |
Debbich A, Ben Abdallah A, Maatouk M, et al. A spatiotemporal exploration and 3D modeling of blood flow in healthy carotid artery bifurcation from two modalities:ultrasound-doppler and phase contrast MRI[J]. Comput Biol Med, 2020, 118(6): 103644. |
| [29] |
邹美娜, 许小扬, 毕国荣, 等. 血清可溶性CD40配体与急性脑梗死患者颈动脉斑块的关系[J]. 国际神经病学神经外科学杂志, 2020, 47(4): 382-386. |
| [30] |
Bragg F, Li L, Bennett D, et al. Association of random plasma glucose levels with the risk for cardiovascular disease among Chinese adults without known diabetes[J]. JAMA Cardiol, 2016, 1(7): 813-823. DOI:10.1001/jamacardio.2016.1702 |
| [31] |
Kim EY, Lee JW, Yoo HM, et al. The Platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio:which is better as a prognostic factor in gastric cancer?[J]. Ann Surg Oncol, 2015, 22(13): 4363-4370. DOI:10.1245/s10434-015-4518-z |
| [32] |
Ekeløf S, Jensen SE, Rosenberg J, et al. Reduced oxidative stress in STEMI patients treated by primary percutaneous coronary intervention and with antioxidant therapy:a systematic review[J]. Cardiovasc Drugs Ther, 2014, 28(2): 173-181. DOI:10.1007/s10557-014-6511-3 |
| [33] |
Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil-lymphocyte ratio[J]. Clin Appl Thromb Hemost, 2016, 22(5): 405-411. DOI:10.1177/1076029615569568 |
 2020, Vol. 47
2020, Vol. 47