聚合物纳米材料由于其形貌的多样性,在催化、化学分离、生物矿化以及药物传递等领域具有广泛的应用[1-5]. 聚合物纳米材料的制备通常采用选择性溶剂自组装的方法,可得到微球、纤维、片状、囊泡等形貌[6-10]. 但这种方法制备出来的聚合物浓度较低(<1%),并且需要进一步的处理(透析、pH调节等),限制了其大规模应用.
近十年来,发展了一种基于可逆−加成断裂链转移自由基活性聚合(Reversible Addition- Fragmentation chain Transfer,RAFT)的聚合诱导自组装(polymerization-induced self-assembly,PISA)体系,聚合物纳米材料固含质量分数可高达50%[11-13]. PISA可分为RAFT水相乳液聚合和RAFT分散聚合. 根据文献的报道,大部分RAFT水相乳液聚合只能得到球形形貌[14-17]. 而RAFT分散聚合可在水[18-20]、乙醇[21-23]、非极性溶剂[24-26]甚至聚乙二醇[27]介质中制备多种形貌(如微球、纤维、囊泡等)的聚合物纳米材料,适用条件广泛. 近期本课题组将光引发聚合引入RAFT聚合诱导自组装中,可在室温下制备环境响应和生物相关的聚合物纳米材料[28-31],并进一步开发了一种酶催化耗氧光聚合诱导自组装[32-33].
虽然可以通过PISA制备多种嵌段共聚物,但所制备的聚合物纳米材料的形貌仅限于微球、纤维、囊泡等常见形貌. 为了制备特殊形貌的聚合物纳米材料,科学家们探讨了多组分嵌段共聚物(包括BAB/AB、AB/B)的聚合物诱导自组装行为. Gao等[34]报导了以两种大分子RAFT试剂调控BAB/AB型嵌段共聚物的聚合诱导自组装,得到多孔的聚合物囊泡. 近期本课题组将小分子RAFT试剂引入聚合诱导自组装,开发出基于嵌段共聚物/均聚物的PISA体系,研究发现均聚物的存在促进了高级形貌的转变[35-37]. Shi等[38]报道了通过种子分散聚合制备含有二茂铁基团的三嵌段共聚物囊泡,所制备的囊泡表面具有氧化还原响应的可切换开关式气孔. He等[39]通过种子分散聚合制备了PDAM-b-PS-b-P4VP三嵌段共聚物多腔体纳米材料,增加4VP的DP可以扩大P4VP的微相范围.
Shi等[40]报道了在mPEG45-PSt的聚合诱导自组装中引入亲溶剂共聚单体4VP,随着[4VP]0/[St]0比例的增加,聚合物形貌由原先的囊泡逐渐向片状、纤维、微球形貌等低级形貌转变,说明亲溶剂单体的引入在一定程度上可以调控PISA形貌. 本文在嵌段共聚物/均聚物的聚合诱导自组装基础上,加入亲溶剂共聚单体4VP,研究表明亲溶剂共聚单体4VP的引入增加了均聚物的亲溶剂性,均聚物从囊泡PS核里面迁移至囊泡表面,使得原先多孔囊泡形貌发生变化,考察其反应动力学,并探讨不同反应条件对聚合物形貌的影响.
1 实验部分 1.1 实验原料苯乙烯(St,99%,上海阿拉丁)和4-乙烯基吡啶(4VP,96%,Aladdin)用碱性氧化铝(上海阿拉丁)过柱提纯并储存于4 ℃;聚乙二醇单甲醚(mPEG45,2 000 g/mol,上海阿拉丁);二环己基碳二亚胺(dicyclohexylcarbodiimide,DCC,上海阿拉丁);4-二甲氨基吡啶(4-dimethylaminopyridine,DMAP,上海阿拉丁);无水二氯甲烷(超干纯,上海阿拉丁);对苯二酚(上海阿拉丁);偶氮二异丁腈(AIBN,上海阿拉丁)经乙醇重结晶并储存于4 ℃;S-正十二烷基-S’-(2-甲基-2-丙酸基)三硫代碳酸酯(DDMAT)根据文献[41]制备;甲醇(分析纯)、正己烷(分析纯),天津市大茂化学试剂厂;去离子水.
1.2 实验步骤 1.2.1 mPEG45-DDMAT大分子RAFT试剂的合成将20.0 g mPEG45 (10 mmol)和7.29 g DDMAT (20 mmol)加至干燥的100 mL圆底烧瓶中,加40 mL无水二氯甲烷搅拌溶解,冷却至0 ℃,接着用10 mL无水二氯甲烷溶解4.12 g DCC (20 mmol)和0.244 g DMAP (2 mmol),并逐滴加入圆底烧瓶中,在干燥密封条件下酯化48 h. 反应完后过滤除去不溶物,滤液在正己烷中沉淀析出,反复洗涤多次,接着用硅胶层析柱法过柱提纯,最后在45 ℃下真空干燥24 h得到黄色固体粉末.
1.2.2 热引发聚合诱导自组装以[mPEG45-DDMAT]/[DDMAT]=1/1(摩尔比),[St]0/[4VP]0=4/1(摩尔比),单体质量分数为15%,目标DP为200的合成为例:将1.20 g St(11.5 mmol)、0.30 g 4VP(2.9 mmol)、0.085 g mPEG45-DDMAT (0.036 mmol)、0.013 g DDMAT(0.036 mmol)、0.0039 g AIBN(0.024 mmol)、6.81 g甲醇/1.70 g水(80/20,质量比)依次加入25 mL的圆底烧瓶中搅拌溶解. 在室温下通氮气20 min后密封,然后在70 ℃油浴锅中反应36 h,反应结束后至于冰水浴中终止反应.
1.2.3 热引发聚合诱导自组装动力学研究[mPEG45-DDMAT]/[DDMAT]=1/1(摩尔比),[St]0/[4VP]0=5/1(摩尔比),单体质量分数为15%,目标DP=200的反应动力学:将1.25 g St(12 mmol)、0.25 g 4VP(2.4 mmol)、0.085 g mPEG45-DDMAT(0.036 mmol)、0.013 g DDMAT(0.036 mmol)、0.0039 g AIBN(0.024 mmol)、6.81 g甲醇/1.70 g水(80/20,质量比)依次加入25 mL的圆底烧瓶中搅拌溶解. 在室温下通氮气20 min后密封,在70 ℃油浴锅中反应36 h. 在氮气氛围下使用注射器在不同时间段抽取样品,所抽取样品用冰水冷却,暴露于空气中并加入少量对苯二酚终止反应. 最后通过1HNMR测其单体转化率.
1.3 仪器与表征使用JEM-1400PLUS 120 kV 透射电镜(TEM)观察聚合物形貌,样品用甲醇/水溶液稀释100倍,滴至铜网1 min后用滤纸吸去过量溶液. 聚合物外部形貌特征通过Hitachi S4800扫描电镜(SEM)观察,样品稀释后滴至云母片上,干燥后喷金. 1HNMR图谱通过BrukerAvance III 400 MHz NMR核磁共振仪测定,样品用少量甲醇稀释,测试溶剂使用CDCl3,聚合物溶液的核磁图谱如图1所示.
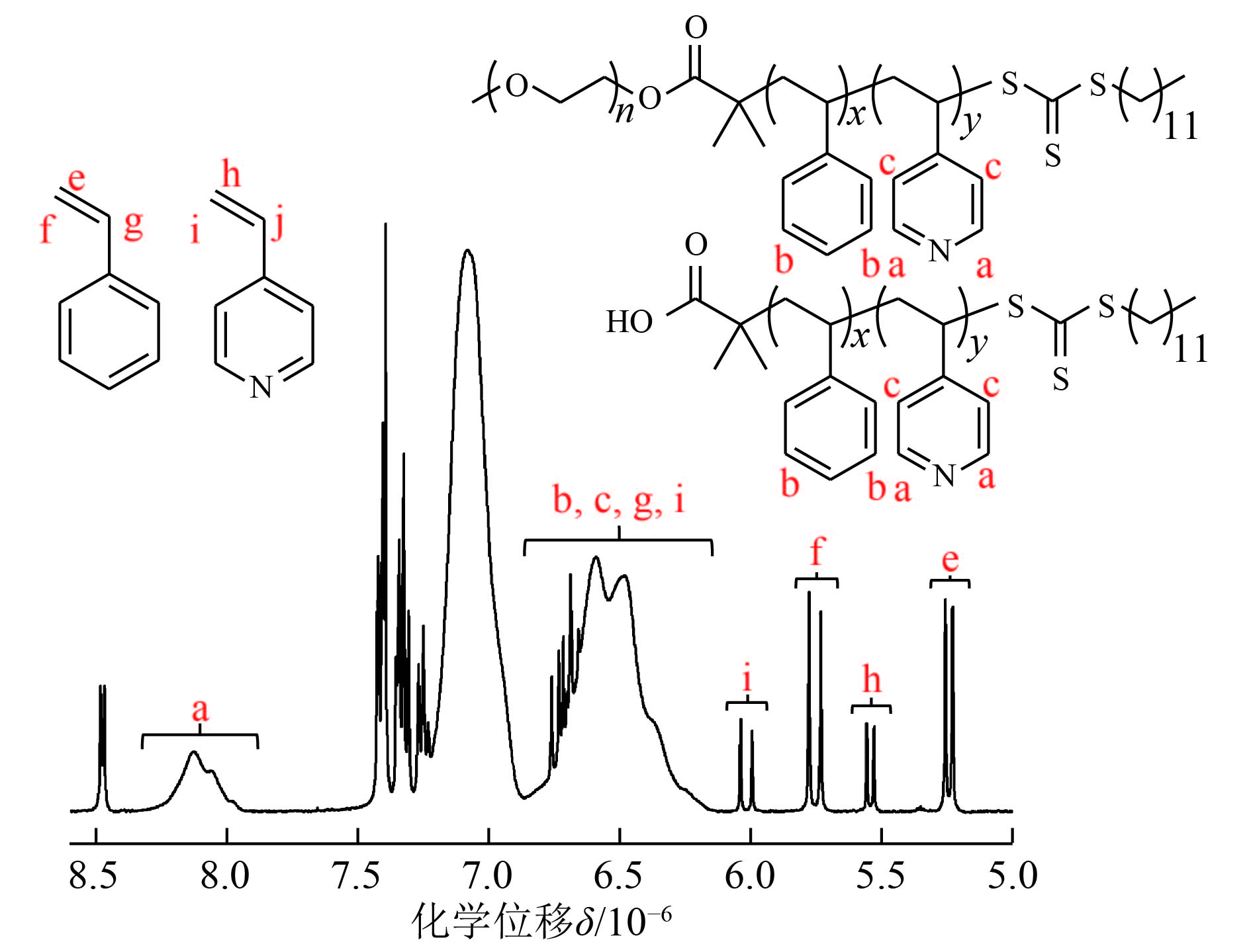
|
图 1 St和4VP热引发RAFT分散聚合后聚合物溶液的1H NMR图 Figure 1 The 1H NMR spectra of the polymerization solution after the RAFT dispersion polymerization of 4VP and St |
单体转化率由式(1)~(3)计算得到:
| ${\rm{Conversio}}{{\rm{n}}_{4{\rm{VP}}}} = \frac{{{I_{7.9 \sim 8.4}}}}{{{I_{7.9 \sim 8.4}} + {I_{5.97 \sim 6.06}} + {I_{5.5 \sim 5.58}}}} \times 100\% ,$ | (1) |
| $\begin{split}&{\rm{Conversio}}{{\rm{n}}_{{\rm{St}}}}=\\ &\displaystyle\frac{{{I_{6.1 \sim 6.85}} - {I_{7.9 \sim 8.4}} - {I_{5.97 \sim 6.06}} - {I_{5.69 \sim 5.82}}}}{{{I_{6.1 \sim 6.85}} - {I_{7.9 \sim 8.4}} - {I_{5.97 \sim 6.06}} + {I_{5.69 \sim 5.82}}}} \times 100\% ,\end{split}$ | (2) |
| $\begin{split}&{\rm{Conversio}}{{\rm{n}}_{{\rm{St}}/4{\rm{VP}}}} = \\&\displaystyle\frac{{{n_{{\rm{St}}}} \times {\rm{Conversio}}{{\rm{n}}_{{\rm{St}}}} + {n_{4{\rm{VP}}}} \times {\rm{Conversio}}{{\rm{n}}_{4{\rm{VP}}}}}}{{{n_{{\rm{St}}}} + {n_{4{\rm{VP}}}}}} \times 100\% ,\end{split}$ | (3) |
其中,nSt和n4VP分别为所加单体St和4VP的摩尔量.
2 结果与讨论 2.1 mPEG45-DDMAT大分子RAFT试剂的合成图2为DDMAT、mPEG45、mPEG45-DDMAT的1HNMR核磁共振图谱.
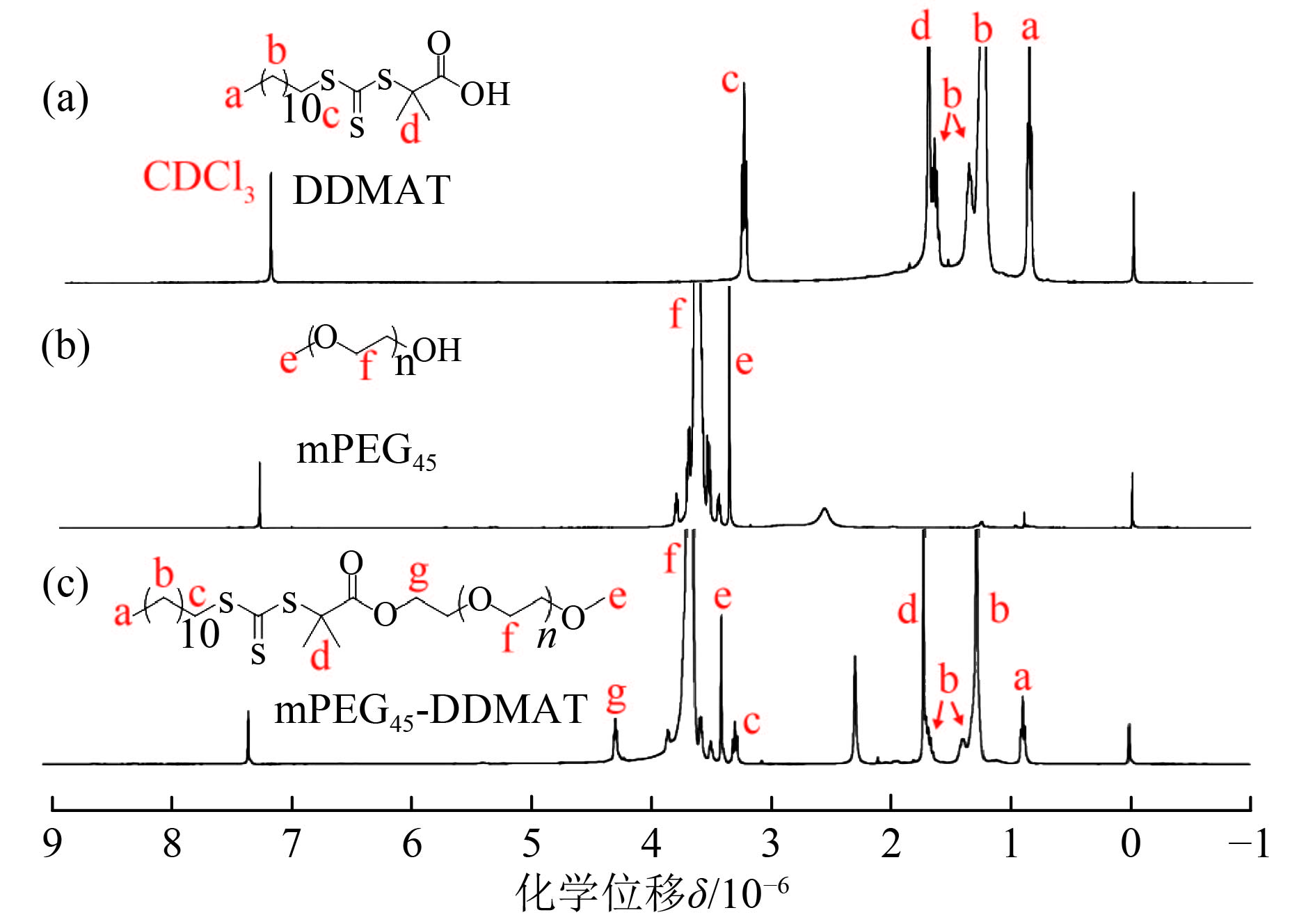
|
图 2 DDMAT、mPEG45、mPEG45-DDMAT的1HNMR核磁共振图谱 Figure 2 1H NMR spectra of DDMAT, mPEG45, and mPEG45-DDMAT |
在图2(c)中δ=4.25×10–6处新增的峰为mPEG45-DDMAT酯键旁边亚甲基氢的信号峰,证明了mPEG45-DDMAT的形成. 酯化率可以通过δ=0.88×10–6和δ=3.38×10–6这两处的信号峰积分面积比例计算,结果说明有96%的mPEG45转化为mPEG45-DDMAT.
2.2 热引发RAFT分散聚合合成mPEG45-P(St-co-4VP)/P(St-co-4VP)如图3所示,mPEG45-DDMAT为大分子RAFT试剂,DDMAT和4VP分别为小分子RAFT试剂和亲溶剂单体,St作为成核单体,在70 ℃的甲醇/水(80/20,质量比)溶剂中反应36 h,单体的转化率可以由1HNMR核磁氢谱信号峰积分面积求出.
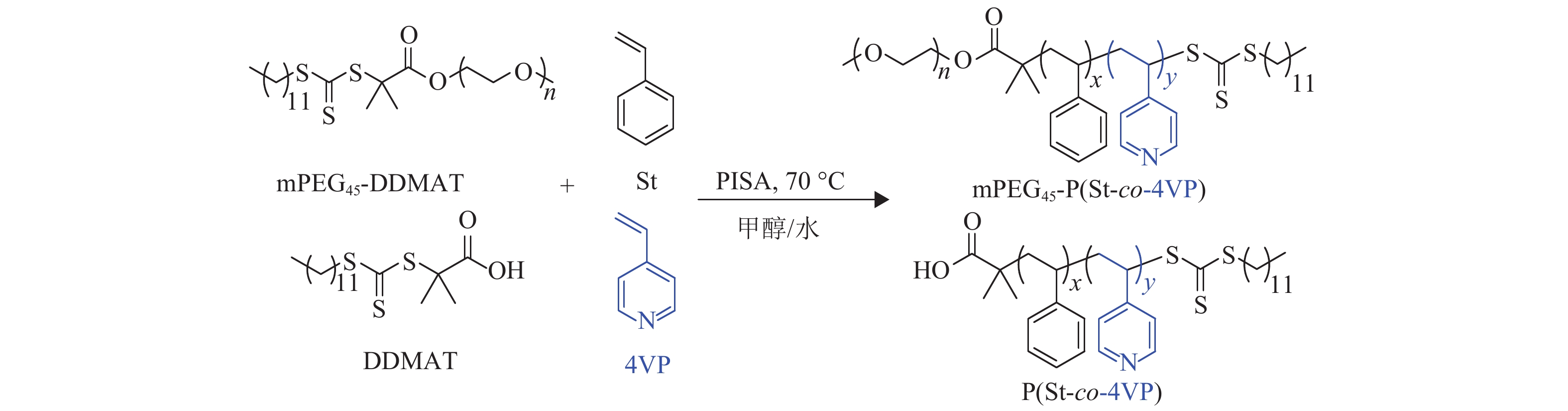
|
图 3 mPEG45-DDMAT和DDMA调控下St和4VP在70 ℃甲醇/水溶液中的热引发RAFT分散聚合 Figure 3 RAFT dispersion polymerization of styrene and 4-vinylpyridine mediated by mPEG45-DDMAT and DDMAT in a methanol/water mixture at 70 ℃ |
图4为[St]0/[4VP]0=5/1(摩尔比),[mPEG45-DDMAT]/DDMAT=1/1(摩尔比),目标聚合DP=200的热引发聚合诱导自组装动力学曲线. 在图4(a)中可以看出,在反应的早期阶段,St的聚合速率略低于4VP的聚合速率,反应16 h后,St的聚合速率超过且略高于4VP的聚合速率. 总体来说,St和4VP的聚合速率比较接近,说明4VP单体相对均一分散于成核链段中. 在RAFT分散聚合过程中,反应在1.5 h使变浑浊,5 h有蓝光现象,11 h开始呈白色,白度随时间逐渐加深,反应25 h转化率可达91%且逐渐持平. 在图4(b)的聚合诱导自组装动力学对数曲线中可明显划分为3个阶段,第1阶段是0~5 h,该阶段为P(St-co-4VP)从溶剂析出的过程;第2阶段是5~16 h,随着反应的进行,具有一定聚合度的mPEG45-P(St-co-4VP)对P(St-co-4VP)起到了增溶作用,球形胶束在这阶段形成,未反应的单体进入胶束中溶剂化成核链段,导致内部单体浓度较高,聚合速率增长迅速. 第3阶段是16~36 h,聚合物形貌进一步转变,聚合速率进一步增加.
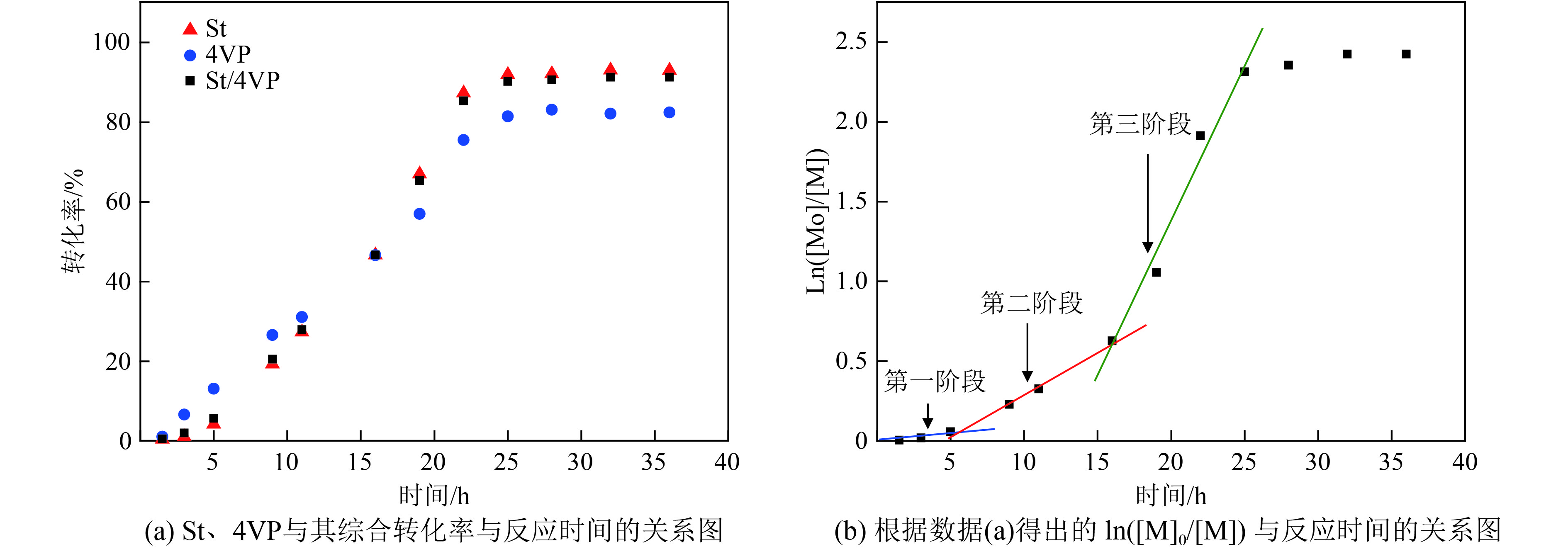
|
图 4 St和4VP在甲醇/水溶液中的热引发RAFT聚合诱导自组装动力学曲线 Figure 4 Kinetics of polymerization of St and 4VP at 70 ℃ at 15% monomer concentration in a methanol/water mixture ([mPEG45-DDMAT]/[DDMAT]=1/1,[St]0/[4VP]0=5/1,单体质量分数为15%) ([mPEG45-DDMAT]/[DDMAT]=1/1, [St]0/[4VP]0=5/1) |
图5为[St]0/[4VP]0=6/1(摩尔比),[mPEG45-DDMAT]/[DDMAT]=1/1(摩尔比),目标DP分别为100、150、200,250的RAFT聚合诱导自组装聚合物纳米微粒的透射电镜图(TEM). 当目标DP=100时,为微球形貌;当目标DP=150时,可观察到微球与囊泡的混合形貌;当目标DP=200时,得到表面凹陷、内部宽松多腔体的复合囊泡;当目标DP=250时,可观察到表面凹陷、内部紧致多腔体的复合囊泡. 随着mPEG45-P(St-co-4VP)与P(St-co-4VP)的DP的增加,成核链段所占比体积增加,促进高级形貌的转变. 另一方面,mPEG45-P(St-co-4VP)与P(St-co-4VP)的DP的增加使得P(St-co-4VP)占有范围增加,有利于复合囊泡的形成. 根据TEM图可以看出,复合囊泡的形成分为两个阶段:(1) 第1阶段为经典的聚合诱导自组装过程,mPEG45-P(St-co-4VP)和P(St-co-4VP)刚开始由纳米粒子演变为囊泡;(2) 随着聚合的进一步进行,囊泡进一步聚集与融合形成复合囊泡. 当4VP不存在的情况下,由于PSt不溶于反应溶液(甲醇/水),因此会被限制在胶束核内. 然而,当亲溶剂单体4VP引入PSt的均聚物/嵌段共聚物聚合诱导自组装,会对最终组装体的形貌产生巨大的影响. 随着聚合度的增加,P(St-co-4VP)以及P(St-co-4VP)链段的亲溶剂性增加,将使得内部的P(St-co-4VP)迁移至表层,由于P(St-co-4VP)没有mPEG45稳定链段,为了降低聚合物胶体的表面能从而提高其稳定性,囊泡与囊泡之间开始聚集并且发生塌缩融合,逐渐形成复合囊泡. 随着聚合度进一步增加,复合囊泡的内部结构变得越来越紧密.
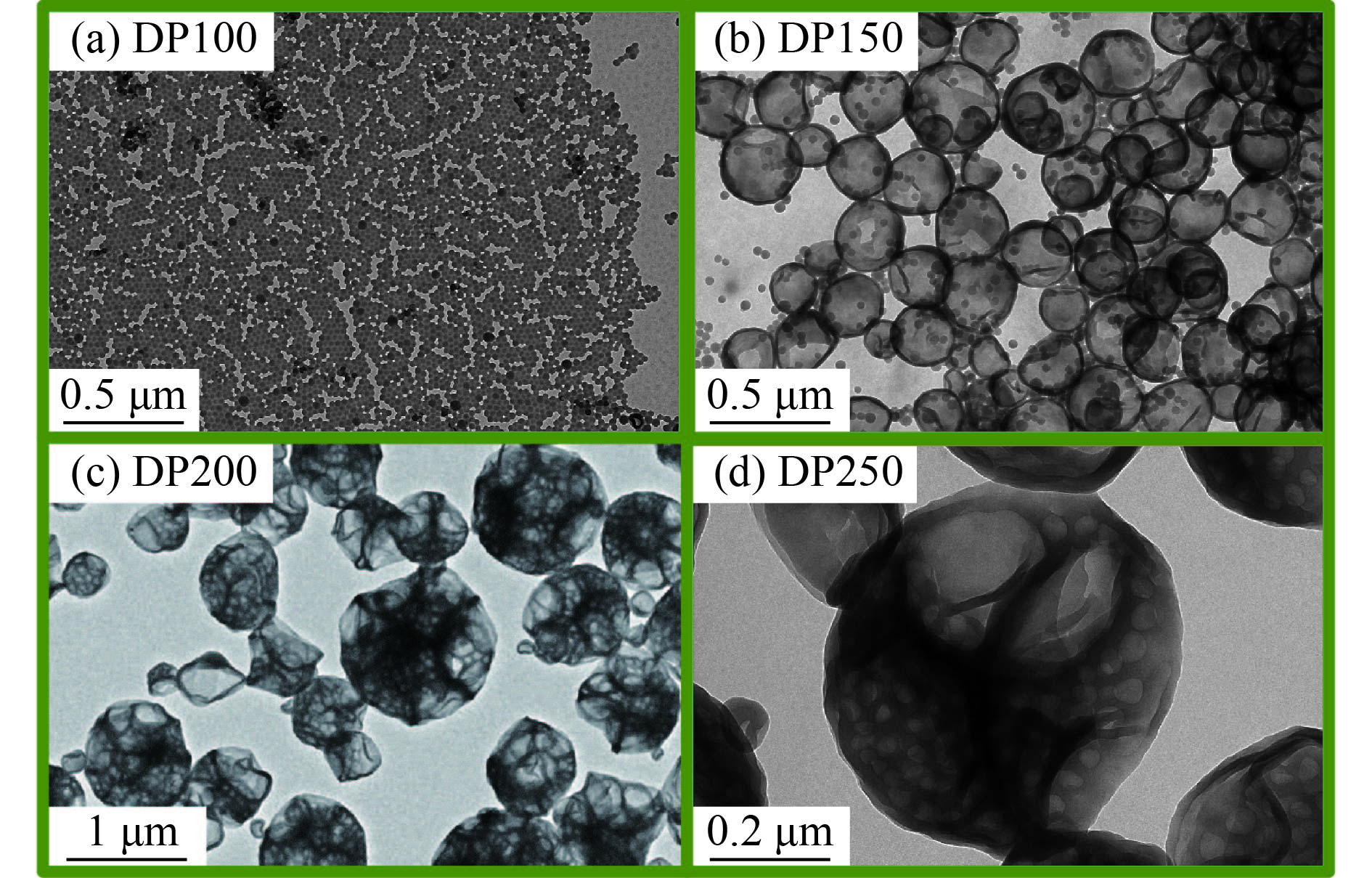
|
图 5 不同目标DP条件下聚合物纳米粒子的TEM图 Figure 5 TEM images of polymer nano-objects with varying target DPs. |
图6的透射电镜图(TEM)和扫描电镜图(SEM)展示了在[mPEG45-DDMAT]/[DDMAT]=1/1(摩尔比),目标DP=200,不同[St]0/[4VP]0摩尔比的条件下所得到的聚合物纳米材料的内部及外观形貌图. 在无4VP亲溶剂单体的引入时,所得到的是表面光滑内部多孔囊泡,如图6(a)所示;当加入少量4VP亲溶剂单体,[St]0/[4VP]0=6/1时,发现原先光滑的表面发生了塌陷,形成表面蜂窝状、内部紧致多腔体的复合囊泡;当[St]0/[4VP]0=5/1时,复合囊泡整体开始变得松弛,表面窝槽尺寸变小;当4VP亲溶剂单体量继续增加,[St]0/[4VP]0=4/1时,窝槽尺寸进一步减小,大量且均一地分布在复合囊泡表面,内部结构紧致;4VP亲溶剂单体量进一步增加至[St]0/[4VP]0=3/1,得到了花瓣状的褶皱形貌;当[St]0/[4VP]0= 2/1时,无复杂形貌,只观察到片状结构. 4VP亲溶剂单体的引入使得成核链段发生从疏溶剂到亲溶剂的转变,随着4VP比例的增大,原先处于胶束内部的P(St-co-4VP)开始迁移到表层,由于P(St-co-4VP)没有mPEG45稳定链段,为了降低聚合物囊泡的表面能稳定聚合物胶体,囊泡发生塌陷,随着4VP量的增加塌陷程度增加,最后复合囊泡完全解离成片状.
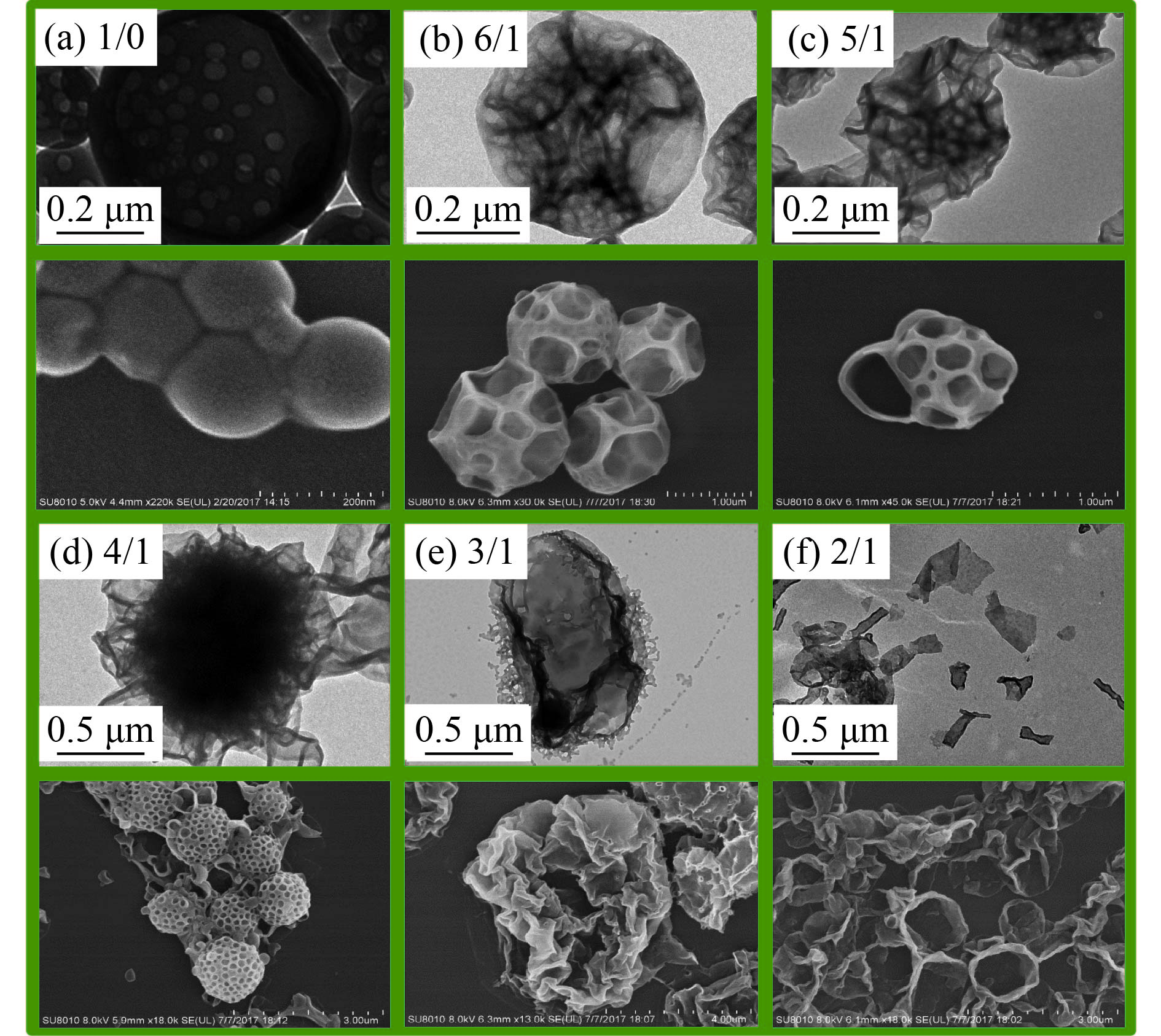
|
图 6 不同[St]0/[4VP]0摩尔比条件下聚合物纳米微粒的TEM和SEM图 Figure 6 TEM and SEM images of polymer nano-objects with varying [St]0/[4VP]0 molar ratios |
mPEG45-P(St-co-4VP)与P(St-co-4VP)的摩尔比由mPEG45-DDMAT和DDMAT的摩尔比决定. 为了进一步研究形貌形成机理,本文还探讨了[mPEG45-DDMAT]/[DDMAT]的比例对自组装形貌的影响.图7为[St]0/[4VP]0=4/1(摩尔比),目标DP=200,不同[mPEG45-DDMAT]/[DDMAT]摩尔比的条件下通过RAFT聚合诱导自组装制备的聚合物纳米材料的透射电镜图(TEM). 当[mPEG45-DDMAT]/ [DDMAT]=4/1或3/1的时候,可以得到塌陷的囊泡形貌,并且囊泡与囊泡之间的聚集程度很低,分布较为均匀;当[mPEG45-DDMAT]/[DDMAT]=2/1时,囊泡聚集程度大大增加;当mPEG45-DDMAT和DDMAT的摩尔比为1/1时,得到的是内部松弛多腔体结构的复合囊泡,尺寸较为稳定;当小分子RAFT试剂DDMAT量进一步增加至[mPEG45-DDMAT]/[DDMAT]=1/2时,复合囊泡外部轮廓变得相对平整,内部多孔且结构紧致. 增加DDMAT的比例即增加了最终产物中P(St-co-4VP)的比例,使得更多的P(St-co-4VP)暴露在囊泡表层,为了降低胶体表面能提高稳定性,引起大量囊泡的聚集,逐渐演变成上述的复合囊泡.
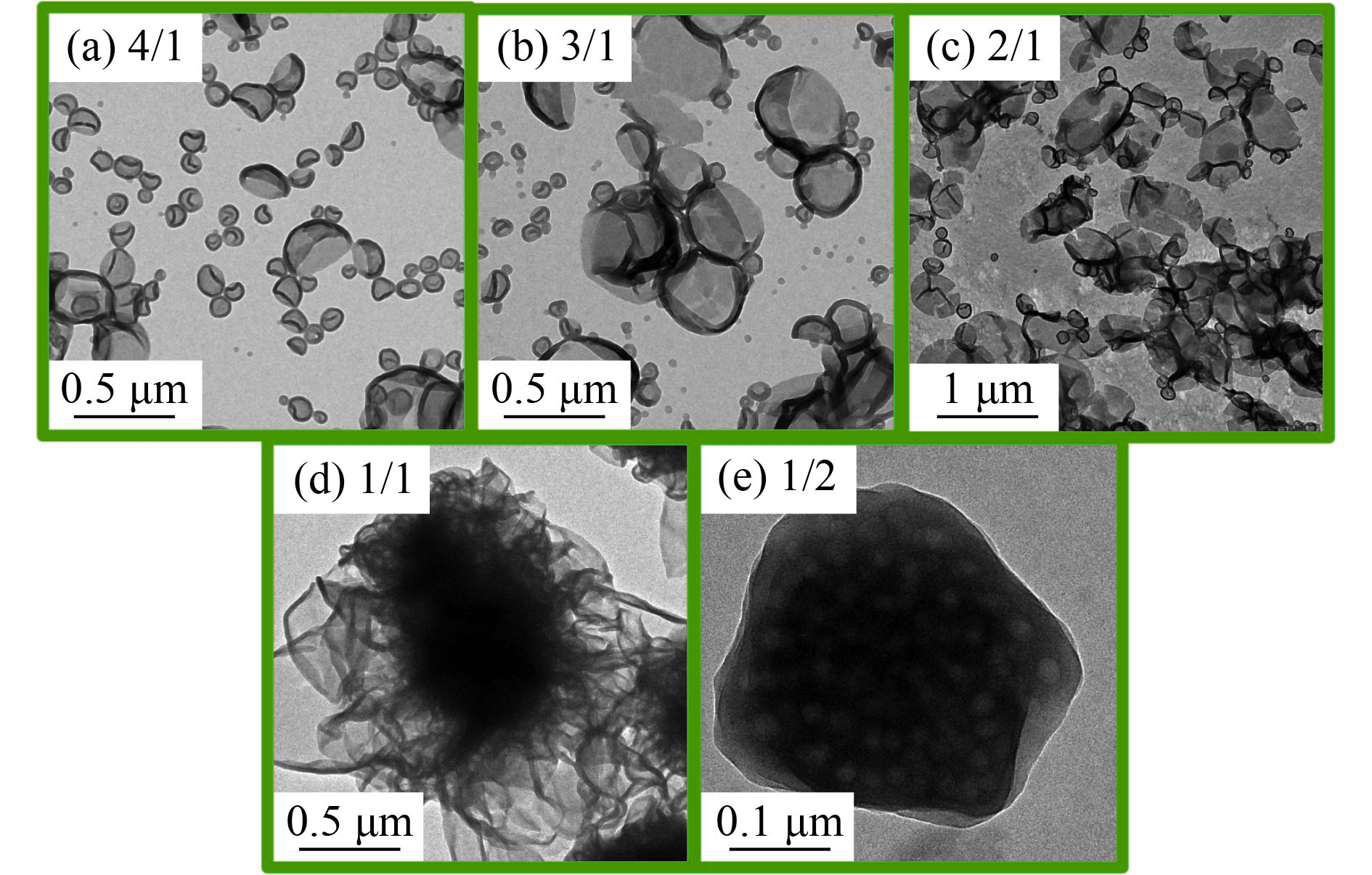
|
图 7 不同[mPEG45-DDMAT]/[DDMAT]摩尔比条件下聚合物纳米微粒的TEM图 Figure 7 TEM images of polymer nano-objects with varying [mPEG45-DDMAT]/[DDMAT] molar ratios |
本文以mPEG45-DDMAT和DDMAT为RAFT试剂,调控St和4VP在70 ℃下以甲醇/水作为溶剂的热引发RAFT分散聚合. 动力学研究表明反应过程分3个阶段:(1) P(St-co-4VP)从溶剂析出的过程;(2) 具有一定聚合度的mPEG45-P(St-co-4VP)对P(St-co-4VP)起到了增容作用,未反应的单体进入胶束,导致内部单体浓度较高,提高聚合速率;(3) 聚合物形貌进一步转变,聚合速率增加. 本文还探讨了不同DP、不同[St]0/[4VP]0摩尔比、不同[mPEG45-DDMAT]/[DDMAT]摩尔比对聚合物形貌的影响:(1) 随着mPEG45-P(St-co-4VP)与P(St-co-4VP)的聚合度的增加,成核链段所占体积比增加,促进高级形貌的转变;另一方面mPEG45-P(St-co-4VP)与P(St-co-4VP)的DP增加使得P(St-co-4VP)的比例增加,有利于复合囊泡的形成. (2) 改变[St]0/[4VP]0比例,聚合物形貌发生从多孔囊泡(1/0)到复合囊泡(6/1-3/1)至片状结构(2/1)的转变. 因为4VP为亲溶剂单体,其聚合物也具有一定的亲溶剂性,随着4VP比例的增加,原先处于胶束内部的P(St-co-4VP)开始迁移到表层,由于P(St-co-4VP)没有mPEG45稳定链段,为了降低聚合物囊泡的表面能,使得囊泡发生塌陷,随着4VP量的增加塌陷程度增加,最后复合囊泡完全解离成片状. (3) 改变[mPEG45-DDMAT]/[DDMAT]摩尔比,聚合物从相对均匀分散的塌陷囊泡(4/1-3/1),逐渐聚集(2/1),最终形成尺寸相对稳定、具有特殊形貌的复合囊泡(1/1-1/2). 增加DDMAT的比例即增加了产物中P(St-co-4VP)的比例,使得更多的P(St-co-4VP)迁移至囊泡表层,为了降低囊泡表面能稳定胶体,使得大量囊泡发生聚集,逐渐演变为复合囊泡. 吡啶基团与贵金属纳米粒子具有很强的作用力,因此可以用于制备有机/无机杂化聚合物纳米材料,本论文所制备的复合囊泡具有很大的比表面积,因此在催化等领域具有潜在的应用价值.
| [1] |
WANG Z, VAN OERS M C M, RUTJES F P J T, et al. Polymersome colloidosomes for enzyme catalysis in a biphasic system[J].
Angewandte Chemie International Edition, 2012, 51(43): 10746-10750.
DOI: 10.1002/anie.v51.43. |
| [2] |
LOUZAO I, VAN HEST J C M. Permeability effects on the efficiency of antioxidant nanoreactors[J].
Biomacromolecules, 2013, 14(7): 2364-2372.
DOI: 10.1021/bm400493b. |
| [3] |
LIU T, HU J, JIN Z, et al. Two-photon ratiometric fluorescent mapping of intracellular transport pathways of pH-responsive block copolymer micellar nanocarriers[J].
Advanced Healthcare Materials, 2013, 2(12): 1576-1581.
DOI: 10.1002/adhm.v2.12. |
| [4] |
LEE J S, FEIJEN J. Polymersomes for drug delivery: design, formation and characterization[J].
Journal of Controlled Release, 2012, 161(2): 473-483.
DOI: 10.1016/j.jconrel.2011.10.005. |
| [5] |
NING Y, FIELDING L A, ANDREWS T S, et al. Sulfate-based anionic diblock copolymer nanoparticles for efficient occlusion within zinc oxide[J].
Nanoscale, 2015, 7(15): 6691-6702.
DOI: 10.1039/C5NR00535C. |
| [6] |
付黎黎, 柏宇豪, 张雪超, 等. RAFT光引发分散聚合制备单分散PMMA微球[J].
广东工业大学学报, 2017, 34(2): 34-39.
FU L L, BAI Y H, ZHANG X C, et al. Synthesis of monodisperse PMMA microspheres by photoinitiated RAFT dispersion polyneerization[J]. Journal of Guangdong University of Technology, 2017, 34(2): 34-39. DOI: 10.12052/gdutxb.160029. |
| [7] |
MAI Y, EISENBERG A. Self-assembly of block copolymers[J].
Chemical Society Reviews, 2012, 41(18): 5969-5985.
DOI: 10.1039/c2cs35115c. |
| [8] |
DU J Z, TANG Y Q, LEWIS A L, et al. pH-sensitive vesicles based on a biocompatible zwitterionic diblock copolymer[J].
Journal of the American Chemical Society, 2005, 127(51): 17982-17983.
DOI: 10.1021/ja056514l. |
| [9] |
KITA-TOKARCZYK K, GRUMELARD J, HAEFELE T, et al. Block copolymer vesicles - using concepts from polymer chemistry to mimic biomembranes[J].
Polymer, 2005, 46(11): 3540-3563.
DOI: 10.1016/j.polymer.2005.02.083. |
| [10] |
BLANAZS A, MASSIGNANI M, BATTAGLIA G, et al. Tailoring macromolecular expression at polymersome Surfaces[J].
Advanced Functional Materials, 2009, 19(18): 2906-2914.
DOI: 10.1002/adfm.v19:18. |
| [11] |
SUN J, HONG C, PAN C. Recent advances in RAFT dispersion polymerization for preparation of block copolymer aggregates[J].
Polymer Chemistry, 2013, 4(4): 873-881.
DOI: 10.1039/C2PY20612A. |
| [12] |
RIEGER J. Guidelines for the synthesis of block copolymer particles of various morphologies by RAFT dispersion polymerization[J].
Macromolecular Rapid Communications, 2015, 36(16): 1458-1471.
DOI: 10.1002/marc.201500028. |
| [13] |
DERRY M J, FIELDING L A, ARMES S P. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization[J].
Progressin Polymer Science, 2016, 52: 1-18.
DOI: 10.1016/j.progpolymsci.2015.10.002. |
| [14] |
CUNNINGHAM V J, ALSWIELEH A M, THOMPSON K L, et al. Poly(glycerol monomethacrylate)-poly(benzyl methacrylate) diblock copolymer nanoparticles via raft emulsion polymerization: synthesis, characterization, and interfacial activity[J].
Macromolecules, 2014, 47(16): 5613-5623.
DOI: 10.1021/ma501140h. |
| [15] |
CHARLEUX B, DELAITTRE G, RIEGER J, et al. Polymerization-induced self-assembly: from soluble macromolecules to block copolymer nano-objects in one step[J].
Macromolecules, 2012, 45(17): 6753-6765.
DOI: 10.1021/ma300713f. |
| [16] |
SUN L, HONG L, WANG C. Facile fabrication of water dispersible latex particles with homogeneous or chain-segregated surface from RAFT polymerization using a mixture of two macromolecular chain transfer agents[J].
Macromolecular Rapid Communications, 2016, 37(8): 691-699.
DOI: 10.1002/marc.v37.8. |
| [17] |
RIEGER J, STOFFELBACH F, BUI C, et al. Amphiphilic poly(ethylene oxide) macromolecular RAFT agent as a stabilizer and control agent in ab initio batch emulsion polymerization[J].
Macromolecules, 2008, 41(12): 4065-4068.
DOI: 10.1021/ma800544v. |
| [18] |
TAN J, LIU D, ZHANG X, et al. Facile preparation of hybrid vesicles loaded with silica nanoparticles via aqueous photoinitiated polymerization-induced self-assembly[J].
RSC Advances, 2017, 7(37): 23114-23121.
DOI: 10.1039/C7RA02770B. |
| [19] |
LIU G, QIU Q, SHEN W, et al. Aqueous dispersion polymerization of 2-methoxyethyl acrylate for the synthesis of biocompatible nanoparticles using a hydrophilic RAFT polymer and a redox initiator[J].
Macromolecules, 2011, 44(13): 5237-5245.
DOI: 10.1021/ma200984h. |
| [20] |
ZHOU W, QU Q, XU Y, et al. Aqueous polymerization-induced self-assembly for the synthesis of ketone-functionalized nano-objects with low polydispersity[J].
ACS Macro Letters, 2015, 4(5): 495-499.
DOI: 10.1021/acsmacrolett.5b00225. |
| [21] |
WAN W, HONG C, PAN C. One-pot synthesis of nanomaterials via RAFT polymerization induced self-assembly and morphology transition[J].
Chemical Communications, 2009(39): 5883-5885.
DOI: 10.1039/b912804b. |
| [22] |
HE W, SUN X, WAN W, et al. Multiple morphologies of PAA-b-PSt assemblies throughout RAFT dispersion polymerization of styrene with PAA Macro-CTA[J].
Macromolecules, 2011, 44(9): 3358-3365.
DOI: 10.1021/ma2000674. |
| [23] |
ZHANG W, HONG C, PAN C. Fabrication of spaced concentric vesicles and polymerizations in RAFT dispersion polymerization[J].
Macromolecules, 2014, 47(5): 1664-1671.
DOI: 10.1021/ma402497y. |
| [24] |
FIELDING L A, LANE J A, DERRY M J, et al. Thermo-responsive diblock copolymer worm gels in non-polar solvents[J].
Journal of the American Chemical Society, 2014, 136(15): 5790-5798.
DOI: 10.1021/ja501756h. |
| [25] |
RATCLIFFE L P D, MCKENZIE B E, LE BOUEDEC G M D, et al. Polymerization-induced self-assembly of all-acrylic diblock copolymers via RAFT dispersion polymerization in alkanes[J].
Macromolecules, 2015, 48(23): 8594-8607.
DOI: 10.1021/acs.macromol.5b02119. |
| [26] |
PEI Y, THURAIRAJAH L, SUGITA O R, et al. RAFT dispersion polymerization in nonpolar media: polymerization of 3-phenylpropyl methacrylate in n-tetradecane with poly(stearyl methacrylate) homopolymers as macro chain transfer agents[J].
Macromolecules, 2015, 48(1): 236-244.
DOI: 10.1021/ma502230h. |
| [27] |
GAO C, ZHOU H, QU Y, et al. In situ synthesis of block copolymer nanoassemblies via polymerization-induced self-assembly in poly(ethylene glycol)[J].
Macromolecules, 2016, 49(10): 3789-3798.
DOI: 10.1021/acs.macromol.6b00688. |
| [28] |
TAN J, SUN H, YU M, et al. Photo-PISA: shedding light on polymerization-induced self-assembly[J].
ACS Macro Letters, 2015, 4(11): 1249-1253.
DOI: 10.1021/acsmacrolett.5b00748. |
| [29] |
TAN J, HUANG C, LIU D, et al. Alcoholic photoinitiated polymerization-induced self-assembly (Photo-PISA): a fast route toward poly(isobornyl acrylate)-based diblock copolymer nano-objects[J].
ACS Macro Letters, 2016, 5(8): 894-899.
DOI: 10.1021/acsmacrolett.6b00439. |
| [30] |
TAN J, BAI Y, ZHANG X, et al. Room temperature synthesis of poly(poly(ethylene glycol) methyl ether methacrylate)-based diblock copolymer nano-objects via photoinitiated polymerization-induced self-assembly (Photo-PISA)[J].
Polymer Chemistry, 2016, 7(13): 2372-2380.
DOI: 10.1039/C6PY00022C. |
| [31] |
YEOW J, XU J, BOYER C. Polymerization-induced self-assembly using visible light mediated photoinduced electron transfer-reversible addition-fragmentation chain transfer polymerization[J].
ACS Macro Letters, 2015, 4(9): 984-990.
DOI: 10.1021/acsmacrolett.5b00523. |
| [32] |
TAN J, LIU D, BAI Y, et al. Enzyme-assisted photoinitiated polymerization-induced self-assembly: an oxygen-tolerant method for preparing block copolymer nano-objects in open vessels and multiwell plates[J].
Macromolecules, 2017, 50(15): 5798-5806.
DOI: 10.1021/acs.macromol.7b01219. |
| [33] |
NG G, YEOW J, XU J, et al. Application of oxygen tolerant PET-RAFT to polymerization-induced self-assembly[J].
Polymer Chemistry, 2017, 8(18): 2841-2851.
DOI: 10.1039/C7PY00442G. |
| [34] |
GAO C, WU J, ZHOU H, et al. Self-assembled blends of AB/BAB block copolymers prepared through dispersion RAFT polymerization[J].
Macromolecules, 2016, 49(12): 4490-4500.
DOI: 10.1021/acs.macromol.6b00771. |
| [35] |
TAN J, HUANG C, LIU D, et al. Polymerization-induced self-assembly of homopolymer and diblock copolymer: a facile approach for preparing polymer nano-objects with higher-order morphologies[J].
ACS Macro Letters, 2017, 6(3): 298-303.
DOI: 10.1021/acsmacrolett.7b00134. |
| [36] |
YUAN B, HE X, QU Y, et al. In situ synthesis of a self-assembled AB/B blend of poly(ethylene glycol)-b-polystyrene/polystyrene by dispersion RAFT polymerization[J].
Polymer Chemistry, 2017, 8(14): 2173-2181.
DOI: 10.1039/C7PY00339K. |
| [37] |
ZHU A, LV X, SHEN L, et al. Polymerization-induced cooperative assembly of block copolymer and homopolymer via RAFT dispersion polymerization[J].
ACS Macro Letters, 2017, 6(3): 304-309.
DOI: 10.1021/acsmacrolett.7b00069. |
| [38] |
SHI P, QU Y, LIU C, et al. Redox-responsive multicompartment vesicles of ferrocene-containing triblock terpolymer exhibiting on off switchable pores[J].
ACS Macro Letters, 2016, 5(1): 88-93.
DOI: 10.1021/acsmacrolett.5b00928. |
| [39] |
HE X, QU Y, GAO C, et al. Synthesis of multicompartment nanoparticles of a triblock terpolymer by seeded RAFT polymerization[J].
Polymer Chemistry, 2015, 6(35): 6386-6393.
DOI: 10.1039/C5PY01041A. |
| [40] |
SHI P, ZHOU H, GAO C, et al. Macro-RAFT agent mediated dispersion copolymerization: a small amount of solvophilic co-monomer leads to a great change[J].
Polymer Chemistry, 2015, 6(27): 4911-4920.
DOI: 10.1039/C5PY00697J. |
| [41] |
LAI J T, FILLA D, SHEA R. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents[J].
Macromolecules, 2002, 35(18): 6754-6756.
DOI: 10.1021/ma020362m. |
 2018, Vol. 35
2018, Vol. 35

