文章信息
- 高鑫, 刘祥萱, 王煊军, 朱左明.
- GAO Xin, LIU Xiang-xuan, WANG Xuan-jun, ZHU Zuo-ming.
- 改性Cu2O光催化剂的研究进展
- Progress in Research on Modified Cu2O Photocatalyst
- 材料工程, 2016, 44(1): 120-128
- Journal of Materials Engineering, 2016, 44(1): 120-128.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2016.01.019
-
文章历史
- 收稿日期: 2014-12-08
- 修订日期: 2015-11-04
2. 第二炮兵装备研究院, 北京 100085
2. The Second Artillery Armament Institute, Beijing 100085, China
废水中的污染物结构复杂、性质稳定,常规处理方法如物理、化学、生物法不能完全将其去除[1]。高级氧化法是目前去除废水中有机污染物的主要方法,具有很强的氧化性能,但相对复杂、化学品消耗大、处理成本较高[2]。光催化技术作为一种行之有效的方法对环境污染物具有较好的处理效果,成为废水处理研究的热点。
TiO2和ZnO是目前最常用的光催化剂,但二者禁带宽度在3.0~3.2eV之间,仅能在紫外光照射下激发,只有不到5%的太阳能被利用[3, 4, 5, 6, 7]。而P型半导体Cu2O禁带宽度仅为2.17eV左右,在可见光范围内被激发,已经广泛应用于光催化降解废水、分解水制备H2、太阳能电池、气敏传感器等[8, 9, 10, 11]。另外,Cu2O无毒,制备成本低,储量丰富,是一种非常有潜力的光催化材料。Cu2O的理论太阳能利用率为14%~20%左右,但是已报道的仅为2%[12, 13]。此外,Cu2O光催化效果不高,主要原因如下:一是光引发的电子-空穴对很容易复合[14, 15];二是Cu2O不稳定[16, 17],较容易在空气中和液相中被氧化或还原。为了解决以上问题,对Cu2O进行改性已经成为目前的研究热点。美国Laser Dynamics实验室合成了Cu2O-Au复合物,利用飞秒发射探针技术追踪Cu2O-Au纳米结构中载流子寿命,发现载流子寿命随Cu2O壳厚度的增加而增加,但是当没有Au时载流子寿命缩短[18]。Zhang等将Cu2O纳米线覆以C层保护,20nm厚的C层能使Cu2O在1200s照射后光电流密度仅衰减19.7%(无C层保护衰减量为87.4%),稳定性提高600%[19]。
近年来,关于Cu2O光催化剂的综述不在少数,但是专门就Cu2O改性研究的综述报道较少,因此,本文从离子改性和半导体复合两个方面叙述了Cu2O改性的研究现状,介绍了Cu2O光催化过程中的稳定性,并对其研究方向进行了展望。
1 Cu2O电子与结构性能Cu2O常温下很稳定,加热到1800℃时才发生分解,反应如下:
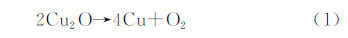
室温下空气干燥时,Cu2O本身是稳定的,但当空气中有水分时很容易被氧化,形成黑色CuO:
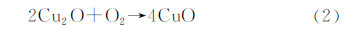
Cu2O晶体结构为赤铜矿型,赤铜矿结构为简单立方Pn3m空间群,属等轴晶系。Cu2O是一种典型的金属缺位P型半导体,Cu3d和O2p的轨道杂化以及晶体结构内部的Cu2 +缺陷,显著提高了空穴的导通性,因此具有良好的光催化性能[20]。
2 Cu2O的改性Cu2O改性主要针对以下三方面:(1)改性后使其具有合适的导带/价带边缘电势;(2)增加载流子的流动性,阻止电子-空穴对的复合;(3)减少光蚀,提高Cu2O的稳定性。
2.1 离子改性 2.1.1 金属离子改性合理的金属离子改性可使Cu2O吸收光谱范围[21, 22]拓展、抑制电子-空穴的复合以提高光催化反应的量子效率[22, 23, 24],增强Cu2O对目标反应物的吸附,从而提高其光催化性能。改性的金属离子种类包括Au[22, 25, 26],Ag[27, 28, 29, 30],Zn[31],Cu[32, 33, 34],Pt[35]等。
2.1.1.1 金属离子改性机理金属掺杂的Cu2O在光照激发后产生电子(e-)-空穴(h+)对,由于金属-半导体界面的肖特基势垒作用,金属作为电子接收器,e-从半导体导带转移至改性的金属上,h+则留在半导体表面[36, 37],延长电子-空穴的复合时间,这样e-和h+再分别作用产生具有较强氧化性的OH·,提高光催化性能[22],原理如图 1所示。此外,贵金属纳米颗粒的等离子体激元共振效应能够增加入射光子的吸收,拓展Cu2O的光谱吸收范围[38]。
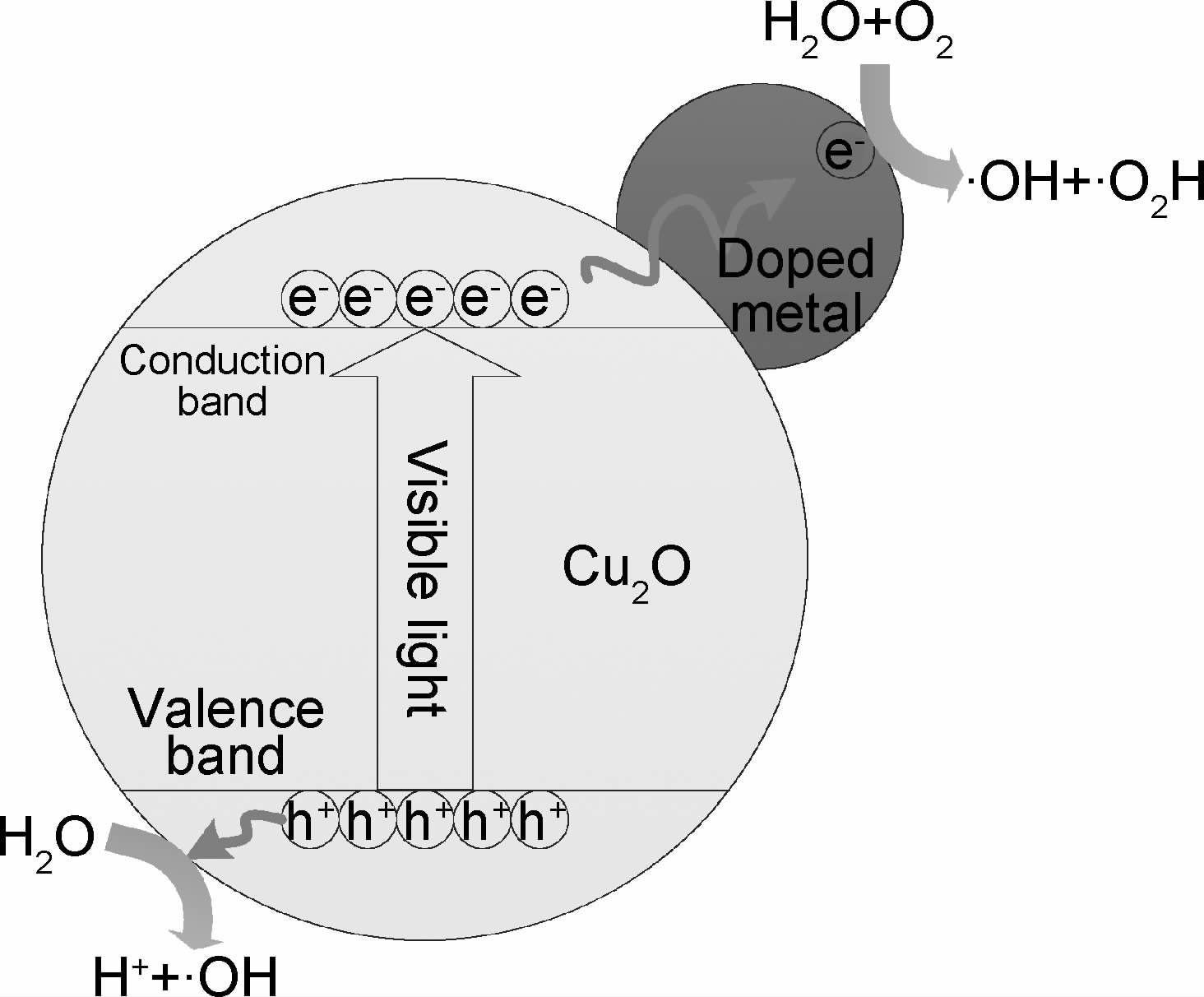 |
图 1 金属改性的Cu2O中电子-空穴传递路径 Fig.1 Vectorial transfer of electrons-holes (e-/h+) in metal doped Cu2O |
Au改性的Cu2O异质结构中由于Au颗粒良好的导电性使Cu2O表面的光引发电子快速转移,从而实现电子-空穴分离[39],此外,Au,Ag等贵金属颗粒改性产生的表面等离子共振现象加速了光生电子的产生[29, 39]。Lin等[27]通过调整Ag@Cu2O核壳异质结中Cu2O壳的厚度可以使催化剂的光吸收范围扩展至整个可见光范围或某一特定范围。瞬态吸收光谱表明,等离子体引起的共振能量转移和直接电子转移两种机制促成了从金属到半导体的表面等离子体能量传递,从而导致Cu2O电荷分离。
金属离子改性在一定程度上可改变Cu2O的能带结构进而改变其光物理性能。光致发光光谱表明,掺杂Zn后的Cu2O能够很容易被光照激发,产生大量载流子[31]。
2.1.1.2 金属离子改性量影响金属离子改性量会影响Cu2O的光催化活性。改性后Cu2O光催化剂表面的粗糙度、厚度、透光率都会影响光催化膜的活性[22]。另外,金属离子改性后,会引入一定量的缺陷,这些缺陷可作为电子-空穴的复合中心,由此降低光催化性能。合适的Zn掺杂量被证实能够明显地提升光引发载流子的寿命[31]。Zhang等[29]发现复合物中Ag量过多时发生Ag颗粒团聚,捕获光电子的活性位点数目减少,Cu2O光催化活性降低。此外,复合物中金属含量过多会阻碍Cu2O的可见光吸收,降低光子利用效率[29, 40]。
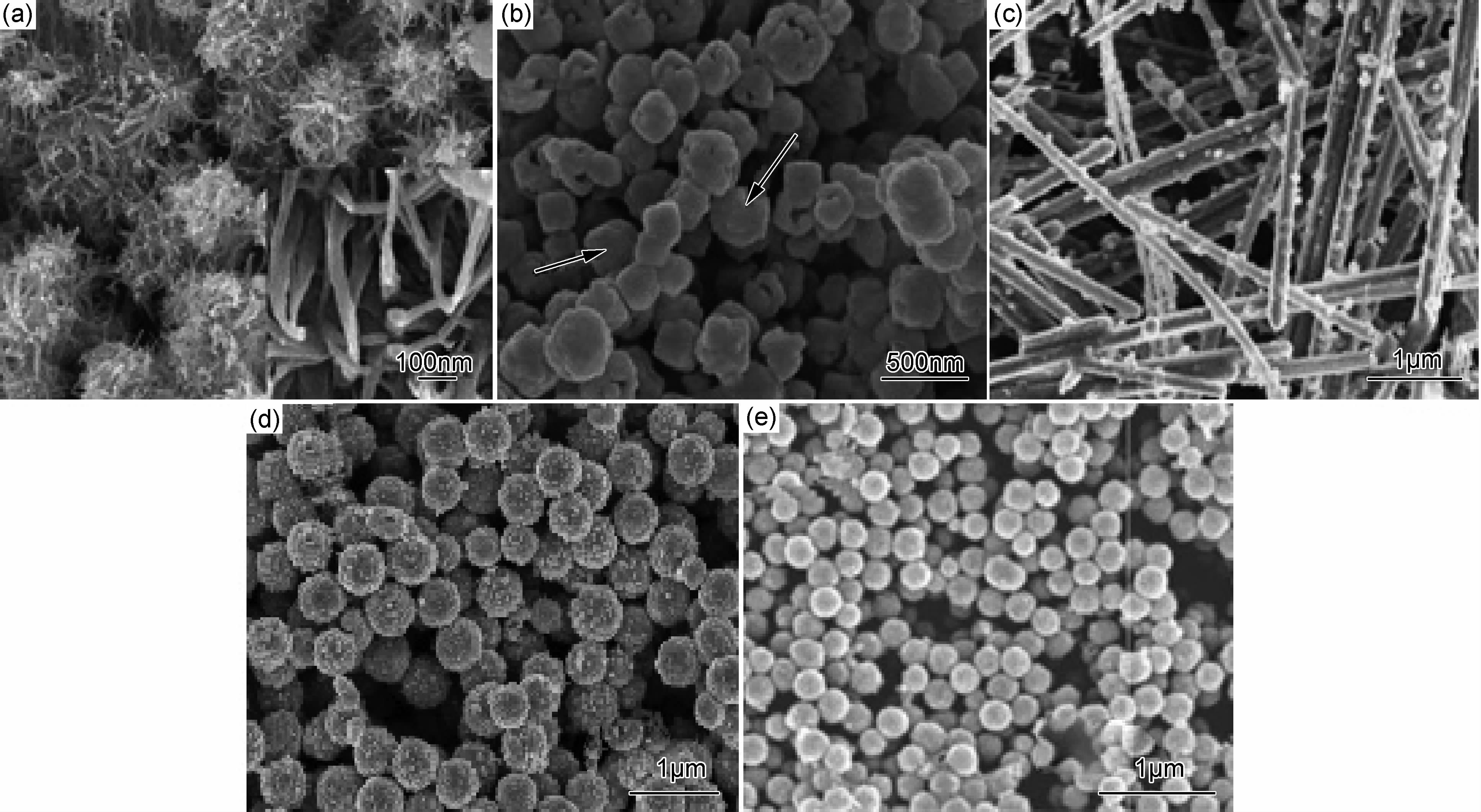
2.1.1.3 金属离子改性形貌研究金属改性的Cu2O光催化剂的形貌不仅会因比表面积的改变而影响对目标降解物的吸附,而且不同形貌立体空间的位置还会影响光催化剂光谱的吸收能力,图 2给出了不同形貌Cu2O光催化剂的SEM图。花朵状Cu2O/Cu纳米复合物[32]和中空的Cu2O/Cu纳米球[33]光催化活性均比纳米颗粒和实心球状的高,这是因为花朵状和中空的Cu2O/Cu拥有大的比表面积,不仅提高了产物的吸附和解吸,而且吸光能力也得到增强。一维纳米结构如纳米线、纳米管、纳米带等具有各向异性并且能在最小尺度内实现电子和激子的有效传递,因此在光催化材料制备中引起很大关注。Xiong等[23]研究发现,与核-壳Ag@Cu2O和Cu2O纳米球相比,一维Ag@Cu2O纳米线可见光下降解甲基橙(MO)活性更高,独特的一维核-壳纳米结构在提高其光催化性能方面具有重要作用。
非金属C,N,B,Si等改性的Cu2O光催化剂已经得到广泛研究,尤其C族改性的非常多,如活性炭[42]、碳纳米管(CNTs)[43, 44]、碳纳米纤维(CNFs)[45, 46]、石墨烯[47, 48, 49, 50, 51]。据已有报道,Cu2O与CNFs,CNTs复合后,其光催化活性都比纯的Cu2O要好。与CNFs相比,CNTs比表面积更大,但是分散性较差且制备成本高。近期,石墨烯由于以下优势而逐渐受到重视:1)比表面积大(理论值达2600m2/g)[52],提高催化剂表面吸附能力,此外,石墨烯作为良好的基底使Cu2O颗粒分散更均匀[53];2)单原子厚度的石墨烯透光率高,利于照射光的吸收[48];3)拥有二维π共轭结构,石墨烯作为电子接收器有效分离电子-空穴[54]。CNTs和CNFs易团聚,而石墨烯制备过程产生的缺陷和空位能够降低导电性从而影响电子传导,因此Zeng等[55]将不同C族物质结合起来与Cu2O复合得到了更好的光催化效果,认为在石墨烯/CNTs-Cu2O复合物中,CNTs作为1D的电子传导通道,石墨烯作为巨大的2D电子传导场地,建立了电荷高效分离网络,由此极大地提高了光催化效果。
此外,一些具有特殊性质的高分子化合物越来越受到青睐,如聚苯胺、壳聚糖等。聚苯胺(PANI)具有特殊的电子-空穴传递特性,光照射PANI引发π-π*跃迁,光引发电子转移至Cu2O导带,而空穴则由Cu2O转移至PANI的π轨道,从而使电荷有效分离[56, 57]。壳聚糖(CS)对金属离子有良好的配位络合能力,能够作为良好的基质材料来定位及控制无机纳米颗粒的生长。Cu2O/CS的特殊表面能够增强催化剂对染料和氧分子的吸附,Cu2O包裹在交联的CS内,光引发电子被吸附的O2捕获,提高其电子-空穴的分离效率[58, 59]。
氮化硼(BN)、氮化碳(C3N4)与Cu2O复合也崭露头角,复合后催化活性均比纯Cu2O高。Huang及其小组制备了Cu2O@h-BN(二维氮化硼)复合物,发现它能用于对硝基苯酚转换成氨基苯酚的反应,Cu2O@h-BN表现出很高的活性,h-BN本身不能完成此转换,但是能吸附对硝基苯酚离子,利于反应进行[60]。瞬态光电流测量表明g-C3N4(类石墨氮化碳)质量分数为10%的Cu2O光电流分别是g-C3N4和Cu2O的4.2倍和14.4倍[61]。C3N4与Cu2O结合时,光引发电子由Cu2O转移到C3N4,空穴则由C3N4转移到Cu2O,使电子-空穴有效分离[61, 62]。
2.2 半导体复合改性半导体复合是指复合两种不同禁带宽度的半导体,由于半导体的价带、导带和带隙能不一致而发生交迭,从而提高光生电子和空穴的分离率,扩展纳米Cu2O的光谱响应范围;因此,与单一的半导体相比,复合半导体表现出更好的稳定性和催化活性,克服了单一半导体催化剂量子效率低的缺点。
半导体复合常见的有两种类型,即MxOy/Cu2O和MxSy/Cu2O(M代表半导体元素)。如TiO2/Cu2O[63, 64, 65, 66, 67, 68, 69],WO3/Cu2O[70, 71, 72, 73],ZnO/Cu2O[74, 75, 76],Ru2O/Cu2O[77],BiVO4/Cu2O[78, 79],SnO2/Cu2O[80],MoS2/Cu2O[81],CuxSy/Cu2O[41]等。
2.2.1 与MxOy半导体复合 Cu2O价带空穴电位低于H2O和OH-的氧化电位,因此纯的Cu2O不能将H2O和OH-氧化生成 OH·,只能通过导带电子将吸附的O2还原为O-2,O-2进一步与水和电子作用生成H2O2和OH·。若将价带空穴电位高于H2O和OH-氧化电位的半导体与其复合,不仅可以通过导带电子作用产生 OH·,也可以通过价带空穴氧化H2O和OH-产生 OH·。OH·有很强的氧化性,可将大多数有机污染物氧化成无机物,使有机染料降解。WO3和ZnO价带空穴电位高于H2O和OH-的氧化电位,宋继梅等将WO[70]3和ZnO[74]分别与Cu2O结合,发现复合物光催化效果均比纯Cu2O要好,且WO3和ZnO摩尔分数为10%时的效果最好。
此外,半导体复合能够提高光生电子-空穴的分离效率,从而使光催化性能提高。半导体复合中载流子的传递机制有两种路径,如图 3所示。一种机制是光照后,Cu2O与复合的半导体同时被激发产生电子(e-)-空穴(h+)对,Cu2O上的e-转移至复合的半导体导带,而h+则由复合的半导体转移至Cu2O的价带,如图 3(a)所示。另一种机制是光照后,只有Cu2O被激发产生电子(e-)-空穴(h+)对,Cu2O导带e-转移至复合的半导体上,如图 3(b)所示。
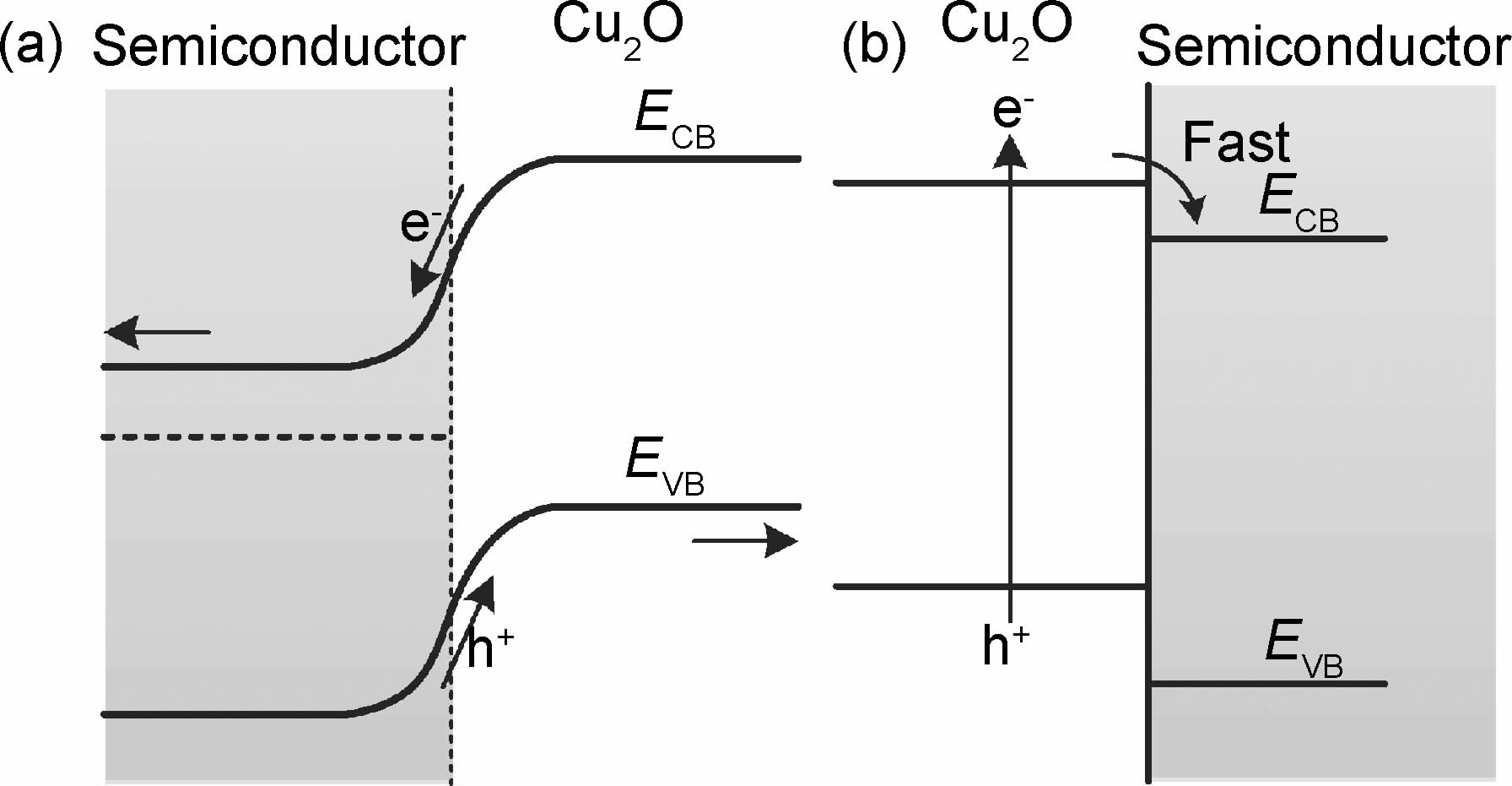 |
图 3 半导体复合中载流子的传递机制 (a)Cu2O与半导体 同时激发产生电子-空穴对;(b)仅Cu2O激发产生电子-空穴对 Fig.3 Transport mechanism of carriers in coupled semiconductors (a)e-/h+ are excited simultaneously from Cu2O and semiconductor; (b)e-/h+ are excited only from Cu2O |
复合的半导体能够作为电子捕获剂[63, 71],有效抑制光生电子-空穴对的复合速率,从而提高Cu2O的光催化性能。同时,Cu2O与复合的半导体能带结构的匹配,可以使光引发电子由Cu2O导带转移到复合半导体的导带,而空穴则由复合的半导体价带转移到Cu2O价带[64, 73, 75, 76, 78, 79]。此外,复合的半导体还可以作为助催化剂,降低反应的活化能[77]。
2.2.2 与MxSy半导体复合Cu2O与MxSy型半导体复合研究的报道相对较少。MoS2具有类似于石墨的层状结构,在利用太阳能制备H2中,MoS2可以作为贵金属(如Pt)的替代品助催化。Zhao等[81]采用MoS2修饰Cu2O得到MoS2@Cu2O,MoS2质量分数达到1.0%时光电流密度可达0.17mA/cm2,是纯Cu2O的8倍,并且连续工作9h后催化剂仅损失7%,稳定性得到很好的提升。纳米MoS2能够接受电子,可作为还原H+的活性位点,MoS2作为助催化剂能够降低电化学质子还原的超电势,抑制光诱导的腐蚀。
铜的硫化物由于在化学计量组成、价态、纳米晶体形态、复杂结构的变化及其独特性能而引起广泛关注。Yang等利用柯肯德尔效应制备了核-壳结构的Cu2O/Cu7.2S[41]4,Liu等[82]合成了Cu2O/Cu31S16核壳结构,Cu31S16保护并稳定Cu2O,阻止电子-空穴之间的复合,所以比单纯的Cu2O光催化效果好且更稳定。
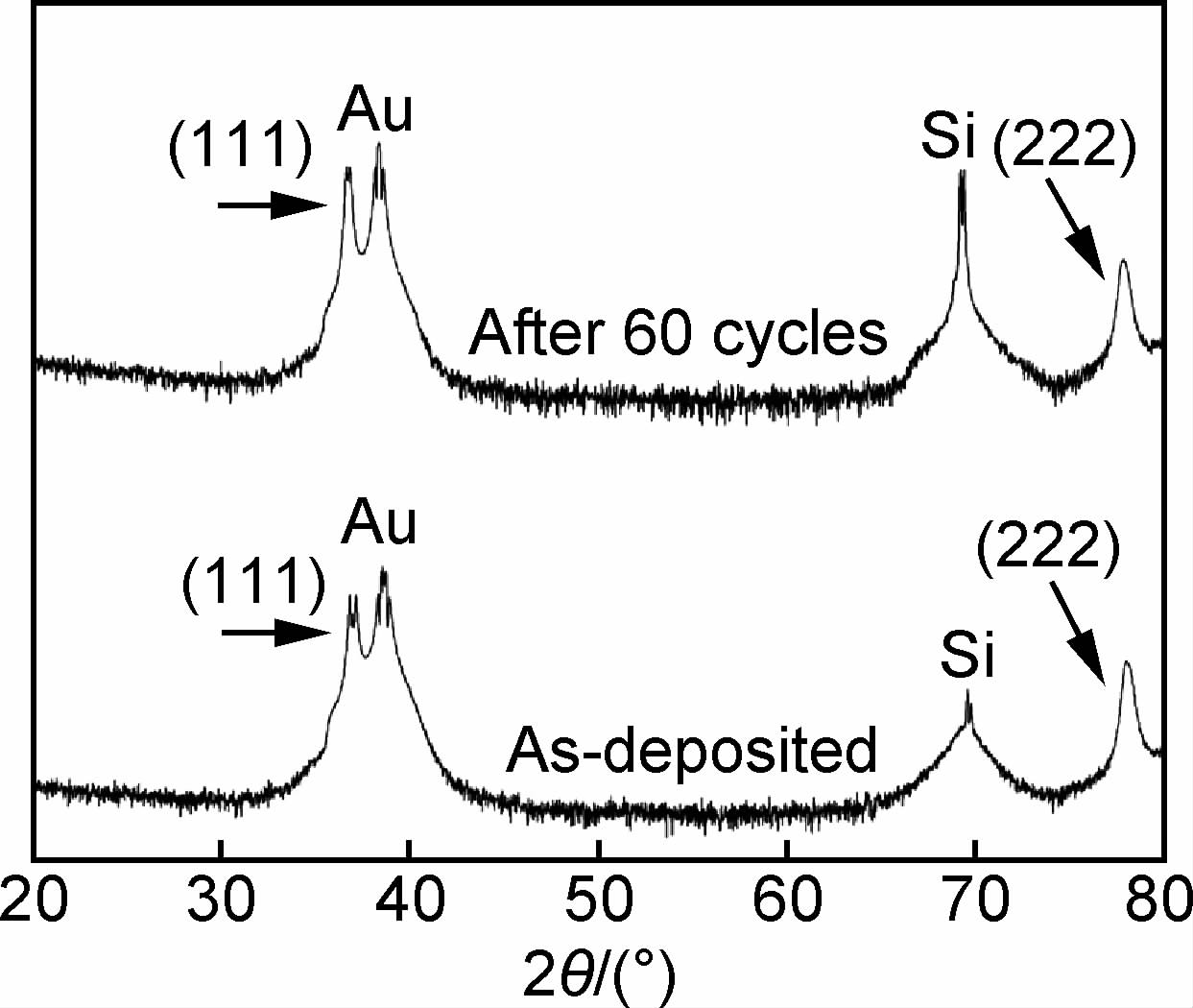
2.3 Cu2O稳定性尽管Cu2O显示出优异的光催化性能,但光腐蚀一直是Cu2O应用所面临的技术难题。Cu2O中的Cu+处于中间价态,很可能被空穴氧化为Cu2+或被电子还原为Cu。科研人员进行了大量Cu2O稳定性方面的研究。Zheng等[83]发现在光催化降解甲基橙(MO)的过程中,Cu2O的{100}和{110}晶面逐渐消失,并且向{111}晶面转化,而由{111}晶面为主导的Cu2O材料具有更好的稳定性。Wu等[84]对Cu2O进行重复循环极化和UV-Vis照射,发现Cu2O形貌由致密的三角形转变为稳定的网状拉长树叶形,其XRD图谱如图 4所示,始终以{111}晶面为主,因此认为{111}最稳定。
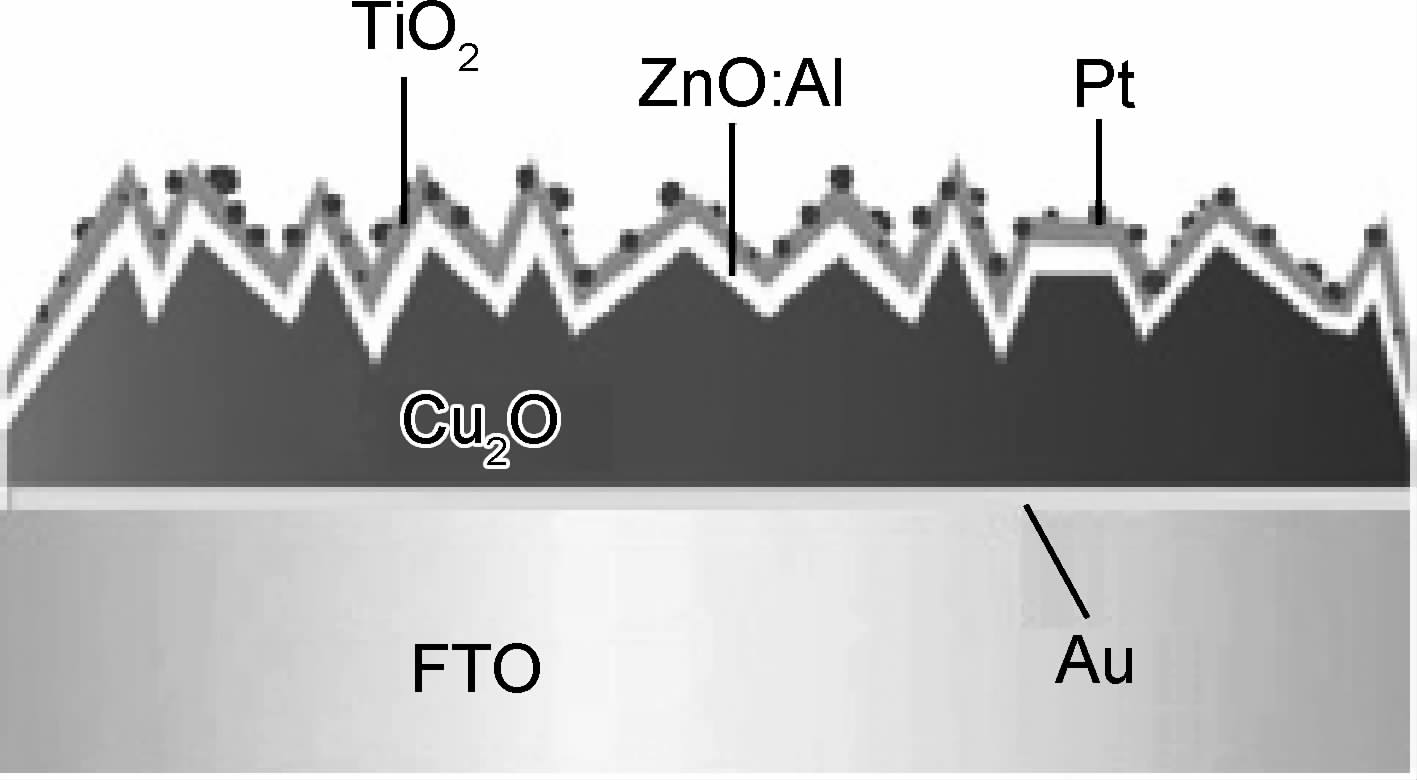
Paracchino等[85]采用原子层沉积法(Atomic Layer Deposition,ALD)在Cu2O上沉积超薄膜(Cu2O/Al:ZnO(20nm)/TiO2(10nm)/Pt),组成为20nm厚Al掺杂ZnO,10nm厚TiO2,再沉积Pt纳米颗粒,如图 5所示。尽管光照20min后光电流衰减,但是XPS测试证实Cu2O没有发生光蚀,光电流衰减是因为TiO2膜中形成了Ti3+。保护层的高导电性能够使Cu2O上的光电子转移到电解质中,降低Cu2O的自还原反应。后来,Paracchino等[35]又对Cu2O/Al:ZnO(20nm)/TiO2
(10nm)/Pt电极稳定性进行了研究,优化沉积温度、电解液pH值、TiO2退火温度,通过合理选择电解液并且尽可能使保护层晶化程度提高,经10h的测试使稳定性维持在62%以上。在Cu2O上覆盖保护层成为改善其稳定性的研究热点[19, 63, 81],但是覆盖的保护层必须具备两点:一是合适的带隙,二是化学稳定[35]。尽管Cu2O光催化稳定性研究已取得一定的进展,但效果还有待进一步提高。
3 改性Cu2O研究展望尽管改性后的Cu2O光催化性能得到一定程度的改善,但距离其实际应用仍存在较大的差距,如图 6所示。
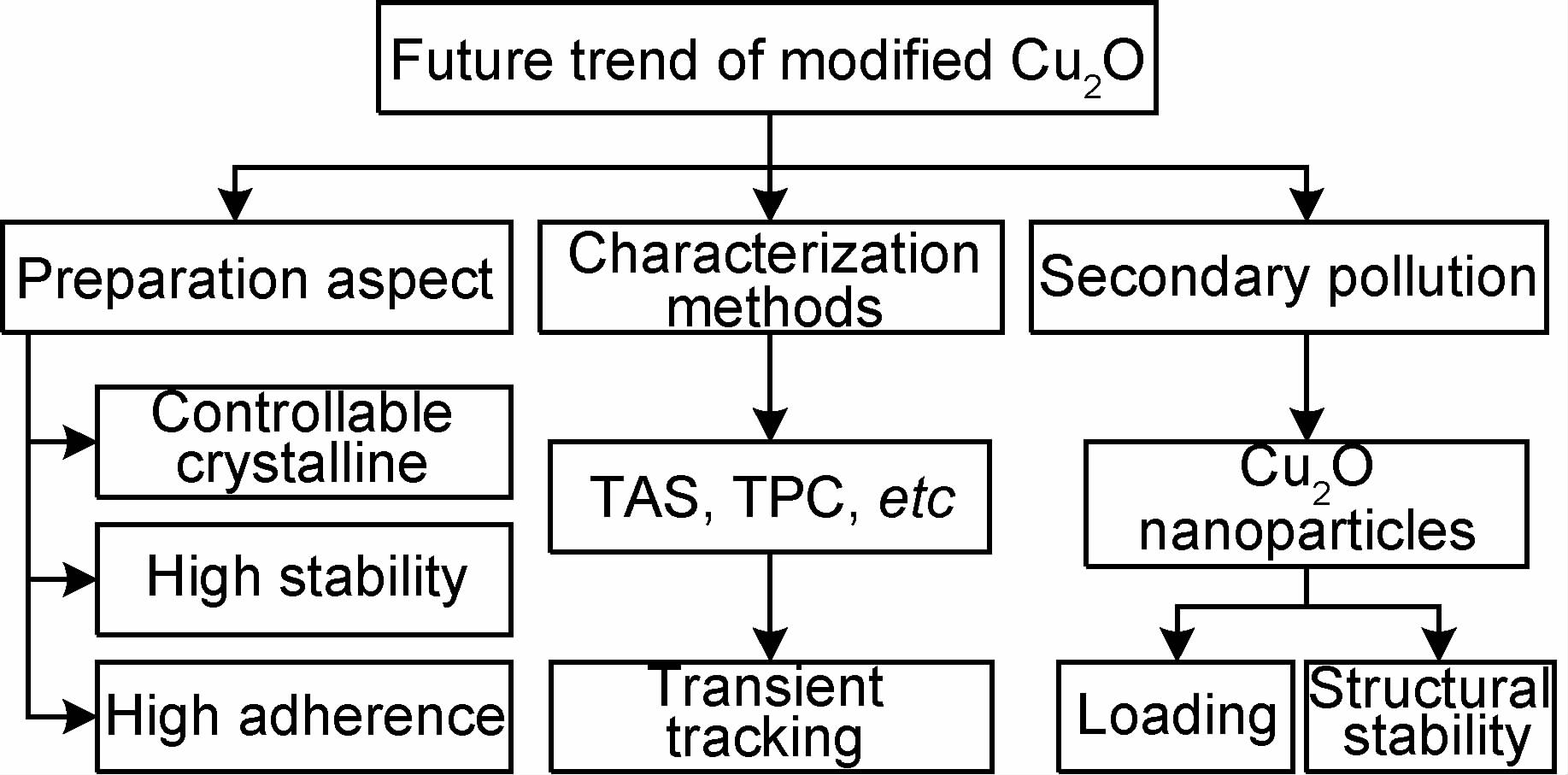 |
图 6 改性Cu2O研究的发展趋势 Fig.6 Future trends of modified Cu2O |
光催化活性与物质的表面形貌关系密切[86],而表面特性又与合理的制备方法不可分割,因此,为了获得比表面积大、催化活性高、稳定性好的光催化剂,不同的制备方法被应用到Cu2O的制备,如水(溶剂)热法、化学气相沉积法、溶胶-凝胶法、电沉积法、磁控溅射法等。但制备过程中,做到晶体形貌可控、沉积的膜致密、与基底连接紧密、纳米尺寸镀膜却很难。此外,选择制备技术时还要兼顾费用、效果、大规模应用等因素,因此,要达到以上效果的制备技术还需要进一步研究。
3.2 表征手段目前,Cu2O光催化过程中载流子的动力学特性尚未清楚,严重阻碍了Cu2O光催化技术的发展,因此,运用先进的表征手段从微秒到秒的时间范围内来追踪光引发空穴和电子的最终去向十分重要。瞬态吸收光谱(Transient Absorbance Spectra,TAS)、瞬态光电流(Transient Photo Current,TPC)等先进技术的应用将会极大地促进Cu2O光催化技术的发展。
3.3 二次污染问题光催化降解废水过程中,Cu2O纳米颗粒遗落在水体环境中造成二次污染,影响水生生物。同时,改性Cu2O引入的阴阳离子也会对水中生态系统产生影响。例如,非金属N的引入会造成水体的富营养化,S与细胞色素中的Fe反应会抑制微生物呼吸。此外,金属元素Pb,Zn,Cu等重金属在水体中不可降解,不断富集,甚至造成水生生物灭绝。为避免二次污染发生,除应使Cu2O负载化以利于回收外,还应加强制备技术的研究以增强材料在使用过程中的稳定性。
4 结束语对Cu2O的改性研究是解决它在实际应用中的限制的必要途径,金属/非金属改性、半导体复合改性是目前主要的改性方法,并取得了一定的进展,但光蚀和电子-空穴复合率高仍然是制约Cu2O光催化活性的主要问题。今后的研究一方面应侧重在改性机理方面加强对Cu2O光催化过程中载流子动力学研究,获得能够弥补Cu2O光催化过程中存在缺陷的改性材料;另一方面要完善Cu2O光催化性能评估体系,寻求更加合理的光催化性能评估方法和模型,使现有的单一指标评价模式转变为综合评价模式,更具实际意义。
| [1] | ZHANG J, WU Y, XING M, et al. Development of modified N-doped TiO2 photocatalyst with metals, nonmetals and metal oxides[J]. Energy & Environmental Science, 2010, 3(6):715-726. |
| [2] | MARTINEZ-HUITLE C A, FERRO S. Electrochemical oxidation of organic pollutants for the wastewater treatment:direct and indirect processes[J]. Chemical Society Reviews, 2006, 35(12):1324-1340. |
| [3] | GOTO H, HANADA Y, OHNO T, et al. Quantitative analysis of superoxide ion and hydrogen peroxide produced from molecular oxygen on photoirradiated TiO2 particles[J]. Journal of Catalysis, 2004, 225(1):223-229. |
| [4] | ZHENG Y, CHEN C, ZHAN Y, et al. Luminescence and photocatalytic activity of ZnO nanocrystals:correlation between structure and property[J]. Inorganic Chemistry, 2007, 46(16):6675-6682. |
| [5] | YU J, YU X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres[J]. Environmental Science & Technology, 2008, 42(13):4902-4907. |
| [6] | PERIYAT P, BAIJU K V, MUKUNDAN P, et al. High temperature stable mesoporous anatase TiO2photocatalyst achieved by silica addition[J]. Applied Catalysis A:General, 2008, 349(1):13-19. |
| [7] | KUMAR A, MATHUR N. Photocatalytic degradation of aniline at the interface of TiO2 suspensions containing carbonate ions[J]. Journal of Colloid and Interface Science, 2006, 300(1):244-252. |
| [8] | 曹春华,肖玲.交联壳聚糖/Cu2O复合颗粒可见光催化脱色活性艳红[J].环境工程学报, 2014,8(4):1482-1486. CAO Chun-hua, XIAO Ling. Photocatalytic decolorization of reactive brilliant red by cross-linked chitosan/Cu2O composite particles[J]. Chinese Journal of Environmental Engineering, 2014,8(4):1482-1486. |
| [9] | 郝彦忠,孙宝,罗冲,等. ZnO纳米管有序阵列与Cu2O纳米晶核壳结构的光电化学性能及全固态纳米结构太阳电池研究[J].高等学校化学学报, 2014, 35(1):127-133. HAO Yan-zhong, SUN Bao, LUO Chong, et al. Photoelectrochemistry of core-shell nanostructure of ordered ZnO nanotube array and Cu2O nanocrystals and performance of the all oxide solid state nanostructure solar cell[J]. Chemical Journal of Chinses Universities, 2014, 35(1):127-133. |
| [10] | ZHANG Z Z, CHEN X H, ZHANG X W, et al. Synthesis of Cu2O/La2CuO4 nanocomposite as an effective heterostructure photocatalyst for H2 production[J]. Catalysis Communications, 2013,36(21):20-24. |
| [11] | 赵晓慧,孙金妮,李其林.基于氧化亚铜纳米立方体的无酶葡萄糖传感器的研制[J].化学传感器, 2013, 33(3):49-54. ZHAO Xiao-hui, SUN Jin-ni, LI Qi-lin, et al. The preparation of nonenzymatic glucose sensor based on the Cu2O nanocubes[J]. Chemical Sensors, 2013, 33(3):49-54. |
| [12] | OLSEN L C, ADDIS F W, MILLER W. Experimental and theoretical studies of Cu2O solar cells[J]. Solar Cells, 1982, 7(3):247-279. |
| [13] | MITTIGA A, SALZA E, SARTO F, et al. Heterojunction solar cell with 2% efficiency based on a Cu2O substrate[J]. Applied Physics Letters, 2006, 88(16):163502-163502-2. |
| [14] | HUANG L, PENG F, YU H, et al. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion[J]. Solid State Sciences, 2009, 11(1):129-138. |
| [15] | HAN C, LI Z, SHEN J. Photocatalytic degradation of dodecyl-benzenesulfonate over TiO2-Cu2O under visible irradiation[J]. Journal of Hazardous Materials, 2009, 168(1):215-219. |
| [16] | OSTERLOH F E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting[J]. Chemical Society Reviews, 2013, 42(6):2294-2320. |
| [17] | ZHENG Z, HUANG B, WANG Z, et al. Crystal faces of Cu2O and their stabilities in photocatalytic reactions[J]. The Journal of Physical Chemistry C, 2009, 113(32):14448-14453. |
| [18] | MAHMOUD M A, QIAN W, EL-SAYED M A. Following charge separation on the nanoscale in Cu2O-Au nanoframe hollow nanoparticles[J]. Nano Lett, 2011, 11:3285-3289. |
| [19] | ZHANG Z H, DUA R, ZHANG L B, et al. Carbon-layer-protected cuprous oxide nanowire arrays for efficient water reduction[J]. ACS Nano, 2013, 7(2):1709-1717. |
| [20] | LIN X, XING J, WANG W, et al. Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3:a strategy for the design of efficient combined photocatalysts[J]. The Journal of Physical Chemistry C, 2007, 111(49):18288-18293. |
| [21] | LI J, CUSHING S K, BRIGHT J, et al. Ag@Cu2O core-shell nanoparticles as visible-light plasmonic photocatalysts[J]. ACS Catalysis, 2012, 3(1):47-51. |
| [22] | PAN Y, DENG S, POLAVARAPU L, et al. Plasmon-enhanced photocatalytic properties of Cu2O nanowire-Au nanoparticle assemblies[J]. Langmuir, 2012, 28(33):12304-12310. |
| [23] | XIONG J, LI Z, CHEN J, et al. Facile synthesis of highly efficient one-dimensional plasmonic photocatalysts through Ag@Cu2O core-shell heteronanowires[J]. ACS Applied Materials & Interfaces, 2014, 6(18):15716-15725. |
| [24] | SHANG Y, CHEN Y, SHI Z, et al. Synthesis and visible light photocatalytic activities of Au/Cu2O heterogeneous nanospheres[J]. Acta Physico-Chimica Sinica, 2013, 29(8):1819-1826. |
| [25] | WANG W C, LYU L M, HUANG M H. Investigation of the effects of polyhedral gold nanocrystal morphology and facets on the formation of Au-Cu2O core-shell heterostructures[J]. Chemistry of Materials, 2011, 23(10):2677-2684. |
| [26] | 谢海泉,郭戈,闫家伟,等.金负载对立方体纳米氧化亚铜光催化性能的影响[J].现代化工, 2010, 30(9):50-52. XIE Hai-quan, GUO Ge, YAN Jia-wei, et al. Effect of Au-loading cubic nano cuprous oxide on photo catalytic performance[J]. Modern Chemical Industry, 2010, 30(9):50-52. |
| [27] | LIN X, ZHOU R, ZHANG J, et al. A novel one-step electron beam irradiation method for synthesis of Ag/Cu2O nanocomposites[J]. Applied Surface Science, 2009, 256(3):889-893. |
| [28] | YANG J, LI Z, ZHAO C, et al. Facile synthesis of Ag-Cu2O composites with enhanced photocatalytic activity[J]. Materials Research Bulletin, 2014, 60:530-536. |
| [29] | ZHANG W, YANG X, ZHU Q, et al. One-pot room temperature synthesis of Cu2O/Ag composite nanospheres with enhanced visible-light-driven photocatalytic performance[J]. Industrial & Engineering Chemistry Research, 2014, 53(42):16316-16323. |
| [30] | WEI S, SHI J, REN H, et al. Fabrication of Ag/Cu2O composite films with a facile method and their photocatalytic activity[J]. Journal of Molecular Catalysis A:Chemical, 2013, 378(11):109-114. |
| [31] | ZHANG L, JING D, GUO L, et al. In-situ photochemical synthesis of Zn-doped Cu2O hollow microcubes for high efficient photocatalytic H2 production[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(6):1446-1452. |
| [32] | ZHOU B, WANG H, LIU Z, et al. Enhanced photocatalytic activity of flowerlike Cu2O/Cu prepared using solvent-thermal route[J]. Materials Chemistry and Physics, 2011, 126(3):847-852. |
| [33] | ZHOU B, LIU Z, ZHANG H, et al. One-pot synthesis of Cu2O/Cu self-assembled hollow nanospheres with enhanced photocatalytic performance[J]. Journal of Nanomaterials, 2014,2014(1):1-8. |
| [34] | 何星存,梁伟夏,黄智,等.可见光响应的"Cu核-Cu2O壳"型光催化剂性能的研究[J].现代化工, 2006, 25(11):38-40. HE Xing-cun, LIANG Wei-xia, HUANG Zhi, et al. Study on photocatalytic properties of visible light responsive "Cu core and Cu2O shell" type of catalyst[J]. Modern Chemical Industry, 2006, 25(11):38-40. |
| [35] | PARACCHINO A, MATHEWS N, HISATOMI T, et al. Ultrathin films on copper (I) oxide water splitting photocathodes:a study on performance and stability[J]. Energy & Environmental Science, 2012, 5(9):8673-8681. |
| [36] | HOFFMANN M R, MARTIN S T, CHOI W, et al. Environmental applications of semiconductor photocatalysis[J]. Chemical Reviews, 1995, 95(1):69-96. |
| [37] | LI X Z, LI F B. Study of Au/Au3+-TiO2 photocatalysts toward visible photo oxidation for water and wastewater treatment[J]. Environmental Science & Technology, 2001, 35(11):2381-2387. |
| [38] | SCHAADT D M, FENG B, YU E T. Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles[J]. Applied Physics Letters, 2005, 86(6):063106-063106-3. |
| [39] | SHANG Y, CHEN Y, SHI Z B, et al. Synthesis and visible light photocatalytic activities of Au/Cu2O heterogeneous nanospheres[J]. Acta Physico-Chimica Sinica, 2013, 29(8):1819-1826. |
| [40] | LIN X, ZHOU R, ZHANG J, et al. A novel one-step electron beam irradiation method for synthesis of Ag/Cu2O nanocomposites[J]. Applied Surface Science, 2009, 256(3):889-893. |
| [41] | YANG Z, ZHANG D, ZHANG W, et al. Controlled synthesis of cuprous oxide nanospheres and copper sulfide hollow nanospheres[J]. Journal of Physics and Chemistry of Solids, 2009, 70(5):840-846. |
| [42] | 刘丽丽,臧德利,邓霞,等.氧化亚铜-活性炭复合光催化材料的制备与表征[J].化学与粘合, 2013,35(4):14-16. LIU Li-li, ZANG De-li, DENG Xia, et al. Preparation and characterization of Cu2O/AC composite photocatalysts[J]. Chemistry and Adhesion, 2013,35(4):14-16. |
| [43] | SONG S Q, RAO R C, YANG H X, et al. Cu2O/MWCNTs prepared by spontaneous redox:growth mechanism and superior catalytic activity[J]. J Phys Chem C, 2010, 114:13998-14003. |
| [44] | 许龙山,陈小华,吴玉蓉,等. "内嵌式"碳纳米管/氧化亚铜复合球的制备及性能研究[A]. 2011中国功能材料科技与产业高层论坛论文集[C].重庆:中国仪器仪表学会, 2011.815-819. |
| [45] | WANG Y, LIU L, CAI Y, et al. Preparation and photocatalytic activity of cuprous oxide/carbon nanofibres composite films[J]. Applied Surface Science, 2013, 270(14):245-251. |
| [46] | 王元前,刘琳,姚菊明.溶剂热法制备碳纳米纤维负载Cu2O光催化材料[J].浙江理工大学学报, 2013, 30(2):139-143. WANG Yuan-qian, LIU Lin, YAO Ju-ming. Preparation of carbon nanofiber-loaded Cu2O photocatalytic materials with solvothermal method[J]. Journal of Zhejiang Institute of Science and Technology, 2013, 30(2):139-143. |
| [47] | WANG A L, LI X S, ZHAO Y B, et al. Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo-catalytic performances[J]. Powder Technology, 2014, 261:42-48. |
| [48] | GAO Z Y, LIU J L, XU F, et al. One-pot synthesis of grapheme-cuprous oxide composite with enhanced photocatalytic activity[J]. Solid State Sciences, 2012, 14(2):276-280. |
| [49] | LI B, LIU T, HU L, et al. A facile one-pot synthesis of Cu2O/RGO nanocomposite for removal of organic pollutant[J]. Journal of Physics and Chemistry of Solids, 2013, 74(4):635-640. |
| [50] | NIU Z G. Reduced graphene oxide-cuprous oxide hybrid nanopowders:hydrothermal synthesis and enhanced photocatalytic performance under visible light irradiation[J]. Materials Science in Semiconductor Processing, 2014, 23:78-84. |
| [51] | ABULIZI A, YANG G H, ZHU J J. One-step simple sonochemical fabrication and photocatalytic properties of Cu2O-rGO composites[J]. Ultrasonics Sonochemistry, 2014, 21(1):129-135. |
| [52] | GEIM A K, NOVOSELOV K S. The rise of graphene[J]. Nature Materials, 2007, 6(3):183-191. |
| [53] | HUANG L, PENG F, YU H, et al. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion[J]. Solid State Sciences, 2009, 11(1):129-138. |
| [54] | MENG X B, GENG D S, LIU J, et al. Controllable synthesis of graphene-based titanium dioxide nanocomposites by atomic layer deposition[J]. Nanotechnology, 2011, 22(16):165602-165611. |
| [55] | ZENG B, CHEN X, NING X, et al. Electrostatic-assembly three-dimensional CNTs/rGO implanted Cu2O composite spheres and its photocatalytic properties[J]. Applied Surface Science, 2013, 276(3):482-486. |
| [56] | CHEN J Y, ZHOU P J, LI J L, et al. Studies on the photocatalytic performance of cuprous oxide/chitosan nanocomposites activated by visible light[J]. Carbohydrate Polymers, 2008, 72(1):128-132. |
| [57] | CAO C, XIAO L, LIU L, et al. Visible-light photocatalytic decolorization of reactive brilliant red X-3B on Cu2O/crosslinked-chitosan nanocomposites prepared via one step process[J]. Applied Surface Science, 2013, 271(6):105-112. |
| [58] | WANG X F, CHEN G M, ZHANG J. Synthesis and characterization of novel Cu2O/PANI composite photocatalysts with enhanced photocatalytic activity and stability[J]. Catalysis Communications, 2013,31:57-61. |
| [59] | MOHAMED R M, AAZAM E S. Preparation and characterization of core-shell polyaniline/mesoporous Cu2O nanocomposites for the photocatalytic oxidation of thiophene[J]. Applied Catalysis A:General, 2014, 480(8):100-107. |
| [60] | HUANG C, YE W, LIU Q, et al. Dispersed Cu2O octahedrons on h-BN nanosheets for p-nitrophenol reduction[J]. ACS Applied Materials & Interfaces, 2014, 6(16):14469-14476. |
| [61] | PENG B Y, ZHANG S S, YANG S Y, et al. Synthesis and characterization of g-C3N4/Cu2O composite catalyst with enhanced photocatalytic activity under visible light irradiation[J]. Materials Research Bulletin, 2014, 56:19-24. |
| [62] | TIAN Y L, CHANG B B, FU J, et al. Graphitic carbon nitride/Cu2O heterojunctions:preparation, characterization, and enhanced photocatalytic activity under visible light[J]. Journal of Solid State Chemistry, 2014, 212:1-6. |
| [63] | LIU L, YANG W, LI Q, et al. Synthesis of Cu2O nanospheres decorated with TiO2 nanoislands, their enhanced photoactivity and stability under visible light illumination, and their post-illumination catalytic memory[J]. ACS Applied Materials & Interfaces, 2014, 6(8):5629-5639. |
| [64] | CHU S, ZHENG X, KONG F, et al. Architecture of Cu2O@TiO2 core-shell heterojunction and photodegradation for 4-nitrophenol under simulated sunlight irradiation[J]. Materials Chemistry and Physics, 2011, 129(3):1184-1188. |
| [65] | ZHANG J, LIU W, WANG X, et al. Enhanced decoloration activity by Cu2O@TiO2 nanobelts heterostructures via a strong adsorption-weak photodegradation process[J]. Applied Surface Science, 2013, 282:84-91. |
| [66] | SU X, ZHAO J, LI Y, et al. Solution synthesis of Cu2O/TiO2 core-shell nanocomposites[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2009, 349(1):151-155. |
| [67] | HAN C, LI Z, SHEN J. Photocatalytic degradation of dodecyl-benzenesulfonate over TiO2-Cu2O under visible irradiation[J]. Journal of Hazardous Materials, 2009, 168(1):215-219. |
| [68] | 唐一文,陈志钢,张丽莎,等.纳米Cu2O/TiO2异质结薄膜电极的制备和表征[J].无机材料学报, 2006, 21(2):453-458. TANG Yi-wen, CHEN Zhi-gang, ZHANG Li-sha, et al. Preparation and characterization of nanocrystalline Cu2O/TiO2 heterojunction film electrode[J]. Journal of Inorganic Materials, 2006, 21(2):453-458. |
| [69] | ZHANG J, ZHU H, ZHENG S, et al. TiO2 film/Cu2O microgrid heterojunction with photocatalytic activity under solar light irradiation[J]. ACS Applied Materials & Interfaces, 2009, 1(10):2111-2114. |
| [70] | 宋继梅,王红,张小霞,等. Cu2O-WO3复合物的制备及其光催化性质研究[J].功能材料, 2012, 43(1):28-30. SONG Ji-mei, WANG Hong, ZHANG Xiao-xia, et al. Preparation and photocatalytic activities of Cu2O-WO3 composites[J]. Functional Materials, 2012, 43(1):28-30. |
| [71] | 牛凤兴,付峰,高晓明,等. WO3/Cu2O光催化剂的制备及其光催化降解苯酚的研究[J].精细石油化工, 2014, 31(2):6-9. NIU Feng-xing, FU Feng, GAO Xiao-ming, et al. Preparation of WO3/Cu2O photocatalyst and its application to photocatalytical degradation of phenol[J]. Speciality Petrochemicals, 2014, 31(2):6-9. |
| [72] | WEI S Q, MA Y Y, CHEN Y Y, et al. Fabrication of WO3/Cu2O composite films and their photocatalytic activity[J]. Journal of Hazardous Materials, 2011, 194(5):243-249. |
| [73] | MINGGU L J, NG K H, KADIR H A, et al. Bilayer n-WO3/p-Cu2O photoelectrode with photocurrent enhancement in aqueous electrolyte photoelectrochemical reaction[J]. Ceramics International, 2014, 40(10):16015-16021. |
| [74] | 宋继梅,胡海琴,张小霞,等.氧化亚铜的复合改性及其光催化性质研究[J].中国钼业, 2012, 36(3):38-43. SONG Ji-mei, HU Hai-qin, ZHANG Xiao-xia, et al. Modification of cuprous oxide and its enhanced photocatalytic activities[J]. China Molybdenum Industry, 2012, 36(3):38-43. |
| [75] | LIU K, ZHANG J, GAO H, et al. Photocatalytic property of ZnO microrods modified by Cu2O nanocrystals[J]. Journal of Alloys and Compounds, 2013, 552(9):299-303. |
| [76] | 马玉燕,魏守强,侯微,等. ZnO/Cu2O复合膜的制备及其光催化活性研究[J].电镀与精饰, 2009, 31(12):5-8. MA Yu-yan, WEI Shou-qiang, HOU Wei, et al. Preparation and photocatalytic activity of ZnO/Cu2O composite films[J]. Plating and Finishing, 2009, 31(12):5-8. |
| [77] | BANERJEE T, MUKHERJEE A. Overall water splitting under visible light irradiation using nanoparticulate RuO2 loaded Cu2O powder as photocatalyst[J]. Energy Procedia, 2014,54(54):221-227. |
| [78] | YUAN Q, CHEN L, XIONG M, et al. Cu2O/BiVO4 heterostructures:synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light[J]. Chemical Engineering Journal, 2014, 255(7):394-402. |
| [79] | LI H, HONG W, CUI Y, et al. Enhancement of the visible light photocatalytic activity of Cu2O/BiVO4 catalysts synthesized by ultrasonic dispersion method at room temperature[J]. Materials Science and Engineering:B, 2014,181(2):1-8. |
| [80] | DU Y, ZHANG N, WANG C. Photo-catalytic degradation of trifluralin by SnO2-doped Cu2O crystals[J]. Catalysis Communications, 2010, 11(7):670-674. |
| [81] | ZHAO Y F, YANG Z Y, ZHANG Y X, et al. Cu2O decorated with cocatalyst MoS2 for solar hydrogen production with enhanced efficiency under visible light[J]. The Journal of Physical Chemistry C, 2014, 118(26):14238-14245. |
| [82] | LIU X, LI Z, ZHANG Q, et al. Controllable synthesis and enhanced photocatalytic properties of Cu2O/Cu31S16 composites[J]. Materials Research Bulletin, 2012, 47(9):2631-2637. |
| [83] | ZHENG Z, HUANG B, WANG Z, et al. Crystal faces of Cu2O and their stabilities in photocatalytic reactions[J]. The Journal of Physical Chemistry C, 2009, 113(32):14448-14453. |
| [84] | WU L, TSUI L, SWAMI N, et al. Photoelectrochemical stability of electrodeposited Cu2O films[J]. The Journal of Physical Chemistry C, 2010, 114(26):11551-11556. |
| [85] | PARACCHINO A, LAPORTE V, SIVULA K, et al. Highly active oxide photocathode for photoelectrochemical water reduction[J]. Nature Materials, 2011, 10(6):456-461. |
| [86] | COLMENARES J C, LUQUE R, CAMPELO J M, et al. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass:an overview[J]. Materials, 2009, 2(4):2228-2258. |
 2016, Vol. 44
2016, Vol. 44