文章信息
- 王昊, 张辉, 张继华, 赵云峰
- WANG Hao, ZHANG Hui, ZHANG Ji-hua, ZHAO Yun-feng
- 非共价键表面修饰的石墨烯/聚合物复合材料研究进展
- Research Progress on Graphene/Polymer Composites with Non-covalent Surface Modification
- 材料工程, 2018, 46(7): 44-52
- Journal of Materials Engineering, 2018, 46(7): 44-52.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2016.000011
-
文章历史
- 收稿日期: 2015-12-31
- 修订日期: 2017-12-01
2. 清华大学 材料学院新型陶瓷与精细工艺国家重点实验室, 北京 100084
2. State Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
碳纳米材料的研究经历了漫长的过程。1947年Wallace[1]首次提出了纳米石墨电子结构的相关理论,为碳纳米材料的发展奠定基础。自1985年起,零维富勒烯[2]及一维碳纳米管[3]的发现引起了材料界的轰动。2004年Geim等[4]成功制备出单层石墨烯,填补了碳纳米材料中二维材料的空缺,将碳纳米材料领域的研究工作推向高峰。
石墨烯是一种具有sp2杂化结构的单原子层碳纳米材料[5],其具有密度低(0.77mg/m2)[6]、表面积大(约2600m2/g)[7]、高强高模(拉伸模量1.1TPa、弹性模量125GPa)[8]、高电导率(室温电子迁移率200000cm2/(V·s)[9]、低电阻率(10-6Ω·cm)[10]及高热导率(5000W/(m·K))[11]等优异性能。将石墨烯作为功能填料加入聚合物中可以改善聚合物的性能,石墨烯/聚合物复合材料在高性能电池[12]、超级电容器[13]、传感器[14]、储能器件[15]和光电器件[16-17]等领域具有良好的应用前景。
然而,石墨烯与聚合物相容性差,易自聚集,难以在复合材料中发挥其全部性能[18-19]。因此,研究者通常先对石墨烯进行表面修饰再与聚合物复合成型[17, 20-22]。共价键修饰和非共价键修饰是石墨烯表面改性的主要方法[21-22]。共价键修饰可以有效提高石墨烯的表面活性,但存在破坏石墨烯sp2杂化结构、降低石墨烯电学及热学性能等缺点[23-26]。与共价键修饰相比,非共价键修饰可以在活化石墨烯表面性质的同时保护其结构,更利于石墨烯在聚合物复合材料中的性能发挥[20-22, 26-27]。本文主要介绍石墨烯非共价键修饰的研究进展,总结并讨论非共价键修饰的石墨烯对聚合物复合材料性能的影响。
1 石墨烯的制备石墨烯的制备方法可以分为机械剥离法[4]、液相剥离法[28]、电化学剥离法[29]、化学气相沉积法[30]、外延生长法[31]、碳纳米管切割法[32]及化学氧化还原法[33]。采用非氧化还原法制备的石墨烯结构完整、性能良好,但这些方法制备效率较低;采用化学氧化还原方法制备的石墨烯缺陷相对较多,但该方法可以实现石墨烯的宏量制备,且制备过程中易产生含氧官能团,有利于进行后续表面修饰。作为复合材料填充体应用的石墨烯常采用化学氧化还原法制备。
2 石墨烯的非共价键表面修饰石墨烯的非共价键表面修饰,即利用一些有机物与石墨烯平面六元环结构间存在的疏水吸附[34-35]、π-π或阳离子-π[36-37]、氢键[38-39]等物理相互作用,使其附着于石墨烯表面,同时通过有机物具有的特殊官能团或化学结构改变石墨烯的表面性质。
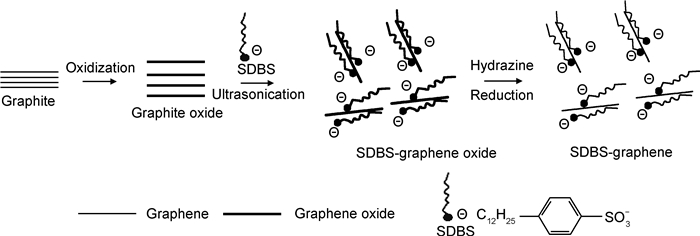
2.1 疏水吸附作用两亲性分子是一类同时具有疏水段和亲水段结构的有机物。通过疏水段与石墨烯结合,两亲性分子的亲水段可以改变石墨烯的表面性质,使其在水溶液中分散良好[34]。Kundu等[35]将十二烷基苯磺酸钠(SDBS)与氧化石墨烯(GO)混合,并对GO进行化学还原,得到了能在水中稳定分散三个月的石墨烯。如图 1所示,SDBS通过疏水吸附作用有序地排列在石墨烯表面,使石墨烯呈现亲水性,同时SDBS阴离子间存在的静电排斥作用使石墨烯片稳定分散。研究表明,采用胆酸钠(SC)[40]、十八烷基三甲基氯化铵(STAC)[41]等两亲性分子均可以有效改变石墨烯的表面性质。
此外,极性聚合物同样可以改善石墨烯的表面性质。Ren等[42]将氧化石墨在聚丙烯酰胺(PAM)的水溶液中机械剥离并化学还原,提高了石墨烯的水溶液分散性。拉曼光谱和原子力显微镜的表征结果说明,PAM与石墨烯通过疏水吸附作用紧密结合,且PAM分子中的氨基官能团起到改变石墨烯表面性质的作用。Ke等[43]将共聚物Pluronic F127与氧化石墨烯混合,采用水热还原法得到了非共价键修饰的石墨烯。在混合过程中,F127与石墨烯间存在的物理吸附相互作用使聚合物分子易于进入石墨烯片层间,诱导片层剥离,改善石墨烯的分散性。
2.2 π-π或阳离子-π作用具有共轭结构的有机小分子可以与石墨烯间产生π-π堆积相互作用,进而通过有机物的特殊官能团改善石墨烯的表面活性。7, 7, 8, 8-四氰基对苯二醌二甲烷(TCNQ)是一种典型的共轭有机物,其阴离子可以吸附于石墨烯表面,使石墨烯具有亲水性[36]。清华大学的Xu等[44]采用芘丁酸盐修饰石墨烯,使石墨烯能以较高浓度分散于水中。紫外可见光谱中吸收峰的红移及石墨烯/芘丁酸的荧光猝灭效应证明,二者间存在较强的π-π相互作用。Tang等[45]采用工业染料罗丹明-B(RhB)修饰石墨烯,使石墨烯可以均匀分散于聚丙烯醇(PVA)的水溶液中。同样利用π-π堆积相互作用,羧酸六苯并苯衍生物[46]、9-蒽酸[47]、蒽醌[48]、荧光增白剂VBL[49]、纤维素衍生物[50]等一系列共轭结构的有机物均能与石墨烯紧密结合,改变石墨烯的表面性质。
同时,许多研究者也采用具有共轭结构的聚合物改性石墨烯。Stankovich等[51]首次采用聚4-苯乙烯磺酸钠(PSS)原位修饰氧化还原石墨烯(rGO)。随着还原过程的进行,石墨烯sp2杂化的碳六元环结构逐渐恢复,PSS与石墨烯紧密结合,改善石墨烯的表面活性并阻止石墨烯自聚集,使石墨烯可以在水中长时间稳定分散。PPE-SO3-是一种多苯环结构共轭磺酸盐,Yang等[52]采用简单的超声处理方法将这种聚合物盐与石墨烯混合,赋予石墨烯良好的亲水性。紫外可见光谱、荧光光谱及原子力显微镜的测试结果均表明PPE-SO3-分子通过π-π相互作用附着于石墨烯表面。此外,PPE-SO3-的引入使石墨烯展现出特殊的光电性能,PPE-SO3-/石墨烯复合物具有成为理想光电器件材料的潜力。作为高分子聚合物的一种,嵌段聚合物具有较强可设计性,将其引入石墨烯体系,可以通过设计嵌段聚合物的结构调节石墨烯的表面性质。Qi等[53]采用具有线-棒-线结构的三嵌段共聚物(PEG-OPE-PEG)修饰石墨烯。通过π-π相互作用,嵌段共聚物的刚性OPE段吸附于石墨烯表面,同时柔性PEG段赋予石墨烯亲水性。此外,PEG段良好的生物相容性使石墨烯具有作为水溶性药物载体的应用前景。有研究报道表示,采用磺化聚苯胺(SPANI)[54]、聚3-己基噻吩(P3HT)[55]、聚(3, 4-乙烯二氧噻吩):聚磺化苯乙烯(PEDOT:PSS)[56]等共轭聚合物也可以有效改善石墨烯的表面活性。
部分生物大分子同样具有共轭结构,采用这些生物大分子修饰石墨烯不仅可以改善其表面性质还能赋予石墨烯特殊的生物医学性能,拓宽石墨烯的应用领域。如采用芘-羟丙基纤维素(PYR-NHS)修饰的石墨烯可以应用于蛋白质的微缩成像[57],采用肝素修饰的石墨烯具有良好的血液相容性,可以作为药物载体使用[58]。此外,采用DNA[59-60]、牛血清蛋白[61]等修饰石墨烯,均可以使石墨烯具备特殊的性能。
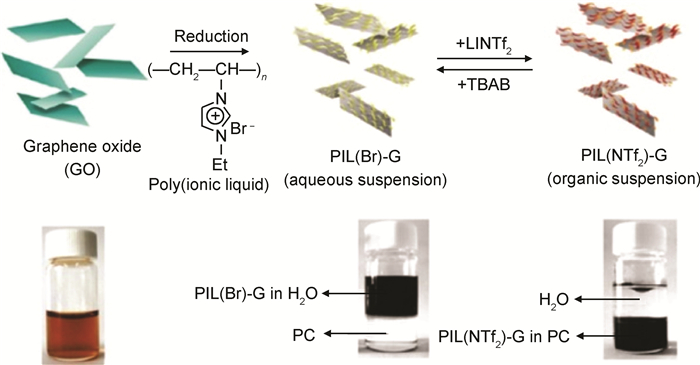
离子液体(IL)是一类化学性质稳定、仅由阴阳离子构成的室温熔融盐[62-63]。除π-π相互作用外,IL的阳离子与石墨烯间还存在阳离子-π相互作用,而阴离子可以改变石墨烯的表面性质。Kim等[64]采用聚乙烯基咪唑溴(PIL(Br))修饰氧化石墨烯并进行原位还原。如图 2所示,通过在体系中先后加入油溶性的双三氟甲磺酰亚氨阴离子或水溶性的溴离子,借助于阴离子交换反应,使石墨烯在水相或有机相中选择性分散,展现出特殊的相转移特性。Yang等[37]采用液相混合的方法制备了苄基离子液体修饰的石墨烯,使石墨烯可以在水、DMSO和DMF等极性溶剂中均匀分散。由于表面能接近石墨烯[65-66],将IL作为溶剂可以促进石墨烯的剥离。Nuvoli等[66]和Wang等[67]将石墨直接在IL中超声剥离,均得到了高浓度石墨烯分散液。相比于其他有机物,IL自身具有较宽的电化学窗口及优异的物理、化学稳定性[62-63],因此采用其修饰石墨烯不仅可以改善石墨烯的表面性质,而且还能赋予石墨烯优异的电化学性能[68-70]。
采用氧化还原法制备的石墨烯的表面仍残留部分含氧基团,一些有机物结构中的极性官能团可以与这些含氧基团形成氢键。Yang等[38]将氧化石墨烯(GO)在聚乙烯醇(PVA)中原位还原,制备出石墨烯-PVA薄膜,红外光谱的峰位移动说明石墨烯与PVA间存在的氢键作用。氧化还原法制备的石墨烯特殊的小范围sp2杂化结构部分限制了π电子的自由移动,使其具有荧光效应。Kundu等[71]分别采用甲基纤维素(MC)和PVA修饰石墨烯,发现聚合物与石墨烯间形成的氢键可以增强石墨烯的荧光效应。
3 非共价键修饰的石墨烯/聚合物复合材料石墨烯/聚合物复合材料的性能不但取决于石墨烯及聚合物基体的自身性能,而且还与二者间的界面结合强度及填料在聚合物基体中的分散性有关[7, 12, 72-73]。先对石墨烯进行表面修饰再与聚合物复合,可以提高石墨烯的分散性及其与聚合物基体的相容性,从而优化复合材料性能[21-22, 74]。通常,非共价键修饰的石墨烯/聚合物复合材料具有较优异的电学性能和力学性能。
3.1 非共价键修饰的石墨烯/聚合物复合材料电学性能在聚合物中加入石墨烯构成复合材料可以改善聚合物的电学性能。添加一定数量的石墨烯后,通过石墨烯和包覆石墨烯周围的聚合物薄层可实现隧道效应,达到导电渗滤阈值,形成导电通路,使复合材料具备导电性质[75]。石墨烯对聚合物材料电导率的影响通过经典渗滤阈值模型计算:
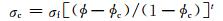
|
(1) |
式中:ϕ为填料体积分数;ϕc为渗滤阈值;σf为填料电导率;σc为复合材料电导率;t为临界指数(通常与复合材料体系的维度有关)。
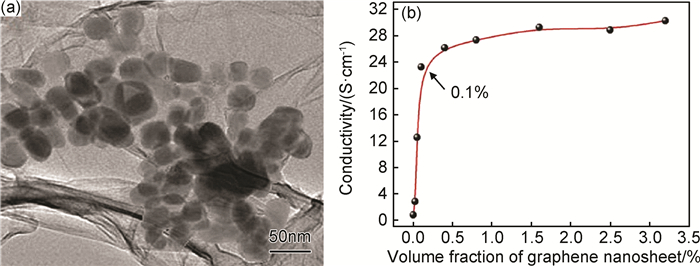
非共价键修饰可以在保护石墨烯结构的同时改善石墨烯的表面活性,使石墨烯可以在聚合物基体中均匀分散,进一步优化石墨烯/聚合物复合材料的电学性能[74]。Khamlich等[76]采用两亲性分子SDS修饰的石墨烯与吡咯单体混合,原位聚合得到聚吡咯(PPy)复合材料(图 3)。如图 3(a)所示,吡咯聚合形成的纳米颗粒通过SDS的疏水吸附作用紧密附着于石墨烯片层表面。这种特殊的结构使石墨烯/石墨烯、石墨烯/PPy间的接触面积明显增加,促进了聚合物导电网络的形成,使复合材料的渗滤阈值仅为0.09%。此外,具有阴离子结构的SDS还可以起到PPy聚阳离子导电掺杂剂的作用,增强聚合物基体自身的导电性能,使复合材料体积分数为3.2%时电导率高达30S/cm。Hsiao等[41]分别采用十八烷基三甲基氯化铵(STAC)修饰的石墨烯(S-GNS)和原始石墨烯(P-GNS)填充聚氨酯(WPU),制备聚合物复合材料。微观形貌观测结果表明,S-GNS与聚合物基体相容性更好,这是由于STAC使石墨烯带有正电荷,可以与带负电荷的WPU分子间产生静电吸附作用。良好的相容性使S-GNS/WPU更易形成导电网络,因此具有更低的导电阈值及更高的电导率。Liu课题组[77]合成了芘基功能化的RAFT引发剂,利用石墨烯与引发剂间的π-π相互作用,使二甲基氨乙基丙烯酸酯、丙烯酸和苯乙烯在石墨烯表面原位聚合,得到纳米复合材料薄膜。几种石墨烯/聚合物薄膜均具有较高的电导率,聚合物分子量的大小、分子链的构象会影响复合材料导电性能。此外,也有报道采用PSS[70, 78]和离子液体[61, 79-80]修饰石墨烯,制备出导电性能良好的聚合物复合材料。
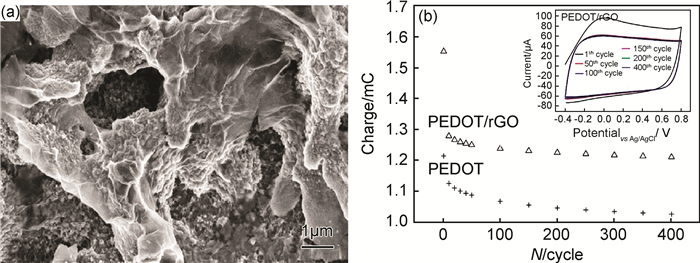
聚吡咯、聚苯胺、聚噻吩等导电聚合物具有较高的电荷储存密度,可以作为超级电容器电极材料使用[81]。但纯导电聚合物材料充放电循环稳定性较差,将表面修饰的石墨烯引入导电聚合物构成复合材料可以有效解决这个问题。Yan等[82]制备了SDBS修饰的石墨烯,将其填充PPy,使复合材料在电流密度为0.1A/g时的单位电容量达到277F/g。在复合材料中,填料-两亲性分子-聚合物分子间较强的相互作用对电荷的稳定储存起到了重要的作用。Damlin等[83]在IL的辅助下将GO与乙烯二氧噻吩(EDOT)混合,通过电化学聚合和还原得到石墨烯/PEDOT复合材料。IL在体系中起到了使石墨烯均匀分散和诱导PEDOT形成多孔结构的作用(图 4(a))。这种三维多孔结构使复合材料的比表面积明显增大,可以实现电解液的高速渗透和快速的氧化还原反应,从而提高电荷的储存能力。如图 4(b)所示,IL和石墨烯的引入使PEDOT材料在400次充放电循环后的电荷储量和稳定性分别提高20%和7%。
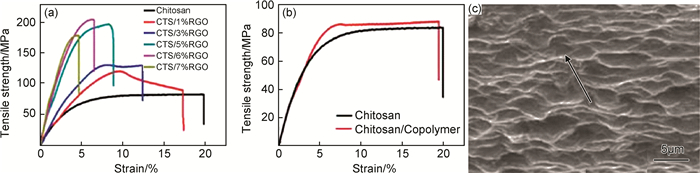
石墨烯极高的理论强度使其在填充量很低时即可改善聚合物材料的力学性能[39, 72]。然而,表面光滑的石墨烯与聚合物分子间较弱的相互作用使二者界面处容易形成应力集中点,限制了复合材料力学性能的进一步提高[84]。进行非共价键修饰可以通过加强分子间相互作用提高石墨烯/聚合物间的界面结合强度[27, 73]。Li等[23]采用PSS修饰的石墨烯填充环氧树脂,构造高性能结构复合材料。PSS提高了石墨烯和树脂基体间的相互作用力,增强了二者的界面结合强度,使材料拉伸时可以在界面处形成有效的应力传递。因此,复合材料的拉伸强度在石墨烯填充量仅为1%(质量分数,下同)时达到89.8MPa,明显高于未经表面修饰的石墨烯/环氧树脂。此外,Halpin-Tsai理想模型的计算拟合结果与实验数据高度吻合,证明表面修饰使石墨烯在聚合物中均匀分散。Wang等[85]采用三嵌段共聚物PEO-PPO-PEO修饰石墨烯,制备了石墨烯/壳聚糖(RGO/CTS)复合材料薄膜。如图 5的应力-应变曲线所示,共聚物修饰的石墨烯填充的复合材料拉伸强度随石墨烯填充量的增加而增加,在填充量为6%时达到最大值。而嵌段共聚物自身无法提高聚合物薄膜的拉伸强度,说明共聚物使石墨烯在体系中均匀分散及石墨烯自身优异的力学性能是聚合物材料力学性能提高的重要因素。此外,由于刚性嵌段共聚物的修饰,聚合物包裹的石墨烯片层沿薄膜水平方向排列,这种高度取向的结构使复合材料内的石墨烯可以更有效地承载径向应力,有助于提高聚合物薄膜的面内强度。利用类似机理,也有研究者采用共轭有机物罗丹明B[45]、两亲性分子[86]修饰的石墨烯填充聚合物材料,改善了复合材料的力学性能。
非共价键修饰的石墨烯/聚合物复合材料还具有优异的热学、电磁学、摩擦学性能。如Xiong等[87]将离子液体修饰的氧化石墨烯填充溴化丁基橡胶,增强了石墨烯和橡胶间界面结合强度,使声子散射程度降低,因此,复合材料在填充量为4%时热导率较纯聚合物提高了30%。复合材料电磁屏蔽强度的提高与填料导电网络的形成有关。Hsiao等[41]采用十八烷基三甲基氯化铵修饰的石墨烯填充聚氨酯,提高了石墨烯填料的分散性,使聚合物在8.2~12.4GHz范围内具备32dB的电磁屏蔽强度,明显优于未修饰的石墨烯/聚氨酯对照组。Saurin等[88]采用IL修饰的石墨烯填充环氧树脂,复合材料的摩擦因数较纯环氧树脂降低了27%。在体系中,IL优异的自润滑性能及其诱导石墨烯的均匀分散是树脂耐摩擦磨损性能提高的主要原因。
4 结束语对石墨烯进行非共价键修饰,可以在不破坏石墨烯结构的基础上增强石墨烯的分散性、提高石墨烯的表面活性。经过非共价键修饰的石墨烯可以优化聚合物复合材料的电学、力学等性能。石墨烯的非共价键修饰具有保护石墨烯结构、修饰工艺简单易行等优点。然而,相比于共价键修饰,非共价键修饰仍存在修饰物与石墨烯间相互作用力较弱、修饰结构易被破坏等缺点。针对上述问题设计新型结构的修饰物,增强修饰物与石墨烯间的非共价键相互作用强度,并进一步优化石墨烯性能将是今后石墨烯表面修饰领域研究的重点。对于非共价键修饰的石墨烯-聚合物复合材料,今后的研究工作需要围绕以下三方面展开:(1)通过设计修饰物的分子结构控制石墨烯-聚合物界面结合强度,进而精确调控聚合物复合材料的宏观性能;(2)通过混合或加工工艺控制石墨烯在聚合物基体中的排列方式,使聚合物复合材料展现出特殊性能;(3)开发新型制备及加工技术,实现石墨烯-聚合物复合材料低成本、高质量、大规模、绿色无污染的制备。
近年来,关于石墨烯及其聚合物复合材料的研究工作愈发火热,也取得了丰硕的研究成果。然而,时至今日,石墨烯及石墨烯复合材料仍未大规模应用于工业生产。关于石墨烯及其衍生物工业化的推广将是众多研究者们共同的任务。
| [1] | WALLACE P R. The band theory of graphite[J]. Physical Review, 1947, 71 (9): 622–634. DOI: 10.1103/PhysRev.71.622 |
| [2] | KROTO H W, HEATH J R, O'BRIEN S C, et al. C60:buckminsterfullerene[J]. Nature, 1985, 318 (6042): 162–163. DOI: 10.1038/318162a0 |
| [3] | LIJIMA S. Helical microtubules of graphitic carbon[J]. Nature, 1991, 354 (6348): 56–58. DOI: 10.1038/354056a0 |
| [4] | MOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306 (5696): 666–669. |
| [5] | GEIM A K, NOVOSELOV K S. The rise of graphene[J]. Nature Materials, 2007, 6 (3): 183–191. DOI: 10.1038/nmat1849 |
| [6] | VERMA D, GOPE P C, SHANDILYAA, et al. Mechanical-thermal-electrical and morphological properties of graphene reinforced polymer composites:a review[J]. Transactions of the Indian Institute of Metals, 2014, 67 (6): 803–816. DOI: 10.1007/s12666-014-0408-5 |
| [7] | RAO C N R, SOOD A K, VOGGU R, et al. Some novel attributes of graphene[J]. Journal of Physical Chemistry Letters, 2010, 1 (2): 572–580. DOI: 10.1021/jz9004174 |
| [8] | LEE C, WEI X D, KYSAR J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science, 2008, 321 (5887): 385–388. DOI: 10.1126/science.1157996 |
| [9] | BOLOTIN K I, SIKES K J, JIANG Z, et al. Ultrahigh electron mobility in suspended graphene[J]. Solid State Communications, 2008, 146 (9/10): 351–355. |
| [10] | AVOURIS P, CHEN Z H, PEREBEINOS V. Carbon-based electronics[J]. Nature Nanotechnology, 2007, 2 (10): 605–615. DOI: 10.1038/nnano.2007.300 |
| [11] | BALANDINAA, GHOSH S, BAO W Z, et al. Superior thermal conductivity of single-layer graphene[J]. Nano Letters, 2008, 8 (3): 902–907. DOI: 10.1021/nl0731872 |
| [12] | KIM K S, ZHAO Y, JANG H, et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes[J]. Nature, 2009, 457 (7230): 706–710. DOI: 10.1038/nature07719 |
| [13] | LIU C G, YU Z N, NEFF D, et al. Graphene-based supercapacitor with an ultrahigh energy density[J]. Nano Letters, 2010, 10 (12): 4863–4868. DOI: 10.1021/nl102661q |
| [14] | WU S X, HE Q Y, TAN C L, et al. Graphene-based electrochemical sensors[J]. Small, 2013, 9 (8): 1160–1172. DOI: 10.1002/smll.201202896 |
| [15] | PUMERA M. Graphene-based nanomaterials for energy storage[J]. Energy & Environmental Science, 2011, 4 (3): 668–674. |
| [16] | KUILLA T, BHADRA S, YAO D H, et al. Recent advances in graphene based polymer composites[J]. Progress in Polymer Science, 2010, 35 (11): 1350–1375. DOI: 10.1016/j.progpolymsci.2010.07.005 |
| [17] | RAMANATHAN T, ABDALA A A, STANKOVICH S, et al. Functionalized graphene sheets for polymer nanocomposites[J]. Nature Nanotechnology, 2008, 3 (6): 327–331. DOI: 10.1038/nnano.2008.96 |
| [18] | LI D, MULLER M B, GIJLE S, et al. Processable aqueous dispersions of graphene nanosheets[J]. Nature Nanotechnology, 2008, 3 (2): 101–105. DOI: 10.1038/nnano.2007.451 |
| [19] | SI Y C, SAMULSKI E T. Synthesis of water soluble graphene[J]. Nano Letters, 2008, 8 (6): 1679–1682. DOI: 10.1021/nl080604h |
| [20] | LAYEK R K, NANDI A K. A review on synthesis and properties of polymer functionalized graphene[J]. Polymer, 2013, 54 (19): 5087–5103. DOI: 10.1016/j.polymer.2013.06.027 |
| [21] | KUILA T, BOSE S, MISHRA A K, et al. Chemical functionalization of graphene and its applications[J]. Progress in Materials Science, 2012, 57 (7): 1061–1105. DOI: 10.1016/j.pmatsci.2012.03.002 |
| [22] | SARAVANAN N, RAJASEKAR R, MAHALAKSHMI S, et al. Graphene and modified graphene-based polymer nanocomposites-a review[J]. Journal of Reinforced Plastics and Composites, 2014, 33 (12): 1158–1180. DOI: 10.1177/0731684414524847 |
| [23] | LI Y C, TANG J G, HUANG L J, et al. Facile preparation, characterization and performance of noncovalently functionalized graphene/epoxy nanocomposites with poly(sodium 4-styrenesulfonate)[J]. Composites Part A:Applied Science and Manufacturing, 2015, 68 : 1–9. DOI: 10.1016/j.compositesa.2014.09.016 |
| [24] | SCHWAMB T, BURG B R, SCHIRMER N C, et al. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures[J]. Nanotechnology, 2009, 20 (40): 405704. DOI: 10.1088/0957-4484/20/40/405704 |
| [25] |
张力, 吴俊涛, 江雷. 石墨烯及其聚合物纳米复合材料[J].
化学进展, 2014, 26 (4): 560–571.
ZHANG L, WU J T, JIANG L. Graphene and its polymer nanocomposites[J]. Progress in Chemistry, 2014, 26 (4): 560–571. |
| [26] |
范彦如, 赵宗彬, 万武波, 等. 石墨烯非共价键功能化及应用研究进展[J].
化工进展, 2011, 30 (7): 1509–1520.
FAN Y R, ZHAO Z B, WAN W B, et al. Research progress of non-covalent functionalization and applications of graphene[J]. Chemical Industry and Engineering Progress, 2011, 30 (7): 1509–1520. |
| [27] |
唐征海, 郭宝春, 张立群, 等. 石墨烯/橡胶纳米复合材料[J].
高分子学报, 2014 (7): 865–877.
TANG Z H, GUO B C, ZHANG L Q, et al. Graphene/rubber nanocomposites[J]. Acta Polymerica Sinica, 2014 (7): 865–877. DOI: 10.11777/j.issn1000-3304.2014.14084 |
| [28] | LOTYA M, HERNANDEZ Y, KING P J, et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions[J]. Journal of American Chemical Society, 2009, 131 (10): 3611–3620. DOI: 10.1021/ja807449u |
| [29] | LIU N, LUO F, WU H X, et al. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite[J]. Advanced Functional Materials, 2008, 18 (10): 1518–1525. DOI: 10.1002/(ISSN)1616-3028 |
| [30] | SHEN J M, FENG Y T. Formation of flower-like carbon nano-sheet aggregations and their electrochemical application[J]. Journal of Physical Chemistry C, 2008, 112 (34): 13114–13120. DOI: 10.1021/jp802285c |
| [31] | BERGER C, SONG Z M, LI X B, et al. Electronic confinement and coherence in patterned epitaxial graphene[J]. Science, 2006, 312 (5777): 1191–1196. DOI: 10.1126/science.1125925 |
| [32] | JIAO L Y, ZHANG L, WANG X R, et al. Narrow graphene nanoribbons from carbon nanotubes[J]. Nature, 2009, 458 (7240): 877–880. DOI: 10.1038/nature07919 |
| [33] | STANKOVICH S, DIKIN D A, PINER R D, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide[J]. Carbon, 2007, 45 (7): 1558–1565. DOI: 10.1016/j.carbon.2007.02.034 |
| [34] | SMITH R J, LOTYA M, COLEMAN J N. The importance of repulsive potential barriers for the dispersion of graphene using surfactants[J]. New Journal of Physics, 2010, 12 (12): 125008. DOI: 10.1088/1367-2630/12/12/125008 |
| [35] | KUNDU A, LAYEK R K, NANDI A K. Enhanced fluorescent intensity of graphene oxide-methyl cellulose hybrid in acidic medium:sensing of nitro-aromatics[J]. Journal of Materials Chemistry, 2012, 22 (16): 8139–8144. DOI: 10.1039/c2jm30402c |
| [36] | HAO R, QIAN W, ZHANG L H, et al. Aqueous dispersions of TCNQ-anion-stabilized graphene sheets[J]. Chemical Communications, 2008, 48 : 6576–6578. |
| [37] | YANG Y K, HE C E, PENG R G, et al. Non-covalently modified graphene sheets by imidazolium ionic liquids for multifunctional polymer nanocomposites[J]. Journal of Materials Chemistry, 2012, 12 (12): 5666–5675. |
| [38] | YANG X M, LI L, SHANG S M, et al. Synthesis and characterization of layer-aligned poly(vinyl alcohol)/graphene nanocomposites[J]. Polymer, 2010, 51 (15): 3431–3435. DOI: 10.1016/j.polymer.2010.05.034 |
| [39] | CHANG H X, WANG G F, YANG A, et al. A transparent, flexible, low-temperature, and solution-processible graphene composite electrode[J]. Advanced Functional Materials, 2010, 20 (17): 2893–2902. DOI: 10.1002/adfm.201000900 |
| [40] | DE S, KING P J, LOTYA M, et al. Flexible, transparent, conducting films of randomly stacked graphene from surfactant-stabilized, oxide-free graphene dispersions[J]. Small, 2010, 6 (3): 458–464. DOI: 10.1002/smll.v6:3 |
| [41] | HSIAO S T, MA C M, TIEN H W, et al. Using a non-covalent modification to prepare a high electromagnetic interference shielding performance graphene nanosheet/water-borne polyurethane composite[J]. Carbon, 2013, 60 : 57–66. DOI: 10.1016/j.carbon.2013.03.056 |
| [42] | REN L L, LIU T X, GUO J, et al. A smart pH responsive graphene/polyacrylamide complex via noncovalent interaction[J]. Nanotechnology, 2010, 21 (33): 335701. DOI: 10.1088/0957-4484/21/33/335701 |
| [43] | KE Q Q, LIU Y Q, LIU H J, et al. Surfactant-modified chemically reduced graphene oxide for electrochemical supercapacitors[J]. RSC Advances, 2014, 4 (50): 26398–26406. DOI: 10.1039/C4RA03826F |
| [44] | XU Y X, BAI H, LU G W, et al. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets[J]. Journal of American Chemical Society, 2008, 130 (18): 5856–5857. DOI: 10.1021/ja800745y |
| [45] | TANG Z H, LEI Y D, GUO B C, et al. The use of rhodamine B-decorated graphene as a reinforcement in polyvinyl alcohol composites[J]. Polymer, 2012, 53 (2): 673–680. DOI: 10.1016/j.polymer.2011.11.056 |
| [46] | GHOSH A, RAO K V, GEORGE S J, et al. Noncovalent functionalization, exfoliation, and solubilization of graphene in water by employing a fluorescent coronene carboxylate[J]. Chemistry-A European Journal, 2010, 16 (9): 2700–2704. DOI: 10.1002/chem.200902828 |
| [47] | KHANRA P, UDDIN M E, KIM N H, et al. Electrochemical performance of reduced graphene oxide surface-modified with 9-anthracene carboxylic acid[J]. RSC Advances, 2015, 5 (9): 6443–6451. DOI: 10.1039/C4RA12356E |
| [48] | AN N, ZHANG F H, HU Z G, et al. Non-covalently functionalizing a graphene framework by anthraquinone for high-rate electrochemical energy storage[J]. RSC Advances, 2015, 5 (30): 23942–23951. DOI: 10.1039/C4RA16092D |
| [49] | TANG Z H, ZENG C F, LEI Y D, et al. Fluorescent whitening agent stabilized graphene and its composites with chitosan[J]. Journal of Materials Chemistry, 2011, 21 (43): 17111–17118. DOI: 10.1039/c1jm13239c |
| [50] | YANG Q, PAN X J, HUANG F, et al. Fabrication of high-concentration and stable aqueous suspensions of graphene nanosheets by noncovalent functionalization with lignin and cellulose derivatives[J]. Journal of Physical Chemistry C, 2010, 114 (9): 3811–3816. DOI: 10.1021/jp910232x |
| [51] | STANKOVICH S, PINER R D, CHEN X Q, et al. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate)[J]. Journal of Materials Chemistry, 2006, 16 (2): 155–158. DOI: 10.1039/B512799H |
| [52] | YANG H F, ZHANG Q X, SHAN C S, et al. Stable, conductive supramolecular composite of graphene sheets with conjugated polyelectrolyte[J]. Langmuir, 2010, 26 (9): 6708–6712. DOI: 10.1021/la100365z |
| [53] | QI X Y, PU K Y, LI H, et al. Amphiphilic graphene composites[J]. Angew Chem Int Ed Engl, 2010, 49 (49): 9426–9429. DOI: 10.1002/anie.201004497 |
| [54] | BAI H, XU Y X, ZHAO L, et al. Non-covalent functionalization of graphene sheets by sulfonated polyaniline[J]. Chemical Communications, 2009, 13 : 1667–1669. |
| [55] | LIU Q, LIU Z F, ZHANG X Y, et al. Polymer photovoltaic cells based on solution-processable graphene and P3HT[J]. Advanced Functional Materials, 2009, 19 (6): 894–904. DOI: 10.1002/adfm.v19:6 |
| [56] | KUMAR S, KUMAR S, SRIVASTAVA S, et al. Reduced graphene oxide modified smart conducting paper for cancer biosensor[J]. Biosensors & Bioelectronics, 2015, 73 : 114–122. |
| [57] | KODALIV K, SCRIMGEOUR J, KIM S, et al. Nonperturbative chemical modification of graphene for protein micropatterning[J]. Langmuir, 2011, 27 (3): 863–865. DOI: 10.1021/la1033178 |
| [58] | LEE D Y, KHATUN Z, LEE J H, et al. Blood compatible graphene/heparin conjugate through noncovalent chemistry[J]. Biomacromolecules, 2011, 12 (2): 336–341. DOI: 10.1021/bm101031a |
| [59] | LV W, GUO M, LIANG M H, et al. Graphene-DNA hybrids:self-assembly and electrochemical detection performance[J]. Journal of Materials Chemistry, 2010, 20 (32): 6668–6673. DOI: 10.1039/c0jm01066a |
| [60] | PATIL A J, VICKERY J L, SCOTT T B, et al. Aqueous stabilization and self-assembly of graphene sheets into layered bio-nanocomposites using DNA[J]. Advanced Materials, 2009, 21 (31): 3159–3164. DOI: 10.1002/adma.v21:31 |
| [61] | LIU J B, FU S H, YUAN B, et al. Toward a universal "adhesive nanosheet" for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide[J]. Journal of American Chemical Society, 2010, 132 (21): 7279–7281. DOI: 10.1021/ja100938r |
| [62] | SEDDON K R. Ionic liquids:a taste of the future[J]. Nature Materials, 2003, 2 (6): 363–365. DOI: 10.1038/nmat907 |
| [63] | TORIMOTO T, TSUDA T, OKAZAKI K, et al. New frontiers in materials science opened by ionic liquids[J]. Advanced Materials, 2010, 22 (11): 1196–1221. DOI: 10.1002/adma.v22:11 |
| [64] | KIM T, LEE H, KIM J, et al. Synthesis of phase transferable graphene sheets using ionic liquid polymers[J]. ACS Nano, 2010, 4 (3): 1612–1618. DOI: 10.1021/nn901525e |
| [65] | RESTOLHO J, MATA J L, SARAMAGO B. On the interfacial behavior of ionic liquids:surface tensions and contact angles[J]. Journal of Colloid and Interface Science, 2009, 340 (1): 82–86. DOI: 10.1016/j.jcis.2009.08.013 |
| [66] | NUVOLI D, VALENTINI L, ALZARI V, et al. High concentration few-layer graphene sheets obtained by liquid phase exfoliation of graphite in ionic liquid[J]. Journal of Materials Chemistry, 2011, 21 (10): 3428–3431. DOI: 10.1039/C0JM02461A |
| [67] | WANG X Q, FULVIO P F, BAKER G A, et al. Direct exfoliation of natural graphite into micrometre size few layers graphene sheets using ionic liquids[J]. Chemical Communications, 2010, 46 (25): 4487–4489. DOI: 10.1039/c0cc00799d |
| [68] | KIM T, KANG H C, TUNG T T, et al. Ionic liquid-assisted microwave reduction of graphite oxide for supercapacitors[J]. RSC Advances, 2012, 2 (23): 8808–8812. DOI: 10.1039/c2ra21400h |
| [69] | KIM J, KIM S. Preparation and electrochemical property of ionic liquid-attached graphene nanosheets for an application of supercapacitor electrode[J]. Electrochimica Acta, 2014, 119 : 11–15. DOI: 10.1016/j.electacta.2013.11.187 |
| [70] | MAO L, LI Y, CHI C Y, et al. Conjugated polyfluorene imidazolium ionic liquids intercalated reduced graphene oxide for high performance supercapacitor electrodes[J]. Nano Energy, 2014, 6 (10): 119–128. |
| [71] | KUNDU A, LAYEK R K, KUILA A, et al. Highly fluorescent graphene oxide-poly(vinyl alcohol) hybrid:an effective material for specific Au3+ ion sensors[J]. ACS Applied Materials & Interfaces, 2012, 4 (10): 5576–5582. |
| [72] |
樊玮, 张超, 刘天西. 石墨烯/聚合物复合材料的研究进展[J].
复合材料学报, 2013, 30 (1): 14–21.
FAN W, ZHANG C, LIU T X. Recent progress in graphene/polymer composite[J]. Acta Materiae Compositae Sinica, 2013, 30 (1): 14–21. |
| [73] |
汤龙程, 万艳君, 高晓宇, 等. 石墨烯/聚合物纳米复合材料研究进展[J].
科技导报, 2013, 31 (27): 71–79.
TANG L C, WAN Y J, GAO X Y, et al. Recent advances in graphene/polymer nanocomposites[J]. Science & Technology Review, 2013, 31 (27): 71–79. DOI: 10.3981/j.issn.1000-7857.2013.27.011 |
| [74] | POTTS J R, DREYER D R, BIELAWSKI C W, et al. Graphene-based polymer nanocomposites[J]. Polymer, 2011, 2 (1): 5–25. |
| [75] | STANKOVICH S, DIKIN D A, DOMMETT G H B, et al. Graphene-based composite materials[J]. Nature, 2006, 442 (7100): 282–286. DOI: 10.1038/nature04969 |
| [76] | KHAMLICH S, BARZEGAR F, NURU Z Y, et al. Polypyrrole/graphene nanocomposite:high conductivity and low percolation threshold[J]. Synthetic Metals, 2014, 198 : 101–106. DOI: 10.1016/j.synthmet.2014.10.004 |
| [77] | CUI L, LIU J Q, WANG R, et al. A facile "graft from" method to prepare molecular-level dispersed graphene-polymer composites[J]. Journal of Polymer Science, Part A:Polymer Chemistry, 2012, 50 (21): 4423–4432. DOI: 10.1002/pola.v50.21 |
| [78] | LUO J, JIANG S S, LI UR, et al. Synthesis of water dispersible polyaniline/poly(styrenesulfonic acid) modified graphene composite and its electrochemical properties[J]. Electrochimica Acta, 2013, 96 : 103–109. DOI: 10.1016/j.electacta.2013.02.072 |
| [79] | ZHOU X S, WU T B, HU B J, et al. Synthesis of graphene/polyaniline composite nanosheets mediated by polymerized ionic liquid[J]. Chemical Communications, 2010, 46 (21): 3663–3665. DOI: 10.1039/c0cc00049c |
| [80] | TUNG T T, KIM T Y, SHIM J P, et al. Poly(ionic liquid)-stabilized graphene sheets and their hybrid with poly(3, 4-ethylenedioxythiophene)[J]. Organic Electronics, 2011, 12 (12): 2215–2224. DOI: 10.1016/j.orgel.2011.09.012 |
| [81] | SNOOK G A, KAO P, BESTA S. Conducting-polymer-based supercapacitor devices and electrodes[J]. Journal of Power Sour-ces, 2011, 196 (1): 1–12. DOI: 10.1016/j.jpowsour.2010.06.084 |
| [82] | YAN X S, ZHANG X D, LIU H L, et al. Fabrication of SDBS intercalated-reduced graphene oxide/polypyrrole nanocomposites for supercapacitors[J]. Synthetic Metals, 2014, 196 : 1–7. DOI: 10.1016/j.synthmet.2014.06.025 |
| [83] | DAMLIN P, SUOMINEN M, HEINONEN M, et al. Non-covalent modification of graphene sheets in PEDOT composite materials by ionic liquids[J]. Carbon, 2015, 93 : 533–543. DOI: 10.1016/j.carbon.2015.05.055 |
| [84] | ROY N, SENGUPTA R, BHOWMICK A K. Modifications of carbon for polymer composites and nanocomposites[J]. Progress in Polymer Science, 2012, 37 (6): 781–819. DOI: 10.1016/j.progpolymsci.2012.02.002 |
| [85] | WANG X L, BAI H, YAO Z Y, et al. Electrically conductive and mechanically strong biomimetic chitosan/reduced graphene oxide composite films[J]. Journal of Materials Chemistry, 2010, 20 (41): 9032–9036. DOI: 10.1039/c0jm01852j |
| [86] | LIAN H Q, LI S X, LIU K L, et al. Study on modified graphene/butyl rubber nanocomposites:I preparation and characterization[J]. Polymer Engineering and Science, 2011, 51 (11): 2254–2260. DOI: 10.1002/pen.v51.11 |
| [87] | XIONG X G, WANG J Y, JIA H B, et al. Structure, thermal conductivity, and thermal stability of bromobutyl rubber nanocomposites with ionic liquid modified graphene oxide[J]. Polymer Degradation and Stability, 2013, 98 (11): 2208–2214. DOI: 10.1016/j.polymdegradstab.2013.08.022 |
| [88] | SAURIN N, SANES J, BERMUDEZ M D. Effect of graphene and ionic liquid additives on the tribological performance of epoxy resin[J]. Tribology Letters, 2014, 56 (1): 133–142. DOI: 10.1007/s11249-014-0392-2 |
 2018, Vol. 46
2018, Vol. 46