2 中国科学院大学, 北京 100049;
3 中国科学院地球科学研究院, 北京 100029)
对出土的骨骼和牙齿等生物矿化材料进行同位素分析,是研究古食谱、古人类迁移行为及古环境重建的重要手段[1]。特别是牙齿最外层高度矿化的牙釉质,成分在青少年时代形成后不再改变,不易受外界污染,是同位素分析的理想材料[2~4]。
目前,牙釉质的同位素分析多是利用牙钻钻取10~20 mg牙釉质粉末进行取样,结果反映的是牙釉质生长过程中沉积的同位素平均信号[5~10]。但在发育过程中,牙釉质呈节律性生长,组织结构上表现为周期性生长线,造成了牙釉质不同部位同位素信号存在差异[11~14]。这无疑要求同位素研究必须具有更高的空间分辨率。近年来,随着高分辨率仪器发展,如激光剥蚀多接收电感耦合等离子体质谱(LA-MC-ICP- MS)和二次离子质谱(SIMS),使得牙釉质的高分辨率同位素分析成为可能[15~19]。认识牙釉质生长过程与生长结构,结合研究目的确定采样策略,这对同位素分析和数据解释尤为重要。
1 牙釉质生长过程牙齿从开始发育到形成一个完整的特定形态,需要经历起始期、蕾状期、帽状期,钟状期和分泌期[20~22]。在口腔形成初期,口腔上皮细胞迅速增生开始形成牙板,其深部覆盖着较厚的间充质细胞层(图 1a)。几周后牙齿发育进入蕾状期,牙板向深部间充质细胞层延伸,在其末端细胞增生并逐渐开始发育成釉器(图 1b)。与此同时,在成釉器的外周,间充质层细胞不断增生,形成牙乳头和牙囊细胞,它们是牙本质和牙髓腔的雏形。帽状期初期,成釉器形成,牙乳头和牙囊体积逐渐增大。帽状期后期,成釉器的外层细胞分化成内釉上皮细胞,为形成釉质基质作准备(图 1c)。
进入钟状期,成釉器进入成熟期,内折变深,牙冠的雏形初步形成。后期时,牙釉质和牙本质组织才真正开始形成(图 1d)。首先,牙乳头细胞分化为成牙本质细胞并分泌前期牙本质基质。在EDJ(Enamel-Dentin Junction,牙釉质与牙本质交界)初始牙本质形成后,又会诱导内釉上皮细胞逐渐分化为成釉细胞并分泌牙釉质基质,牙釉质开始形成(图 1e)。
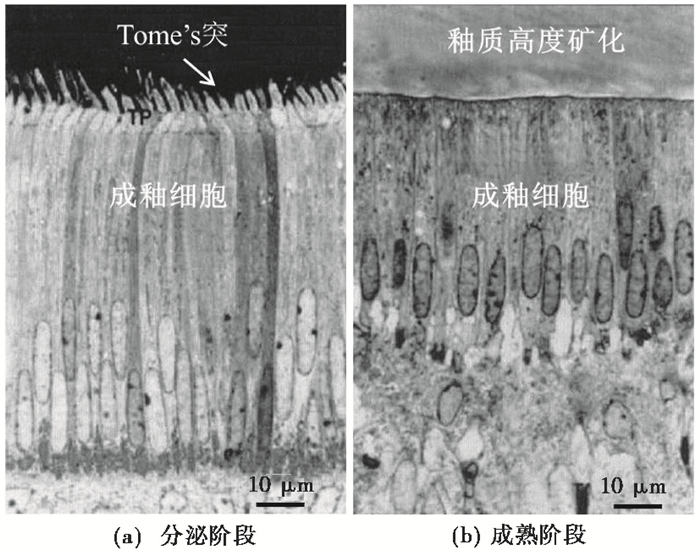
牙釉质的形成一般分为两个阶段:分泌阶段和成熟阶段(图 2)。分泌阶段形成的牙釉质矿化度非常低,只有1/ 3是矿物质;成熟阶段则是在分泌阶段基础上进行去有机质和去水过程,牙釉质二次结晶,釉质高度矿化,矿物质成分高达96 % [20~21]。
|
图 2 釉质形成过程示意图 (a)釉质分泌阶段:成釉细胞分泌的蛋白质基质填充在托姆斯突上,大量微晶在基质上沉积,逐渐形成釉柱;(b)釉质成熟阶段:釉质基质发生去有机质和去水过程,釉质高度矿化)(据文献[23]改编) Fig. 2 Two main stages of enamel development. (a)During enamel secretion, the ameloblasts secrete protein matrix into the Tomes' process and a large number of enamel crystallites are seeded in the matrix, gradually forming cross-striations; (b)Enamel maturation involves the degradation and removal of enamel matrix proteins and water, and secondary mineralization (adapted from reference[23]) |
内釉上皮细胞分化的成釉细胞在釉质生长过程中起着至关重要的作用[23]。釉质的形成离不开成釉细胞的分泌活动,同时也随着成釉细胞的死亡而停止生长[21],因而可以用成釉细胞在釉质不同生长阶段的活动来理解釉质的生长过程。
在牙釉质分泌阶段,成釉细胞的主要活动是分泌蛋白质基质,并在基质上沉积羟基磷酸盐微晶(直径约30 nm),逐步形成富含有机质、低矿化度的釉质[21~23](图 2a)。这一过程首先发生在EDJ层,之后成釉细胞离开EDJ层进行下一次分泌活动,直到OES层(Outer Enamel Surface,牙釉质最外层)时停止分泌活动[22]。成熟阶段是牙釉质高度矿化过程,这一时期成釉细胞主要作用是分解有机质、去水,并发生多次变形来调节和运输离子进出釉质流体的速度,以补充矿化需要的离子[23~24](图 2b)。例如,钙离子在成熟阶段通过成釉细胞进入釉质流体的速度明显增加,比第一阶段快4倍左右[25]。因此,牙釉质成熟阶段是记录同位素信号的主要时期。
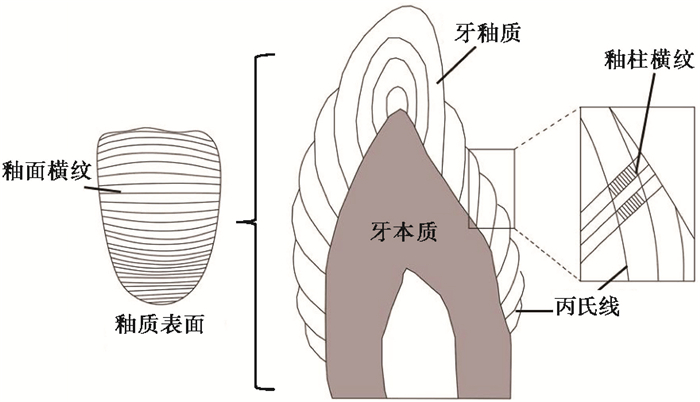
2 牙釉质生长结构牙釉质是从釉质本质交界向釉质表层、齿尖向齿颈方向生长,呈叠瓦状一层层渐增,在釉质内部则表现出不同周期的生长层结构,包括釉柱横纹、丙氏线、釉面横纹和纹层线4种[11, 26]。
釉柱横纹与釉柱长轴方向垂直,在釉柱上呈规律性重复排列。在偏振光显微镜下呈现明暗相间条带,每一个明暗带组合代表一天。釉柱横纹可能反映釉柱中有机物和无机物含量、密度的变化,其形成与成釉细胞周期性活动有关,因为成釉细胞代谢速度会影响釉质基质分泌过程,进而影响釉柱矿物质的组成和密度[21, 27]。
第二种结构具有更长的生长周期,称为丙氏线(图 3)。在光镜下观察釉质横切面,丙氏线呈同心环状排列,犹如树轮结构。而在纵切面上,丙氏线的延展方向与成釉细胞的运动方向一致,即自EDJ向OES方向生长。丙氏线实际上是成釉细胞沉积前端的等时线,反映成釉细胞更长的代谢周期[21]。丙氏线在齿尖处呈环形排列,之后向齿颈方向生长逐渐呈平行排列。不同物种丙氏线生长周期存在差异,如灵长类丙氏线周期为2~12天[28],犀牛牙釉质丙氏线周期为7天[11, 16]。对同一个体而言,不同类型牙齿的丙氏线生长周期是一致的。丙氏线的生长周期可以用相邻丙氏线之间的釉柱横纹数计算得到[27]。
|
图 3 釉柱横纹、丙氏线与釉面横纹结构(据文献[1, 27]改编) Fig. 3 Structures of cross-striations, retzius lines and perikymata(adapted from references[1, 27]) |
当丙氏线不断向外生长至最外层时,在釉质表面出现平行排列的线纹,即为釉面横纹。它的生长周期与丙氏线相同,生长方向也是从齿尖向齿颈方向生长(图 3)。相邻釉面横纹间距存在差异,这与釉质沉积速率有关[20~21]。研究表明,现代人在齿尖处的釉面横纹相邻间距30~35 μm,齿颈处间距15~20 μm,说明齿尖釉质沉积速率比齿颈快[27]。
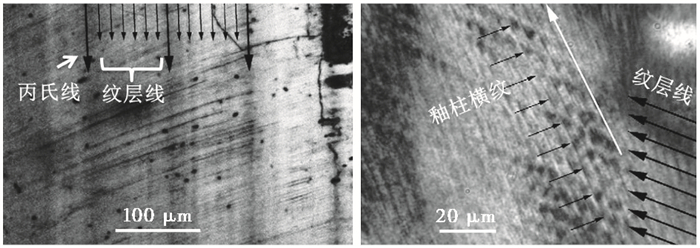
还有一种与丙氏线平行的生长线,也是釉质沉积前端的等时线,但其生长周期更短,称为纹层线(lamination)。有学者通过荧光标记实验证实了哺乳动物纹层线的生长周期为一天[26]。因此,另一种计算丙氏线生长周期的方法是通过相邻丙氏线之间的纹层线数量来确定[11, 16]。纹层线与釉柱横纹周期都为一天,但两者可以通过排列方向来区别(图 4)。
|
图 4 纹层线与丙氏线、釉柱横纹的关系[11] Fig. 4 Transmitted light microscopy of cross-striations, Retzius lines and laminations[11] |
可以看出,单齿在釉质内部表现出不同周期的生长层结构,而在釉质表层表现为齿尖向齿颈逐渐递增的生长序列[29]。在齿列上,不同类型牙齿的萌发顺序存在差异,在形成时间上则存在重叠期[30]。例如,猪的第一下颌切齿在出生2~3个月后萌发,大约在17个月后形成;第二下颌切齿萌发于出生后6~10月,约24个月后形成;第三臼齿萌发于出生后2个月,18个月后才完全形成[31]。因此,对同一个体齿列上的不同牙齿取样,可以建立更长的同位素时间框架,同时也能弥补牙齿形态决定的季节性信息不全的缺陷。单齿或齿列的生长序列在分析动物与人的季节性行为中有重要应用[32]。
3 采样方法 3.1 微钻系列采样动物在不同时期摄入的同位素通过新陈代谢进入动物的牙釉质中,并按照生长顺序,保存在不同位置;同时,牙齿的生长结构提供了相对的时间序列[29]。通过动物牙釉质微钻系列采样分析,可以精细重现牙齿发育过程中同位素组成的变化。
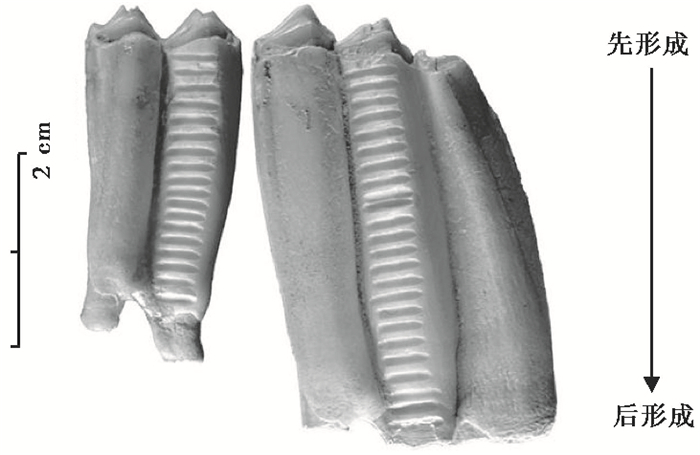
具体而言,用金刚石或者碳化钨钻头的牙钻对牙釉质表层从齿尖到齿颈进行系列采样,采样间距通常为1~5 mm(图 5)。微钻取样后用热电离质谱仪(TIMS)测试锶同位素组成,用同位素比值质谱仪(IRMS)测试碳、氧同位素组成。根据动物样本牙齿的形成时间建立整个采样剖面的时间框架,从而获取同位素季节变化信息。考虑到牙齿大小和形成时间,一般选择第二臼齿(M2)或者第三臼齿(M3)作为研究对象。目前也有研究根据不同牙齿类型形成时间存在重叠期,来建立同位素的年际变化曲线[31, 33]。
|
图 5 红铜时代绵羊牙釉质的系列采样图(据文献[34]改编) Fig. 5 Intra-tooth sequential sampling in early Eneolithic sheep (adapted from reference[34]) |
系列采样法是根据牙齿的形成时间来建立同位素变化的时间框架,但牙釉质生长序列的变化难以对应绝对的时间标尺,造成时间精度不够高;同时,该方法样品损耗大,多用于高齿冠牙齿的同位素分析。近年来,随着激光剥蚀多接收电感耦合等离子体质谱仪(LA-MC-ICP- MS、laser-ablation PIMMS)、二次离子质谱(SIMS)、离子探针技术(SHRIMP Ⅱ)等仪器的发展,高分辨率同位素分析逐渐成为可能[15~18]。与微钻系列采样法相比,这些方法的空间分辨率更高,能揭示牙齿内部同位素组成更细微和复杂的变化[35];同时,微区采样方法相对于TIMS和IRMS分析所需样品量少,适合珍贵样品分析[36]。虽然微区采样方法目前尚处于起步阶段,但在考古研究中有重要意义和研究前景[37~39]。
可以利用牙釉质生长层的结构来精确建立微区采样点时间框架。例如,丙氏线和釉质横纹(或纹层线)数量能确定釉质本质交界到釉质表层形成时间,精度达到天。釉面横纹在精确建立时间框架时无需对样品进行抛光,将样品损耗降到最低,但由于釉面横纹在牙釉质表层,形成时间上属于釉质形成的后期。实际研究中,若难以观测釉质内部的生长层结构,可根据牙釉质生长速率以及横截面宽度,估算釉质的形成时间[16~17]。
LA-MC-ICP- MS、SIMS、SHRIMP Ⅱ等仪器的空间分辨率能到达微米级[15~18],为获得原位信息提供了可能。研究中通常根据样品特性来选择采样方式。若抛光面为样品的横截面(图 6a),选取EDJ层向OES层方向微区采样;若抛光面为纵切面(图 6b),一般从EDJ层向OES层或者从齿尖向齿颈方向微区采样(图 6)。若根据釉面横纹建立时间框架,则顺着釉面横纹生长方向沿釉质表层微区采样[40]。
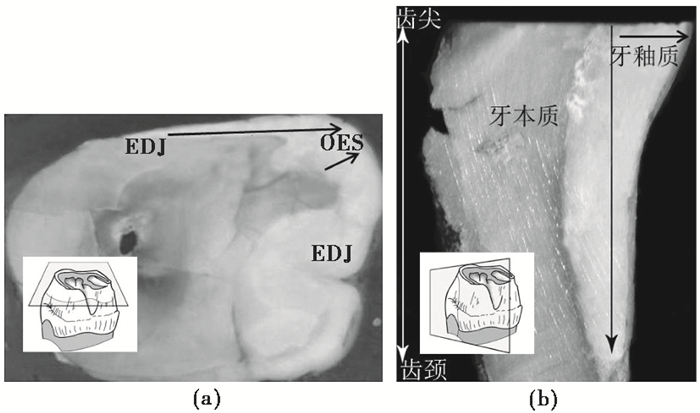
|
图 6 牙釉质横截面(a)和纵切面(b)采样方式 据文献[16~17]改编 Fig. 6 Microsampling along the longitudinal and the transversal section of enamel(adapted from references[16~17]) |
如前所述,通过动物牙釉质高空间分辨率采样,可以精细重现牙齿发育过程中同位素组成的变化。而牙釉质的碳、氧、锶等同位素可以记录饮食结构、气候变化、空间迁移等信息[41~45]。将两者结合,基于牙釉质结构的同位素高分辨率分析为研究季节性行为或气候季节变化提供了全新手段,在古环境和考古中的应用日趋广泛[46~49]。
4.1 古食谱重建在考古中,牙釉质的碳同位素主要反映个体幼年时期的饮食结构。因植物光合作用途径差异,C3植物的δ13C比值为- 32 ‰ ~- 24 ‰,C4植物的δ13C比值为- 10 ‰ ~- 14 ‰ [50]。而不同组织对碳同位素的吸收过程会发生分馏作用,人类牙釉质的碳同位素相对于食物会富集14.1 ‰ [51],食草动物牙釉质的碳同位素相对于食物富集13 ‰ [52]。在实际应用中,牙釉质的碳同位素变化可以反映不同地区人类和动物的饮食差异[53~54]。
在考古研究中,南方古猿饮食结构的变化一直备受关注[50]。传统的碳同位素分析和牙齿微痕分析无法精确地提供南方古猿在生命周期内的饮食变化信息[55~56]。Sponheimer等[40]首次借助激光剥蚀气相色谱-同位素比值质谱(LA-GC/IRMS)对4个南方古猿粗壮种牙釉质表层进行了碳同位素微区采样,为研究南方古猿饮食的季节和年际变化提供了依据。研究者根据釉面横纹的条数建立时间标尺,最终建立碳同位素的季节和年际变化(图 7a)。结果表明[54],4个南方古猿的釉面横纹总数最小值为39,其釉面横纹的生长周期大约为7天,推测采样剖面的形成时间至少大于半年;碳同位素结果显示,4个个体牙釉质碳同位素均呈现季节和年际变化,极差变化在1.3 ‰ ~5.2 ‰之间,表明南方古猿饮食结构存在变化,并不挑食;其中SKX5939个体在92条釉面横纹间,牙釉质碳同位素从- 2.7 ‰逐渐降低到- 7.9 ‰,说明在这574天内,该个体的饮食结构由C4植物为主向C3植物为主转变(图 7b)。
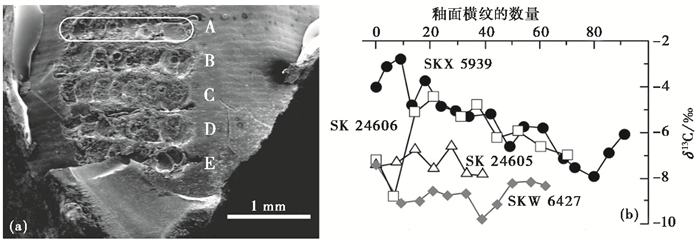
|
图 7 4个南方古猿粗壮种牙釉质微区采样及碳同位素变化 (a)南方古猿SKX5939部分采样示意图(轨迹A~E之间有22条釉面横纹,根据釉面横纹周期为7,表明A~E样品形成需154天);(b)南方古猿牙齿生长序列上碳同位素变化[40] Fig. 7 Laser ablation carbon isotope analysis of tooth enamel in four Paranthropus robustus individuals. (a)A portion of the enamel of SKX 5939, on which the total number of perikymata between the first and last ablation samples shown(A to E)is 22, meaning that the interval represented by these samples is approximately 154 days(22×7); (b)δ13C of multiple ablation samples along the growth axes of teeth[40] |
近期,Stacklyn等[51]对中国南方龙骨洞和岩亮洞出土的早更新世大熊猫和其他哺乳动物牙釉质进行了间距为1~3 mm的微钻系列取样,并通过碳同位素分析重建早更新世大熊猫饮食结构。碳同位素结果显示[51],两个洞穴中不同大熊猫个体的δ13C变化范围分别为- 17.8 ‰ ~- 19.7 ‰和- 17.2 ‰ ~- 19.8 ‰,表明早更新大熊猫以纯C3植物为食。而大熊猫在牙釉质生长序列上的δ13C变化仅为0.6 ‰,远小于其他哺乳动物,表明其饮食变化并不显著。
4.2 古环境重建牙釉质中的碳、氧元素是通过环境中的水和食物进入个体体内,其同位素组成继承着环境信息,是恢复古环境和古气候的重要指标[57~59]。植物的碳同位素组成受到其生长环境的影响,如温度、水分状况、大气CO2等状况都会影响植物中δ13C变化[60~61],这种变化会通过食物链保存在动物牙釉质中[62]。一些研究主要利用动物牙釉质的碳同位素反映地质时期C3和C4植被分布[59, 63]以及年平均降水量变化[64]。人体牙釉质的氧同位素主要来源于饮用的大气降水[65],后者受到温度、纬度等环境因素的影响[66],使得牙釉质的δ18O同位素记录着丰富的环境信息[67~68]。目前,多数研究利用动物牙釉质δ18O值重建大气降水氧同位素[69~70]和古温度[71~72]。
新仙女木(YD)是发生在末次冰消期升温过程中北半球的一次急剧降温事件[73~78]。在YD事件发生前后,地中海东部地区人类社会结构与经济结构发生了转型,即人类逐渐从采集狩猎群向农耕群体发展,表明这种转型与YD事件的气候波动有关[79~80]。已有的研究显示,该地区在YD时期气候变冷,但其干湿变化一直是学界争议问题[81~83]。在黎凡特南部地区,Hartman等[58]选取新仙女木时期之前的纳吐夫文化早期HC洞遗址(EN HC)与新仙女木时期的纳吐夫文化晚期HTC洞遗址(LN HTC)的瞪羊第三臼齿,分析了其齿尖到齿颈的碳、氧同位素组成,重建了当时环境的干湿变化。结果显示,纳吐夫文化晚期瞪羊牙釉质的氧同位素比早期偏正约1.14 ‰ ~1.32 ‰ (图 8a),表明YD事件期间温度降低;而瞪羊牙釉质的碳同位素值接近,且季节变化一致(图 8b),说明YD事件期间未出现降水显著减少的现象,澄清了农业起源的气候环境背景。
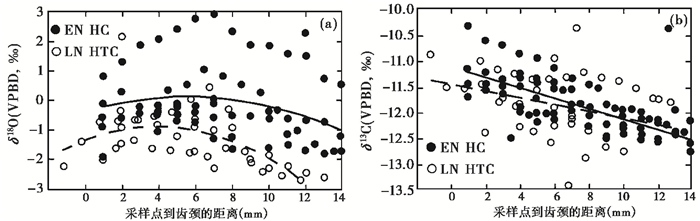
|
图 8 纳吐夫文化早期HC洞穴(EN HC)和晚期HTC洞穴(LN HTC)瞪羊牙釉质碳氧同位素变化 (a)瞪羊牙釉质氧同位素系列采样结果;(b)瞪羊M3牙釉质碳同位素系列采样结果[58] Fig. 8 Sequential δ18O and δ13C data of gazelles teeth from EN HC and LN HTC. (a)Sequential sampling results of gazelle δ13C values; (b)Sequential sampling results of gazelle δ18O values[58] |
始新世-渐新世(E/O)界限的降温事件,是新生代气候演化过程中最显著的变冷事件之一[84]。目前对始新世-渐新世(E/O)界线古气候变冷事件的研究主要来自海洋沉积物记录,而来自陆地的E/O事件记录相对较少[85~86]。为探讨该气候降温事件在北美地区的气候响应,Zanazzi等[69]对加拿大萨斯喀彻温省西南部始新世-渐新世地层中出土的犀牛和马牙化石进行了间隔为1.25 mm的微钻系列取样。结果显示,渐新世(- 9.0±0.3 ‰)和始新世(- 8.8±0.3 ‰)的牙釉质碳同位素差异并不显著,表明E/O转变前后该地区大气降水不存在显著差异[69]。而渐新世的牙釉质氧同位素序列变化较始新世更加显著,说明在E/O转变之后季节性温度变幅增强。此外,与低纬度的美国大草原同时期记录对比发现[85],萨斯喀彻温省牙釉质碳同位素值小幅偏高,氧同位素在E/O转变前后的季节变化更加强烈,这说明E/O转变前后在高纬地区气候更加干旱,且季节性温度变幅较低纬度更加敏感。
4.3 示踪古人类及动物迁移行为牙釉质中的锶(87Sr/86Sr)[87~88]、氧元素主要用于动物与人的迁移行为研究[89]。不同地区的锶同位素背景值取决于基岩年龄和Rb/Sr比值。锶元素在表生环境中同位素分馏非常小。在岩石风化、成壤和食物链过程中,87Sr/86Sr基本保持不变。而且锶原子半径与钙相似,在牙齿形成过程中,锶以类质同象形式替换羟磷灰石中的钙保存下来[90~91]。因此个体牙釉质锶同位素组成能与其生活地区保持一致。通过对比个体牙釉质的87Sr/86Sr比值与当地锶同位素背景值差异,判断个体迁移行为[43]。牙釉质的氧同位素继承大气降水的氧同位素,呈现出明显的地域差异,也可以反映迁移行为[92]。
研究灭绝动物的迁移行为以及迁移距离,对评估动物灭绝假说、探讨人类利用和获取动物资源模式以及古气候变化等问题至关重要[93]。在实际研究中,准确判断灭绝动物的迁移距离需要高分辨率的同位素信息。Hoppe等[93]的研究表明,佛罗里达州北部和中部遗址中的猛犸象牙釉质87Sr/86Sr比值(>0.710)均高于当地锶同位素的比值(0.7080~0.7097),推测猛犸象存在迁移行为;对北部Page-Ladson遗址猛犸象牙齿进一步系列采样研究发现,87Sr/86Sr比值存在季节变化(0.7078~0.7121),且一年内大部分时间接近佐治亚州南部和阿巴拉契亚山脉的87Sr/86Sr背景值(0.7087~0.7144)。这表明佛罗里达州北部和中部的猛犸象在釉质形成过程中多次向北迁移进入佐治亚州,迁移距离至少为120~300 km(图 9)。
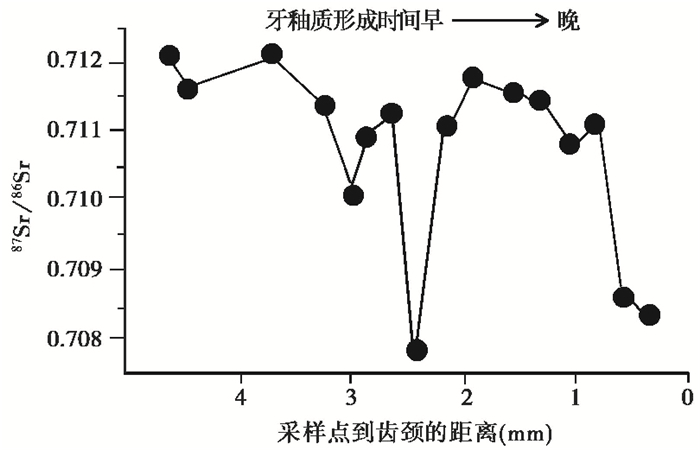
|
图 9 佛罗里达州北部Page-Ladson遗址的猛犸象牙齿87Sr/86Sr比值[93] Fig. 9 87Sr/86Sr ratios of microsamples from Page-Ladson mastodon[93] |
最近,Lugli等[16]利用LA-MC-ICP- MS对中更新世(约580 ka)两枚犀牛牙齿(RH-IS26和RH-IS30)进行了锶同位素微区分析。研究者根据丙氏线和纹层线确定RH-IS26样品的时间框架,根据犀牛生长速率及釉质厚度确定RH-IS30样品的时间框架。在采样方式上,Lugli等[16]对RH-IS30样品的横截面进行轨迹A和轨迹B线扫描取样(图 10a),对RH-IS26纵切面进行轨迹C、轨迹D和轨迹E迹线扫描取样(图 10b),采集两个样品锶同位素的微区信息。结果表明,两个犀牛样品在牙釉质生长方向上均存在87Sr/86Sr值的周期变化,其中RH-IS30牙齿87Sr/86Sr均值是0.70951±0.00014,RH-IS26牙齿87Sr/86Sr均值是0.70976±0.00015,两者均高于当地锶同位素背景值(0.70918±0.00013和0.70934±0.00009)。较高的87Sr/86Sr值正好能对应附近火山区87Sr/86Sr背景值,推测犀牛经常活跃于火山土发育地区,并存在季节性迁移行为[16]。
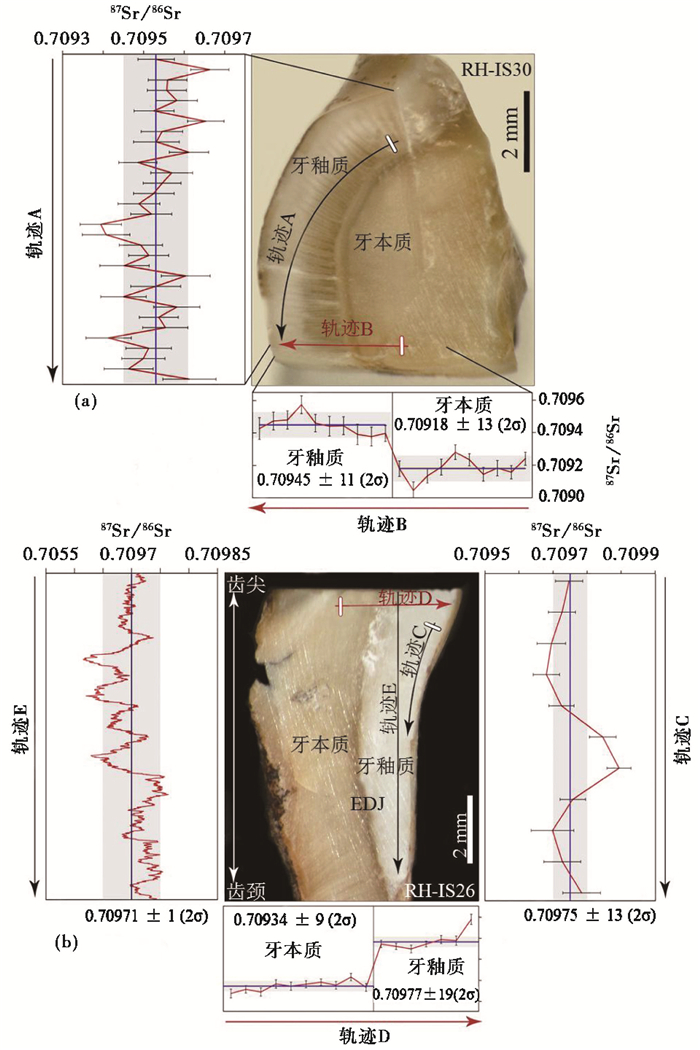
|
图 10 两个犀牛牙釉质样品LA-MC-ICP- MS采样方法及锶同位素分析[16] (a)RH-IS30样品采样方式;(b)RH-IS26样品采样方式 Fig. 10 In situ87Sr/86Sr analysis of two rhinos tooth enamel by LA-MC-ICP- MS[16]. (a)Sampling of sample RH-IS30; (b)Sampling of sample RH-IS26 |
尼安德人是否存在长距离的迁移行为,一直是考古界讨论的热点问题[35, 94]。过去的研究主要基于遗址中石器以及外来墓葬品证据间接反映尼安德特人的迁移距离(<5 km)[95]。Richards等[35]利用laser-ablation PIMMS对希腊Lakonis遗址中4万年前尼安德特人个体的M3牙齿进行了微区取样,发现牙釉质的锶同位素比值均高于当地锶同位素比值背景值(石灰岩地区),结合整个区域的锶同位素地质背景值,推测其迁移范围至少为20 km。
除了重建古食谱、古环境、古人类与动物迁移行为,牙釉质高空间分辨率分析还能反映动物的季节性繁殖季节[96]、人类狩猎方式[33]及畜牧管理[97]等考古学问题。动物季节性繁殖行为是影响游牧经济的重要因素,其变化特征能通过动物牙釉质氧同位素的季节变化反映出来[98]。若动物在同一季节繁殖,牙釉质氧同位素序列的变化趋势应一致。南非史前时期Kasteelberg遗址绵羊M2和M3牙釉质微钻系列采样分析结果表明,氧同位素序列均存在两种变化趋势,这两种趋势相位差约为半个周期,直观地表明该遗址羊群有两个繁殖期,除了传统认为的秋季,另一个在春季[29]。
综上所述,通过微钻系列取样和高分辨率微区取样的同位素分析可以获得季节变化信息,可以研究动物的迁移、饮食等行为特征,同时也能认识早期人类的狩猎行为、游牧民族迁移活动以及生业方式等。甚至可以解决更加细微的考古学问题,比如区别动物是季节性自然迁移还是游牧活动造成的人为迁移[93]。
5 存在的问题可以看出,牙釉质的采样方式和采样部位对同位素分析和解译极为重要。尽管系列采样的微钻取样和高空间分辨率的微区取样在同位素研究中取得了很大进展,但也存在不少问题。
首先,必须注意,上述提及的生长层结构形成于釉质基质分泌阶段,而釉质矿化以及釉质记录的同位素信号大部形成于成熟阶段[11, 13]。最近的研究表明,釉质的沉积方向在第一阶段(釉质分泌阶段)和第二阶段(釉质成熟阶段)很有可能存在差异,这将会导致生长层结构记录的同位素信号与其釉质基质形成的时间不同步[15]。Hoppe等[98]、Trayler和Kohn[14]发现,马牙、牛科动物等牙釉质在第一阶段相对于EDJ层为低角度沉积,而第二阶段的沉积方向独立于第一阶段,如马牙的第二阶段釉质内层和中间层呈高角度沉积。针对该问题,目前已有学者提出可以平行于成熟阶段釉质沉积方向进行采样来解决[14]。
其次,系列采样方式分析的前提是假设釉质的生长速率恒定,但实际上釉质在齿尖的生长速率要远大于齿颈[98],如Bendrey等[99]计算出马的牙釉质从齿尖到齿颈生长速率是呈指数递减。因此,等间距的采样势必会导致分析大部分齿尖形成时输入的同位素而不具代表性。针对这个问题,在系列采样过程中,建议在生长速率较快的齿尖部位加密采样,同时也需要关注不同动物牙齿的生长模式与速率,以获得更为精确的同位素季节信息。
再者,学术界已经认识到,LA-MC-ICP- MS等微区分析虽然在空间分辨率上较TIMS、IRMS等系列采样法有很大提高,但分析精度远不如后者[100~101]。以牙釉质样品的锶同位素测试为例,由于多种同质异位素的存在(尤其是40Ca31P16O和40Ar31P16O的干扰),造成LA-MC-ICP- MS与TIMS的偏差(ΔLA-TIMS)可高达500~1500 ppm,特别是低Sr含量的样品偏差尤为明显[102~105]。目前研究主要从两方面改善LA-MC-ICP- MS分析精度:其一,通入氮气来减少氧化物离子的产生,以降低含氧同素异形体的干扰(40Ca31P16O和40Ar31P16O),从而缩小ΔLA-TIMS偏差[106];其二,通过一系列Sr含量已知的生物磷灰石标样建立87Sr/86Sr准确度(测量值/真实值)与1/88Sr曲线,利用其线性变化区间校正样品,提高低锶含量样品的测试精度[102]。
近期,我们首次尝试利用LA-MC-ICP- MS对罗布泊一号墓地出土的1枚马牙和吉尔赞喀勒墓地4枚人牙进行了微区采样。结果显示,高锶含量(Sr约8000 ppm)的马牙釉质LA测试值为0.71046±0.00012 (2σ),与TIMS测定值(0.71045±0.00001 (2σ))基本保持一致,而低锶含量(92~800 ppm)的人牙LA测试值与TIMS值偏差较大(ΔLA-TIMS=0.00145±0.00103 (2σ))。现有研究表明,人牙釉质样品的这种偏差可能与40Ca31P16O干扰有关[102, 106]。对此,我们在前人的基础上[102]选择鲨鱼牙齿、磷灰石和人牙(88Sr信号强度大于1.5 V)作为标样,建立了87Sr/86Sr准确度与1/88Sr曲线来校正40Ca31P16O干扰。经校正后,LA测试精度显著提高,所有人牙釉质样品ΔLA-TIMS=0.00034±0.00016 (2σ),88Sr信号强度大于1.5 V的样品ΔLA-TIMS=0.000298±0.00019 (2σ),且两个低锶含量样品(锶含量<150 ppm)的ΔLA-TIMS均小于460 ppm。本研究初步改善了LA-MC-ICP- MS人类牙釉质Sr同位素的分析精度,但LA测定值仍偏离真实值,未来将进一步对人牙Sr同位素校正方法进行探讨。
总结来说,尽管在实际应用中存在一些问题,基于牙釉质生长结构的同位素分析为考古研究提供了一个直接、重要而全新的方法,这有助于我们更深入认识古代动物与人类的行为特征。
致谢: 感谢中国科学院地质与地球物理研究所岩石圈演化国家重点实验LA-MC-ICP- MS实验室杨岳衡研究员,郭利成、杨雷和李睿同学在实验分析和数据处理中的帮助,感谢审稿专家和编辑部杨美芳老师、赵淑君老师提出的宝贵修改意见和建议。
| [1] |
Bentley R A. Strontium isotopes from the earth to the archaeological skeleton:A review[J]. Journal of Archaeological Method and Theory, 2006, 13(3): 135-187. DOI:10.1007/s10816-006-9009-x |
| [2] |
Price T D, Johnson C M, Ezzo J A, et al. Residential mobility in the prehistoric southwest United States:A preliminary study using strontium isotope analysis[J]. Journal of Archaeological Science, 1994, 21(3): 315-330. DOI:10.1006/jasc.1994.1031 |
| [3] |
Wright L E. Identifying immigrants to Tikal, Guatemala:Defining local variability in strontium isotope ratios of human tooth enamel[J]. Journal of Archaeological Science, 2005, 32(4): 555-566. DOI:10.1016/j.jas.2004.11.011 |
| [4] |
Zhang X, Burton J, Jin Z, et al. Isotope studies of human remains from Mayutian, Yunnan Province, China[J]. Journal of Archaeological Science, 2014, 50: 414-419. DOI:10.1016/j.jas.2014.08.001 |
| [5] |
Bäckström Y, Price T D. Social identity and mobility at a pre-industrial mining complex, Sweden[J]. Journal of Archaeological Science, 2016, 66: 154-168. DOI:10.1016/j.jas.2016.01.004 |
| [6] |
Bentley R A, Bickle P, Fibiger L, et al. Community differentiation and kinship among Europe's first farmers[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(24): 9326-9330. DOI:10.1073/pnas.1113710109 |
| [7] |
尹若春, 张居中, 杨晓勇. 贾湖史前人类迁移行为的初步研究——锶同位素分析技术在考古学中的运用[J]. 第四纪研究, 2008, 28(1): 50-57. Yin Ruochun, Zhang Juzhong, Yang Xiaoyong. Preliminary study of prehistoryic human migration based on Sr isotope analysis from Jiahu relics[J]. Quaternary Sciences, 2008, 28(1): 50-57. DOI:10.3321/j.issn:1001-7410.2008.01.006 |
| [8] |
赵春燕, 杨杰, 袁靖, 等. 河南省偃师市二里头遗址出土部分动物牙釉质的锶同位素比值分析[J]. 中国科学:地球科学, 2012, 42(7): 1011. Zhao Chunyan, Yang Jie, Yuan Jing, et al. Strontium isotope analysis of archaeological fauna at the Erlitou site[J]. Science China:Earth Sciences, 2012, 42(7): 1011. |
| [9] |
赵春燕, 何驽. 陶寺遗址中晚期出土部分人类牙釉质的锶同位素比值分析[J]. 第四纪研究, 2014, 34(1): 66-72. Zhao Chunyan, He Nu. Strontium isotope analysis of archaeological skeletons from the middle-later period of the Taosi site[J]. Quaternary Sciences, 2014, 34(1): 66-72. DOI:10.3969/j.issn.1001-7410.2014.01.09 |
| [10] |
赵春燕, 王明辉, 叶茂林. 青海喇家遗址人类遗骸的锶同位素比值分析[J]. 人类学学报, 2016, 35(2): 212-222. Zhao Chunyan, Wang Minghui, Ye Maolin. Strontium isotope analysis of human teeth and bones from the Lajia site in Qinghai Province[J]. Acta Anthropologica Sinica, 2016, 35(2): 212-222. |
| [11] |
Tafforeau P, Bentaleb I, Jaeger J J, et al. Nature of laminations and mineralization in rhinoceros enamel using histology and X-ray synchrotron microtomography:Potential implications for palaeoenvironmental isotopic studies[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2007, 246(2-4): 206-227. DOI:10.1016/j.palaeo.2006.10.001 |
| [12] |
Passey B H, Cerling T E. Tooth enamel mineralization in ungulates:Implications for recovering a primary isotopic time-series[J]. Geochimica et Cosmochimica Acta, 2002, 66(18): 3225-3234. DOI:10.1016/S0016-7037(02)00933-X |
| [13] |
Zazzo A, Balasse M, Patterson W P. High-resolution δ13C intratooth profiles in bovine enamel:Implications for mineralization pattern and isotopic attenuation[J]. Geochimica et Cosmochimica Acta, 2005, 69(14): 3631-3642. DOI:10.1016/j.gca.2005.02.031 |
| [14] |
Trayler R B, Kohn M J. Tooth enamel maturation reequilibrates oxygen isotope compositions and supports simple sampling methods[J]. Geochimica et Cosmochimica Acta, 2017, 198: 32-47. DOI:10.1016/j.gca.2016.10.023 |
| [15] |
Blumenthal S A, Cerling T E, Chritz K L, et al. Stable isotope time-series in mammalian teeth:In situδ18O from the innermost enamel layer[J]. Geochimica et Cosmochimica Acta, 2014, 124: 223-236. DOI:10.1016/j.gca.2013.09.032 |
| [16] |
Lugli F, Cipriani A, Peretto C, et al. In situ high spatial resolution 87Sr/86Sr ratio determination of two Middle Pleistocene(c.a. 580 ka)Stephanorhinus hundsheimensis teeth by LA-MC-ICP-MS[J]. International Journal of Mass Spectrometry, 2017, 412: 38-48. DOI:10.1016/j.ijms.2016.12.012 |
| [17] |
Aubert M, Williams I S, Boljkovac K, et al. In situ oxygen isotope micro-analysis of faunal material and human teeth using a SHRIMP Ⅱ:A new tool for palaeo-ecology and archaeology[J]. Journal of Archaeological Science, 2012, 39(10): 3184-3194. DOI:10.1016/j.jas.2012.05.002 |
| [18] |
Lewis J, Coath C D, Pike A W G. An improved protocol for 87Sr/86Sr by laser ablation multi-collector inductively coupled plasma mass spectrometry using oxide reduction and a customised plasma interface[J]. Chemical Geology, 2014, 390: 173-181. |
| [19] |
白滨, 王旭. 小哺乳动物化石牙齿釉质碳、氧同位素测试方法简介及应用前景[J]. 古脊椎动物学报, 2013, 51(3): 242-251. Bai Bin, Wang Xu. A review of methods in carbon and oxygen isotopic analyses of tooth enamel from small fossil mammals[J]. Vertebrata PalAsiatica, 2013, 51(3): 242-251. |
| [20] |
Hillson S. Dental Anthropology[M]. Cambridge: Cambridge University Press, 1996: 118-125.
|
| [21] |
Hillson S. Teeth[M]. Cambridge: Cambridge University Press, 2005: 207-255.
|
| [22] |
于世凤. 口腔组织病理学——第5版[M]. 北京: 人民卫生出版社, 2004: 23-59. Yu Shifeng. Oral Pathology——Fifth Edition[M]. Beijing: People's Medical Publishing House, 2004: 23-59. |
| [23] |
Smith C E. Cellular and chemical events during enamel maturation[J]. Critical Reviews in Oral Biology & Medicine, 1998, 9(2): 128-161. |
| [24] |
Simmer J P, Hu J C C. Expression, structure, and function of enamel proteinases[J]. Connective Tissue Research, 2002, 43(2-3): 441-449. DOI:10.1080/03008200290001159 |
| [25] |
Humphrey L T, Dean M C, Jeffries T E, et al. Unlocking evidence of early diet from tooth enamel[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(19): 6834-6839. DOI:10.1073/pnas.0711513105 |
| [26] |
Smith T M, Tafforeau P. New visions of dental tissue research:tooth development, chemistry, and structure[J]. Evolutionary Anthropology:Issues, News, and Reviews, 2008, 17(5): 213-226. DOI:10.1002/evan.20176 |
| [27] |
Katzenberg M A, Saunders S R. Biological Anthropology of the Human Skeleton[M]. Hoboken: John Wiley & Sons, 2011: 237-263.
|
| [28] |
Fukuhara T. Comparative anatomical studies of the growth lines in the enamel of mammalian teeth[J]. Acta Anatomica Nipponica, 1959, 34: 322-332. |
| [29] |
Balasse M, Smith A B, Ambrose S H, et al. Determining sheep birth seasonality by analysis of tooth enamel oxygen isotope ratios:The Late Stone Age site of Kasteelberg(South Africa)[J]. Journal of Archaeological Science, 2003, 30(2): 205-215. DOI:10.1006/jasc.2002.0833 |
| [30] |
Slater P A, Hedman K M, Emerson T E. Immigrants at the Mississippian polity of Cahokia:Strontium isotope evidence for population movement[J]. Journal of Archaeological Science, 2014, 44: 117-127. DOI:10.1016/j.jas.2014.01.022 |
| [31] |
Frémondeau D, Cucchi T, Casabianca F, et al. Seasonality of birth and diet of pigs from stable isotope analyses of tooth enamel(δ18O, δ13C):A modern reference data set from Corsica, France[J]. Journal of Archaeological Science, 2012, 39(7): 2023-2035. DOI:10.1016/j.jas.2012.04.004 |
| [32] |
Price T D, Meiggs D, Weber M J, et al. The migration of Late Pleistocene reindeer:Isotopic evidence from northern Europe[J]. Archaeological and Anthropological Sciences, 2015, 9(3): 371-394. |
| [33] |
Tornero C, Balasse M, Bǎlǎşescu A, et al. The altitudinal mobility of wild sheep at the Epigravettian site of Kalavan 1(Lesser Caucasus, Armenia):Evidence from a sequential isotopic analysis in tooth enamel[J]. Journal of Human Evolution, 2016, 97: 27-36. DOI:10.1016/j.jhevol.2016.05.001 |
| [34] |
Tornero C, Bǎlǎşescu A, Ughetto-Monfrin J, et al. Seasonality and season of birth in early Eneolithic sheep from Cheia(Romania):Methodological advances and implications for animal economy[J]. Journal of Archaeological Science, 2013, 40(11): 4039-4055. DOI:10.1016/j.jas.2013.05.013 |
| [35] |
Richards M, Harvati K, Grimes V, et al. Strontium isotope evidence of Neanderthal mobility at the site of Lakonis, Greece using laser-ablation PIMMS[J]. Journal of Archaeological Science, 2008, 35(5): 1251-1256. DOI:10.1016/j.jas.2007.08.018 |
| [36] |
Copeland S R, Sponheimer M, de Ruiter D J, et al. Strontium isotope evidence for landscape use by early hominins[J]. Nature, 2011, 474(7349): 76-78. DOI:10.1038/nature10149 |
| [37] |
Balter V, Braga J, Télouk P, et al. Evidence for dietary change but not landscape use in South African early hominins[J]. Nature, 2012, 489(7417): 558-560. DOI:10.1038/nature11349 |
| [38] |
Lugli F, Cipriani A, Arnaud J, et al. Suspected limited mobility of a Middle Pleistocene woman from Southern Italy:Strontium isotopes of a human deciduous tooth[J]. Scientific Reports, 2017, 7(1): 8615. DOI:10.1038/s41598-017-09007-5 |
| [39] |
Glykou A, Eriksson G, Storå J, et al. Intra-and inter-tooth variation in strontium isotope ratios from prehistoric seals by laser ablation multi-collector inductively coupled plasma mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2018, 32(15): 1215-1224. DOI:10.1002/rcm.8158 |
| [40] |
Sponheimer M, Passey B H, de Ruiter D J, et al. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus[J]. Science, 2006, 314(5801): 980-982. DOI:10.1126/science.1133827 |
| [41] |
Amiot R, Wang X, Zhou Z, et al. Oxygen isotopes of East Asian dinosaurs reveal exceptionally cold Early Cretaceous climates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(13): 5179-5183. DOI:10.1073/pnas.1011369108 |
| [42] |
Brookman T H, Ambrose S H. Seasonal variation in kangaroo tooth enamel oxygen and carbon isotopes in Southern Australia[J]. Quaternary Research, 2012, 78(2): 256-265. DOI:10.1016/j.yqres.2012.05.011 |
| [43] |
Price T D, Frei R, Bäckström Y, et al. Origins of inhabitants from the 16th century Sala(Sweden)silver mine cemetery-A lead isotope perspective[J]. Journal of Archaeological Science, 2017, 80: 1-13. DOI:10.1016/j.jas.2017.01.013 |
| [44] |
Borić D, Price T D. Strontium isotopes document greater human mobility at the start of the Balkan Neolithic[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(9): 3298-3303. DOI:10.1073/pnas.1211474110 |
| [45] |
Szabó P, Kocsis L, Vennemann T, et al. Pliocene-Early Pleistocene climatic trends in the Italian Peninsula based on stable oxygen and carbon isotope compositions of rhinoceros and gomphothere tooth enamel[J]. Quaternary Science Reviews, 2017, 157: 52-65. DOI:10.1016/j.quascirev.2016.11.003 |
| [46] |
Britton K, Grimes V, Dau J, et al. Reconstructing faunal migrations using intra-tooth sampling and strontium and oxygen isotope analyses:A case study of modern caribou(Rangifer tarandus granti)[J]. Journal of Archaeological Science, 2009, 36(5): 1163-1172. DOI:10.1016/j.jas.2009.01.003 |
| [47] |
Makarewicz C A, Arbuckle B S, Öztan A. Vertical transhumance of sheep and goats identified by intra-tooth sequential carbon(δ13C)and oxygen(δ18O)isotopic analyses:Evidence from Chalcolithic Köşk Höyük, Central Turkey[J]. Journal of Archaeological Science, 2017, 86: 68-80. DOI:10.1016/j.jas.2017.01.003 |
| [48] |
Suraprasit K, Bocherens H, Chaimanee Y, et al. Late Middle Pleistocene ecology and climate in Northeastern Thailand inferred from the stable isotope analysis of Khok Sung herbivore tooth enamel and the land mammal cenogram[J]. Quaternary Science Reviews, 2018, 193: 24-42. DOI:10.1016/j.quascirev.2018.06.004 |
| [49] |
Stevens R E, Balasse M, O'Connell T C. Intra-tooth oxygen isotope variation in a known population of red deer:Implications for past climate and seasonality reconstructions[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2011, 301(1-4): 64-74. DOI:10.1016/j.palaeo.2010.12.021 |
| [50] |
Cerling T E, Manthi F K, Mbua E N, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(26): 10501-10506. DOI:10.1073/pnas.1222568110 |
| [51] |
Stacklyn S, Wang Y, Jin C Z, et al. Carbon and oxygen isotopic evidence for diets, environments and niche differentiation of Early Pleistocene pandas and associated mammals in South China[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2017, 468: 351-361. DOI:10.1016/j.palaeo.2016.12.015 |
| [52] |
Passey B H, Cerling T E, Schuster G T, et al. Inverse methods for estimating primary input signals from time-averaged isotope profiles[J]. Geochimica et Cosmochimica Acta, 2005, 69(16): 4101-4116. DOI:10.1016/j.gca.2004.12.002 |
| [53] |
Swift J A, Molle G, Conte E. Coastal subsistence and settlement at the Hane dune site, Ua Huka(Marquesas Islands):New insights from Pacific rat(Rattus exulans)stable isotope analysis[J]. Journal of Archaeological Science:Reports, 2017, 15: 161-168. DOI:10.1016/j.jasrep.2017.07.020 |
| [54] |
Martyn R E V, Garnsey P, Fattore L, et al. Capturing Roman dietary variability in the catastrophic death assemblage at Herculaneum[J]. Journal of Archaeological Science:Reports, 2018, 19: 1023-1029. DOI:10.1016/j.jasrep.2017.08.008 |
| [55] |
Sponheimer M, Lee-Thorp J, de Ruiter D, et al. Hominins, sedges, and termites:New carbon isotope data from the Sterkfontein valley and Kruger National Park[J]. Journal of Human Evolution, 2005, 48(3): 301-312. DOI:10.1016/j.jhevol.2004.11.008 |
| [56] |
Scott R S, Ungar P S, Bergstrom T S, et al. Dental microwear texture analysis shows within-species diet variability in fossil hominins[J]. Nature, 2005, 436(7051): 693. DOI:10.1038/nature03822 |
| [57] |
Sarkar A, Mukherjee A D, Bera M K, et al. Oxygen isotope in archaeological bioapatites from India:Implications to climate change and decline of Bronze Age Harappan civilization[J]. Scientific Reports, 2016, 6: 26555. DOI:10.1038/srep26555 |
| [58] |
Hartman G, Bar-Yosef O, Brittingham A, et al. Hunted gazelles evidence cooling, but not drying, during the Younger Dryas in the Southern Levant[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(15): 3997-4002. DOI:10.1073/pnas.1519862113 |
| [59] |
Hynek S A, Passey B H, Prado J L, et al. Small mammal carbon isotope ecology across the Miocene-Pliocene boundary, Northwestern Argentina[J]. Earth and Planetary Science Letters, 2012, 321-322: 177-188. DOI:10.1016/j.epsl.2011.12.038 |
| [60] |
Kohn M J. Carbon isotope compositions of terrestrial C3 plants as indicators of(paleo)ecology and(paleo)climate[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(46): 19691-19695. DOI:10.1073/pnas.1004933107 |
| [61] |
Farquhar G D, O'Leary M H, Berry J A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves[J]. Functional Plant Biology, 1982, 9(2): 121-137. DOI:10.1071/PP9820121 |
| [62] |
Kohn M J, Cerling T E. Stable isotope compositions of biological apatite[J]. Reviews in Mineralogy and Geochemistry, 2002, 48(1): 455-488. DOI:10.2138/rmg.2002.48.12 |
| [63] |
Arppe L, Kaakinen A, Passey B H, et al. Small mammal tooth enamel carbon isotope record of C4 grasses in Late Neogene China[J]. Global and Planetary Change, 2015, 133: 288-297. DOI:10.1016/j.gloplacha.2015.09.003 |
| [64] |
Amiot R, Wang X, Zhou Z H, et al. Environment and ecology of East Asian dinosaurs during the Early Cretaceous inferred from stable oxygen and carbon isotopes in apatite[J]. Journal of Asian Earth Sciences, 2015, 98: 358-370. DOI:10.1016/j.jseaes.2014.11.032 |
| [65] |
Bryant J D, Froelich P N. A model of oxygen isotope fractionation in body water of large mammals[J]. Geochimica et Cosmochimica Acta, 1995, 59(21): 4523-4537. DOI:10.1016/0016-7037(95)00250-4 |
| [66] |
Dansgaard W. Stable isotopes in precipitation[J]. Tellus Series B:Chemical and Physical Meteorology, 1964, 16(4): 436-468. |
| [67] |
Longinelli A. Oxygen isotopes in mammal bone phosphate:A new tool for paleohydrological and paleoclimatological research?[J]. Geochimica et Cosmochimica Acta, 1984, 48(2): 385-390. DOI:10.1016/0016-7037(84)90259-X |
| [68] |
Janssen R, Joordens J C A, Koutamanis D S, et al. Tooth enamel stable isotopes of Holocene and Pleistocene fossil fauna reveal glacial and interglacial paleoenvironments of hominins in Indonesia[J]. Quaternary Science Reviews, 2016, 144: 145-154. DOI:10.1016/j.quascirev.2016.02.028 |
| [69] |
Zanazzi A, Judd E, Fletcher A, et al. Eocene-Oligocene latitudinal climate gradients in North America inferred from stable isotope ratios in perissodactyl tooth enamel[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2015, 417: 561-568. DOI:10.1016/j.palaeo.2014.10.024 |
| [70] |
Amiot R, Lécuyer C, Buffetaut E, et al. Latitudinal temperature gradient during the Cretaceous Upper Campanian-Middle Maastrichtian:δ18O record of continental vertebrates[J]. Earth and Planetary Science Letters, 2004, 226(1-2): 255-272. DOI:10.1016/j.epsl.2004.07.015 |
| [71] |
Skrzypek G, Sadler R, Wi As'1 niewski A. Reassessment of recommendations for processing mammal phosphate δ18O data for paleotemperature reconstruction[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2016, 446: 162-167. DOI:10.1016/j.palaeo.2016.01.032 |
| [72] |
Pryor A J E, Stevens R E, O'Connell T C, et al. Quantification and propagation of errors when converting vertebrate biomineral oxygen isotope data to temperature for palaeoclimate reconstruction[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2014, 412: 99-107. DOI:10.1016/j.palaeo.2014.07.003 |
| [73] |
Severinghaus J P, Sowers T, Brook E J, et al. Timing of abrupt climate change at the end of the Younger Dryas interval from thermally fractionated gases in polar ice[J]. Nature, 1998, 391(6663): 141-146. DOI:10.1038/3434 |
| [74] |
Alley R B, Clark P U. The deglaciation of the Northern Hemisphere:A global perspective[J]. Annual Review of Earth & Planetary Sciences, 1999, 27(1): 149-182. |
| [75] |
Wang Y J, Cheng H, Edwards R L, et al. A high-resolution absolute-dated Late Pleistocene monsoon record from Hulu cave, China[J]. Science, 2001, 294(5550): 2345-2348. DOI:10.1126/science.1064618 |
| [76] |
崔梦月, 洪晖, 孙晓双, 等. 福建仙云洞石笋记录的新仙女木突变事件结束时的缓变特征[J]. 第四纪研究, 2018, 38(3): 711-719. Cui Mengyue, Hong Hui, Sun Xiaoshuang, et al. The gradual change characteristics at the end of the Younger Dryas event inferred from a speleothem record from Xianyun cave, Fujian Province[J]. Quaternary Sciences, 2018, 38(3): 711-719. |
| [77] |
刘晶晶, 张江勇, 陈云如, 等. 基于叶蜡正构烷烃重建的南海及周边地区植被类型[J]. 第四纪研究, 2016, 36(3): 553-563. Liu Jingjing, Zhang Jiangyong, Chen Yunru, et al. Vegetation changes recorded by leaf-wax n-alkanes around the South China Sea[J]. Quaternary Sciences, 2016, 36(3): 553-563. |
| [78] |
申改慧, 丁国强, 阳小兰, 等. 白洋淀地区全新世以来的气候环境变化[J]. 第四纪研究, 2018, 38(3): 756-768. Shen Gaihui, Ding Guoqiang, Yang Xiaolan, et al. Holocene climate and environmental change in the Baiyangdian area[J]. Quaternary Sciences, 2018, 38(3): 756-768. |
| [79] |
Byrd B F. Reassessing the emergence of village life in the Near East[J]. Journal of Archaeological Research, 2005, 13(3): 231-290. DOI:10.1007/s10814-005-3107-2 |
| [80] |
Bar-Yosef O. The Natufian culture in the Levant, threshold to the origins of agriculture[J]. Evolutionary Anthropology:Issues, News, and Reviews, 1998, 6(5): 159-177. DOI:10.1002/(ISSN)1520-6505 |
| [81] |
Affek H P, Bar-Matthews M, Ayalon A, et al. Glacial/interglacial temperature variations in Soreq cave speleothems as recorded by 'clumped isotope' thermometry[J]. Geochimica et Cosmochimica Acta, 2008, 72(22): 5351-5360. DOI:10.1016/j.gca.2008.06.031 |
| [82] |
Liu T, Broecker W S, Stein M. Rock varnish evidence for a Younger Dryas wet period in the Dead Sea basin[J]. Geophysical Research Letters, 2013, 40(10): 2229-2235. DOI:10.1002/grl.50492 |
| [83] |
Gvirtzman G, Wieder M. Climate of the last 53, 000 years in the eastern Mediterranean, based on soil-sequence stratigraphy in the coastal plain of Israel[J]. Quaternary Science Reviews, 2001, 20(18): 1827-1849. DOI:10.1016/S0277-3791(01)00008-7 |
| [84] |
Zachos J, Pagani M, Sloan L, et al. Trends, rhythms, and aberrations in global climate 65 Ma to present[J]. Science, 2001, 292(5517): 686-693. DOI:10.1126/science.1059412 |
| [85] |
Zanazzi A, Kohn M J, MacFadden B J, et al. Large temperature drop across the Eocene-Oligocene transition in Central North America[J]. Nature, 2007, 445(7128): 639. DOI:10.1038/nature05551 |
| [86] |
肖国桥, 张仲石, 姚政权. 始新世-渐新世气候转变研究进展[J]. 地质论评, 2012, 58(1): 91-105. Xiao Guoqiao, Zhang Zhongshi, Yao Zhengquan. The Eocene-Oligocene climate transition:Review of recent progress[J]. Geological Review, 2012, 58(1): 91-105. DOI:10.3969/j.issn.0371-5736.2012.01.009 |
| [87] |
Ericson J E. Strontium isotope characterization in the study of prehistoric human ecology[J]. Journal of Human Evolution, 1985, 14(5): 503-514. DOI:10.1016/S0047-2484(85)80029-4 |
| [88] |
Hajj F, Poszwa A, Bouchez J, et al. Radiogenic and "stable" strontium isotopes in provenance studies:A review and first results on archaeological wood from shipwrecks[J]. Journal of Archaeological Science, 2017, 86: 24-49. DOI:10.1016/j.jas.2017.09.005 |
| [89] |
Buzon M R, Bowen G J. Oxygen and carbon isotope analysis of human tooth enamel from the New Kingdom site of Tombos in Nubia[J]. Archaeometry, 2010, 52(5): 855-868. DOI:10.1111/arch.2010.52.issue-5 |
| [90] |
Price T D, Burton J H, Bentley R A. The characterization of biologically available strontium isotope ratios for the study of prehistoric migration[J]. Archaeometry, 2002, 44(1): 117-135. DOI:10.1111/arch.2002.44.issue-1 |
| [91] |
Stanish C, Tantaleán H, Knudson K. Feasting and the evolution of cooperative social organizations circa 2300 BP in Paracas culture, Southern Peru[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(29): E6716-E6721. DOI:10.1073/pnas.1806632115 |
| [92] |
Evans J, Stoodley N, Chenery C. A strontium and oxygen isotope assessment of a possible fourth century immigrant population in a Hampshire cemetery, southern England[J]. Journal of Archaeological Science, 2006, 33(2): 265-272. DOI:10.1016/j.jas.2005.07.011 |
| [93] |
Hoppe K A, Koch P L, Carlson R W, et al. Tracking mammoths and mastodons:Reconstruction of migratory behavior using strontium isotope ratios[J]. Geology, 1999, 27(5): 439-442. DOI:10.1130/0091-7613(1999)027<0439:TMAMRO>2.3.CO;2 |
| [94] |
Holliday T W, Falsetti A B. Lower limb length of European early modern humans in relation to mobility and climate[J]. Journal of Human Evolution, 1995, 29(2): 141-153. DOI:10.1006/jhev.1995.1050 |
| [95] |
Féblot-Augustins J. Mobility strategies in the late Middle Palaeolithic of central Europe and western Europe:elements of stability and variability[J]. Journal of Anthropological Archaeology, 1993, 12(3): 211-265. DOI:10.1006/jaar.1993.1007 |
| [96] |
Frémondeau D, De Cupere B, Evin A, et al. Diversity in pig husbandry from the Classical-Hellenistic to the Byzantine periods:An integrated dental analysis of Düzen Tepe and Sagalassos assemblages(Turkey)[J]. Journal of Archaeological Science:Reports, 2017, 11: 38-52. DOI:10.1016/j.jasrep.2016.11.030 |
| [97] |
Marciniak A, Evans J, Henton E, et al. Animal husbandry in the early and middle Neolithic settlement at Kopydłowo in the Polish lowlands:A multi-isotope perspective[J]. Archaeological and Anthropological Sciences, 2017, 9(7): 1461-1479. DOI:10.1007/s12520-017-0485-6 |
| [98] |
Hoppe K A, Stover S M, Pascoe J R, et al. Tooth enamel biomineralization in extant horses:Implications for isotopic microsampling[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2004, 206(3): 355-365. |
| [99] |
Bendrey R, Vella D, Zazzo A, et al. Exponentially decreasing tooth growth rate in horse teeth:Implications for isotopic analyses[J]. Archaeometry, 2015, 57(6): 1104-1124. DOI:10.1111/arcm.v57.6 |
| [100] |
杨岳衡, 吴福元, 谢烈文, 等. 地质样品Sr同位素激光原位等离子体质谱(LA-MC-ICP-MS)测定[J]. 岩石学报, 2009, 25(12): 3431-3441. Yang Yueheng, Wu Fuyuan, Xie Liewen, et al. In-situ Sr isotope measurement of natural geological samples by LA-MC-ICP-MS[J]. Acta Petrologica Sinica, 2009, 25(12): 3431-3441. |
| [101] |
Passey B H, Cerling T E. In situ stable isotope analysis(δ13C, δ18O)of very small teeth using laser ablation GC/IRMS[J]. Chemical Geology, 2006, 235(3): 238-249. |
| [102] |
Horstwood M S A, Evans J A, Montgomery J. Determination of Sr isotopes in calcium phosphates using laser ablation inductively coupled plasma mass spectrometry and their application to archaeological tooth enamel[J]. Geochimica et Cosmochimica Acta, 2008, 72(23): 5659-5674. DOI:10.1016/j.gca.2008.08.016 |
| [103] |
Woodhead J, Swearer S, Hergt J, et al. In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS:Interference corrections, high spatial resolution and an example from otolith studies[J]. Journal of Analytical Atomic Spectrometry, 2005, 20(1): 22-27. DOI:10.1039/b412730g |
| [104] |
Copeland S R, Sponheimer M, Lee-Thorp J, et al. Strontium isotope ratios in fossil teeth from South Africa:Assessing laser ablation MC-ICP-MS analysis and the extent of diagenesis[J]. Journal of Archaeological Science, 2010, 37(7): 1437-1446. DOI:10.1016/j.jas.2010.01.003 |
| [105] |
Willmes M, Kinsley L, Moncel M H, et al. Improvement of laser ablation in situ micro-analysis to identify diagenetic alteration and measure strontium isotope ratios in fossil human teeth[J]. Journal of Archaeological Science, 2016, 70: 102-116. DOI:10.1016/j.jas.2016.04.017 |
| [106] |
Lewis J, Coath C D, Pike A W G. An improved protocol for 87Sr/86Sr by laser ablation multi-collector inductively coupled plasma mass spectrometry using oxide reduction and a customised plasma interface[J]. Chemical Geology, 2014, 390: 173-181. DOI:10.1016/j.chemgeo.2014.10.021 |
2 University of Chinese Academy of Sciences, Beijing 100049;
3 Institutes of Earth Science, Chinese Academy of Sciences, Beijing 100029)
Abstract
Stable isotope analysis of tooth enamel is widely used to reconstruct paleodietary, paleoenvironment and human migration patterns in the past. Previous studies mainly focus on the bulk sampling of enamel, which reflects the average isotopic signals during enamel formation. Recently, interest has focused on the possibilities of microsampling to reconstruct seasonal migration patterns of animals, which can be divided into two sampling strategies:(1) Intra-tooth sequential sampling. This sampling typically is based on evenly spaced sampling along the growth axis of the tooth in order to obtain a temporal sequence of isotopic changes recorded in enamel. Although this method is widely applied in paleoenvironment and archeology studies, it lacks precise time-series information and requires a large amount of samples. (2) High spatial resolution sampling, which is using high spatial resolution instruments for in situ isotopic analysis and is regarded as a micro-destructive approach. Based on enamel incremental features, this method potentially can accurately capture seasonal fluctuations of isotopic compositions in enamel, but it has lower precision and accuracy. High spatial resolution sampling has a great potential in reconstructing paleoenvironment, paleodietary and human migration patterns in archaeology studies although it is at initial stage. 2019, Vol.39
2019, Vol.39

