2. 哈尔滨工业大学 城市水资源与水环境国家重点实验室,黑龙江 哈尔滨 150090
2. State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China
环境污染和能源短缺已经成为当今世界面临的两大问题[1]。由于光催化过程是利用丰富且可持续的太阳能或光能进行催化反应,因而近年来光催化技术得到了迅速发展,并广泛应用于环境修复和能源转换领域[2-5]。然而,大多数光催化材料存在着禁带宽度宽、可见光利用率低、电子空穴对复合速度快等缺点,导致光催化效率较低[6],不利于光催化技术在工程中进行应用。
金属有机框架(MOFs)是以金属离子或团簇为节点,以有机配体为连接体,通过两者的配位作用自组装形成的新型晶态多孔材料,是近年来的研究热点之一[7-10]。由于具有超高的比表面积、稳定的多孔结构、多变的功能性等独特性质,它在气体吸附与分离、传感、生物医药、质子传导和催化等领域得到了广泛的应用[11-14]。然而,由于本征MOFs的禁带宽度普遍较大,导致其对可见光吸收范围较小,这一瓶颈限制了MOFs材料在光催化领域的进一步应用[15]。
铋基半导体作为一种新型可见光响应光催化剂,其带隙通常在2.0 ~ 3.0 eV之间,被认为很适合用于去除环境介质中的污染物质以及催化合成新型能源,近年来也引起了越来越多研究者的关注[16]。目前,为了解决本征铋基半导体光生空穴−电子对快速复合的不足,通常是通过组分调节、形貌控制和构建异质结结构等策略来进行改性,例如与贵金属纳米粒子、TiO2、g-C3N4等制备异质结光催化剂[17]。尽管上述复合光催化剂在一定程度上提高了原材料的光催化性能,但存在比表面积小、亲水性差、活性位点不足等问题。由此可见,从铋基半导体和MOFs的微观结构和光学特性出发,设计MOFs/铋基半导体复合材料(MBCs),可将上述两种材料的优势相结合,进而增大与反应物的接触面积、调控带隙宽度、拓展可见光响应范围并提高光生载流子的分离效率,是突破上述瓶颈问题的关键所在。
本文综述了近年来国内外对MBCs的多种制备方法及在环境修复和能源转换等方面的应用进展,以期为光催化技术的大规模推广应用提供新的思路。
1 MBCs的制备根据目前文献中报道的多种MBCs光催化剂的制备过程,可将其制备方法主要归纳为两类:一是先通过水热法/溶剂热法、电化学法、超声法、微波法等前处理方法制备出MOFs材料,再借助水热/溶剂热过程将MOFs与铋基半导体结合,制备出MBCs;二是在制备出MOFs材料后,基于高能球磨法,使MOFs和铋基半导体材料发生低温化学反应,进而形成MBCs复合材料。
1.1 水热/溶剂热法水热/溶剂热合成法是以水或有机溶剂为反应介质,在一定温度和压力条件下,利用密闭环境创造出高温高压的临界反应状态,制得形貌均一的纳米材料的方法。该方法成熟稳定且可精确调控产物的形貌和结晶度等物化性质,但其也存在反应时间较长、反应条件需要高温高压、能耗大等不足[18]。
Bibi等[19]采用水热法制备了BiOBr/NH2-UiO-66(BUN)杂化复合材料,其微观形貌为二维BiOBr纳米片生长在八面体NH2-UiO-66上,与纯BiOBr和纯NH2-UiO-66相比,光催化降解染料的效果分别提高了2.7倍和5.3倍,见图1(a-c)。类似地,Hu等[20]以甘露醇为反应介质,采用溶剂热法制备了NH2-UiO-66/BiOBr(NU/BOB)复合催化剂,见图1(d-f)。从微观形貌的角度,水热法与溶剂热法制备的NH2-UiO-66/BiOBr较为相似,均是二维BiOBr纳米片生长在八面体NH2-UiO-66上。此外,由图1(b)和图1(e)对比可知,溶剂热法合成的BiOBr的纳米片厚度更薄(100 nm),大约为水热法合成BiOBr样品厚度的1/10~1/5,这将更有利于光催化过程中异质结界面上的电子传导行为,进而实现了其对水体中有机污染物四环素和Cr(VI)的有效光催化去除。
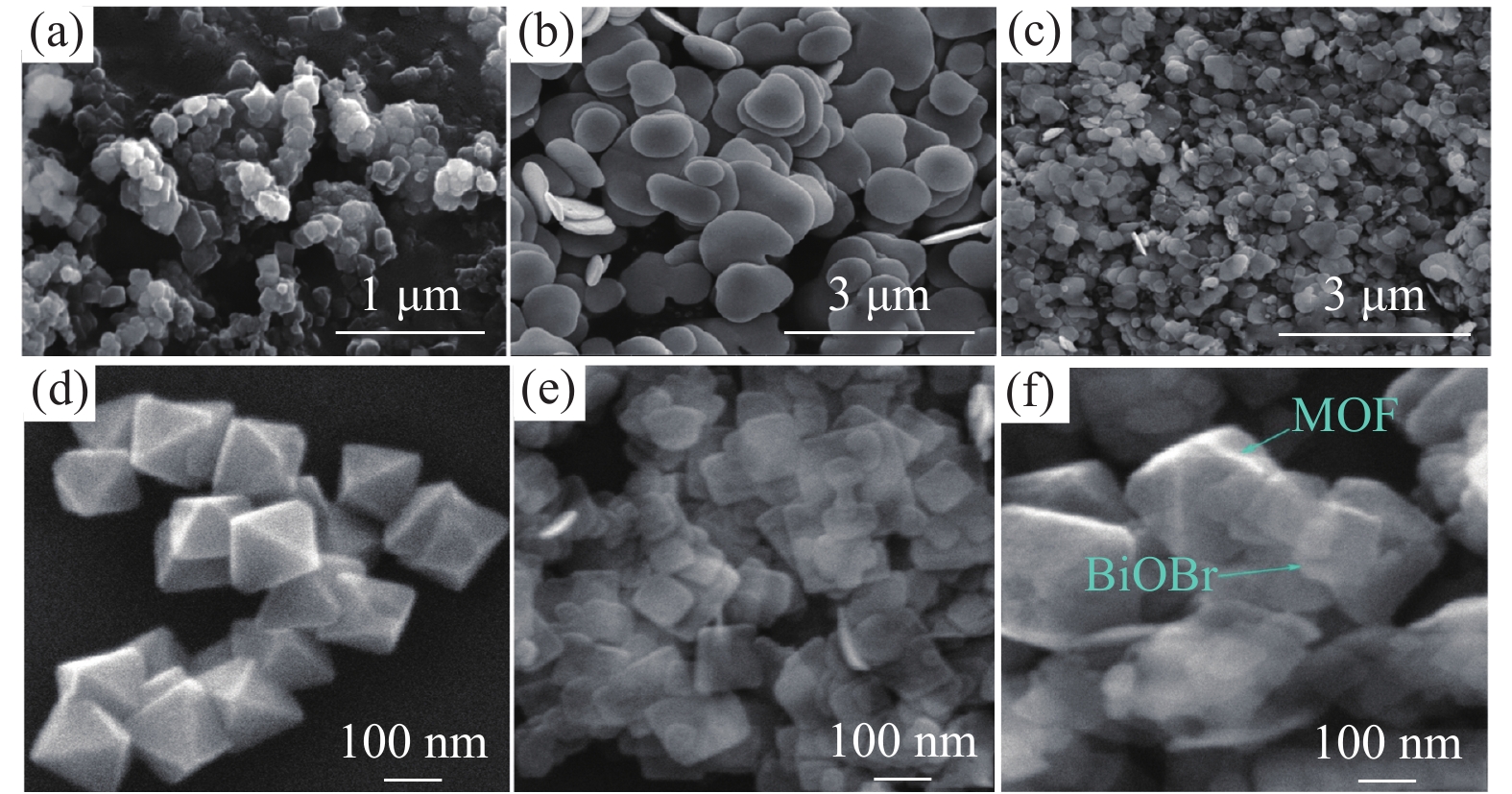
|
图 1 (a) 纯 UiO-66-NH2扫描电镜图[19], (b) 纯 BiOBr扫描电镜图[19], (c) BUN-15扫描电镜图[19], (d) 纯 NH2-UiO-66扫描电镜图[20], (e) 纯 BiOBr扫描电镜图[20], (f) NU/BOB-15扫描电镜图[20] Figure 1 SEM images of (a) UiO-66-NH2[19], (b) BiOBr nanoflakes[19], (c) BUN-15[19], (d) NH2-UiO-66[20], (e) BiOBr[20] and (f) NU/BOB-15[20] |
对于三元MBCs复合材料,也可通过多步水热/溶剂热的方法制备。Khasevani等[21]先制备出MIL-88A(Fe)/g-C3N4二元前驱体材料,随后结合水热原位沉淀法将BiOI引入前驱体中,从而制备得到三元BiOI/MIL-88A(Fe)/g-C3N4核壳催化剂。与二元前驱体相比,其对酸性蓝、罗丹明B和苯酚的光催化活性分别提高了约50%、45%和40%。Askari等[22]通过简单的水热法制备CuWO4/Bi2S3异质结结构,再借助第二步水热/溶剂热法引入ZIF-67构建了双Z型CuWO4/Bi2S3/ZIF67三元催化剂,从而对抗生素具有高效的光催化活性。
1.2 高能球磨法高能球磨法是利用球磨机的转动或振动,通过研磨介质对原材料进行强烈的撞击、研磨、搅拌来制备合成纳米材料的方法。球磨法操作简单、产率高且较为绿色环保[18]。
在MBCs的制备过程中,通常采用将MOFs和铋基半导体前驱体按照一定比例放入罐中进行球磨,其研磨介质普遍采用直径10 mm或20 mm的不锈钢球。Li等[23]按照一定比例将分别用水热法和溶剂热法制备的Bi12O17Cl2和MIL-53(Fe)前驱体倒入行星式研磨罐中,球磨法制备得到球状的MIL-53(Fe) /Bi12O17Cl2复合材料(BMx)。在此基础上,此团队还采用类似方法制备了Bi12O17Cl2/MIL-100(Fe)材料(BMx),这一系列的BMx光催化剂可在90 min可见光照射下将99.2%的Cr(VI)有效还原为Cr(III)。此外,在向BMx催化体系内添加过二硫酸盐后,在可见光作用下还可实现双酚A的有效降解去除[24]。
2 MBCs光催化的应用近年来,MBCs在光催化领域的应用报道逐年增加,主要用于环境修复和能源转换两大方面。具体应用如表1和图2所示。
| 表 1 MBCs在光催化方面的应用 Table 1 Application of MBCs in photocatalysis |
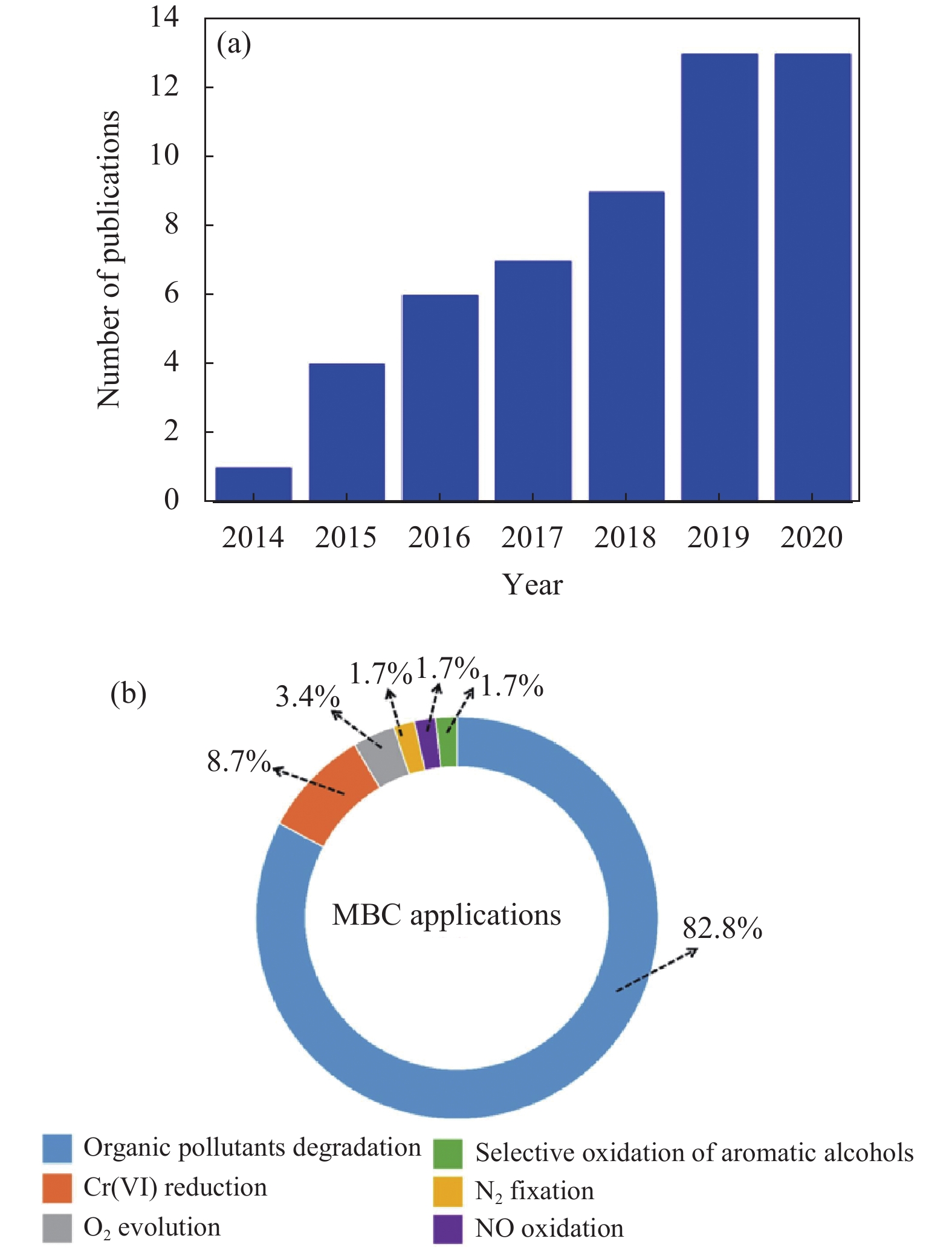
|
图 2 (a) MBC 光催化技术在2014~2020年度的研究论文数量图, (b)多种 MBCs 光催化应用的环形比例图[16] Figure 2 (a) Number of publications of MBC photocatalysts during 2014~2020 and (b) doughnut chart of photocatalytic applications of MBCs[16] |
随着工业的发展,每年有近80万t缺乏预处理的染料废水直接排放到水体中[38]。水体中的染料不仅会对人体造成过敏、皮炎、恶心等毒性影响,而且还具有遗传毒性和致畸、致癌、致突变的影响[39-40]。MBCs复合材料具有优异的光生载流子分离迁移效率和广谱的光吸收能力,是光催化降解有机污染物的理想光催化剂之一。Yang等[41]利用超声法制备了MOF-5/BiOBr复合材料,其对甲基橙的光催化降解实验结果表明,引入MOFs组分可以有效提高本征卤氧化铋的光催化性能。Bibi等[19]通过水热处理使BiOBr沉积在UiO-66-NH2表面形成结合更加紧密的BiOBr/UiO-66-NH2复合异质结结构,其中UiO-66-NH2组分质量分数为15%时,形成的复合材料具有最优的催化活性,在可见光照射2 h后罗丹明B的降解率达83%。
2.1.2 药物和个人护理产品降解药物和个人护理品(Pharmaceutical and Personal Care Products,PPCPs)是新兴的环境污染物之一,其环境风险受到研究者们越来越多的关注[42]。随着PPCPs产量的增长和广泛应用,各种水体环境及土壤底泥中普遍检出抗生素和内分泌干扰物等多种持久性有机污染物[43-46]。Hu等[31]采用一步水热法制备了NH2-MIL-125(Ti)/BiOCl复合材料(NM/BOC),在对四环素(TC)的光催化降解实验表明,NM/BOC-10(NH2-MIL-125(Ti)质量分数为10%)的催化效果最优,分别是纯BiOCl和纯NH2-MIL-125(Ti)活性的3.5倍和35倍。如图3所示,Tang等[30]通过溶剂热法制备了BiOBr/MIL-53(Fe)复合材料(BM),BM-20(MIL-53的质量分数为20%)在100 min的可见光照射下可降解85%卡马西平,其降解动力学常数是纯BiOBr和纯MIL-53(Fe)的15倍。如图4所示,Liang等[32]利用溶剂热−水热法制备了三元异质结BiOI@UIO-66(NH2) @g-C3N4(BiOI@UNCN)。其中BiOI@UNCN-40(BiOI的质量分数为10%)对TC的光催化效果最优,在180 min内可降解80%的TC,其降解动力学常数分别是UIO-66(NH2)、UNCN、BiOI和BiOI@UNCN-20的18.42、4.41、3.06和2.59倍。
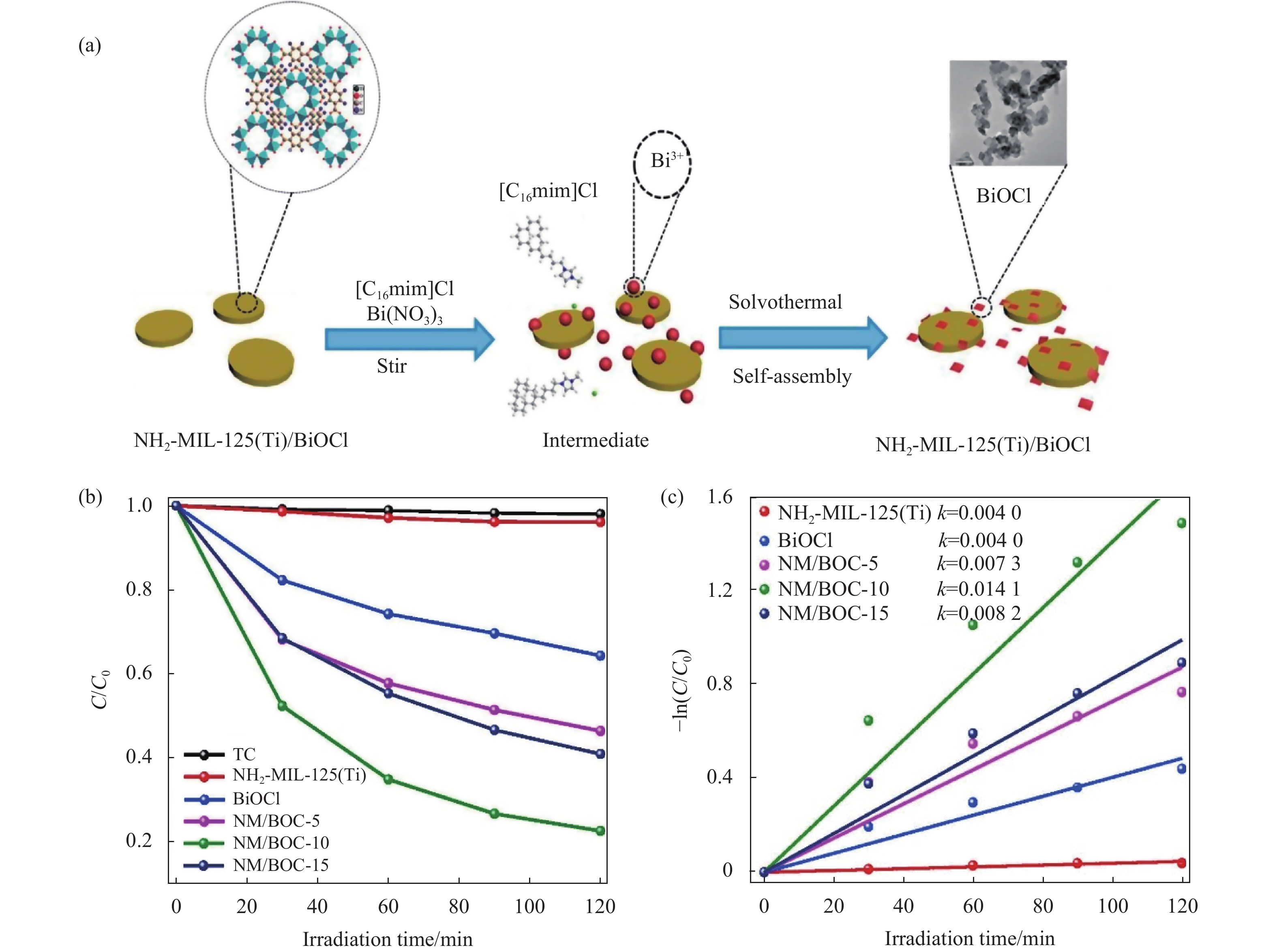
|
图 3 (a) NH2-MIL-125(Ti)/BiOCl复合材料的合成示意图, (b)可见光下光降解TC动力学曲线, (c) 光降解TC的一级拟动力学曲线 [31] Figure 3 (a) Schematic representation of the synthesis of NH2-MIL-125(Ti)/BiOCl composites, (b) Dynamic curves of TC photodegradation under visible light irradiation, (c) Plots of -In(C/C0) versus irradiation time and reaction rate constant k obtained from linear fitting[31] |
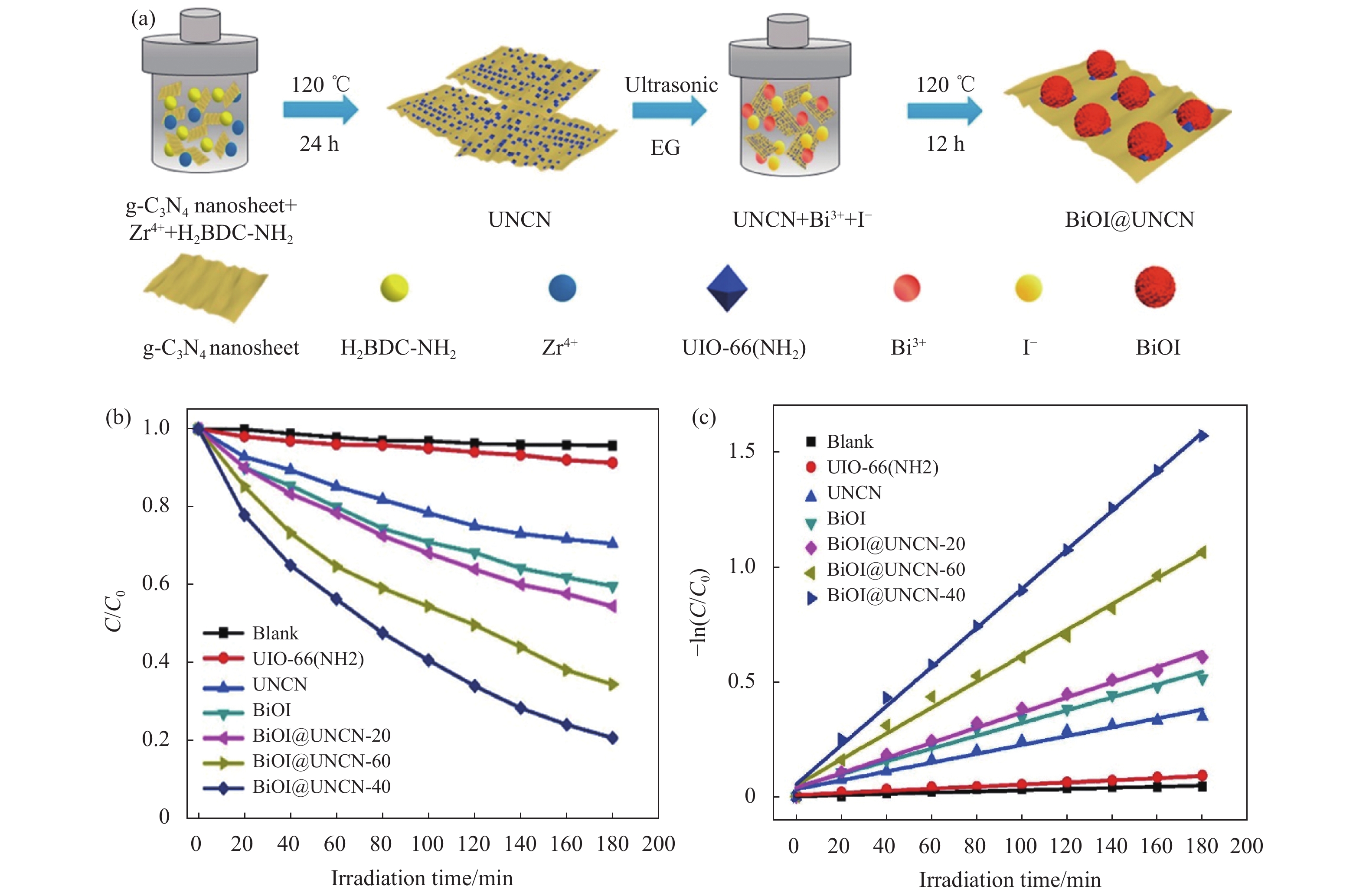
|
图 4 (a) BiOI@UNCN复合材料的合成示意图, (b)可见光下光降解TC动力学曲线, (c) 光降解TC的一级动力学拟合曲线[32] Figure 4 (a) Schematic representation for BiOI@UNCN composites fabrication, (b) TC over the as-prepared samples under visible-light irradiation, (c) The first-order kinetics of TC photocatalytic degradation[32] |
六价铬(Cr(VI))是一种常见的地表水和地下水污染物,来源于制革、电镀、印刷、颜料等制造业的工业废水中,其具有超高毒性、致癌性和高溶解度等特点,对人类和其他生物构成显著的健康威胁[47-48]。Zhao等[34]首次利用水热法合成Bi24O31Br10,并采用球磨法将Bi24O31Br10与BUC-21偶联制备了BUC-21/Bi24O31Br10复合材料,其合成示意图如图5(a) 所示。图5(b)光还原Cr(VI)的实验表明,当Bi24O31Br10与BUC-21的质量比为1:1时(BB-100)催化效果最佳,可在2 h内实现Cr(VI)的彻底去除。此外,该团队还利用球磨法制备球状的MIL-53(Fe)/Bi12O17Cl2复合材料(BMx),可在光照90 min后去除99.2%的Cr(VI),其光催化还原Cr(VI)性能相较于纯MIL-53(Fe)和纯Bi12O17Cl2分别提高了30%和76%[23]。
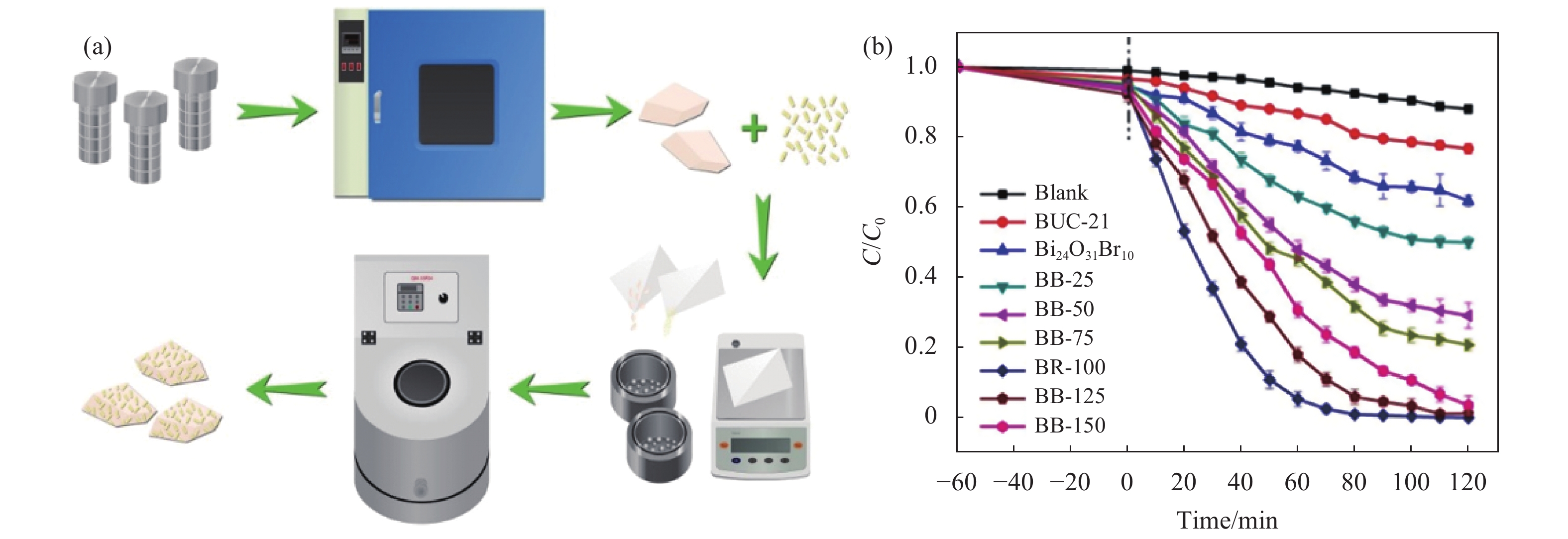
|
图 5 (a) BUC-21/Bi24O31Br10复合材料的合成示意图, (b) Cr (VI)在 pH = 2时的光催化还原反应[34] Figure 5 (a) The procedure for the preparation of BUC-21/Bi24O31Br10 composites, (b) Cr(VI) photoreduction at pH = 2 via numerous photocatalysts[34] |
随着工业的快速发展,能源短缺受到广泛关注[49],而光催化分解水制氢技术可将太阳能转化为可再生的清洁能源,从而有效突破能源短缺的瓶颈问题[50-51]。然而光解水效率主要受到缓慢的析氧动力学的限制,因此开发具有高活性的析氧催化剂以提高光解水效率是至关重要的。Han等[36]采用水热法在十面体BiVO4表面原位生长MIL-100(Fe)纳米粒子,制备了新型复合材料MIL-100(Fe)@BiVO4,其形貌及制备过程如图6(a-e)所示。光催化分解水实验表明,当MIL-100(Fe)的负载量为8%时,MIL-100(Fe)@BiVO4复合材料表现出最优的光催化析氧性能,是纯BiVO4催化活性的4.3倍。其光催化性能的提高主要是由于MIL-100(Fe)与BiVO4之间形成了紧密的异质结界面,促使了双功能组分间载流子的有效转移和传导。

|
图 6 (a) BiVO4扫描电镜图, (b) MIL-100(Fe)@BiVO4扫描电镜图, (c) 选定区域1的扫描电镜图, (d) 选定区域2的EDX, (e) MIL-100(Fe)@BiVO4 的制备过程及光催化机理[36], (f) ZIF-8透射电镜图, (g) and (i) Bi4O5Br2/ZIF-8 (30%)透射电镜图, (h) Bi4O5Br2纳米片透射电镜图, (j) 光催化固氮活性, (k) Bi4O5Br2/ZIF-8 (30%)光催化机理[37] Figure 6 (a) SEM images of BiVO4, (b) MIL-100(Fe)@ BiVO4, (c) SEM image of selected area 1, (d) EDX analysis of selected area 1, (e) Schematic Preparation Process of MIL-100(Fe)@ BiVO4 and the Proposed Photocatalytic Mechanism, (f) TEM micrographs of ZIF-8, (g) and (i) Bi4O5Br2/ZIF-8 (30%), (h) Bi4O5Br2 nanoparticles, (j) Photocatalytic N2 fixation activities of as-prepared materials, (k) Photocatalytic mechanism of Bi4O5Br2/ZIF-8 (30%)[37] |
为了解决全球能源和环境问题,一个能利用太阳能的人工光合作用系统是必不可少的,其中氨作为化肥原料及潜在的氢载体和燃料是现代工业农业中不可或缺的能源物质[52-53]。传统合成氨采用哈伯-博世工艺,但其不仅需要400~600 °C高温和20~40 MPa高压环境,而且在合成氨过程中还会产生大量二氧化碳气体排放[54-55]。因此,基于光催化技术,在常温常压下利用N2和H2O合成NH3是一种绿色、可持续的合成方法。如图6(f-j)所示,Liu等[37]将亲水性Bi4O5Br2沉积在疏水性ZIF-8表面,形成一种具有气液固三相反应界面的Bi4O5Br2/ZIF-8复合光催化剂,其光催化合成NH3的速率为327.338 μmol·L−1·h−1·g−1,是Bi4O5Br2合成效率的3.6倍。这一优异活性的来源是,独特的三相界面结构使N2从气相直接输送到光催化反应界面,取代了N2通过液相进行扩散的过程,这种快速供给的N2能有效地捕获光生电子,从而提高光生电子的利用效率和固氮活性,其机理图如图6(k)所示。
3 结论与展望本文综述了MBCs的两种主要制备方法以及其在光催化环境修复和能源转换领域中的应用,主要包括有机染料的降解、PPCPs的降解和Cr(VI)还原、光分解水制氢和合成氨。总体而言,MBCs复合结构的构建增加了催化反应的活性位点,扩展了光谱范围并抑制了光生载流子复合,从而提高了光催化性能。然而,MOFs/铋基复合材料的研究工作仍需要进一步的深入,尤其是在MBCs的制备改性方法、光催化效能的提高、氧化降解有机物机制以及光催化全解水机制方面有待进一步研究。
(1) MBCs的制备方法到目前为止仍不够完善,不论是水热/溶剂热法还是球磨法都有不足之处,因此,需要探索新方法来改进MBCs的制备技术。
(2) 目前,MBCs制备过程中通常采用有机溶剂和高温处理,因此有必要评估各种MBCs制备方法对环境的影响。
| [1] |
WU T, LIU X, LIU Y, et al. Application of QD-MOF composites for photocatalysis: energy production and environmental remediation[J].
Coordination Chemistry Reviews, 2020, 403: 213097.
DOI: 10.1016/j.ccr.2019.213097. |
| [2] |
OLA O, MAROTO-VALER M M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction
[J].
Journal of Photochemistry and Photobiology C:Photochemistry Reviews, 2015, 24: 16-42.
DOI: 10.1016/j.jphotochemrev.2015.06.001. |
| [3] |
TRELLU C, MOUSSET E, PECHAUD Y, et al. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: a critical review[J].
Journal of Hazardous Materials, 2016, 306: 149-174.
DOI: 10.1016/j.jhazmat.2015.12.008. |
| [4] |
REDDY P A K, REDDY P V L, KWON E, et al. Recent advances in photocatalytic treatment of pollutants in aqueous media[J].
Environment International, 2016, 91: 94-103.
DOI: 10.1016/j.envint.2016.02.012. |
| [5] |
MAEDA K, TERAMURA K, LU D L, et al. Photocatalyst releasing hydrogen from water- enhancing catalytic performance holds promise for hydrogen production by water splitting in sunlight[J].
Nature, 2006, 440(7082): 295-295.
DOI: 10.1038/440295a. |
| [6] |
LIANG Q, LIU X, ZENG G, et al. Surfactant-assisted synthesis of photocatalysts: mechanism, synthesis, recent advances and environmental application[J].
Chemical Engineering Journal, 2019, 372: 429-451.
DOI: 10.1016/j.cej.2019.04.168. |
| [7] |
LI H, EDDAOUDI M, O'KEEFFE M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J].
Nature, 1999, 402(6759): 276-279.
DOI: 10.1038/46248. |
| [8] |
YAGHI O M, O'KEEFFE M, OCKWIG N W, et al. Reticular synthesis and the design of new materials[J].
Nature, 2003, 423(6941): 705-714.
DOI: 10.1038/nature01650. |
| [9] |
FURUKAWA H, CORDOVA K E, O'KEEFFE M, et al. The chemistry and applications of metal-organic frameworks[J].
Science, 2013, 341(6149): 1230444.
|
| [10] |
黄刚, 陈玉贞, 江海龙. 金属有机骨架材料在催化中的应用[J].
化学学报, 2016, 74(02): 113-129.
HUANG G, CHEN Y Z, JIANG H L. Metal-organic frameworks for catalysis[J]. Acta Chimica Sinica, 2016, 74(02): 113-129. DOI: 10.6023/A15080547. |
| [11] |
WENG H, YAN B. Flexible Tb(III) functionalized cadmium metal organic framework as fluorescent probe for highly selectively sensing ions and organic small molecules[J].
Sensors and Actuators B-Chemical, 2016, 228: 702-708.
DOI: 10.1016/j.snb.2016.01.101. |
| [12] |
ZHU J, XIA T, CUI Y, et al. A turn-on MOF-based luminescent sensor for highly selective detection of glutathione[J].
Journal of Solid State Chemistry, 2019, 270: 317-323.
DOI: 10.1016/j.jssc.2018.11.032. |
| [13] |
AL-NADDAF Q, ROWNAGHI A A, REZAEI F. Multicomponent adsorptive separation of CO2, CO, CH4, N2, and H2 over core-shell zeolite-5A@MOF-74 composite adsorbents
[J].
Chemical Engineering Journal, 2020, 384: 123251.
|
| [14] |
SUN H, YU X, MA X, et al. MnOx-CeO2 catalyst derived from metal-organic frameworks for toluene oxidation
[J].
Catalysis Today, 2020, 355: 580-586.
DOI: 10.1016/j.cattod.2019.05.062. |
| [15] |
LI X, ZHU Q L. MOF-based materials for photo- and electrocatalytic CO2 reduction
[J].
EnergyChem, 2020, 2(3): 100033.
DOI: 10.1016/j.enchem.2020.100033. |
| [16] |
ZHAO C, PAN X, WANG Z, et al. 1 + 1 > 2: a critical review of MOF/bismuth-based semiconductor composites for boosted photocatalysis[J].
Chemical Engineering Journal, 2021, 417: 128022.
|
| [17] |
HE R, XU D, CHENG B, et al. Review on nanoscale Bi-based photocatalysts[J].
Nanoscale Horizons, 2018, 3(5): 464-504.
DOI: 10.1039/C8NH00062J. |
| [18] |
陈丹丹, 衣晓虹, 王崇臣. 机械化学法制备金属-有机骨架及其复合物研究进展[J].
无机化学学报, 2020, 36(10): 1805-1821.
CHEN D D, YI X H, WANG C C. Preparation of metal-organic frameworks and their composites using mechanochemical methods[J]. Chinese Journal of Inorganic Chemistry, 2020, 36(10): 1805-1821. DOI: 10.11862/CJIC.2020.212. |
| [19] |
BIBI R, SHEN Q, WEI L, et al. Hybrid BiOBr/UiO-66-NH2 composite with enhanced visible-light driven photocatalytic activity toward RhB dye degradation
[J].
RSC Advances, 2018, 8(4): 2048-2058.
DOI: 10.1039/C7RA11500H. |
| [20] |
HU Q, CHEN Y, LI M, et al. Construction of NH2-UiO-66/BiOBr composites with boosted photocatalytic activity for the removal of contaminants
[J].
Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 579: 123625.
DOI: 10.1016/j.colsurfa.2019.123625. |
| [21] |
KHASEVANI S G, GHOLAMI M R. Engineering a highly dispersed core@shell structure for efficient photocatalysis: a case study of ternary novel BiOI@MIL-88A(Fe)@g-C3N4 nanocomposite
[J].
Materials Research Bulletin, 2018, 106: 93-102.
DOI: 10.1016/j.materresbull.2018.05.024. |
| [22] |
ASKARI N, BEHESHTI M, MOWLA D, et al. Fabrication of CuWO4/Bi2S3/ZIF67 MOF: a novel double Z-scheme ternary heterostructure for boosting visible-light photodegradation of antibiotics
[J].
Chemosphere, 2020, 251: 126453.
DOI: 10.1016/j.chemosphere.2020.126453. |
| [23] |
LI H, ZHAO C, LI X, et al. Boosted photocatalytic Cr(VI) reduction over Z-scheme MIL-53(Fe)/Bi12O17Cl2 composites under white light
[J].
Journal of Alloys and Compounds, 2020, 844: 156147.
DOI: 10.1016/j.jallcom.2020.156147. |
| [24] |
ZHAO C, WANG J, CHEN X, et al. Bifunctional Bi12O17Cl2/MIL-100(Fe) composites toward photocatalytic Cr(VI) sequestration and activation of persulfate for bisphenol A degradation
[J].
Science of the Total Environment, 2021, 752: 141901.
DOI: 10.1016/j.scitotenv.2020.141901. |
| [25] |
YANG Z, DING J, FENG J, et al. Preparation of BiVO4/MIL‐125 (Ti) composite with enhanced visible‐light photocatalytic activity for dye degradation
[J].
Applied Organometallic Chemistry, 2018, 32(4): e4285.
DOI: 10.1002/aoc.4285. |
| [26] |
何云鹏, 金雪阳, 李文卓, 等. Bi2WO6/UiO-66复合材料的制备及其光催化性能
[J].
无机化学学报, 2019, 35(6): 996-1004.
HE Y P, JIN X Y, LI W Z, et al. Synthesis and photocatalytic properties of Bi2WO6/UiO-66 composite [J]. Chinese Journal of Inorganic Chemistry, 2019, 35(6): 996-1004. |
| [27] |
李梦佳, 妥小军, 李小妹, 等. BiVO4/MIL-100(Fe)复合材料光催化降解结晶紫
[J].
精细化工, 2020, 37(1): 33-38.
LI J M, TUO X J, LI X M, et al. Photocatalytic degradation of crystal violet using BiVO4/MIL-100(Fe) composites [J]. Fine Chemicals, 2020, 37(1): 33-38. |
| [28] |
KHASEVANI S G, GHOLAMI M R. Evaluation of the reaction mechanism for photocatalytic degradation of organic pollutants with MIL-88A/BiOI structure under isible light irradiation[J].
Research on Chemical Intermediates, 2019, 45(3): 1341-1356.
DOI: 10.1007/s11164-018-3681-9. |
| [29] |
ASKARI N, BEHESHTI M, MOWLA D, et al. Fabrication of CuWO4/Bi2S3/ZIF-67 MOF: a novel double Z-scheme ternary heterostructure for boosting visible-light photodegradation of antibiotics
[J].
Chemosphere, 2020, 251: 126453.
DOI: 10.1016/j.chemosphere.2020.126453. |
| [30] |
TANG L, LV Z Q, XUE Y C, et al. MIL-53 (Fe) incorporated in the lamellar BiOBr: Promoting the visible-light catalytic capability on the degradation of rhodamine B and carbamazepine[J].
Chemical Engineering Journal, 2019, 374: 975-982.
DOI: 10.1016/j.cej.2019.06.019. |
| [31] |
HU Q, DI J, WANG B, et al. In-situ preparation of NH2-MIL-125(Ti)/BiOCl composite with accelerating charge carriers for boosting visible light photocatalytic activity
[J].
Applied Surface Science, 2019, 466: 525-534.
DOI: 10.1016/j.apsusc.2018.10.020. |
| [32] |
LIANG Q, CUI S, JIN J, et al. Fabrication of BiOI@UIO-66(NH2)@g-C3N4 ternary Z-scheme heterojunction with enhanced visible-light photocatalytic activity
[J].
Applied Surface Science, 2018, 456: 899-907.
DOI: 10.1016/j.apsusc.2018.06.173. |
| [33] |
綦毓文, 魏砾宏, 石冬妮, 等. UiO-66/BiVO4复合光催化剂的制备及其对四环素的光解
[J].
中国环境科学, 2021, 41(3): 1162-1171.
QI Y W, WEI L H, SHI D N, et al. Preparation of UiO-66/BiVO4 composite photocatalyst and its photodegradation of tetracycline [J]. China Environmental Science, 2021, 41(3): 1162-1171. DOI: 10.3969/j.issn.1000-6923.2021.03.019. |
| [34] |
ZHAO C, WANG Z, LI X, et al. Facile fabrication of BUC-21/Bi24O31Br10 composites for enhanced photocatalytic Cr(VI) reduction under white light
[J].
Chemical Engineering Journal, 2020, 389: 123431.
DOI: 10.1016/j.cej.2019.123431. |
| [35] |
ZHANG S, DU M, KUANG J, et al. Surface-defect-rich mesoporous NH2-MIL-125 (Ti)@Bi2MoO6 core-shell heterojunction with improved charge separation and enhanced visible-light-driven photocatalytic performance
[J].
Journal of Colloid and Interface Science, 2019, 554: 324-334.
DOI: 10.1016/j.jcis.2019.07.021. |
| [36] |
HAN Q, DONG Y, XU C, et al. Immobilization of Metal-Organic Framework MIL-100(Fe) on the Surface of BiVO4: a new platform for enhanced visible-light-driven water oxidation
[J].
ACS Applied Materials & Interfaces, 2020, 12(9): 10410-10419.
|
| [37] |
LIU J X, LI R, ZU X, et al. Photocatalytic conversion of nitrogen to ammonia with water on triphase interfaces of hydrophilic-hydrophobic composite Bi4O5Br2/ZIF-8
[J].
Chemical Engineering Journal, 2019, 371: 796-803.
DOI: 10.1016/j.cej.2019.03.283. |
| [38] |
LOPEZ Y C, VILTRES H, GUPTA N K, et al. Transition metal-based metal-organic frameworks for environmental applications: a review[J].
Environmental Chemistry Letters, 2021: 1-40.
|
| [39] |
TARKWA J B, OTURAN N, ACAYANKA E, et al. Photo-Fenton oxidation of Orange G azo dye: process optimization and mineralization mechanism[J].
Environmental Chemistry Letters, 2019, 17(1): 473-479.
DOI: 10.1007/s10311-018-0773-0. |
| [40] |
董振, 刘亮, 郝艳, 等. 偶氮染料废水处理技术的研究进展[J].
水处理技术, 2017, 43(4): 6-10.
DONG Z, LIU L, HAO Y, et al. Research progress on the treatment of azo dye containing wastewater[J]. Technology of Water Treatment, 2017, 43(4): 6-10. |
| [41] |
YANG H M, LIU X, SONG X L, et al. In situ electrochemical synthesis of MOF-5 and its application in improving photocatalytic activity of BiOBr[J].
Transactions of Nonferrous Metals Society of China, 2015, 25(12): 3987-3994.
DOI: 10.1016/S1003-6326(15)64047-X. |
| [42] |
MUGUNTHAN E, SAIDUTTA M B, JAGADEESHBABU P E. Visible light assisted photocatalytic degradation of diclofenac using TiO2-WO3 mixed oxide catalysts
[J].
Environmental Nanotechnology, Monitoring & Management, 2018, 10: 322-330.
|
| [43] |
LI G, NIE X, CHEN J, et al. Enhanced simultaneous PEC eradication of bacteria and antibiotics by facilely fabricated high-activity facets TiO2 mounted onto TiO2 nanotubular photoanode
[J].
Water Research, 2016, 101: 597-605.
DOI: 10.1016/j.watres.2016.06.001. |
| [44] |
KARKMAN A, PARNANEN K, LARSSON D G J. Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments[J].
Nature Communications, 2019, 10(1): 80.
DOI: 10.1038/s41467-018-07992-3. |
| [45] |
BANASCHIK R, LUKES P, JABLONOWSKI H, et al. Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues[J].
Water Research, 2015, 84: 127-35.
DOI: 10.1016/j.watres.2015.07.018. |
| [46] |
王雪平, 朱惠斌. 制药工业废水中14种沙星类抗生素的液相色谱分析法[J].
工业水处理, 2019, 39(7): 89-93.
WANG X P, ZHU H B. Liquid chromatographic analysis of 14 kinds of afloxacin antibiotics in pharmaceutical industrial wastewater[J]. Industrial Water Treatment, 2019, 39(7): 89-93. DOI: 10.11894/iwt.2018-0506. |
| [47] |
WANG C C, DU X D, LI J, et al. Photocatalytic Cr(VI) reduction in metal-organic frameworks: a mini-review[J].
Applied Catalysis B:Environmental, 2016, 193: 198-216.
DOI: 10.1016/j.apcatb.2016.04.030. |
| [48] |
王雪瑾, 朱霞萍, 蓝路梅. 镁铝层状超分子化合物去除废水中的六价铬[J].
应用化学, 2017, 34(1): 98-104.
WANG X J, ZHU X P, LAN L M. Efficient removal of Cr(Vl) in wastewater by Mg/Al layered superamolecular compounds[J]. Chinese Journal of Applied Chemistry, 2017, 34(1): 98-104. DOI: 10.11944/j.issn.1000-0518.2017.01.160115. |
| [49] |
HAO X, JIN Z, YANG H, et al. Peculiar synergetic effect of MoS2 quantum dots and graphene on metal-organic frameworks for photocatalytic hydrogen evolution
[J].
Applied Catalysis B:Environmental, 2017, 210: 45-56.
DOI: 10.1016/j.apcatb.2017.03.057. |
| [50] |
BLAKEMORE J D, CRABTREE R H, BRUDVIG G W. Molecular catalysts for water oxidation[J].
Chemical Reviews, 2015, 115(23): 12974-3005.
DOI: 10.1021/acs.chemrev.5b00122. |
| [51] |
李跃军, 曹铁平, 赵艳辉, 等. Bi@Bi2Sn2O7/TiO2等离子体复合纤维的制备及增强的光催化产氢活性
[J].
无机化学学报, 2019, 35(8): 1371-1378.
JI Y J, CAO T P, ZHAO Y H, et al. Preparation of Bi@Bi2Sn2O7/TiO2 plasmonic composite fibers with enhanced photocatalytic hydrogen generation activity [J]. Chinese Journal of Inorganic Chemistry, 2019, 35(8): 1371-1378. DOI: 10.11862/CJIC.2019.187. |
| [52] |
OSHIKIRI T, UENO K, MISAWA H. Plasmon-induced ammonia synthesis through nitrogen photofixation with visible light irradiation[J].
Angewandte Chemie, 2014, 126(37): 9960-9963.
DOI: 10.1002/ange.201404748. |
| [53] |
WANG L, XIA M, WANG H, et al. Greening ammonia toward the solar ammonia refinery[J].
Joule, 2018, 2(6): 1055-1074.
DOI: 10.1016/j.joule.2018.04.017. |
| [54] |
HIRAKAWA H, HASHIMOTO M, SHIRAISHI Y, et al. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide[J].
Journal of the American Chemical Society, 2017, 139(31): 10929-10936.
DOI: 10.1021/jacs.7b06634. |
| [55] |
VAN DER HAM C J M, KOPER M T M, HETTERSCHEID D G H. Challenges in reduction of dinitrogen by proton and electron transfer[J].
Chemical Society Reviews, 2014, 43(15): 5183-5191.
DOI: 10.1039/C4CS00085D. |
 2022, Vol. 39
2022, Vol. 39

